Abstract
Background
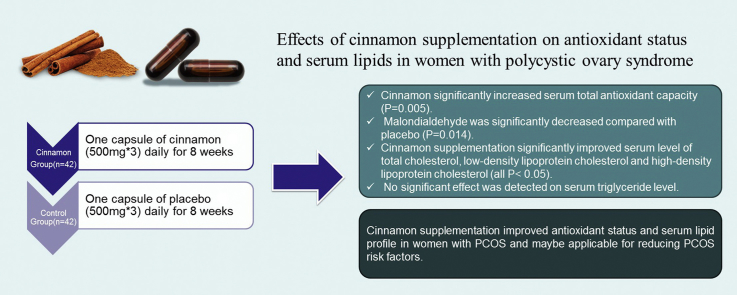
The objectives of study were to investigate the effects of cinnamon supplementation on antioxidant status and serum lipids in women with polycystic ovary syndrome (PCOS).
Methods
This double-blind randomized controlled clinical trial was conducted on 84 overweight or obese PCOS patients; aged 20–38 years. Subjects in cinnamon (n = 42) and placebo (n = 42) groups were given 3 cinnamon capsules (each one contained 500 mg cinnamon) or placebo daily for 8 weeks. Fasting blood samples, anthropometric measurements and dietary intake data were gathered at the beginning and at the end of the study. Independent t test, paired t test and analysis of covariance were used to analyze of data.
Results
Cinnamon significantly increased serum total antioxidant capacity (P = 0.005). Malondialdehyde was significantly decreased compared with placebo (P = 0.014). Cinnamon supplementation significantly improved serum level of total cholesterol, low-density lipoprotein cholesterol and high-density lipoprotein cholesterol (all P < 0.05). No significant effect was detected on serum triglyceride level.
Conclusions
Cinnamon supplementation improved antioxidant status and serum lipid profile in women with PCOS and may be applicable for reducing PCOS risk factors.
Keywords: Polycystic ovary syndrome, Cinnamon, Total antioxidant capacity, Malondialdehyde, Serum lipids
Graphical abstract
1. Introduction
Polycystic ovary syndrome (PCOS) is a common metabolic and reproductive condition in women with an estimated prevalence of 6% to as high as 26%.1, 2 This disorder is accompanied with irregular menstrual cycle, chronic anovulation and hyperandrogenism. PCOS is associated with complications in different health aspects, including obesity, insulin resistance, infertility, diabetes and atherosclerosis.3 Oxidative stress (OS) as one of the main causes of molecular damage to cellular is increased in women with the PCOS. Obesity and insulin resistance play a vital role in the pathogenesis of PCOS and subsequently increased OS in these patients. In addition, the resultant oxidative stress induces an inflammatory environment furthering elevated insulin resistance and contributing to hyperandrogenism,4 dyslipidemia, hypertension and etc.5, 6 Numerous investigations have revealed that oxidative stress level is significantly increased in patients with PCOS compared with the normal ones. Fenkci et al revealed that patients with PCOS had higher OS and increased OS is related to hyperandrogenism status.7 In another study, Desai et al displayed that the OS is also present in non-obese women with PCOS.8
PCOS is a condition with a significant decrease in serum antioxidant. Therefore, antioxidant supplementation may be effective in these patients. Antioxidants are considered significant agents in the healthy body. Many studies have displayed that the use of antioxidants, as well as herbal agents, might help to reduce OS.9 Furthermore, medicinal herbs are expected to have a similar degree of efficacy without the side effects related to conventional medication.
Cinnamomum zeylanicum, is an herbaceous plant, belonging to the Lauraceae family. It is one of the most important spices used by people all over the world. Different flavonoids and Polyphenols isolated from cinnamon have free-radical-scavenging activities and antioxidant properties.10 These compounds have been revealed to decrease oxidative stress in a dose-dependent manner through the inhibition of 5-lipoxygenase.11 Antihyperlipidemic and antioxidant activity of cinnamon has been proven in experimental studies.12, 13, 14, 15, 16, 17 In animal study, Shalaby et al indicated that consumption of cinnamon aqueous extract improved activity of tissue antioxidant enzymes in obese diabetic rat.18 Additionally, in the study by Kim et al, cinnamon lowered total cholesterol and triglyceride levels in diabetic mice.19 Roussel et al showed that supplementation of 250 mg/day of an aqueous extract of cinnamon increased the plasma levels of thiol group and decreased malondialdehyde compared to those of placebo in patients with impaired fasting blood glucose for 12 weeks.20
Although some studies have reported the effects of cinnamon on oxidative stress and lipids profile in several diseases,5, 6, 21 Its possible effects on serum antioxidant status and lipids profile of women with PCOS have not been studied. Therefore, we initiated a study to assess the effects of cinnamon supplementation on oxidative stress including serum total antioxidant capacity (TAC), malondialdehyde (MDA) and lipids in women with PCOS.
2. Material and methods
2.1. Design study
A total of 84 women with PCOS aged 20–38 years with a BMI between 25–40 kg/m2 were enrolled in this double-blind, randomized, controlled clinical trial from the Gynecology clinic, Mohheb Yas Hospital in Tehran, Iran from October 2015 to February 2016. The sample size was determined based on the information acquired from the study by Kort et al for IR.22 Considering 95% confidence interval and 80% power, the sample size was computed to be 32 per group. This number was increased to 42 per group to accommodate the anticipated dropout rate.
The diagnosis of PCOS was established according to 2003 Rotterdam criteria, which require at least two of three features for diagnosis: chronic amenorrhea or oligo-amenorrhea, clinical and/or biochemical features of hyperandrogenism and polycystic ovaries by ultrasonography.1 Study exclusion criteria included: thyroid disorders, hyperprolactinemia, diabetes mellitus, pregnancy and lactation, liver or kidney diseases, Cushing syndrome, cardiovascular diseases, seizure and cerebrovascular disorder, hypertension, the use of medications such as insulin sensitizers, insulin, B-blockers, cholesterol-lowering drugs and dietary supplements, smoking, current treatment of infertility, inhaled corticosteroid use, following a specific diet and regular exercise (>2 weeks) and allergy to cinnamon. The Ethical Committee of Tabriz University of Medical Science approved the study protocol and was registered on the Iranian Registry of Clinical Trials website (identifier: IRCT201508173664N14). Written informed consent was gained from each subject. The participants were randomly allocated into two groups using a block randomization procedure with matched subjects in each block based on age and BMI. Subjects were questioned to continue their usual dietary intakes and physical activity during the study.
A general questionnaire was completed for each patient. Body weight was measured using a scale (Seca, Hamburg, Germany), without shoes and wearing light clothing. Height was measured using a mounted tape without shoes. BMI was calculated as the weight in kilogram divided by the height in meters squared. Information about daily energy and macronutrient intakes were obtained by 24-h recall method for 3 d, including 2 d during the week and 1 during the weekend. A three day average for energy and macronutrient intakes of all subjects was analyzed by Nutritionist 4 software (First Databank Inc., San Bruno, CA).
Cinnamon bark was provided from the Iranian Institute of medicinal plants, Tehran, Iran. Cinnamon barks were grinded with a plant tissue grinder. Each capsule containing approximately of 500 mg cinnamon powder was manufactured on October 2015. Subjects in the treatment group received three capsules of cinnamon and control group subjects received three placebo capsules (wheat flour) that they were required to take daily for 8 weeks. The compliance of the volunteers with the study protocol was monitored via phone interviews once per week and also by counting returned capsules every 2 weeks.
2.2. Blood sampling and biochemical assays
Blood samples (5 ml) were collected after a 12-h overnight fasting, in the morning. The serum samples were separated from whole blood by centrifugation at 2606.8 ×g for 10 min (Beckman Avanti J-25; Beckman Coulter, Brea, CA, USA). The serum samples were frozen immediately at −70 °C until assay. Serum total cholesterol (TC), triacylglycerol (TG) and high-density lipoprotein cholesterol (HDL-C) were measured using the standard enzymatic methods by Pars Azmun kit (Karaj, Iran). Low-density lipoprotein cholesterol (LDL-C) concentration was determined by the Friedewald formula: LDL − C = TC − (HDL − C + TG/5).23 Measurement of TAC in serum was performed by using the colorimetric method with commercial kits (TAC, RANDOX kits; UK).24, 25, 26 The serum MDA level was estimated by using a reaction with thiobarbituric acid as a thiobarbituric acid reactive substance to produce a pink colored complex. Next, its fluorescence intensity was measured at 547 nm with excitation at 525 nm by a spectrofluorimeter (model SFM 25 A; Kontron, Milan, Italy).27 All anthropometric, dietary intakes, blood sampling and biochemical measurements were assessed again at the end of intervention period in both groups.
2.3. Statistical analyses
The collected data were analyzed using the statistical software SPSS, version 22. (SPSS Inc., Chicago, IL, USA) and the results are expressed as means ± SD. The normality of the distribution of variables was checked by Kurtosis–Skewness test. The baseline measurements and dietary intakes of subjects in two groups were compared using independent samples t test and chi-square test for quantitative and qualitative variables, respectively. Analysis of covariance (ANCOVA) was used to identify any differences between the two groups after intervention, adjusting for baseline measurements and confounders (BMI and energy changes during study). The changes in anthropometric measurements, energy and nutrient intakes and blood parameters of the participants between the beginning and end of the trial were compared by paired samples t test. The percentage of changes in variables after intervention was determined with the formula: [(after values − before values)/before values] × 100. Results with P < 0.05 were considered as statistically significant.
3. Results
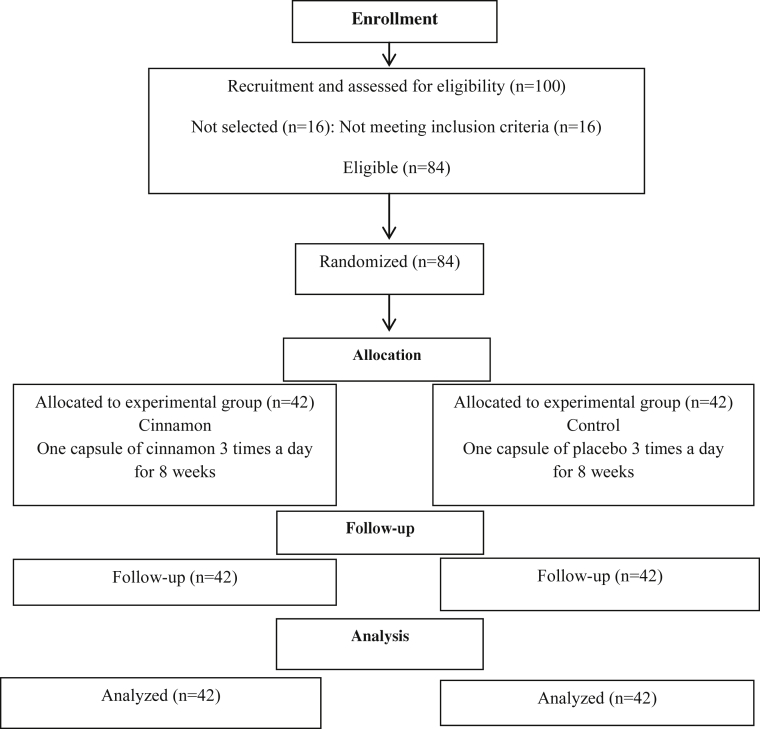
All of the patients (42 patients in cinnamon group and 42 patients in placebo group) completed the study (Fig. 1). Compliance was good, with more than 94% of the supplements prescribed being consumed during the study period. No side effects were reported from participants during study period.
Fig. 1.
Participant's flow diagram.
General and biochemical characteristics of participants at the beginning and end of the study are shown in Table 1. There were no significant differences between groups in BMI and daily energy intake in the beginning of the study and after 8 weeks of intervention. Significant differences were seen between the two groups in serum levels of MDA at baseline. Levels of TAC were not different between two groups at baseline. Serum TAC levels significantly change in cinnamon group after intervention compared to their baseline values (P = 0.001). Variations in MDA levels were not significant (P = 0.102) compare to baseline value. Serum levels of TAC and MDA did not alter significantly in control group. Results of ANCOVA test showed statistically significant differences between two studied groups in serum TAC (P = 0.005) and MDA (P = 0.014) at the end of the study, adjusted for BMI and energy intake. Serum levels of TAC significantly increased in the cinnamon group by 9.28 % at the end of the study. Significant decrease in serum levels of MDA by 7.87% was obtained in cinnamon group over the 8 week in comparison to baseline values.
Table 1.
General characteristics and antioxidant status of the women with PCOS at baseline and after 8-week of intervention.
| variable | Measurement period | Cinnamon group (n = 42) | Control group (n = 42) | MD (95 % CI), p value |
|---|---|---|---|---|
| Age (y) | Baseline | 29.26 (6.14) | 30.17 (6.69) | |
| BMI (kg/m2) | Baseline After intervention |
30.75 (5.04) 30.62 (4.99) |
31.61 (4.84) 31.60 (4.87) |
|
| Energy (kcal/d) | Baseline After intervention |
1651.2(251.09) 1602.4(265.73) | 1749.3 (265.38) 1696 (264.71) |
|
| TAC (mmol/L) | Baseline After intervention MD (95% CI), P-valueb |
1.66 (0.32) 1.83 (0.42) 0.165 (0.07–0.25), 0.001 |
1.79 (0.39) 1.73 (0.32) −0.055 (−0.18 to 0.07),0.38 |
−0.126 (−0.266 to 0.013), 0.076a 0.225 (0.06–0.38), 0.005c |
| MDA (nmol/mL) | Baseline After intervention MD (95% CI), P-valueb |
2.33 (0.63) 2.16 (0.75) −0.173 (−0.38 to 0.03), 0.107 |
2.06 (0.56) 2.26 (0.73) 0.197 (−0.02 to 0.41),0.07 |
0.269 (0.006–0.53),0.044a −0.385 (−0.68 to −0.08), 0.014c |
BMI body mass index, MDA malondialdehyde, TAC total antioxidant status, MD mean difference, CI confidence interval.
The results are described as means ± Standard Deviation (SD).
MD (95% CI), P-value is reported based on the analysis of independent sample t test.
MD (95% CI), P-value is reported based on the analysis of paired sample t test.
MD (95% CI), P-value is reported based on the analysis of covariance.
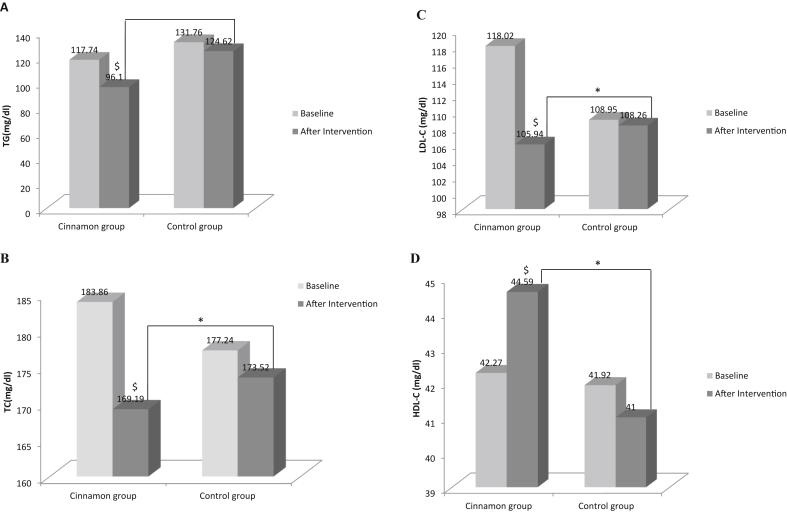
Fig. 2(A–D) illustrates changes in serum levels of TG, TC, LDL-C and HDL-C of studied groups and during 8-wk period of study. No significant differences were seen in serum levels of lipids between the two groups at baselines. As shown in Fig. 2(B–D), significant differences were seen between two studied groups in serum levels of TC, LDL-C and HDL-C at the end of the study adjusted for energy, BMI and baseline values (P < 0.05). Changes in serum TG (P = 0.71) was not significant. (Fig. 2A). Serum levels of TG, TC and LDL-C significantly decreased in the intervention group by 18.24 % (vs. 5.42 % decrease in control group), 7.73 % (vs. 2.10 % decrease in control group) and 10.24 % (vs. 0.63 % decrease in control group), respectively, at the end of the study in comparison to baseline values. (data not shown).
Fig. 2.
Effects of cinnamon on (A) triglyceride, (B) cholesterol levels, (C) low density lipoprotein and (D) High density lipoprotein. Data were present as means ± SE for 42 PCOS patients in each group. $P < 0.05 for within group comparisons (paired sample t test); *P < 0.05 for between group comparisons (ANCOVA adjusted for baseline value, BMI and energy intake daily).
4. Discussion
Cinnamon has been used as a traditional herbal medicine for centuries. This spice has been found to have strong antioxidant, antihyperlipidemia5 and anti-inflammatory properties. To our knowledge, only few studies revealed antioxidant effects of cinnamon. Based on our literature review, this is the first report concerning the effects of cinnamon consumption on antioxidant status and serum lipid levels in women with PCOS.
Based on results BMI and daily energy intake of subjects did not change significantly in any of groups throughout the study, therefore, these variables could not be accounted as confounding factors in interpreting biochemical findings.
Exposure to endocrine and metabolic disturbances such as hyperandrogenism, hyperinsulinemia and dyslipidemia might be responsible for PCOS-associated oxidative stress.28 Reactive oxygen species react with lipids causing peroxidative changes that result in elevated lipid peroxidation products such as MDA.29 TAC is an indicator of the overall protective effect of antioxidants in body fluids, on cell membranes and other components of cells against oxidative injury.30 According to our results, cinnamon supplementation caused a significant increase in TAC levels and decreased MDA levels compared with placebo group. Our findings also were similar with results of other studies.31, 32 Salih indicated that consumption of 1 g/day of cinnamon improved oxidative stress markers among poorly controlled type 2 diabetes patients for 12 weeks.32 In an experimental study, Sariözkan demonstrated that cinnamon bark oil had protective effect on oxidant/antioxidant balance, in 88 adult diabetic male rats for 10 weeks.33 Various studies reported that ether, aqueous and metabolic extracts of cinnamon have considerable antioxidant activities.34, 35 Faix et al revealed that considerably lower lipid peroxidation in plasma and duodenal epithelium of chicks fed the diet supplemented with 0.10% of cinnamon essential oil.36
It was revealed that cinnamon has high levels of different phytochemicals compounds with free radical scavenger actions, such as epicatechin, camphene, eugenol, gamma-terpinene, phenol, salicylic acid and tannins.37 Proanthocyanidins, which are high in cinnamon, are plant metabolites with antioxidant activity.38 Hence, cinnamon is rich in antioxidants. These components inhibit peroxynitrite-induced nitration and fatty acid as well as lipid peroxidation in the β-carotene-linoleic acid system in vitro models.35 They display a protective capacity against irradiation induced lipid peroxidation in liposomes and quench hydroxyl radicals and hydrogen peroxide.39 The ethanolic extract of cinnamon has been demonstrated to decrease the carbon tetrachloride induced lipid peroxidation and as a result, a fall occurs in the markers of oxidative stress such as MDA.40
Our results demonstrated that cinnamon supplementation had antioxidant capability and improved the oxidative stress in the studied subjects. It was possible that the elevate in TAC in our cinnamon treated group might be due to their decreased consumption for free radical detoxification or utilization which was approved by following reduction in serum MDA and improvement in TAC levels.
Our findings indicated that supplementation of cinnamon significantly decreased serum levels of TC and LDL-C and increased HDL-C levels. These results are in agreement with findings of some others studies.41, 42, 43 Soliman showed that supplementation with 1.5 g/day and 3 g/day of cinnamon for 45 days, significantly decreased TG, TC and LDL-C levels in patients with type 2 diabetes.44 Khadem et al showed that 1.5 g/day of cinnamon for 8 weeks, improved lipid profiles in type 2 diabetic patients.45 In animal study, Sambaiah demonstrated that cinnamon reduced serum TC level in rats even after increasing the intake up to 5 times the normal intake.46 Quin et al showed that cinnamon administration (50 mg/kg daily) for 8 weeks decreased TG, TC, chylomicron-apoB48 and VLDL-apoB100 in Wistar rats fed on a high-fructose diet to induce insulin resistance.47 Recent study suggested that cinnamon extract may improve insulin action via increasing glucose uptake, perhaps through enhancing the insulin-signaling pathway in skeletal muscle.38
It was proposed that antihyperlipidemic activity of cinnamon might be due to its high contents of polyphenols inhibiting the intestinal absorption of cholesterol with subsequent hypocholesterolemic activity.18 In another study, cinnamaldehyde changed serum biochemical factors associated with lipolysis, such as glycerol and free fatty acid levels and increased adipose tissue lipolysis in high-fat diet-fed mice.48, 49 Moreover, Kim et al confirmed that cinnamon administration regulated lipid metabolism via the up-regulation of PPARα expression.19 Sheng et al reported that PPARα expression increased the cellular uptake of fatty acids liberated from fat tissues. PPARα ligands also increased the expression of the lipoprotein lipase gene, resulting in the anti-hyperlipidemia effect.28 However, some studies did not find any significant change in lipids profile after cinnamon consumption or its different extracts.50, 51
The strengths of the present study were the double blind placebo-controlled design with no drop-outs. However, our study had some limitations including its short study duration of 8 weeks, small sample population and use of a fixed dose of cinnamon. This study also included subjects with BMI ≥ 25 kg/m2. Therefore, the results of our study may not be applicable to underweight or normal weight patients with PCOS and also to other doses of cinnamon or different intervention period. Studies are warranted to evaluate the effects of cinnamon on androgen status, oxidized LDL and other indicators of OS in these patients, too.
In conclusion, results of present study indicated that 1.5 g of cinnamon supplementation for 12 weeks improved antioxidant status and lipid profile in women with PCOS that could be effective for this disease.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We thank the Research Vice-Chancellor of Tabriz University of Medical Sciences, Tabriz, Iran for the financial support (grant number: 5.4.5477). We would like to thank all of the investigators, coordinators and patients who took part in this study. This article was written based on data set of MSc thesis (number 80) registered in Tabriz University of Medical Sciences, Iran.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Szczuko M., Skowronek M., Zapałowska-Chwyć M., Starczewski A. Quantitative assessment of nutrition in patients with polycystic ovary syndrome (PCOS) Rocz Panstw Zakl Hig. 2016;67:419–426. [PubMed] [Google Scholar]
- 2.Ozcan Dag Z., Alpua M., Isik Y., Buturak S.V., Tulmac O.B. The evaluation of temperament and quality of life in patients with polycystic ovary syndrome. Gynecol Endocrinol. 2016;2:1–4. doi: 10.1080/09513590.2016.1254610. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A., Aponte-Mellado A., Premkumar B.J., Shaman A., Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-kataan M.A., Ibrahim M.A., Al-jammas M.H.H., Shareef Y.S., Sulaiman M.A. Serum antioxidant vitamins changes in women with polycystic ovarian syndrome. J Bahrain Med Sci. 2010;22:68–71. [Google Scholar]
- 5.Liu J., Zhang D. The role of oxidative stress in the pathogenesis of polycystic ovary syndrome. Sichuan Da Xue Xue Bao Yi Xue Ban. 2012;43:187–190. [PubMed] [Google Scholar]
- 6.Gong J., Wu D.B., Zhang L.L., Li J., Zhao X., Zhang D. Study on the oxidative stress in the ovaries of a rat model of polycystic ovary. Sichuan Da Xue Xue Bao Yi Xue Ban. 2015;46:238–242. [PubMed] [Google Scholar]
- 7.Fenkci V., Fenkci S., Yilmazer M., Serteser Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil Steril. 2003;80:1. doi: 10.1016/s0015-0282(03)00571-5. [DOI] [PubMed] [Google Scholar]
- 8.Desai V., Rajendra Prasad N., Musturu Manohar S. Stress in non-obese women with polycystic ovarian syndrome. J Clin Diagn Res. 2014;8:CC01–CC03. doi: 10.7860/JCDR/2014/8125.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.X, Soong Y.Y., Lim S.W., Henry C.J. Evaluation of antioxidant capacity of Chinese five-spice ingredients. Int J Food Sci Nutr. 2015;66:289–292. doi: 10.3109/09637486.2015.1007452. [DOI] [PubMed] [Google Scholar]
- 10.Ranasinghe P., Pigera S., Premakumara G.A., Galappaththy P., Constantine G.R., Katulanda P. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): a systematic review. BMC Complement Altern Med. 2013;22:13–275. doi: 10.1186/1472-6882-13-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dugoua J., Seely D., Perri D. From type 2 diabetes to antioxidant activity: a systematic review of the safety and efficacy of common and cassia cinnamon bark. J Physiol Pharmacol. 2007;85:837–847. doi: 10.1139/Y07-080. [DOI] [PubMed] [Google Scholar]
- 12.Macut D., Bjekić-Macut J., Savić-Radojević A. Dyslipidemia and oxidative stress in PCOS. Front Horm Res. 2013;40:51–63. doi: 10.1159/000341683. [DOI] [PubMed] [Google Scholar]
- 13.Shatwan I.A., Ahmed L.A., Badkook M.M. Effect of barley flour, crude cinnamon and their combination on glycemia, dyslipidemia and adipose tissue hormones in type 2 diabetic rats. J Med Food. 2013;16:656–662. doi: 10.1089/jmf.2012.0083. [DOI] [PubMed] [Google Scholar]
- 14.Javed I., Faisal I., Rahman Z. Lipid lowering effect of Cinnamomum zeylanicum in hyperlipidaemic albino rabbits. Pak J Pharm Sci. 2012;25:141–147. [PubMed] [Google Scholar]
- 15.Dehghan G., Shaghaghi M., Jafari A., Mohammadi M., Badalzadeh R. Effect of endurance training and cinnamon supplementation on post-exercise oxidative responses in rats. Mol Biol Res Commun. 2014;3:269–281. [PMC free article] [PubMed] [Google Scholar]
- 16.Khaki A., Khaki A.A., Hajhosseini L., Golzar F.S., Ainehchi N. The anti-oxidant effects of ginger and cinnamon on spermatogenesis dys-function of diabetes rats. Afr J Tradit Complement Altern Med. 2014; 4;11:1–8. doi: 10.4314/ajtcam.v11i4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain S., Sangma T., Shukla S.K., Mediratta P.K. Effect of Cinnamomum zeylanicum extract on scopolamine-induced cognitive impairment and oxidative stress in rats. Nutr Neurosci. 2015;18:210–216. doi: 10.1179/1476830514Y.0000000113. [DOI] [PubMed] [Google Scholar]
- 18.Shalaby M.A., Saifan H.Y. Some pharmacological effects of cinnamon and ginger herbs in obese diabetic rats. J Intercult Ethnopharmacol. 2014;3:144–149. doi: 10.5455/jice.20140818050741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S.H., Choung S.Y. Antihyperglycemic and antihyperlipidemic action of Cinnamomi Cassiae (Cinnamon bark) extract in C57BL/Ks db/db mice. Arch Pharm Res. 2010;33:325–333. doi: 10.1007/s12272-010-0219-0. [DOI] [PubMed] [Google Scholar]
- 20.Roussel A.M., Hininger I., Benaraba R., Ziegenfuss T.N., Anderson R.A. Antioxidant effects of cinnamon extract in people with impaired fasting glucose that are overweight or obese. J Am Coll Nutr. 2009;28:16–21. doi: 10.1080/07315724.2009.10719756. [DOI] [PubMed] [Google Scholar]
- 21.Hamidpour R., Hamidpour M., Hamidpour S., Shahlari M. Cinnamon from the selection of traditional applications to its novel effects on the inhibition of angiogenesis in cancer cells and prevention of Alzheimer's disease, and a series of functions such as antioxidant, anticholesterol,antidiabetes, antibacterial, antifungal, nematicidal, acaracidal, and repellent activities. J Tradit Complement Med. 2015;5:66–70. doi: 10.1016/j.jtcme.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kort D.H., Lobo R.A. Preliminary evidence that cinnamon improves menstrual cyclist in women with polycystic ovary syndrome: a randomized controlled trial. Am J Obstet Gynecol. 2014;211:4–8. doi: 10.1016/j.ajog.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Ozkaya M., Cakal E., Ustun Y. Effect of metformin on serum visfatin levels in patients with polycystic ovary syndrome. Fertil Steril. 2010;93:880–884. doi: 10.1016/j.fertnstert.2008.10.058. [DOI] [PubMed] [Google Scholar]
- 24.Miller N.J., Rice-Evans C., Davies M.J., Gopinathan V., Milner A. A novel method for measuring antioxidant capacity and its application for monitoring the antioxidant status in premature neonates. Clin Sci. 1993;84:407–412. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- 25.Winterbourn C.C., Hawkins R.E., Brian M., Carrel R.W. Red cell superoxide dismutase activity. J Lab Clin Med. 1975;85:337–350. [PubMed] [Google Scholar]
- 26.Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 27.Del Rio D., Pellegrini N., Colombi B. Rapid fluorimetric method to detect total plasma malondialdehyde with mild derivatization conditions. Clin Chem. 2003;49:690–692. doi: 10.1373/49.4.690. [DOI] [PubMed] [Google Scholar]
- 28.Sheng X., Zhang Y., Gong Z., Huang C., Zang Y.Q. Improved insulin resistance and lipid metabolism by cinnamon extract through activation of peroxisome proliferator-activated receptors. PPAR Res. 2008;58:1348. doi: 10.1155/2008/581348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deepika M.L., Nalini S., Maruthi G. Analysis of oxidative stress status through MN test and serum MDA levels in PCOS women. Pak J Biol Sci. 2014;17:574–577. doi: 10.3923/pjbs.2014.574.577. [DOI] [PubMed] [Google Scholar]
- 30.Tu A.S., Zhong Y., Mao X.G. Changes of serum TOS and TAS levels and their association with apolipoprotein (a) in patients with polycystic ovary syndrome and infertility. Nan Fang Yi Ke Da Xue Xue Bao. 2016;36:405–409. [PubMed] [Google Scholar]
- 31.Prasad K.N., Yang B., Dong X. Flavonoid contents andantioxidant activities from Cinnamomum species. Food Sci Emer Technol. 2009;10:627–632. [Google Scholar]
- 32.Salih A. Anti-diabetic and antioxidant effect of cinnamon in poorly controlled type-2diabetic Iraqi patients: a randomized, placebo-controlled clinical trial. J Intercult Ethnopharmacol. 2016;5:2. doi: 10.5455/jice.20160217044511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sariözkan S., Türk G., Güvenç M. Effects of Cinnamon (C. zeylanicum) bark oil against taxanes-induced damages in sperm quality, testicular and epididymal oxidant/antioxidant balance, testicular apoptosis, and sperm DNA Integrity. Nutr Cancer. 2016;68:481–494. doi: 10.1080/01635581.2016.1152384. [DOI] [PubMed] [Google Scholar]
- 34.Lin C.C., Wu S.J., Chang C.H., Ng L.K. Antioxidant activity of Cinnamomum cassia. Phytother Res. 2003;17:726–730. doi: 10.1002/ptr.1190. [DOI] [PubMed] [Google Scholar]
- 35.Chericoni S., Prieto J.M., Iacopini P., Cioni P., Morelli I. In vitro activity of the essential oil of Cinnamomum zeylanicum and eugenol in peroxynitrite-induced oxidative processes. J Agric Food Chem. 2005;53:4762–4765. doi: 10.1021/jf050183e. [DOI] [PubMed] [Google Scholar]
- 36.Faix S., Faixová Z., Plachá I., Koppe L.J. Effect of Cinnamomum zeylanicum essential oil on antioxidative status in broiler chickens. Acta Vet Hung. 2009;78:411–417. [Google Scholar]
- 37.Lee S.C., Xu W.X., Lin L.Y., Yang J.J., Liu C.T. Chemical composition and hypoglycemic and pancreas-protective effect of leaf essential oil from indigenous cinnamon (Cinnamomum osmophloeum Kanehira) J Agric Food Chem. 2013;61:4905–4913. doi: 10.1021/jf401039z. [DOI] [PubMed] [Google Scholar]
- 38.Anderson R.A., Zhan Z.H., Luo R. Cinnamon extract lowers glucose, insulin and cholesterol in people with elevated serum glucose. J Tradit Complement Med. 2016;6:332–336. doi: 10.1016/j.jtcme.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan K.W., Khong N.M., Iqbal S., Ch'ng S.E., Younas U., Babji A.S. Cinnamon bark deodorised aqueous extract as potential natural antioxidant in meat emulsion system: a comparative study with synthetic and natural food antioxidants. J Food Sci Technol. 2014;51:3269–3276. doi: 10.1007/s13197-012-0818-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moselhy S.S., Ali H.K. Hepatoprotective effect of cinnamon extracts against carbon tetrachloride induced oxidative stress and liver injury in rats. Biol Res. 2009;42:93–98. [PubMed] [Google Scholar]
- 41.Khan R., Khan Z., Shah S. Cinnamon may reduce glucose, lipid and cholesterol level in type 2 diabetic individuals. Pak J Nutr. 2010;9:430–433. [Google Scholar]
- 42.Askari F., Rashidkhani B., Hekmatdoost A. Cinnamon may have therapeutic benefits on lipid profile, liver enzymes, insulin resistance and high-sensitivity C-reactive protein in nonalcoholic fatty liver disease patients. Nutr Res. 2014;34:143–148. doi: 10.1016/j.nutres.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Khan A., Safdar M., Ali Khan M.M., Khattak K.N., Anderson R.A. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26:3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 44.Soliman Ghada Z.A. Effect of Cinnamon bark on glucose and lipids levels of male egyptian type II diabetes mellitus patients. Bull Egypt Soc Physiol Sci. 2007;27:2007. [Google Scholar]
- 45.Khadem H., Haghighian A., Naimi F., Pourghassem GargariAkbar B., Asgharzadeh A., Nemati A. Effect of cinnamon supplementation on blood glucose and lipid levels in type2 diabetic patients. J Tradit Complement Med. 2011;2:1. [Google Scholar]
- 46.Sambaiah K., Srinivasan K. Effect of cumin, cinnamon, ginger, mustard and tamarind in induced hypercholesterolemic rats. Nahrung. 1991;35:47–51. doi: 10.1002/food.19910350112. [DOI] [PubMed] [Google Scholar]
- 47.Qin B., Polansky M.M., Anderson R.A. Cinnamon extract regulates plasma levels of adipose-derived factors and expression of multiple genes related to carbohydrate metabolism and lipogenesis in adipose tissue of fructose-fed rats. Horm Metab Res. 2010;42:187–193. doi: 10.1055/s-0029-1242746. [DOI] [PubMed] [Google Scholar]
- 48.Amin Kamal A., Abd El-Twab Thanaa M. Original Article Oxidative markers, nitric oxide and homocysteine alteration in hypercholesterolimic rats: role of atorvastatine and cinnamon. Int J Clin Exp Med. 2009;2:254–265. [PMC free article] [PubMed] [Google Scholar]
- 49.Khare P., Jagtap S., Jain Y., Bishnoi M. Cinnamaldehyde supplementation prevents fasting-induced hyperphagia, lipid accumulation and inflammation in high-fat diet-fed mice. Article Bio Factors. 2016;42:77. doi: 10.1002/biof.1265. [DOI] [PubMed] [Google Scholar]
- 50.Blevins S.M., Leyva M.J., Brown J., Wright J., Scofield R.H., Aston C.E. Effect of cinnamon on glucose and lipid levels in non-insulin-dependent type 2 diabetes. Diabetes Care. 2007;30:2236–2237. doi: 10.2337/dc07-0098. [DOI] [PubMed] [Google Scholar]
- 51.Wickenberg J., Lindstedt S., Berntorp K., Nilsson J., Hlebowicz J. Ceylon cinnamon does not affect postprandial plasma glucose or insulin in subjects with impaired glucose tolerance. Br J Nutr. 2012;107:1845–1849. doi: 10.1017/S0007114511005113. [DOI] [PubMed] [Google Scholar]