Abstract
Despite the fact that Ficus deltoidea and vitexin played important roles in controlling hyperglycemia, an effective mitigation strategy dealing with cognitive deficit observed in diabetes, little is known about its neuroprotective effects. The study is aimed to determine changes in behavioral, gyrification patterns and brain oxidative stress markers in streptozotocin (STZ)-induced diabetic rats following F. deltoidea and vitexin treatments. Diabetic rats were treated orally with metformin, methanolic extract of F. deltoidea leaves and vitexin for eight weeks. Morris water maze (MWM) test was performed to evaluate learning and memory functions. The patterns of cortical gyrification were subsequently visualized using micro-computed tomography (micro-CT). Quantification of brain oxidative stress biomarkers, insulin, amylin as well as serum testosterone were measured using a spectrophotometer. The brain fatty acid composition was determined using gas chromatography (GC). Biochemical variation in brain was estimated using Fourier transform infrared (FT-IR) spectroscopy. Results showed that oral administration of F. deltoidea extract and vitexin to diabetic rats attenuated learning and memory impairment, along with several clusters of improved gyrification. Both treatments also caused a significant increase in the superoxide dismutase (SOD) and glutathione peroxidase (GPx) values, as well as a significant reduction of TBARS. Strikingly, improvement of cortical gyrification, spatial learning and memory are supported by serum testosterone levels, fatty acid composition of brain and FT-IR spectra.
Keywords: Diabetes, F. deltoidea, Vitexin, Cognition, Gyrification, Micro-computed tomography
Graphical abstract
1. Introduction
There is a considerable amount of scientific data that consistently links diabetes mellitus (DM) to neurological consequences, in particular, to cognitive function.1, 2, 3 It is now recognized that oxidative stress is a key factor linking DM with cognitive impairment in experimental animals and humans.4, 5, 6 Excessive levels of reactive oxygen species (ROS) by hyperglycemia cause damage to cellular components such as DNA, neuronal proteins and lipid peroxidation.7, 8 Despite intensive glycemic control, evidence of a series of neurobehavioral shifts pertaining to DM9, 10 highlighted the need for interventions to reduce the deleterious effect of diabetes on cognition.
Considering the pathophysiologic mechanism of the cognitive impairments consequences of DM, it is important to determine the pattern and magnitude of this complication. Clinical studies have demonstrated that diabetes not only impairs the integration of neural systems, but also alters the brain anatomy.11 As cortical gyrification patterns are thought to orchestrate cognitive functions,12 the combined analysis of data from behavioral and biochemical test as well as micro-CT imaging may provide insight into central aspects of pathology that could be present exclusively in the brain. It is becoming increasingly evident that micro-CT has tremendous potential for general mapping of the mouse and rat brain.13, 14 Micro-CT has emerged as an attractive diagnostic imaging technique that enables noninvasive, high-resolution, in vivo and ex vivo imaging.15 Thus, exploring cortical gyrification using micro-CT imaging technique may provide additional clues with respect to the underlying anatomical feature which correlates cognitive ability and decline.
Approach to analyze biochemical variation in rat brain by FT-IR may establish conclusively the link between the molecular fingerprint and diabetes-related cognitive change.16 FT-IR has proved to be a powerful tool for studying molecules changes that occur in diseases. It possesess many advantages such as very small sample volume requirement, good precision over the entire physiological range, being reagent-free, can rapidly and noninvasively detect changes in the biochemical composition of cells and tissues (at the molecular level).17 These techniques have been used to probe chemical changes in serum,18, 19 plasma,20 brain,21, 22 pancreas,23 urine24 and bone25, 26, 27 of human and animal.
Many efforts have been made to elucidate the mechanisms and therapeutic properties of plant-derived neuroprotective agents. In recent, special interest has been paid to medicinal plants with antioxidant and antidiabetic properties.28 This is due to the fact that hyperglycemia-induced oxidative stress plays a key role in accentuating the progression of cognitive impairment in diabetes. In Malaysia, the decoction of Ficus deltoidea leaves has been extensively used as an herbal medicine to normalize blood sugar, afterbirth tonic to contract the uterus and vaginal muscles as well as a dietary supplement used for treating leucorrhoea in humans.29 In recent, a great deal of attention has been focused on leaves due to the high antioxidant.30, 31 showed the presence of more than 20 varieties of flavonoids in the leaves of F. deltoidea, in which vitexin has recently been isolated, identified, and evaluated.32 Above all, antioxidant and antidiabetic properties of F. deltoidea in animal models has been shown to be associated with vitexin.33 Therefore, this study was designed to characterize the changes in behavioral, cortical gyrification and brain oxidative stress markers following F. deltoidea and vitexin treatment. Accordingly, we considered it is worthwhile to examine whether these changes are related to hormonal profile, fatty acid composition and the pattern of brain FT-IR spectral.
2. Methods
2.1. Plant materials and extract preparations
The leaves of F. deltoidea var. deltoidea were collected from Forest Research Institute Malaysia, Kepong, Malaysia in January 2015. The sample was then deposited at the Herbarium Unit, Universiti Kebangsaan Malaysia, Bangi with a voucher number Herbarium UKMB-40315. The leaves were washed thoroughly and over-dried at 37 ± 5 °C. The dried leaves were finely powdered using an electric grinder. For extraction, 100 g of powdered leaves was soaked in 1 L methanol for three days at room temperature.32 Liquid extracts were concentrated by using a rotary evaporator at 40 °C and subjected to freeze drying for 48 h. The extraction yield calculated was 10.6%. The extracts were kept in tightly closed glass containers and stored at −20 °C until further use.
2.2. Animals
The animal use and experiment protocols involved in the study were approved by the Universiti Putra Malaysia, Animal Care and Use Committee with an approval number: UPM/IACUC/AUP-R090/2014. Thirty four-week-old Sprague Dawley male rats (mean body weight, 80 g) were used. The reason of using younger animals was to minimize the confounding effects of neuronal loss, and other aging changes in rats that might confound the results. It is known that after a period of rapid growth, neuronal loss started to occur as soon as the end of adolescence in rats, or at three months old in normal rats.34 Furthermore, work done by Wang et al35 also showed that four months after STZ treatment in 8–10 week old rats, age-related diabetic encephalopathies and cognitive deficits became very evident. The animals were acclimatized upon arrival for a week and were housed at a density of three per cage in a temperature controlled room (22 ± 1 °C and a 12 h light/dark cycle). The rats were identified with a cage card indicating project number, dose level, group, and animal number. They were had access to standard rat chow (Gold Coin Holdings, Kuala Lumpur, Malaysia) and water ad libitum.
Diabetes-like hyperglycemia was induced experimentally in rats by STZ injection at a dosage of 60 mg/kg of body weight.36, 37 In order to prevent drug-induced hypoglycemic shock, the rats were given prophylactic 5% glucose water for 2 days following STZ injection.38 After a week, animals with fasting blood glucose levels >11 mmol/L were considered diabetic.39
2.3. Experimental design and procedure
The rats were divided into five groups of six rats per treatment group. The treatment group were normal control rats treated with saline (NC), diabetic control rats treated with saline (DC), diabetic rats treated with 1000 mg/kg of metformin (DMET),40 diabetic rats treated with 1000 mg/kg of F. deltoidea (DFD),32 diabetic rats treated with 1 mg/kg b.w. of vitexin (DV).33
Metformin, methanolic extract of F. deltoidea and vitexin were dissolved in saline and treatments were given once daily via oral gavage for eight weeks. At the end of the experiment, all animals were fasted overnight. Animals were then anesthetized with ketamine (80 mg/kg) and xylazine (8 mg/kg), followed by terminal exsanguinations. Blood samples (10–15 ml) were collected via cardiac puncture from the rats into a plain red-top tube containing no anticoagulants (BD Vacutainer®, USA). The blood samples were then centrifuged at 4000 g for 15 min, and serum was stored in aliquots at −80 °C.
2.4. Morris water maze test (MWM)
MWM was carried out from day 51 and continue each day to day 56 of the treatment period as described by Nagapan et al. and Weitzner et al.41, 42 Latencies to find the platform, swim speed and time in each quadrant were recorded using a ceiling-mounted camera (DSR-SR47; Sony Corporation, Tokyo, Japan) and analyzed with ANY-maze Video Tracking System Software (Stoelting Co., USA). Prior to the maze test, all animals were examined and verified to be free from physical disabilities and motor function deficits that would affect their maze performance. The test was carried out in two phases:
2.4.1. Spatial acquisition
This phase evaluated the spatial learning abilities of the rats. Rats had daily training for five consecutive days with three trials per day per treatment regime. On each trial, the rat was placed in the water, facing the edge of the pool and located at one of four pseudo-randomly determined start positions as described in Table 1. The rats were given 1 min to locate hidden platform which was placed in the centre of SW quadrant. If the animal failed to find the platform within 1 min, it was physically guided to the platform. The escape latency(s) and path length (cm) to find the platform were measured in each trial and averaged over three trials for each rat.
Table 1.
Depiction of different starting positions in each trail.
| Day | Trial 1 | Trial 2 | Trial 3 |
|---|---|---|---|
| 1 | E | SE | NW |
| 2 | N | NW | E |
| 3 | SE | E | N |
| 4 | E | NW | N |
| 5 | SE | E | NW |
| 6 (probe trial) | NE |
2.4.2. Probe trial
To assess the spatial memory retention, a probe trial was performed 24 h after the last acquisition day. In this trial, the platform was removed and the animal was released from the opposite site of the quadrant and allowed to swim for 1 min. The relative time animal spent in the former platform quadrant was recorded and analyzed.
2.5. In vivo micro CT analysis of brain
Before imaging, rats were anesthetized with 10:1 ratio of ketamine to xylazine and placed vertically to the beam path on its left side. In vivo brain imaging was performed with SkyScan 1176 μCT (Bruker, Kontich, Belgium) at 12 μm spatial resolution, a voltage of 41 kV and a current of 232 μA. Beam hardening effect of the X-rays was minimized by applying a 1 mm aluminum filter, to equalize the energy of the emitted X-rays from the source. The rotation step size used for acquiring images was 0.5 degrees according the methods described by Wathen et al.43 Total scan time for the brain was approximately 20 min. The images were reconstructed using NRecon software (Bruker-MicroCT) to generate grayscale images. The brain region of interest (ROI) was first defined. For standardization purpose, brain ROIs were created using the largest circles that could be enclosed within the endosteal envelope on the 2D slices. The transverse, coronal and sagittal slices were then displayed to generate anatomy specific data sets.
2.6. Preparation of tissue homogenates
One hundred milligram of brain tissue was homogenized in 5–10 ml/g of homogenizing buffer (50 mM Tris-HCl, 5 mM EDTA, 1 mM DTT pH 7.5) using a Teflon pestle (Glass-Col, USA) at 900 rpm as described previously by Saxena et al.44 The homogenates were centrifuged at 9,000×g in a refrigerated centrifuge (4 °C) for 10 min to remove nuclei and debris. The supernatant obtained was used for biochemical assays and FT-IR analysis. Protein concentration was estimated by the method of Lowry,45 using bovine serum albumin as the standard.
2.7. Estimation of TBARS levels in brain
The levels of MDA equivalents were determined in pancreas by TBARS assay kit (Cayman, MI, USA) as describe by Hardwick et al.46 The absorbance was determined spectrophotometrically at a wavelength of 540 nm using a spectrophotometer.
2.8. Assessment of enzymatic activities GPx and SOD in brain
GPx and SOD activity was measured using assay kit (Cayman, MI, USA). The experimental procedures were carried out according to the manufacturer's instructions.
2.9. Estimations of testosterone, insulin and amylin levels
Serum levels of testosterone were measured using commercial RIA kits (Labor Diagnostika Nord GmbH & Co, KG, Nordhorn, Germany) while insulin levels in brain were determined by Enzyme Linked Immunosorbent Assay kit specific for rat insulin (Cloud-Clone Corp., Houston, USA) as described by Zhang et al.47 The amylin level in the brain was determined by using Rat Amylin Enzyme Immunoassay Kit (RayBiotech Incorporation, GA). All samples were run in triplicate and the optical density was read at 450 nm with microplate reader (Epoch Microplate Spectrophotometer, BioTek, USA).
2.10. Fatty acid analysis
Fats from the brain were extracted according to the methods described by Hajjar et al.48 According to this method, 1 ml of serum were thawed at room temperature for 30 min and extracted using the Folch method (chloroform:methanol, 2:1, v/v) containing butylated hydroxytoluene as antioxidant. Then, fatty acids methyl esters (FAME) were prepared using 0.66 N potassium hydroxide (KOH) in methanol and 14% methanolic boron trifluoride (BF3) (Sigma Chemical Co. St. Louis, Missouri, USA). The FAME were separated with an Agilent 7890A Series GC system (Agilent Technologies, Palo Alto, CA, USA) using a 30 m × 0.25 mm ID (0.20 μm film thickness) Supelco SP-2330 capillary column (Supelco, Inc., Bellefonte, PA, USA). The fatty acid proportions are expressed as percentage of total identified fatty acids. One microlitre of FAME was injected by an auto sampler into the chromatograph, equipped with a split/splitless injector and a flame ionization detector (FID) detector. The injector temperature was programmed at 250 °C and the detector temperature was 300 °C. The column temperature program initiated runs at 100 °C, for 2 min, warmed to 170 °C at 10 °C/min, held for 2 min, warmed to 200 °C at 7.5 °C/min, and then held for 20 min to facilitate optimal separation. Identification of fatty acids was carried out by comparing relative FAME peak retention times of samples to standards obtained from Sigma (St. Louis, MO, USA).
2.11. FT-IR analysis
Infrared spectroscopic experiments were performed using a Bruker 66V FT-IR spectrometer (Bruker Corp., MA, USA) that was equipped with a focal plane array detector. Twenty microliter of brain homogenate was then deposited on a liquid cell (demounted cell) using a pipette according to the method reported by Demir et al.16 All individual FT-IR spectra were recorded in the region 4000–400 cm−1 at room temperature. In order to resolve the overlapped absorption components in FT-IR spectra, second derivative spectra were calculated using Savitzky–Golay algorithm. All spectra processing was performed by using Bruker Spectra software (Version 7.0). The peaks of each spectra curve were then fitted and calculated. The region enriched vibration changes were compared to existing literature database towards providing chemical information on the targeted tissues. Absorptions belonging to fatty acyl chains, proteins and carbohydrates of biological samples are basically available in the 3020–2800 cm−1, 1700–1500 cm−1 and 1200–900 cm−1 spectral intervals, respectively.49
2.12. Statistical analysis
Behavioral data from the training period was analyzed using repeated measures ANOVA. Meanwhile, the one-way ANOVA was used to compare differences among groups for probe trials, brain insulin, amylin, oxidative stress marker, antixioxidant enzymes and fatty acids compositions dataset. If any significant changes were found, post hoc comparisons were performed using Duncan's multiple comparison test. Results were presented as mean ± 1 SD. All analysis was performed at 95% confidence level.
3. Results
3.1. Fasting blood glucose
The means and standard deviations of FBG in entire groups are given in Table 2. The FBG concentrations in the DC animals were increased by 50.65% compared to initial values, confirming the validity of the diabetogenic dose of STZ in this study. The treatment for 8 weeks with either metformin or F. deltoidea or vitexin resulted in a significant reduction in FBG. F. deltoidea and vitexin treatment of the diabetic rats resulted in significantly decreased by 38.03% and 47.85%, respectively compared to pre-treatment values. All treated diabetic groups depict significant changes at week 6 of treatment.
Table 2.
Effect of F. deltoidea and vitexin on fasting blood glucose level (mmol/L) in STZ induced diabetic rats.
| Groups | Fasting blood glucose (mmol/L) ± SD |
% changes | |
|---|---|---|---|
| Before | After | ||
| NC | 4.80 ± 0.30a | 4.93 ± 0.21a | +2.71% |
| DC | 20.00 ± 3.24b | 30.13 ± 2.63b | +50.65% |
| DMET | 29.30 ± 3.70c | 19.83 ± 3.75c | −32.32% |
| DFD | 27.87 ± 6.03c | 17.27 ± 4.97c | −38.03% |
| DV | 30.43 ± 4.07c | 15.87 ± 2.01c | −47.85% |
Values are mean ± 1 SD for 6 rats in each group. Values with different superscripts down the column indicate significant difference at p < 0.05.
3.2. Morris water maze test
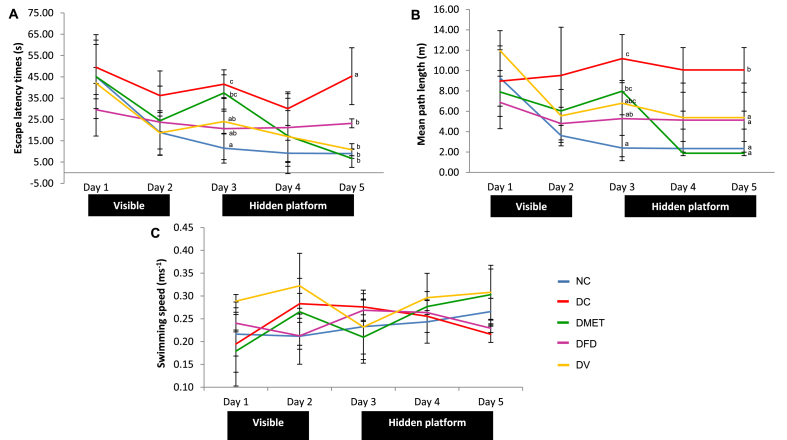
As illustrated in Fig. 1, there was no significant difference between any of the groups on the first day of the training in the water maze. All animals progressively had learned the task by climbing onto the visible platform on the second day, as indicated by decreasing latencies and swim distance. It was observed that the DC, DMET and DV rats had longer latencies on day 3. However, the latencies and swim distance gradually decreased with the days of training for the DMET and DV groups. The DC rats associated with prolonged latency (45.30 ± 13.35 vs 8.97 ± 2.25) and swim distance (10.06 ± 2.20 vs 2.3373 ± 0.70) compared to the NC rats by the end of that session.
Fig. 1.
Effects of 8 weeks daily of administration of F. deltoidea and vitexin on (A) escape latency, (B) mean path length, (C) swimming speed in control and treated diabetic rats, respectively (n = 6, mean ± SD). Day 1- rats were oriented to the maze; Day 2- rats learned to locate the visible platform; Day 3 to 5-rats learned to locate the hidden platform from different or randomized quadrants. Values with different superscripts are significantly different at p < 0.05.
During the probe test, STZ treated animals had longer latencies, higher path length and a lower percentage of time spent in the target quadrant (Table 3). The swim speed to the platform, however, was not significantly different in the DC animals. Remarkably, the DMET, DFD and DV rats had a significant decreased in latencies and path length as compared to DC group. A significant increase in the swim speed was observed in the DMET and DV rats.
Table 3.
Changes during probe trial of various experimental groups.
| Groups | Escape latency times (s) | Mean path length (m) | % of time spent in the target quadrant | Swimming speed (ms−1) |
|---|---|---|---|---|
| NC | 6.97 ± 2.12a | 1.84 ± 0.46a | 33.41 ± 2.99c | 0.267 ± 0.021ab |
| DC | 42.57 ± 2.58c | 8.79 ± 0.33d | 14.04 ± 1.68a | 0.212 ± 0.002a |
| DMET | 10.67 ± 2.08a | 3.25 ± 1.13b | 23.23 ± 2.73b | 0.299 ± 0.045b |
| DFD | 18.87 ± 10.74b | 5.19 ± 2.62c | 12.44 ± 2.56a | 0.280 ± 0.031ab |
| DV | 9.34 ± 0.85a | 3.1 ± 0.26b | 16.56 ± 1.16a | 0.334 ± 0.005b |
Data are presented as mean ± 1SD. Values with different superscripts in a column differed significantly at p < 0.05.
3.3. Micro-computed tomography images of brain
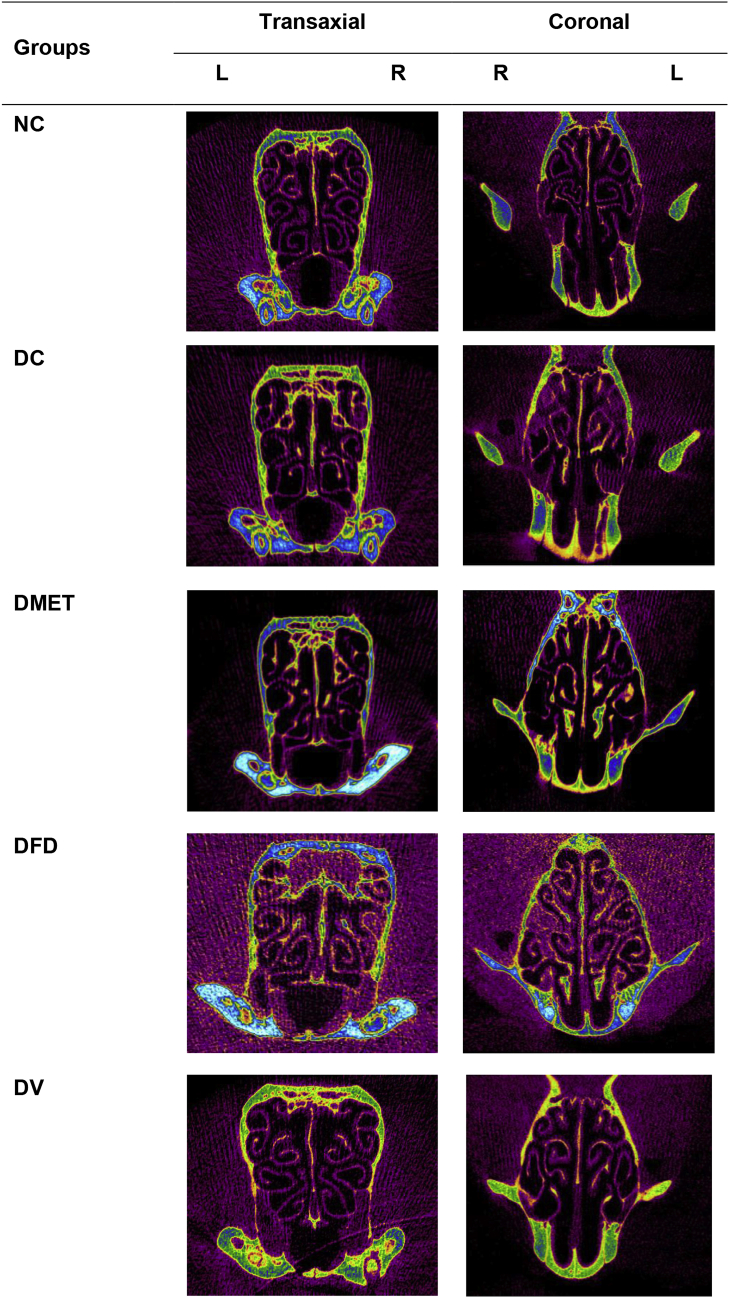
The transaxial and coronal images showed that the DC rats had severe reduction in cortical convolution (Fig. 2). However, increased gyrification, consistent with clear deep sulci was observed in the cortex of DFD and DV rats for over 8 weeks period.
Fig. 2.
Cross-sectional micro-CT images of rat brain. Gyrification abnormalities were observed in the DC and DMET while a clear deep sulcal lines obtained in the cortex of DFD and DV rats. NC: Normal control; DC: diabetic control; DMET: diabetic treated with metformin; DFD: diabetic treated with F. deltoidea; DV: diabetic treated with vitexin; L: left hemisphere and R: right hemisphere. Images are representative of three animals per experimental group.
3.4. Oxidative stress marker and antioxidant enzymes in brain
Table 4 showed that the DC rats had a significant decrease in the activity of SOD and a substantial increase of TBARS. Significant increases in the SOD and GPx values as well as a significant reduction of TBARS were observed in the brain of DFD rats. Similarly, vitexin treatment is associated with a significant increase in the activity of SOD and decrease in TBARs levels, but resulted in a significant decrease in GPx activity.
Table 4.
Oxidative stress marker and antioxidant enzymes of different experimental groups.
| Groups | Oxidative stress marker |
Antioxidant enzymes |
|
|---|---|---|---|
| TBARS (nmol MDA/mg protein) | GPx (U/mg protein) | SOD (mU/mg protein) | |
| NC | 1.61 ± 0.109a | 25.03 ± 0.371b | 13.02 ± 0.891d |
| DC | 44.15 ± 9.655c | 21.69 ± 5.223b | 2.61 ± 0.363a |
| DMET | 20.95 ± 2.493b | 24.09 ± 2.538b | 4.45 ± 0.413b |
| DFD | 10.74 ± 0.662ab | 42.13 ± 0.702c | 6.94 ± 0.663c |
| DV | 9.55 ± 0.385ab | 13.40 ± 3.255a | 4.89 ± 0.208b |
Data are presented as mean ± 1SD. Values with different superscripts in a column differed significantly at p < 0.05.
3.5. Changes in brain insulin, amylin and serum testosterone
It was observed that STZ-induced diabetes caused a significant decrease in the levels of brain insulin, but did not substantially affect the levels of brain amylin and serum testosterone as compared to the NC rats. A significant reduction of insulin levels was also observed in the brain of DFD rats. It is noteworthy that metformin and vitexin resulted in significant increase in the levels of brain insulin. However, only vitexin could lead to significant increase in brain amylin levels as compared to the DC rats. As depicted in Table 5, all groups had experienced testosterone increase after treatment. Interestingly, groups that had shown marked improvement in cognitive performance also had among the highest testosterone levels.
Table 5.
Insulin, amylin and testosterone concentrations of different experimental groups.
| Groups | Insulin (pg/ml) | Amylin (ng/ml) | Testosterone (ng/ml) |
|---|---|---|---|
| NC | 63.15 ± 0.936d | 40.87 ± 1.162b | 1.81 ± 0.088ab |
| DC | 39.13 ± 0.254b | 35.02 ± 0.702a | 0.84 ± 0.370a |
| DMET | 50.25 ± 0.718c | 33.15 ± 4.442a | 2.97 ± 0.627c |
| DFD | 34.99 ± 0.369a | 32.25 ± 0.913a | 2.40 ± 0.166bc |
| DV | 89.49 ± 1.279e | 45.60 ± 0.860b | 3.03 ± 0.436c |
Data are presented as mean ± 1SD. Different superscripts in a column differed significantly at p < 0.05.
3.6. Brain fatty acid changes
As shown in Table 6, the total brain saturated fatty acids (SFA) and monounsaturated fatty acids (MUFAs) were not affected following STZ induction. However, the DC rats had significantly decreased total n-3 PUFA, in particular, α-linolenic acid (ALA) but increased n-6 PUFA compared to controls. Elevation in docosapentaenoic acid (DPA) was also noticed in the brain of DC rats. Similar changes were observed in the animals treated with vitexin. Interestingly, DFD rats had increased brain n-3 PUFA, especially ALA concentrations.
Table 6.
Fatty acid composition (percentage of total identified fatty acids) of the brain of the experimental groups.a
| Fatty acids composition (%) | Groups |
||||
|---|---|---|---|---|---|
| NC | DC | DMET | DFD | DV | |
| Saturated fatty acids (SFA) | |||||
| Palmitic acid (C16:0) | 14.11 ± 2.41 | 19.07 ± 1.21 | 17.98 ± 2.17 | 15.01 ± 5.59 | 20.58 ± 1.79 |
| Heptadecanoic acid (C17:0) | 1.63 ± 2.30 | 4.31 ± 0.04 | 4.32 ± 0.690 | 2.32 ± 2.714 | 4.13 ± 0.183 |
| Stearic acid (C18:0) | 13.09 ± 2.26 | 19.12 ± 0.82 | 17.09 ± 2.07 | 14.73 ± 5.71 | 18.36 ± 1.30 |
| Total SFA | 28.82 ± 6.97 | 42.50 ± 1.71 | 39.39 ± 4.93 | 32.06 ± 12.76 | 43.06 ± 1.97 |
| Monounsaturated fatty acids (MUFA) | |||||
| Oleic acid (C18:1n9) | 14.91 ± 2.15 | 29.06 ± 2.13 | 23.15 ± 7.95 | 19.96 ± 8.26 | 27.91 ± 0.65 |
| Total MUFA | 14.91 ± 2.15 | 29.06 ± 2.13 | 23.15 ± 7.95 | 19.96 ± 8.26 | 27.91 ± 0.65 |
| n-6 PUFA | |||||
| Linoleic acid (C18:2n6) | 0.00 ± 0.00 | 2.39 ± 2.08 | 2.14 ± 1.18 | 1.45 ± 1.34 | 4.03 ± 1.94 |
| Arachidonic acid (C20:4n6) | 5.80 ± 1.30 | 8.05 ± 0.75 | 8.03 ± 0.55 | 6.64 ± 2.62 | 8.58 ± 0.45 |
| total n-6 PUFA | 5.80 ± 1.30a | 10.43 ± 2.23bc | 10.17 ± 0.63bc | 8.09 ± 2.71ab | 12.60 ± 1.60c |
| n-3 PUFA | |||||
| α-Linolenic acid (C18:3n3) | 43.35 ± 14.63c | 6.92 ± 2.28a | 15.66 ± 12.86ab | 31.70 ± 7.42bc | 4.31 ± 1.54a |
| Docosapentaenoic acid (C22:5n3) | 1.09 ± 1.55a | 3.19 ± 0.17b | 3.36 ± 0.16b | 2.09 ± 0.96ab | 3.21 ± 0.12b |
| Docosahexaenoic acid (C22:6n3) | 6.02 ± 2.66 | 7.90 ± 1.42 | 8.27 ± 0.81 | 7.30 ± 3.64 | 8.90 ± 0.65 |
| Total n-3 PUFA | 50.46 ± 10.42b | 18.01 ± 1.03a | 27.29 ± 13.51a | 41.10 ± 3.65b | 16.43 ± 1.66a |
| n-6: n-3 | 0.12 ± 0.05a | 0.59 ± 0.16bc | 0.43 ± 0.24abc | 0.32 ± 0.30ab | 0.78 ± 0.15c |
Values are mean ± 1 SD at n = 3. Values with different superscripts in a row differed significantly at p < 0.05.
The data are expressed as the percentage of total identified fatty acids.
3.7. FT-IR spectroscopy
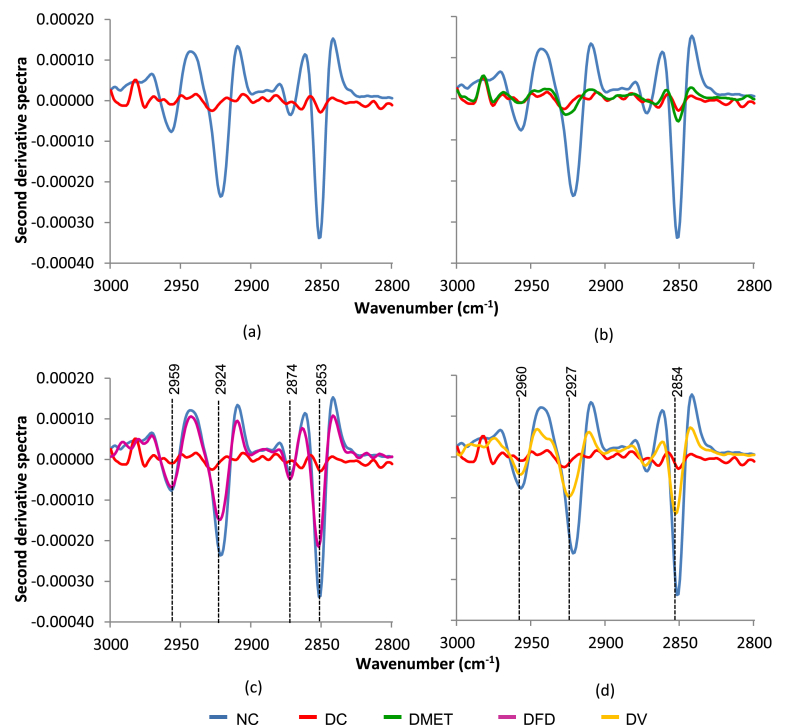
The FT-IR spectroscopy revealed that the brain tissues from different groups are distinct at three regions of infrared (IR) spectra. It was noticed that the most dominant peak corresponds to CH2 stretch of lipid-rich regions had decreased to levels far below normal in the DC and DMET groups as illustrated in Fig. 3. Interestingly, the brain of DFD and DV rats associated with the reappearance of the sharp IR peaks in the lipid-rich region.
Fig. 3.
Second derivative spectra for brain tissue in the regions of 3000–2800 cm−1.
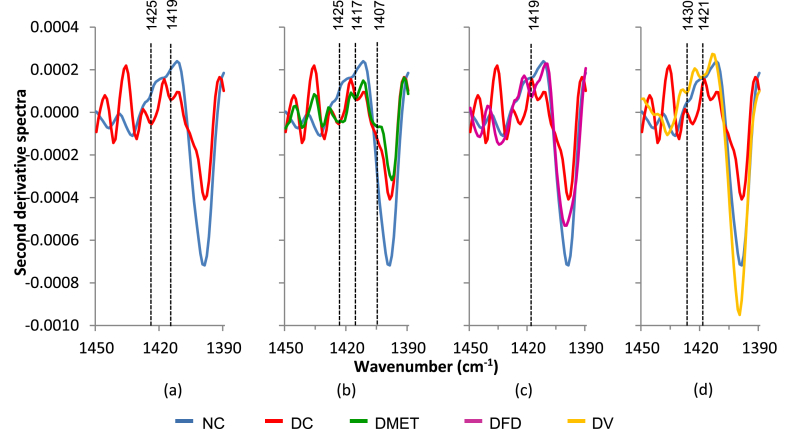
The appearance of two additional methyl peaks at 1425 and 1419 cm−1 was observed in the DC rats. Meanwhile, three new peaks appeared in the DMET group. However, as seen in Fig. 4(c and d), the IR spectra of DFD and DV groups appear less affected by STZ injection.
Fig. 4.
Second derivative spectra for brain tissue in the regions of 1450–1390 cm−1.
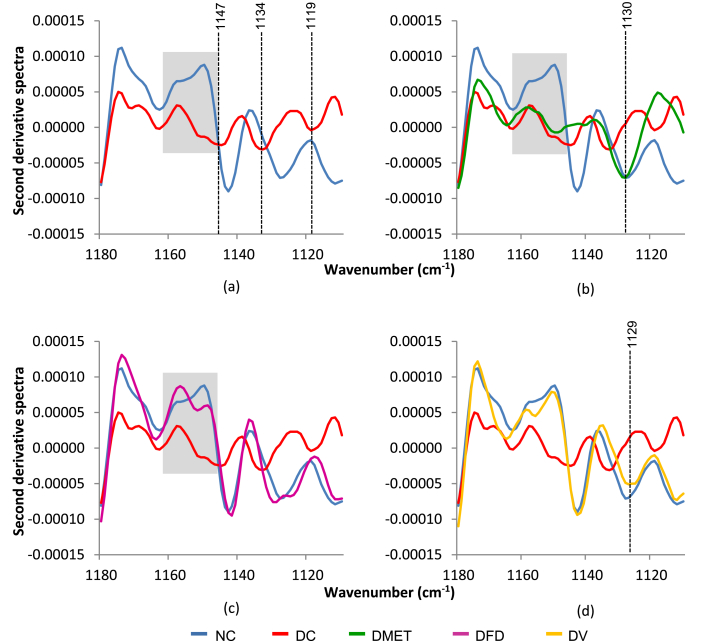
The differences in IR spectra between groups were also observed in the range of 1180–1110 cm−1. The brain of DC rats displayed a decreased in the intensity of sugar vibrations as compared to the NC rats, with a slight shift for three peaks as depicted in Fig. 5(a). More important, the DC, DMET and DFD groups had led to the distortion of the peak approximately at 1151–1160 cm−1, implying some structural changes of carbohydrates. However, the treatment with F. deltoidea had significantly induced the reappearance of the peak at 1143 and 1131 cm−1, likely similar to that observed in the NC group [Fig. 5(c)]. Meanwhile, Fig. 5(d) showed that the DV group displayed similar vibrational frequencies, in which some differences in the absorption intensities were observed, accompanied by shifts in the peak position.
Fig. 5.
Second derivative spectra 5 for brain tissue in the regions of 1180–1110 cm−1.
4. Discussion
The results of the present study demonstrate, for the first time, that F. deltoidea and vitexin improve both spatial learning and memory retention in diabetic rats. Transaxial and coronal views from micro-CT imaging showed that the DFD and DV rats associated with increased cortical gyrification (Fig. 2). Also, it was noticed that these improvements may to some extent be linked with brain oxidative stress markers, hormonal changes and FT-IR spectra.
Behavior predominantly reflects structural and functional of the brain.50 Herein, the MWM test was employed to assess spatial learning and memory. We showed convincing evidence that STZ-induced diabetic rats are associated with disturbance in spatial learning and memory functions. Regardless of the location of the swim-start, the DC rats required longer time to find the submerged platform and learned its location, indicating a decrease of spatial learning ability.51 These results were consistent with previous studies demonstrating that STZ induced diabetic rats associated with higher mean in latencies although the swim speeds did not differ significantly to the NC rats.52, 53 It is intriguing that the DMET, DFD and DV rats recorded a shorter latency in both acquisition phase and probe trial than that observed in the DC rats. Several reports had described that any significant decrease in latency and mean path length and increase in the percentage of time spent in the target quadrant indicate improvement of learning and memory.41, 54, 55, 56 This could be explained by the fact that most of the treated animals had a greater tendency to swim away from the wall, cross swimming to the target quadrant of the pool and decreased floating behavior during spatial navigation. It was well corresponded to the previous studies that behavioral aspects can be enhanced by plant extract or active compound with potent learning and memory enhancing effects.57, 58, 59
Working towards the objectives of the visualizing structure of rat brain, X-ray micro-CT was used for non-invasive determination of cortical convolution. The data clearly indicated that brain gyrification was severely decreased in rats exposed to STZ. It has been suggested by Palaniyappan60 that lower gyrification are related to a reduction in cortical thickness. Indeed, thickness reduction of prefrontal cortical regions has been reported in T1DM61 and T2DM patients.62 In fact, the association of cortical thinning and cognitive decline has been reported earlier.63, 64 Although there is disagreement regarding the structure-function relations of brain areas and cognition,65 but, cognitive abilities and sensorimotor skills are associated with the size and extent of folding of the cerebral cortex in most animal species.66, 67
One of the most striking findings of this study is the association of F. deltoidea and vitexin treatments with the pattern of cortical gyrification. Results showed gyrification of the DFD and DV rats improved in parallel to the behavioral transition. This observation supports the idea that increased cortical gyrification can lead to better learning-memory performance.68 Therefore, it is conceivable that cortical gyrification and sulcal spans could facilitate the early diagnosis of cognitive decline69 and restoration of cognitive impairment in diabetes would largely depend on cortical gyrification.
Despite activation of oxidative stress can be regarded as an early predictor of tissue damage,70, 71 literature on the link between brain oxidative stress and cortical gyrification are more limited. The present study found that the activity of SOD decreased while the levels of TBARS increased in the brain of DC rats. These data strengthen the fact that antioxidant depletion can cause oxidative damage, in turn, may contribute to the physiological and behavioral changes.72 Interestingly, it was observed that TBARS decreased while SOD increased in the brain of animals with improved learning and memory. This finding is in agreement with the other studies showing that increased cognitive performance in STZ induced diabetic rats are related to the improvement of brain antioxidant markers.73, 74 Treatment with antioxidants has been proven to ameliorate depressive-like behavior in diabetic rats.75 In fact,76 demonstrated that several plants extract improves behavioral deficits via enhancement of antioxidant capacities.
At first glance, it was thought that cortical gyrification would be better related to SOD activity, but it soon appeared that the brain with greater gyrification are associated with increases in both GPx and SOD activity. As pointed out by Yu et al,77 the balance between the main antioxidant enzymes are more important than any single one for the treatment of ROS-related diseases, thus provide insight into the relationship between gyrification and brain antioxidants activities.
Neuroprotective roles of insulin have been outlined in previous reviews.78 According to the results summarized in Table 5, the DC brains had lower insulin levels. Similar findings have been obtained in other rat models of diabetes.79, 80 These data imply that brain insulin content could have a significant influence on cognitive ability and behavior.81, 82, 83 Indeed, improvement in behavior has been reported in STZ-induced diabetic rats treated with insulin,84 thereby supporting the notion that insulin affects spatial learning and memory abilities.
It is interesting to note that treatment with metformin and vitexin are capable of inducing brain insulin levels in diabetic rats. However, the increment was negated by F. deltoidea. This is not surprising because it has been observed that metformin rapidly crossed the blood-brain barrier and have specific pharmacological effects on the central nervous system of rat.85 It is conceivable that metformin and vitexin inhibit learning and memory impairments, at least in part, by enhancing glucose metabolism and promoting neurotransmission. Another possibility is that metformin and vitexin can reduce the accumulation of β-amyloid (Aβ) plaque in the brains, thereby reversing cognitive decline.86 Vandal87 demonstrated that a single insulin injection decreased soluble Aβ levels, thus prompt us to examine whether insulin could influence amylin levels in the brain. Nevertheless, the possibility of F. deltoidea to restore cognitive function cannot formally exclude. Perhaps the most convincing explanation is that F. deltoidea has only modest blood-brain barrier penetration and/or utilize different mechanisms to improve learning and memory.
Clinical and animal studies have recently looked at the unique relationship between amylin and cognition.88, 89 Similar to the finding by Qiu,90 the concentration of amylin was markedly decreased in the DC rats. It is worth mentioning that amylin hormone potently enhances the translocation of Aβ peptide from the brain into blood.91, 92 Therefore, it is conceivable that learning and memory deficits in STZ-treated rat were somehow related to Aβ aggregation, manifestation of brain amylin deficiency.93, 94
As depicted in Table 5, vitexin significantly increased brain amylin. This finding is particularly important because amylin has been shown to improve glucose metabolism95 and enhances neural regeneration,96 which may support, in part, the improvement in learning and memory were seen in the DV group. Although amylin deposition in the brain is linked to neuro-inflammatory response and neurologic deficits,97 some aspects of the neuro-inflammatory response can be beneficial to central nervous system.98 It has been reported that early inflammatory response mediated by chemokine signaling involved in neurogenesis and even axonal regeneration.99 Therefore, it is reasonable to suggest that amylin hormone could be beneficial to cognitive function.
While the effects of testosterone on brain behavioral functions and cognitive abilities are still debated, we found all treatment groups that had shown marked improvement in cognitive performance exhibited a significant increase in serum testosterone levels. However, there were no statistically significant differences between the DC and NC rats.100 The potential of F. deltoidea to increase the secretion of testosterone in male diabetic rats has been reported earlier.101 Interestingly, it has been reported that testosterone attenuates cognitive decline in animals by mitigating brain oxidative stress.102 Although others have argued that current clinical evidence is inconclusive,103, 104 low endogenous levels of testosterone-related cognitive decline and neuropsychiatric symptoms have been reported in several studies.105, 106 The discovery raises the possibility that elevated circulating testosterone may influence cognitive performance in diabetic rats, but his hypothesis warrants further investigation.
It is well established that PUFA regulate several processes within the brain.107 In fact, recent studies revealed that circulating testosterone levels is dictated in part by n-6 PUFA.108 The present work showed that vitexin significantly increased the value of brain n-6 PUFA as compared to NC, which would be beneficial in attenuating a cognitive decline in STZ-induced diabetic rats. This hypothesis is strengthened by the fact that linoleic acid is able to prevent both apoptosis and necrosis in a rat model of spinal cord ischemia.109 In aged rats, the fluidity of biological membranes, hippocampal plasticity and spatial cognition improved with arachidonic acid.110 In fact, arachidonic acid was also reported to be a survival molecule for glial cells.111 Tang112 later showed that both linoleic acid and arachidonic acid reduced in vitro neurotoxicity of 1-methyl-4-phenylpyridinium in PC12 cells. Based on this interpretation, results suggest that n-6 PUFAs exert neuroprotective effects in the brain of STZ-induced diabetic rats.
Strengthening the hypothesis that behavioral impairment and gyrification destruction is secondary to diabetes, FT-IR results showed that the intensity of the lipid methylene band in the brain IR spectra attenuated following STZ induction. It is important to note that lipids regulate synaptic throughput in neurons by determining the localization and function of proteins in the cell membrane.113 In fact, it was hypothesized that smooth brains gyrate are related to lipids.114 Kingsbury115 provide evidence that extracellular lipids induced cortical folding in up to ∼85% by increasing the number of cortical cell number. Thus, it appears that brain function, structure and behavioral performance directly related to the lipid composition of the brain. These findings aligned well with previous work that showed diabetes apparently causes lipid perturbation in the prefrontal cortex, hippocampus, and amygdala.75, 116 The fact that diabetes induces myelin abnormalities by altering lipid pattern strengthening the importance of methylene vibrations in maintaining brain function.117 Intriguingly, the frequency of the intense band was markedly improved in the brain of DFD and DV rat. Although it seems implausible to suggest that the absence of lipid methylene signal from FT-IR spectrum would reflect to brain structure and function, but decreased in two major structural lipid classes has been reported in Alzheimer's disease.118 Therefore, FT-IR signals might be very relevant in grading brain lipid and could be the important markers for defining learning and memory impairments in diabetes.
One particularly interesting finding was the vibrations of methyl groups of protein in the brain of DC rats appeared to split at a different frequency with lower intensity as compared to the NC rats. It seems likely that injection of STZ associated with protein aggregation in the brain of diabetic rats. Supporting this view,119 demonstrated high protein aggregation in the diabetic rat brain. Amyloid formation provides a simple explanation of observing protein aggregation. Indeed, our data showed that there were obvious reduction of amylin levels in diabetic animals with peak splitting at 1450–1390 cm−1. Recent studies suggest that alterations in brain cortical anatomy significantly correlated with deposition of Aβ,120 thus raising the potential of methyl groups vibrations as a promising biomarker to supporting structure-function relations of the brain.121 Interestingly, the vibrations of methyl groups of DFD and DV groups that had shown marked improvement in the cortical gyrification look reasonably similar to the NC rats.
While the impact of diabetes on the pattern of gyrification is not readily explained, this study suggests the relationship between FT-IR spectra and brain structure-function. There is evidence that the brain of DC rats was associated with lower intensity and peak distortion in the region of carbohydrate. Similar distortion at 1160–1151 cm−1 was observed in the brain of DMET and DFD rats. It is important to note that remarkable spectral differences may indicate structural changes.122 Nikonenko123 demonstrated that the absorption bands in the 1175–1140 cm−1 spectral range is related to the 1 → 4 glycosidic linkage formation in polysaccharides. Therefore, our results suggest that the peak distortion could be related to the position of α and β glycosidic bond conformations. Reportedly, carbohydrate structure is one of the important factors determining the insulin concentrations.124 Earlier work done by Rossini and Soeldner125 shown that the α-glucose anomer produces a greater insulin release than β-glucose in various animal models. Interestingly, these results were paralleled by a decrease in brain insulin content, suggesting FT-IR as vibration spectroscopic method that sensitive to the anomeric structure.126 Further work is needed, however, to characterize the relationships between inversion of the glycosidic bond and brain structure-function.
Collectively, these results suggest that restoring the redox balance, brain insulin and amylin levels showed a tendency to improve STZ-induced learning and memory impairments. This is supported by good agreement between pathological changes and FT-IR peaks. Further studies are required to confirm the specific effects and mechanisms of brain fatty acid compositions on its structure and functions.
5. Conclusion
It is concluded that F. deltoidea and vitexin improves learning ability among STZ-treated rats. This improvement is associated with reduction of oxidative stress and positive changes in the brain topography. It is believed that reduction of brain sugar is an important factor that mediated all these improvements.
Conflict of interests
The authors declare that they have no competing interests.
Acknowledgement
This research was supported by grants from the Ministry of Science, Technology and Innovation (SF: 100-RMI/SF 16/6/2 (7/2015)), Ministry of Higher Education (MOE FRGS: 600-RMI/FRGS 5/3 (5/2014)), Faculty of Applied Sciences, Universiti Teknologi MARA (UiTM) and Faculty of Veterinary Medicine, Universiti Putra Malaysia. We thank Laboratory Animal Facility and Management (LAFAM), UiTM for the postoperative care of the animals.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Biessels G.J., Gispen W.H. The impact of diabetes on cognition: what can be learned from rodent models? Neurobiol Aging. 2005;1:36–41. doi: 10.1016/j.neurobiolaging.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 2.McCrimmon R.J., Ryan C.M., Frier B.M. Diabetes and cognitive dysfunction. Lancet. 2012;16:2291–2299. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- 3.Ho N., Sommers M.S., Lucki I. Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neurosci Biobehav Rev. 2013;37(8):1346–1362. doi: 10.1016/j.neubiorev.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kodl C.T., Seaquist E.R. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29(4):494–511. doi: 10.1210/er.2007-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu X., Guo L., Tian G. Diabetes cognitive impairments and the effect of traditional Chinese herbs. Evid Based Complement Altern Med. 2013;2013:649396. doi: 10.1155/2013/649396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X., Zhao L. Calycosin ameliorates diabetes-induced cognitive impairments in rats by reducing oxidative stress via the PI3K/Akt/GSK-3β signaling pathway. Biochem Biophys Res Commun. 2016;473(2):428–434. doi: 10.1016/j.bbrc.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Demaurex N., Scorran L. Reactive oxygen species are NOXious for neurons. Nat Neurosci. 2009;12:819–820. doi: 10.1038/nn0709-819. [DOI] [PubMed] [Google Scholar]
- 8.Piras S., Furfaro A.L., Domenicotti C. RAGE expression and ROS generation in neurons: differentiation versus damage. Oxid Med Cell Longev. 2016;2016:9348651. doi: 10.1155/2016/9348651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox D.J., Kovatchev B.P., Gonder-Frederick L.A. Relationships between hyperglycemia and cognitive performance among adults with type 1 and type 2 diabetes. Diabetes Care. 2005;28(1):71–77. doi: 10.2337/diacare.28.1.71. [DOI] [PubMed] [Google Scholar]
- 10.van der Ven N.C., Hogenelst M.H., Tromp-Wever A.M. Short-term effects of cognitive behavioural group training (CBGT) in adult Type 1 diabetes patients in prolonged poor glycaemic control. A randomized controlled trial. Diabet Med. 2005;22(11):1619–1623. doi: 10.1111/j.1464-5491.2005.01691.x. [DOI] [PubMed] [Google Scholar]
- 11.Roriz-Filho J., Sá-Roriz T.M., Rosset I. (Pre)diabetes, brain aging, and cognition. Biochim Biophys Acta. 2009;1792(5):432–443. doi: 10.1016/j.bbadis.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Luders E., Narr K.L., Bilder R.M. Mapping the relationship between cortical convolution and intelligence: effects of gender. Cereb Cortex. 2008;18(9):2019–2026. doi: 10.1093/cercor/bhm227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayasaka N., Nagai N., Kawao N. In vivo diagnostic imaging using micro-CT: sequential and comparative evaluation of rodent models for hepatic/brain ischemia and stroke. PLoS One. 2012;7(2):e32342. doi: 10.1371/journal.pone.0032342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saito S., Murase K. Ex vivo imaging of mouse brain using micro-CT with non-ionic iodinated contrast agent: a comparison with myelin staining. Br J Radiol. 2012;85(1019) doi: 10.1259/bjr/13040401. e973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobrivojević M., Bohaček I., Erjavec I., Gorup D., Gajović S. Computed microtomography visualization and quantification of mouse ischemic brain lesion by nonionic radio contrast agents. Croat Med J. 2013;54(1):3–11. doi: 10.3325/cmj.2013.54.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demir P., Akkas S.B., Severcan M., Zorlu F., Severcan F. Ionizing radiation induces structural and functional damage on the molecules of rat brain homogenate membranes: a Fourier transform infrared (FT-IR) spectroscopic study. Appl Spectrosc. 2015;69(1):154–164. doi: 10.1366/13-07154. [DOI] [PubMed] [Google Scholar]
- 17.Xavier S., Ramalingam S., Periandy S. Experimental [FT-IR and FT-Raman] analysis and theoretical [IR, Raman, NMR and UV-Visible] investigation on propylbenzene. J Theor Comput Sci. 2014;1:109. [Google Scholar]
- 18.Gajjar K., Trevisan J., Owens G. Fourier-transform infrared spectroscopy coupled with a classification machine for the analysis of blood plasma or serum: a novel diagnostic approach for ovarian cancer. Analyst. 2013;138:3917–3926. doi: 10.1039/c3an36654e. [DOI] [PubMed] [Google Scholar]
- 19.Khanmohammadi M., Ghasemi K., Garmarudi A.B., Ramin M. Diagnostic prediction of renal failure from blood serum analysis by FTIR spectrometry and chemometrics. Spectrochim Acta A Mol Biomol Spectrosc. 2015;136(Pt C):1782–1785. doi: 10.1016/j.saa.2014.10.082. [DOI] [PubMed] [Google Scholar]
- 20.Zhou C.E., Kan C.W. Plasma-assisted regenerable chitosan antimicrobial finishing for cotton. Cellulose. 2014;21:2951. [Google Scholar]
- 21.Dreissig I., Machill S., Salzer R., Krafft C. Quantification of brain lipids by FTIR spectroscopy and partial least squares regression. Spectrochim Acta A Mol Biomol Spectrosc. 2009;71(5):2069–2075. doi: 10.1016/j.saa.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Araki K., Yagi N., Ikemoto Y. Synchrotron FTIR micro-spectroscopy for structural analysis of Lewy bodies in the brain of Parkinson's disease patients. Sci Rep. 2015;5:17625. doi: 10.1038/srep17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan C., Cao W., Cheng C. Research of recognition method of discrete wavelet feature extraction and PNN classification of rats FT-IR pancreatic cancer data. J Anal Methods Chem. 2014;2014:564801. doi: 10.1155/2014/564801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw R.A., Low-Ying S., Leroux M., Mantsch H.H. Toward reagent-free clinical analysis: quantitation of urine urea, creatinine, and total protein from the mid-infrared spectra of dried urine films. Clin Chem. 2000;46:1493–1495. [PubMed] [Google Scholar]
- 25.Boskey A., Camacho N.P. FT-IR imaging of native and tissue-engineered bone and cartilage. Biomaterials. 2007;28(15):2465–2478. doi: 10.1016/j.biomaterials.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kourkoumelis N., Tzaphlidou M. Spectroscopic assessment of normal cortical bone: differences in relation to bone site and sex. Sci World J. 2010;10:402–412. doi: 10.1100/tsw.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z.X., Lloyd A.A., Burket J.C., Gourion-Arsiquaud S., Donnelly E. Altered distributions of bone tissue mineral and collagen properties in women with fragility fractures. Bone. 2016;84:237–244. doi: 10.1016/j.bone.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Aslam M., Sial A.A. Neuroprotective effect of ethanol extract of leaves of Malva parviflora against amyloid-β- (Aβ-) mediated Alzheimer's disease. Int Sch Res Notices. 2014;2014:156976. doi: 10.1155/2014/156976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bunawan H., Amin N.M., Bunawan S.N., Baharum S.N., Mohd Noor N. Ficus deltoidea Jack: a review on its phytochemical and pharmacological importance. Evid Based Complement Altern Med. 2014;2014:902734. doi: 10.1155/2014/902734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Misbah H., Aziz A.A., Aminudin N. Antidiabetic and antioxidant properties of Ficus deltoidea fruit extracts and fractions. BMC Complement Altern Med. 2013;13:118. doi: 10.1186/1472-6882-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Omar M.H., Mullen W., Crozier A. Identification of proanthocyanidin dimers and trimers, flavone C-Glycosides, and antioxidants in Ficus deltoidea, a malaysian herbal tea. J Agric Food Chem. 2011;59(4):1363–1369. doi: 10.1021/jf1032729. [DOI] [PubMed] [Google Scholar]
- 32.Farsi E., Ahmad M., Hor S.Y., Ahamed M.B., Yam M.F., Asmawi M.Z. Standardized extract of Ficus deltoidea stimulates insulin secretion and blocks hepatic glucose production by regulating the expression of glucose-metabolic genes in streptozitocin-induced diabetic rats. BMC Complement Altern Med. 2014;14:220. doi: 10.1186/1472-6882-14-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choo C.Y., Sulong N.Y., Man F., Wong T.W. Vitexin and isovitexin from the Leaves of Ficus deltoidea with in-vivo α-glucosidase inhibition. J Ethnopharmacol. 2012;142(3):776–781. doi: 10.1016/j.jep.2012.05.062. [DOI] [PubMed] [Google Scholar]
- 34.Morterá P., Herculano-Houzel S. Age-related neuronal loss in the rat brain starts at the end of adolescence. Front Neuroanat. 2012;6:45. doi: 10.3389/fnana.2012.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J.Q., Yin J., Song Y.F. Brain aging and AD-like pathology in streptozotocin-induced diabetic rats. J Diabetes Res. 2014;2014:796840. doi: 10.1155/2014/796840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Motyl K., McCabe L.R. Streptozotocin, type I diabetes severity and bone. Biol Proced Online. 2009;11:296–315. doi: 10.1007/s12575-009-9000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J., Zhang S., Zhang L., Wang R., Wang M. Effects of L-3-n-butylphthalide on cognitive dysfunction and NR2B expression in hippocampus of streptozotocin (STZ)-induced diabetic rats. Cell Biochem Biophys. 2015;71(1):315–322. doi: 10.1007/s12013-014-0200-5. [DOI] [PubMed] [Google Scholar]
- 38.Gurukar M.S.A., Mahadevamma S., Chilkunda N.D. Renoprotective effect of Coccinia indica fruits and leaves in experimentally induced diabetic rats. J Med Food. 2013;16(9):839–846. doi: 10.1089/jmf.2012.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong Y., Jing T., Meng Q. Studies on the antidiabetic activities of cordyceps militaris extract in diet-streptozotocin-induced diabetic Sprague-dawley rats. BioMed Res Int. 2014;2014:160980. doi: 10.1155/2014/160980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tahara A., Matsuyama-Yokono A., Nakano R., Someya Y., Shibasaki M. Hypoglycaemic effects of antidiabetic drugs in streptozotocin-nicotinamide-induced mildly diabetic and streptozotocin-induced severely diabetic rats. Basic Clin Pharmacol Toxicol. 2008;103(6):560–568. doi: 10.1111/j.1742-7843.2008.00321.x. [DOI] [PubMed] [Google Scholar]
- 41.Nagapan G., Goh Y.M., Razak I.S.A., Nesaretnam K., Ebrahimi M. The effects of prenatal and early postnatal tocotrienol-rich fraction supplementation on cognitive function development in male offspring rats. BMC Neurosci. 2013;14:77. doi: 10.1186/1471-2202-14-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weitzner D.S., Engler-Chiurazzi E.B., Kotilinek L.A., Ashe K.H., Reed M.N. Morris water maze test: optimization for mouse strain and testing environment. J Vis Exp. 2015;100 doi: 10.3791/52706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wathen C.A., Foje N., van Avermaete T. In vivo X-ray computed tomographic imaging of soft tissue with native, intravenous, or oral contrast. Sensors (Basel) 2013;13(6):6957–6980. doi: 10.3390/s130606957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saxena A.K., Abdul-Majeed S.S., Gurtu S., Mohamed W.M. Investigation of redox status in chronic cerebral hypoperfusion-induced neurodegeneration in rats. Appl Transl Genom. 2015;5:30–32. doi: 10.1016/j.atg.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 46.Hardwick R.N., Fisher C.D., Canet M.J., Lake A.D., Cherrington N.J. Diversity in antioxidant response enzymes in progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos. 2010;38(12):2293–2301. doi: 10.1124/dmd.110.035006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y., Sun X., Sun X. Molecular characterization of insulin resistance and glycolytic metabolism in the rat uterus. Sci Rep. 2016;6:30679. doi: 10.1038/srep30679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hajjar T., Goh Y.M., Rajion M.A., Vidyadaran S., Li T.A., Ebrahimi M. Alterations in neuronal morphology and synaptophysin expression in the rat brain as a result of changes in dietary n-6: n-3 fatty acid ratios. Lipids Health Dis. 2013;12:113. doi: 10.1186/1476-511X-12-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petibois C., Déléris G., Piccinini M., Cestelli-Guidi M., Marcelli A. A bright future for synchrotron imaging. Nat Photonics. 2009;3:179. [Google Scholar]
- 50.Nuñez S.C., Roussotte F., Sowell E.R. Focus on: structural and functional brain abnormalities in fetal alcohol spectrum disorders. Alcohol Res Health. 2011;34(1):121–132. [PMC free article] [PubMed] [Google Scholar]
- 51.Vorhees C.V., Williams M.T. Assessing spatial learning and memory in rodents. ILAR J. 2014;55(2):310–332. doi: 10.1093/ilar/ilu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Isik A.T., Celik T., Ulusoy G. Curcumin ameliorates impaired insulin/IGF signalling and memory deficit in a streptozotocin-treated rat model. Age (Dordr) 2009;31(1):39–49. doi: 10.1007/s11357-008-9078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu X., Mo Y., Gong J. Puerarin ameliorates cognitive deficits in streptozotocin-induced diabetic rats. Metab Brain Dis. 2016;31(2):417–423. doi: 10.1007/s11011-015-9779-5. [DOI] [PubMed] [Google Scholar]
- 54.Costa R., Tamascia M.L., Nogueira M.D., Casarini D.E., Marcondes F.K. Handling of adolescent rats improves learning and memory and decreases anxiety. J Am Assoc Lab Anim Sci. 2012;51(5):548–553. [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan Z., Wang M., Yan B. An enriched environment improves cognitive performance in mice from the senescence-accelerated prone mouse 8 strain: role of upregulated neurotrophic factor expression in the hippocampus. Neural Regen Res. 2012;7(23):1797–1804. doi: 10.3969/j.issn.1673-5374.2012.23.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee B., Sur B., Shim J., Hahm D.H., Lee H. Acupuncture stimulation improves scopolamine-induced cognitive impairment via activation of cholinergic system and regulation of BDNF and CREB expressions in rats. BMC Complement Altern Med. 2014;14:338. doi: 10.1186/1472-6882-14-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nahata A., Patil U.K., Dixit V.K. Effect of Evolvulus alsinoides Linn. on learning behavior and memory enhancement activity in rodents. Phytother Res. 2010;24(4):486–493. doi: 10.1002/ptr.2932. [DOI] [PubMed] [Google Scholar]
- 58.Jivada N., Rabieib Z. A review study on medicinal plants used in the treatment of learning and memory impairments. Asian Pac J Trop Biomed. 2014;4(10):780–789. [Google Scholar]
- 59.Rabiei Z., Rafieian-Kopaei M., Heidarian E., Saghaei E., Mokhtari S. Effects of Zizyphus jujube extract on memory and learning impairment induced by bilateral electric lesions of the nucleus Basalis of Meynert in rat. Neurochem Res. 2014;39(2):353–360. doi: 10.1007/s11064-013-1232-8. [DOI] [PubMed] [Google Scholar]
- 60.Palaniyappan L., Liddle P.F. Aberrant cortical gyrification in schizophrenia: a surface-based morphometry study. J Psychiatry Neurosci. 2012;37(6):399–406. doi: 10.1503/jpn.110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lyoo I.K., Yoon S., Jacobson A.M. Prefrontal cortical deficits in type 1 diabetes mellitus: brain correlates of comorbid depression. Arch Gen Psychiatry. 2012;69(12):1267–1276. doi: 10.1001/archgenpsychiatry.2012.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wennberg A.M., Spira A.P., Pettigrew C. Blood glucose levels and cortical thinning in cognitively normal, middle-aged adults. J Neurol Sci. 2016;365:89–95. doi: 10.1016/j.jns.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Segura B., Baggio H.C., Marti M.J. Cortical thinning associated with mild cognitive impairment in Parkinson's disease. Mov Disord. 2014;29(12):1495–1503. doi: 10.1002/mds.25982. [DOI] [PubMed] [Google Scholar]
- 64.Koerte I.K., Mayinger M., Muehlmann M. Cortical thinning in former professional soccer players. Brain Imaging Behav. 2016;10(3):792–798. doi: 10.1007/s11682-015-9442-0. [DOI] [PubMed] [Google Scholar]
- 65.Fields R.D. Changes in brain structure during learning: fact or artifact? Reply to Thomas and Baker. Neuroimage. 2013;73:260–264. doi: 10.1016/j.neuroimage.2012.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen C., Omiya Y. Brain asymmetry in cortical thickness is correlated with cognitive function. Front Hum Neurosci. 2014;8:877. doi: 10.3389/fnhum.2014.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun T., Hevner R.F. Growth and folding of the mammalian cerebral cortex: from molecules to malformations. Nat Rev Neurosci. 2014;15(4):217–232. doi: 10.1038/nrn3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gautam P., Anstey K.J., Wen W., Sachdev P.S., Cherbuin N. Cortical gyrification and its relationships with cortical volume, cortical thickness, and cognitive performance in healthy mid-life adults. Behav Brain Res. 2015;287:331–339. doi: 10.1016/j.bbr.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 69.Liu T., Lipnicki D.M., Zhu W. Cortical gyrification and sulcal spans in early stage Alzheimer's disease. PLoS One. 2012;7(2):e31083. doi: 10.1371/journal.pone.0031083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Acar A., Akil E., Alp H. Oxidative damage is ameliorated by curcumin treatment in brain and sciatic nerve of diabetic rats. Int J Neurosci. 2012;122(7):367–372. doi: 10.3109/00207454.2012.657380. [DOI] [PubMed] [Google Scholar]
- 71.Wang G., Chen L., Pan X. The effect of resveratrol on beta amyloid-induced memory impairment involves inhibition of phosphodiesterase-4 related signaling. Oncotarget. 2016;7(14):17380–17392. doi: 10.18632/oncotarget.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumsta C., Thamsen M., Jakob U. Effects of oxidative stress on behavior, physiology, and the redox thiol proteome of Caenorhabditis elegans. Antioxid Redox Signal. 2011;14(6):1023–1037. doi: 10.1089/ars.2010.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khan R.A., Khan M.R., Sahreen S. Brain antioxidant markers, cognitive performance and acetylcholinesterase activity of rats: efficiency of Sonchus asper. Behav Brain Funct. 2012;8:21. doi: 10.1186/1744-9081-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Z., Chen H., Wu P. Flos Puerariae extract ameliorates cognitive impairment in streptozotocin-induced diabetic mice. Evid Based Complement Altern Med. 2015;2015:873243. doi: 10.1155/2015/873243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Réus G.Z., Dos Santos M.A., Abelaira H.M. Antioxidant treatment ameliorates experimental diabetes-induced depressive-like behavior and reduces oxidative stress in brain and pancreas. Diabetes Metab Res Rev. 2016;32(3):278–288. doi: 10.1002/dmrr.2732. [DOI] [PubMed] [Google Scholar]
- 76.Soumyanath A., Zhong Y.P., Henson E. Centella asiatica extract improves behavioral deficits in a mouse model of Alzheimer's disease: investigation of a possible mechanism of action. Int J Alzheimers Dis. 2012;2012:381974. doi: 10.1155/2012/381974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu H., Ge Y., Wang Y. A fused selenium-containing protein with both GPx and SOD activities. Biochem Biophys Res Commun. 2007;358(3):873–878. doi: 10.1016/j.bbrc.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 78.Yu L.Y., Pei Y. Insulin neuroprotection and the mechanisms. Chin Med J Engl. 2015;128(7):976–981. doi: 10.4103/0366-6999.154323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baskin D.G., Stein L.J., Ikeda H. Genetically obese Zucker rats have abnormally low brain insulin content. Life Sci. 1985;36(7):627–633. doi: 10.1016/0024-3205(85)90166-3. [DOI] [PubMed] [Google Scholar]
- 80.Grünblatt E., Salkovic-Petrisic M., Osmanovic J., Riederer P., Hoyer S. Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem. 2007;101(3):757–770. doi: 10.1111/j.1471-4159.2006.04368.x. [DOI] [PubMed] [Google Scholar]
- 81.Erion R., Sehgal A. Regulation of insect behavior via the insulin-signaling pathway. Front Physiol. 2013;4:353. doi: 10.3389/fphys.2013.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kleinridders A., Ferris H., Cai W., Kahn C.R. Insulin action in brain regulates systemic metabolism and brain function. Diabetes. 2014;63(7):2232–2243. doi: 10.2337/db14-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cator L.J., Pietri J.E., Murdock C.C. Immune response and insulin signalling alter mosquito feeding behaviour to enhance malaria transmission potential. Sci Rep. 2015;5:11947. doi: 10.1038/srep11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Farshid A.A., Tamaddonfard E. Histopathological and behavioral evaluations of the effects of crocin, safranal and insulin on diabetic peripheral neuropathy in rats. Avicenna J Phytomed. 2015;5(5):469–478. [PMC free article] [PubMed] [Google Scholar]
- 85.Łabuzek K., Suchy D., Gabryel B., Bielecka A., Liber S., Okopień B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol Rep. 2010;62(5):956–965. doi: 10.1016/s1734-1140(10)70357-1. [DOI] [PubMed] [Google Scholar]
- 86.Matthes F., Hettich M.M., Ryan D.P., Ehninger D., Krauss S. The anti-diabetic drug metformin improves cognitive impairment and reduces amyloid-beta in a mouse model of Alzheimer's disease. Alzheimer's Dement. 2015;11(7):P845. [Google Scholar]
- 87.Vandal M., White P.J., Tremblay C. Insulin reverses the high-fat diet-induced increase in brain Aβ and improves memory in an animal model of Alzheimer disease. Diabetes. 2014;63(12):4291–4301. doi: 10.2337/db14-0375. [DOI] [PubMed] [Google Scholar]
- 88.Moore E.M., Mander A.G., Ames D., AIBL Investigators Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care. 2013;36(10):2981–2987. doi: 10.2337/dc13-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liberini C.G., Borner T., Boyle C.N., Lutz T.A. The satiating hormone amylin enhances neurogenesis in the area postrema of adult rats. Mol Metab. 2016;5(10):834–843. doi: 10.1016/j.molmet.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qiu W.Q., Au R., Zhu H. Positive association between plasma amylin and cognition in a homebound elderly population. J Alzheimers Dis. 2014;42(2):555–563. doi: 10.3233/JAD-140210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ly H., Despa F. Hyperamylinemia as a risk factor for accelerated cognitive decline in diabetes. Expert Rev Proteom. 2015;12(6):575–577. doi: 10.1586/14789450.2015.1104251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu H., Wang X., Wallack M. Intraperitoneal injection of the pancreatic peptide amylin potently reduces behavioral impairment and brain amyloid pathology in murine models of Alzheimer's disease. Mol Psychiatry. 2015;20:252–262. doi: 10.1038/mp.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bitel C.L., Kasinathan C., Kaswala R.H., Klein W.L., Frederikse P.H. Amyloid-β and tau pathology of Alzheimer's disease induced by diabetes in a rabbit animal model. J Alzheimers Dis. 2012;32(2):291–305. doi: 10.3233/JAD-2012-120571. [DOI] [PubMed] [Google Scholar]
- 94.Acharya N.K., Levin E.C., Clifford P.M. Diabetes and hypercholesterolemia increase blood-brain barrier permeability and brain amyloid deposition: beneficial effects of the LpPLA2 inhibitor darapladib. J Alzheimers Dis. 2013;35(1):179–198. doi: 10.3233/JAD-122254. [DOI] [PubMed] [Google Scholar]
- 95.Moreno P., Acitores A., Gutiérrez-Rojas I. Amylin effect in extrapancreatic tissues participating in glucose homeostasis, in normal, insulin-resistant and type 2 diabetic state. Peptides. 2011;32(10):2077–2085. doi: 10.1016/j.peptides.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 96.Trevaskis J.L., Lei C., Koda J.E., Weyer C., Parkes D.G., Roth J.D. Interaction of leptin and amylin in the long-term maintenance of weight loss in diet-induced obese rats. Obesity (Silver Spring) 2010;18(1):21–26. doi: 10.1038/oby.2009.187. [DOI] [PubMed] [Google Scholar]
- 97.Srodulski S., Sharma S., Bachstetter A.B. Neuroinflammation and neurologic deficits in diabetes linked to brain accumulation of amylin. Mol Neurodegener. 2014;9:30. doi: 10.1186/1750-1326-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fuster-Matanzo A., Llorens-Martín M., Hernández F., Avila J. Role of neuroinflammation in adult neurogenesis and Alzheimer disease: therapeutic approaches. Mediat Inflamm. 2013;2013:260925. doi: 10.1155/2013/260925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alunni A., Bally-Cuif L. A comparative view of regenerative neurogenesis in vertebrates. Development. 2016;143(5):741–753. doi: 10.1242/dev.122796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Dam E.W., Dekker J.M., Lentjes E.G. Steroids in adult men with type 1 diabetes: a tendency to hypogonadism. Diabetes Care. 2003;26(6):1812–1818. doi: 10.2337/diacare.26.6.1812. [DOI] [PubMed] [Google Scholar]
- 101.Nurdiana S., Mohd Idzham A.Z., Zanariah A., Mohd Luqman Hakim M.N. Effect of Ficus deltoidea leaves extracts on blood clotting, sperm quality and testosterone level in alloxan-induced male diabetic rats. Int J Pharm Sci Rev Res. 2012;13:111–114. [Google Scholar]
- 102.Pintana H., Pongkan W., Pratchayasakul W., Chattipakorn N., Chattipakorn S.C. Testosterone replacement attenuates cognitive decline in testosterone-deprived lean rats, but not in obese rats, by mitigating brain oxidative stress. Age (Dordr) 2015;37(5):84. doi: 10.1007/s11357-015-9827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Allen K.M., Purves-Tyson T.D., Fung S.J., Weickert S.C. The effect of adolescent testosterone on hippocampal BDNF and TrkB mRNA expression: relationship with cell proliferation. BMC Neurosci. 2015;16:4. doi: 10.1186/s12868-015-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao J.V., Lam T.H., Jiang C. A Mendelian randomization study of testosterone and cognition in men. Sci Rep. 2016;6:21306. doi: 10.1038/srep21306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Beauchet O. Testosterone and cognitive function: current clinical evidence of a relationship. Eur J Endocrinol. 2006;155(6):773–781. doi: 10.1530/eje.1.02306. [DOI] [PubMed] [Google Scholar]
- 106.Hall J.R., Wiechmann A.R., Cunningham R.L., Texas Alzheimer's Research and Care Consortium Total testosterone and neuropsychiatric symptoms in elderly men with Alzheimer's disease. Alzheimers Res Ther. 2015;7(1):24. doi: 10.1186/s13195-015-0107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bazinet R.P., Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15:771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- 108.Erkan L.G., Altinbas B., Guvenc G. Brain thromboxane A2 via arachidonic acid cascade induces the hypothalamic-pituitary-gonadal axis activation in rats. Auton Neurosci. 2015;189:50–55. doi: 10.1016/j.autneu.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 109.Lang-Lazdunski L., Blondeau N., Jarretou G., Lazdunski M., Heurteaux C. Linolenic acid prevents neuronal cell death and paraplegia after transient spinal cord ischemia in rats. J Vasc Surg. 2003;38(3):564–575. doi: 10.1016/s0741-5214(03)00473-7. [DOI] [PubMed] [Google Scholar]
- 110.Fukaya T., Gondaira T., Kashiyae Y. Arachidonic acid preserves hippocampal neuron membrane fluidity in senescent rats. Neurobiol Aging. 2007;28(8):1179–1186. doi: 10.1016/j.neurobiolaging.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 111.Palomba L., Cerioni L., Cantoni O. Arachidonic acid: a key molecule for astrocyte survival to peroxynitrite. Glia. 2009;57(15):1672–1679. doi: 10.1002/glia.20879. [DOI] [PubMed] [Google Scholar]
- 112.Tang K.S. Protective effect of arachidonic acid and linoleic acid on 1-methyl-4-phenylpyridinium-induced toxicity in PC12 cells. Lipids Health Dis. 2014;13:197. doi: 10.1186/1476-511X-13-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Muller S.H., Diaz J.H., Kaye A.D. Clinical applications of intravenous lipid emulsion therapy. J Anesth. 2015;29(6):920–926. doi: 10.1007/s00540-015-2036-6. [DOI] [PubMed] [Google Scholar]
- 114.Price D.J. Lipids make smooth brains gyrate. Trends Neurosci. 2004;27(7):362–364. doi: 10.1016/j.tins.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 115.Kingsbury M.A., Rehen S.K., Contos J.J., Higgins C.M., Chun J. Non-proliferative effects of lysophosphatidic acid enhance cortical growth and folding. Nat Neurosci. 2003;6(12):1292–1299. doi: 10.1038/nn1157. [DOI] [PubMed] [Google Scholar]
- 116.Xia C., Zhu L., Shao W. The effect of hippocampal cognitive impairment and xiap on glucose and lipids metabolism in rats. Cell Physiol Biochem. 2016;38(2):609–618. doi: 10.1159/000438654. [DOI] [PubMed] [Google Scholar]
- 117.Cermenati G., Abbiati F., Cermenati S. Diabetes-induced myelin abnormalities are associated with an altered lipid pattern: protective effects of LXR activation. J Lipid Res. 2012;53(2):300–310. doi: 10.1194/jlr.M021188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wood P. Lipidomics of Alzheimer's disease: current status. Alzheimers Res Ther. 2012;4(1):5. doi: 10.1186/alzrt103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Talaei F., Van Praag V.M., Shishavan M.H., Landheer S.W., Buikema H., Henning R.H. Increased protein aggregation in Zucker diabetic fatty rat brain: identification of key mechanistic targets and the therapeutic application of hydrogen sulfide. BMC Cell Biol. 2014;15:1. doi: 10.1186/1471-2121-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang T., Shi F., Jin Y., Jiang W., Shen D., Xiao S. Abnormal changes of brain cortical anatomy and the association with plasma microrna107 level in amnestic mild cognitive impairment. Front Aging Neurosci. 2016;8:112. doi: 10.3389/fnagi.2016.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Biggar K.K., Li S.S. Non-histone protein methylation as a regulator of cellular signalling and function. Nat Rev Mol Cell Biol. 2015;16(1):5–17. doi: 10.1038/nrm3915. [DOI] [PubMed] [Google Scholar]
- 122.Sudo Y., Furutani Y., Spudich J.L., Kandori H. Early photocycle structural changes in a bacteriorhodopsin mutant engineered to transmit photosensory signals. J Biol Chem. 2007;282(21):15550–15558. doi: 10.1074/jbc.M701271200. [DOI] [PubMed] [Google Scholar]
- 123.Nikonenko N.A., Buslov D.K., Sushko N.I., Zhbankov R.G. Analysis of the structure of carbohydrates with use of the regularized deconvolution method of vibrational spectra. BAÜ Fen Bil Enst Dergisi. 2002;4(2):13–16. [Google Scholar]
- 124.Blom W.A., Stafleu A., de Graaf C., Kok F.J., Schaafsma G., Hendriks H.F. Ghrelin response to carbohydrate-enriched breakfast is related to insulin. Am J Clin Nutr. 2005;81(2):367–375. doi: 10.1093/ajcn.81.2.367. [DOI] [PubMed] [Google Scholar]
- 125.Rossini A.A., Soeldner J.S. Insulin release is glucose anomeric specific in the human. J Clin Investig. 1976;57(4):1083–1088. doi: 10.1172/JCI108351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Synytsya A., Novak M. Structural analysis of glucans. Ann Transl Med. 2014;2(2):17. doi: 10.3978/j.issn.2305-5839.2014.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]