Abstract
Background
There is a variety of experimentally proven medicinal plants having antidiabetic properties but data on herb-drug interaction are very limited. Earlier studies indicated that aqueous extract of turnip leaf (AETL) has hypoglycemic potential in diabetic animals. The present study was conducted to evaluate co-administration effects of AETL and metformin, a commonly used antidiabetic drug, in diabetic rats.
Methods
Metformin at the two different doses (50,100 mg/kg) and AETL at the dose of 400 mg/kg (separately or concurrent with metformin) were orally given to streptozotocin-induced diabetic rats for 4 weeks daily. Fasting blood glucose (FBG) was measured at the times 0, 7, 14, 21 and 28 days after investigation. At the end of study, liver enzymes activity [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)] as well as liver histopathology were evaluated.
Results
Both treatments could significantly decrease FBG levels when they administrated separately. Interestingly, co-administration of AETL and metformin in a dose dependent manner significantly improved hypoglycemic activity of metformin. While neither metformin nor AETL could ameliorate liver alterations alone, but in concomitant therapy they efficiently attenuated liver enzymes elevation and histological damages.
Conclusion
The results of the present study demonstrate that combination of metformin with AETL enhance the prior effectiveness and reduced the latter adverse effects by a synergistic interaction.
Keywords: Turnip, Brassica rapa, Metformin, Interaction, Liver, Rat
Graphical abstract
1. Introduction
Nowadays, due to the global rise in the aging population and obesity, the incidence and prevalence of diabetes are increasing. Since 1980, the number of diabetic patients has quadrupled in the world.1 According to recent WHO report, in 2014, 422 million people with diabetes were estimated and the prevalence of the disease will steadily increase everywhere, particularly in the middle-income countries.2 Based on cost evaluation from a recent systematic review study, it has been projected that the direct cost of diabetes to the world is more than US$ 827 billion, annually.3
There are many herbal medicines suggesting for the treatment of diabetes and some of them have been approved in experimental studies.4, 5 Lots of people thought that herbal products are inherently safe, owing to their natural origin not based on experimentally approved evidence.6 Moreover, despite the fact that there are limited studies in the field of herb-drug interactions, some of patients prefer to use conventional drugs with herbal medicine, concurrently.7, 8 In fact, concomitant use of drug and herbal medicine may sometimes produce interaction effects. Interactions between herbs and drugs may increase or decrease the pharmacological or toxicological effects of either components.9 Therefore, it is imperative to promote credible research on safety and possibility interaction of herbal medicine with synthetic drugs.
Brassica rapa (turnip) has been cultivated and consumed for many centuries across Europe and expanding eventually to central and East of Asia.10 The results of our previous studies showed that turnip root had hypolipidemic potential and its leaf exerted hypoglycemic and renoprotective efficiencies in diabetic rats.11, 12, 13, 14 On the other hand, metformin is a widely consumed oral antdiabetic drug in the world.15 Evidence shows it is most prescribed antidiabetic drug amongst hospital prescriptions. Moreover, metformin plays a pivotal role in most of two-drug, three-drug and four-drug combination therapy in diabetic patients.16 Metformin toxicity is rare, but in all reported cases it was used concurrently with other drugs or herbs.17 It should be noted that metformin also plays a pivotal roles in most of marketed combination antidiabetic drugs like Glucovance, Metaglip, Benformin, Calformin etc.18 Hence, metformin was used as a reference antidiabetic drug to study its concurrent effects with turnip leaf extract in diabetic rats.
2. Objective
As the medicinal plants with antidiabetic properties are widely use separately or in combination with conventional drugs among diabetic patients, this study aimed to evaluate the effects of co-administration of aqueous extract of turnip leaves (AETL) and metformin on blood glucose and liver function markers in diabetic rats.
3. Material and methods
3.1. Plant collection and extract preparation
The turnips (Brassica rapa) were collected during December 2011 from Birjand, South Khorasan province, Iran. The plant was identified by an expert botanist, and a voucher specimen (221) kept in the herbarium of Agricultural Faculty of Birjand University, Birjand, Iran.
Brassica rapa leaves were dried in shade and at the room temperature, then milled by an electric grinder. The powder was macerated in distilled water 1:10 (w/v) on a magnetic stirrer for 2 days at room temperature. Afterwards, the mixture was passed through filter paper (Blue Ribbon, Grade 589, Germany), and concentrated under vacuum evaporator. Subsequently, lyophilized by freeze dryer (Dena Vacuum Industry, model FD-5005-BT, Iran) to produce aqueous extract. The extraction yield was 15.7%.
3.2. Animals
Male Wistar albino rats, weighting 180–220 g procured from the Research Centre of Experimental Medicine at Birjand University of Medical Sciences, Brjand, Iran. The rats were maintained in controlled environment (12 h light/dark cycles and 21–25 °C temperature) and fed with standard laboratory animal pellet diet (Javanneh-Khorasan co, Iran) as well as tap water, ad libitum. The experimental procedures used in the present study were approved by the Ethic Committee of Laboratory Animals at Birjand University of Medical Sciences, Birjand, Iran.
3.3. Chemicals
Streptozotocin (STZ) was purchased from Sigma Chemicals Company (St. Louis, MO, USA) and metformin tablets from Merck Sante' S.A.S.(Lyon, France). The STZ and metformin were freshly dissolved in citrate buffer, pH 4.5 or saline 0.9% for intraperitoneal (IP) and oral administration, respectively.
3.4. Induction and assessment of diabetes
The overnight fasted rats were made diabetic with a single IP injection of 45 mg/kg STZ19 and control normal rats (NC group, n = 8) received vehicle (citrate buffer). Diabetes was confirmed in animals by measuring the fasting blood glucose (FBG) levels three days after the STZ injection. The rats with FBG levels above 350 mg/dl were considered as severe diabetic and used in this study.20
3.5. Experimental design
Based on previous studies, aqueous extract of turnip leaves (AETL) at the dose of 400 mg/kg, as an effective dose, was used in this study.11, 13 After two weeks of diabetes confirmation, the animals were randomly divided into six equal groups (n = 8). Group 1 received saline as diabetic model (DM), groups 2 and 3 received metformin at the doses of 50 mg/kg (MET50) and 100 mg/kg (MET100) respectively, group 4 treated with 400 mg/kg AETL alone (AETL400), groups 5 and 6 received 400 mg/kg AETL plus metformin at the dose of either 50 mg/kg or 100 mg/kg, respectively (AETL + MET50 or AETL+100 MET). Moreover, 8 healthy rats allocated as normal control (CON) group and treated with saline 0.9 %.
Drugs were dissolved in saline (0.9%) solution and orally administrated to the animals using intragastric tube for a period of four weeks. The same volume of saline (0.9%) was administrated to the NC group.
3.6. Measurement of blood glucose and biochemical liver function markers
Weekly measurement of FBG was done using a commercially available digital glucometer (Accu Chek, Germany). At the end of the experimental period, after 14 h fasting and under anesthesia with ketamine:xylazine (65:10 mg/kg), the blood collection was performed from the animals' heart. Plasma was collected to assess the liver function by measuring plasma levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST). The AST and ALT levels were determined by the automatic biochemistry analyzer (Roche Hitachi 912, Japan) and commercially available kits (Pars Azmoon, Iran).
3.7. Histopathology
Following the anesthesia and blood collection, the animals' livers were removed and fixed in 10% formaldehyde in phosphate buffered saline (0.01 M). Tissue samples of the liver were processed for paraffin-embedding and serial sections were made for staining with hematoxilin and eosin dyes. For each rat, three random sections were analyzed under a light microscope (UPLAN FI, Japan). They were evaluated according to a check list semi quantitatively for the degree of histopathological changes (Table 2).
Table 2.
Grading of the histopathological changes in liver sections.
| Alterations | CON | DM | MET50 | 100MET | AETL | AETL + MET50 | AETL + MET100 |
|---|---|---|---|---|---|---|---|
| Limphocyte infiltration | – | ++ | – | – | ++ | – | – |
| Microvesicular deposition fat | – | +++ | + | – | – | – | – |
| Eosinopilic cytoplasm | – | ++ | – | – | + | – | – |
| Hemorrhage | – | ++ | ++ | + | – | + | – |
| Sinusoidal dilatation | – | – | + | + | + | + | + |
Scoring was done as follows for each microscopic field: none (−), low (+), mild (++), severe (+++).
3.8. Statistical analysis
The data of FBG and liver enzymes concentrations were presented as means ± SD. Results were analyzed using one-way ANOVA, followed by Dunnett's post-hoc test to determine significant differences among the means. The level of statistical significance was set at P < 0.05. SPSS 22 for Windows was used to perform the total statistical analysis.
4. Results
4.1. Blood glucose
Table 1 illustrates the effects of four weeks administration of AETL alone or in combination with either 50 or 100 mg/kg metformin on FBG levels in diabetic rats. At the initial time, FBG levels were 448.75 ± 24.52 mg/dL in DM group versus 101.62 ± 8.22 in CON group. There was no significant difference between diabetic groups at this time.
Table 1.
Effects of metformin (MET), aqueous extract of turnip leaves (AETL) or their combination on fasting blood glucose levels in STZ-induced diabetic rats.
| Time groups | Initial | First week | Second week | Third week | Fourth week |
|---|---|---|---|---|---|
| CON | 101.62 ± 8.22a | 100.87 ± 5.74a | 98.14 ± 5.83a | 101.25 ± 4.94a | 99.19 ± 4.21a |
| DM | 448.75 ± 24.52b | 470.75 ± 34.42b | 461.25 ± 32.25b | 463.75 ± 28.26b | 460.37 ± 24.24b |
| MET50 | 436.62 ± 43.74b | 350.75 ± 48.58c | 264.00 ± 18.35d | 304.60 ± 26.7c | 109.12 ± 12.39a |
| MET100 | 456.62 ± 29.73b | 277.75 ± 44.39d,e | 170.21 ± 24.65f | 115.21 ± 11.92a | 104.00 ± 8.17a |
| AETL | 456.00 ± 34.6b | 325.3 ± 15.25e | 259.37 ± 23.36d | 167.0 ± 21.21f | 110.37 ± 5.5a |
| AETL + MET50 | 465.24 ± 36.72b | 327.57 ± 31.19e | 207.24 ± 20.31d | 99.25 ± 10.31a | 91.75 ± 3.42a |
| AETL + MET100 | 493.62 ± 31.13b | 322.59 ± 42.39e | 143.39 ± 12.43 | 99.37 ± 9.93a | 88.56 ± 4.13a |
Each value represents the mean ± S.D. CON: control normal rats; DM: diabetic model group, MET50; Metformin n doses of 50 mg/kg; MET100: Metformin in dose of 100 mg/kg; AETL: aqueous extract of turnip leaves n dose of 400 mg/kg. Different letters (a–f) show significant difference (p < 0.05) within and between groups and same letters shown on-significant difference.
Compared to the DM group, all experimental groups showed gradually decrease in FBG levels during the study period from the first week to the last. At the first week, all experimental groups had significant lower FBG levels than DM group. Comparison between the experimental groups showed, FBG lowering activity of MET50 was only a little lower than MET100 (p = 0.03) while there was no significant difference between the other experimental groups. At the second week, although, there was no significant difference between MET100 and MET100 + AETL (p = 0.16), the FBG comparison between experimental groups showed that the both MET100 and MET100 + AETL presented highest FBG lowering activity than MET50 (p < 0.001, each) AETL (p < 0.001, each) and MET50 + AETL (p = 0.017, p = 0.001 respectively). However, until third week, any of experimental treatments could not normalize FBG levels in diabetic rats. At the third week, the rats treated with MET100 (115.21 ± 11.92), AETL + MET50 (99.25 ± 10.31) and AETL + MET100 (99.37 ± 9.93) had normal FBG levels and there was no significant difference between them. At the fourth week, all of the experimental groups had normal FBG levels.
4.2. Liver enzymes
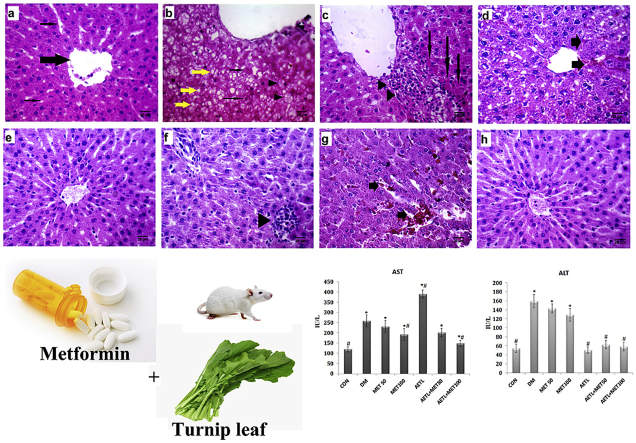
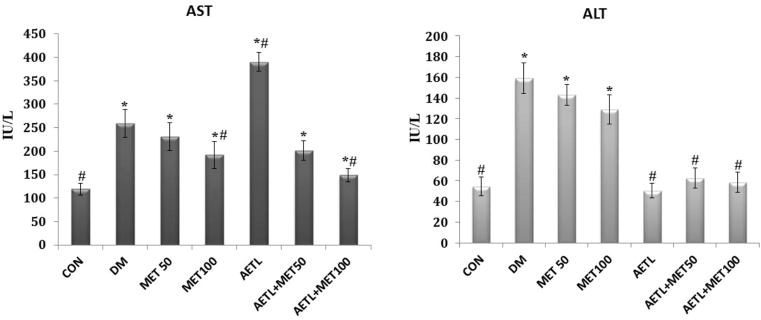
The plasma concentrations of AST and ALT were presented in Fig. 1. Generally, both AST and ALT levels were significantly increased in diabetic rats in comparison with the CON group (p < 0.001).
Fig. 1.
Liver enzymes activity, values are presented as mean ± S.D, n = 8 in each group.CON: control normal rats; DM: diabetic model group, MET50; Metformin n doses of 50 mg/kg; MET100: Metformin in dose of 100 mg/kg; AETL: aqueous extract of turnip leaves n dose of 400 mg/kg. One way ANOVA followed Tukey's post-hoc test, *p < 0.05: compared to normal control group, #p < 0.05: compared to diabetic model group.
Any of interventions could not normalize AST levels in diabetic rats. Not only AETL did not reduce the AST levels, but also it significantly increased its levels more than DM group (p < 0.001). However, compared to DM group, both the MET100 (p = 0.001) and MET100 + AETLcould reduce the AST levels, significantly (p < 0.001).
While treating diabetic rats with metformin (both doses) could not reduce elevated ALT levels, but the groups receiving AETL (alone or in combination with metformin) had normal ALT levels. In this regard, there was no significant difference between CON group and AETL (p = 0.99), AETL+50 MET (p = 0.07) and AETL+100 MET (p = 0.83) groups.
4.3. Liver pathology
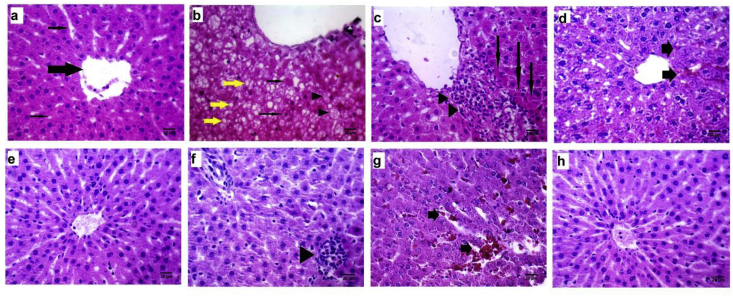
Liver histopathological examination of control group revealed normal structure with distinct hepatocytes, normal sinusoidal spaces and central vein (Fig. 2a). However, in DM group, abnormal morphologies typically some degree of fatty change signs including microvesicular fat vacuoles deposition, ballooning of hepatocytes and apoptosis (councilman bodies) and also mononuclear cell infiltration were observed (Fig. 2b,c). In the animals treated with MET50 or MET100, low degree of fatty changes and sinusoidal hemorrhage without inflammation were observed (Fig. 2d,e). AETL treated rats showed mild infiltration of lymphocytes and numerous activated Kupffer cells (Fig. 2f). Despite the fact that there were some alterations in AETL or MET50 and MET100 alone, mixing of AETL with metformin in the both doses showed normal histological appearance. Only in MET50 + AETL some degrees of sinusoidal hemorrhage were observed (Fig. 2g,h). Table 1 shows the scores of histopathological changes in all groups.
Fig. 2.
(a–h): Effects of aqueous extract of turnip leaves (AETL), metformin (MET) or their combination on the liver of STZ-induced diabetic Wistar rats. (a) normal control liver with normal structure, central vein (thick arrow) and normal sinusoids (thin arrows); (b) liver sections of diabetic model group with typical microvesicular deposited vacuoles (yellow arrow), apoptotic hepatocytes and councilman bodies (thin arrows) and early fatty changes (arrow heads); (c) another section of diabetic model group with spotty necrosis (arrows) and mononuclear cells infiltration (arrow heads); (d) mild sinusoidal dilatation with hemorrhage (arrows) in MET50 group; (e) normal structure of liver of MET100 group with only little Kupffer cells proliferations in sinusoidal spaces; (f) lymphocytes infiltration (arrow head) in liver belongs to AETL group; (g) sinusoidal dilatation with hemorrhage (arrow) in AETL + MET50 group; (h) normal appearance of diabetic rat liver treated with AETL + MET100.
5. Discussion
All diabetic patients require medication to reduce their elevated blood glucose levels. An increasing number of glucose lowering medications is available. Glimepiride, Metformin, Acarbose etc. are the most common oral antidiabetic drugs. However, in some countries these drugs are frequently unavailable or expensive.21 In addition, there is a growing interest in using of herbal medicine in the world.22 Moreover, above all, the use of herbal medicine among diabetic patients receiving antidiabetic pharmacotherapy is common.23 Nowadays, scientists are searching for new types of drug-herb combinations to treat various diseases like diabetes more effective than conventional drugs alone. So providing evidence on the safety and efficiency of herbal medicines including the possibility of interactions with conventional antidiabetic drugs is imperative.
In the current study co-administration of AETL and metformin on blood glucose levels and liver function in diabetic rats was investigated. The results of hypoglycemic activity showed that, as expected, AETL, MET50 and MET100 significantly reduced elevated blood glucose levels when they administrated separately. Interestingly, the hypoglycemic potential of the drug and herb improved when they were co-administrated. The biochemical and histopathological markers regarding liver function revealed that metformin particularly in the high dose (100 mg/kg) ameliorated AST levels and microvesicular alterations while it could not affect ALT levels significantly. In contrast to metformin, AETL treatment caused a dramatic elevation in AST levels and lymphocytes infiltration while it sufficiently ameliorated ALT levels in diabetic animals. However, in concomitant therapy they efficiently attenuated liver enzymes elevation (both AST and ALT) and histological damages.
Metformin is an aminoguanidine hypoglycemic drug that widely used in the management of diabetes. Several studies have shown it has insulin sensitizing effects.24, 25, 26 In one hand, it is well established that therapeutic effects of metformin are attributed to its effects on muscle and liver by increasing glucose uptake and decreasing glucose production, respectively. Also it has been reported that metformin restore the antioxidant status, enzymatic activity and inflammatory parameters in diabetic patients.27 Hypoglycemic activity of AETL is attributed to its high levels of polyphenol compounds (20.88 ± 0.72 mg/g Gallic acid equivalent) stimulating of peripheral glucose uptake in tissues, decreasing liver gluconeogenesis, regulating carbohydrate metabolism, and attenuating intestinal absorption of dietary carbohydrates.11 The synergistic effects on glucose lowering potential observing in this study might be due to the similar hypoglycemic mechanisms of metformin and AETL. Based on these data, it can be concluded that metformin and AETL had synergistic effects in hypoglycemic activity.
The liver plays a pivotal role in regulating glucose levels in different physiological and pathological states such as diabetes. Moreover, many drugs and compounds are metabolized and detoxified in the liver, so it is subject to potential damage from an enormous array of therapeutic and environmental chemicals.28 A huge number of evidence indicates that diabetes is associated with a wide range of liver abnormalities including abnormal glycogen deposition, non-alcoholic fatty liver disease (NAFLD), fibrosis, cirrhosis, hepatocellular carcinomas, abnormal elevated liver enzymes, acute liver disease and viral hepatitis.29, 30, 31
The results of this study demonstrated that diabetic rats presented histological alterations and enzymes elevation in the liver that largely resembled chronic liver disease in humans. The histopathological evaluations showed liver lesions ranged from microvesicular fat deposition to mononuclear cells infiltration. The histological examination showed that metformin and AETL could greatly ameliorate microvesicular alterations when they were used separately. However, some inflammatory infiltrations as well as hemorrhagic signs into sinusoidal spaces were remained in these groups. On the other hand, concurrent administration of metformin with AETL revealed a normal liver structure only with a mild sinusoidal dilatation.
In diabetes, derangement of lipid, protein and carbohydrate metabolism can lead to NAFLD.32 Increased oxidative stress and aberrant inflammatory response are the main causes of liver injury in diabetic patients. These underlying mechanisms lead to up-regulation of pro-apoptotic genes resulting hepatocyte damages.28 Fat droplets deposition is one of the main hepatocellular damages observed in diabetic patients as well as animal models, it is known as NAFLD. Subsequently, some pathological responses including hepatic inflammation, necrosis and fibrosis are observed in diabetes or hyperlipidemic occasions which are symptoms of a condition known as non-alcoholic steato-hepatitis (NASH).33, 34 During early stage of NASH, fat accumulation into hepatocytes leads to pathological feature calling microvesicular steatosis (without nucleus distortion), which then progresses to macrovesicular steatosis that garble the hepatocyte nucleus.35 The other prominent features of liver steatosisare included mild lymphocytic, neutrophilic and other inflammatory infiltration. An increment of fat accumulation in liver causes modification of adipocytokines secretion and it may contribute to mitochondrial oxidative stress and trigger the formation of free radicals in peroxisomes.28 There are strong body of evidence indicates that metformin improves liver function, but not histological response in NAFLD patients.36 In our knowledge, there is no research regarding turnip leaf effects on NAFLD in human or animal studies. However, in study conducted by Mohajeri et al. turnip root extracts ameliorated early hepatic injuries in diabetic rats.37 The difference between these findings may be due to use of different parts of turnip (leaf and root) in above mentioned studies.
In present study, similar to several other investigations, diabetic rats had high level of plasma AST and ATL compared to normal control group. Plasma levels of AST and ALT are the first and foremost indicators in assessing liver injuries. Evidence have shown that in chronic hepatitis and cirrhosis, serum AST levels are higher than ALT; this may reflect hepatic cell necrosis with releasing of mitochondrial AST.38, 39 Metformin only at the dose of 100 mg/kg could decrease AST levels in diabetic rats. The beneficial effects of metformin on liver enzymes activity in diabetic rats have been reported in some studies.40 In addition, the present results in metformin effects on elevated AST and ALT levels are consistent with the findings of Ghadge et al. (2016) who reported metformin had not beneficial effects on elevated ALT levels in diabetic rats.41 On the other hand, monotherapy with AETL significantly increased AST levels even more than diabetic animals while it reduced ALT levels efficiently. Likewise, similar results were observed in early study in which AETL has been administrated to diabetic rats at the same dose.11 Also there is evidence that shows in some occasions, brassica forage (like turnip leaf) crazing can cause hepatotoxicity in dairy cows.42 There are a variety of sulfur-containing glucosinolates (GSLs) in turnip greens.43 Various nitrile and epithionitrile derivatives of GSL found in crop plants have been shown to be hepatic, renal, or gastric toxins in rats.44 Interestingly, combination of metformin with AETL efficiently prevented the hepatic damages in diabetic rats. It can be concluded that metformin and AETL with a selective synergism interaction are able to ameliorate liver alterations in diabetic rats.
6. Conclusion
In summary, the results of this study indicate that concurrent administration of AETL with metformin not only has not agonist effects on glucose lowering activity, but also might increase it, significantly. Also, while single use of metformin and AETL represented some incompetence or toxic effects on liver function markers, respectively, but co-administration of AETL and metformin could ameliorate liver injury by positive interaction.
Conflicts of interest
The authors have no financial or nonfinancial conflicts of interest to declare.
Acknowledgments
We thankfully acknowledge Mr. Pouyan, Birjand University, for confirming identification of the plant.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Collaboration NRF Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roglic G. WHO Global report on diabetes: a summary. Int J Noncommunicable Dis. 2016;1(1):3. [Google Scholar]
- 3.Henriksson M., Jindal R., Sternhufvud C., Bergenheim K., Sörstadius E., Willis M. A systematic review of cost-effectiveness models in type 1 diabetes mellitus. Pharm Econ. 2016;34(6):569–585. doi: 10.1007/s40273-015-0374-8. [DOI] [PubMed] [Google Scholar]
- 4.Ghiravani Z., Hosseini M., Taheri M.H., Fard M.H., Abedini M.R. Evaluation of hypoglycemic and hypolipidemic effects of internal septum of walnut fruit in alloxan-induced diabetic rats. Afr J Tradit Complement Altern Med (AJTCAM) 2016;13(2):94–100. [Google Scholar]
- 5.Saravanamuttu S., Sudarsanam D. Antidiabetic plants and their active ingredients: a review. Int J Pharm Sci Res. 2012;3(10):3639. [Google Scholar]
- 6.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2013;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gopalakrishna R.N., Bannimath G., Huded S.P. Herb-drug interaction: effect of poly-herbal formulation on glibenclamide therapy in patients with Type-2 diabetes mellitus. Pharm Methods. 2017;(1):8. [Google Scholar]
- 8.Agbabiaka T., Wider B., Watson L.K., Goodman C. Concurrent use of prescription drugs and herbal medicinal products in older adults: a systematic review protocol. Syst Rev. 2016;5(1):65. doi: 10.1186/s13643-016-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fugh-Berman A. Herb-drug interactions. Lancet. 2000;355(9198):134–138. doi: 10.1016/S0140-6736(99)06457-0. [DOI] [PubMed] [Google Scholar]
- 10.Dixon G.R. CABI; Wallingford: 2007. Vegetable brassicas and related crucifers. [Google Scholar]
- 11.Hassanpour Fard M., Naseh G., Lotfi N., Hosseini S.M., Hosseini M. Effects of aqueous extract of turnip leaf (Brassica rapa) in alloxan-induced diabetic rats. Avicenna J Phytomed. 2015;5(2):148–156. [PMC free article] [PubMed] [Google Scholar]
- 12.HassanpourFard M., Naseh G., Lotfi N., Hosseini M. Effect of aqueous extract of turnip root on glucose and lipid profile in Alloxan induced diabetic rats. J Gorgan Univ Med Sci. 2015;17(4):36–42. [Google Scholar]
- 13.Hassanzadeh-Taheri M., Hosseini M., Hassanpour-Fard M. Effect of turnip leaf and root extracts on renal function in diabetic rats. Orient Pharm Exp Med. 2016;16(4):279–286. [Google Scholar]
- 14.Vafaei Nejad S., Serki E., Hassanpour Fard M., Hosseini M. Hypolipidemic activity of aqueous extract of turnip (Brassica rapa) root in hyperlipidemic rats. Q Horizon Med Sci. 2015;21(1):45–51. [Google Scholar]
- 15.Rojas L.B.A., Gomes M.B. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr. 2013;5(1):6. doi: 10.1186/1758-5996-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nandy M., Mandal A., Banerjee S., Ray K. A prescription survey in diabetes assessing metformin use in a tertiary care hospital in Eastern India. J Pharmacol Pharmacother. 2012;3(3):273–275. doi: 10.4103/0976-500X.99444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miralles-Linares F., Puerta-Fernandez S., Bernal-Lopez M.R., Tinahones F.J., Andrade R.J., Gomez-Huelgas R. Metformin-induced hepatotoxicity. Diabetes Care. 2012;35(3) doi: 10.2337/dc11-2306. e21–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prabhakar P., Kumar A., Doble M. Combination therapy: a new strategy to manage diabetes and its complications. Phytomedicine. 2014;21(2):123–130. doi: 10.1016/j.phymed.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Hami J., Vafaei-nezhad S., Ghaemi K. Stereological study of the effects of maternal diabetes on cerebellar cortex development in rat. Metab Brain Dis. 2016;31(3):643–652. doi: 10.1007/s11011-016-9802-5. [DOI] [PubMed] [Google Scholar]
- 20.Etuk E. Animals models for studying diabetes mellitus. Agric Biol JN Am. 2010;1(2):130–134. [Google Scholar]
- 21.Cameron A., Ewen M., Ross-Degnan D., Ball D., Laing R. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet. 2009;373(9659):240–249. doi: 10.1016/S0140-6736(08)61762-6. [DOI] [PubMed] [Google Scholar]
- 22.Banerjee J. Medicinal plants with potential hypoglycemic property-a review. J Biomed Pharm Res. 2015;4(1) [Google Scholar]
- 23.Enioutina E.Y., Salis E.R., Job K.M., Gubarev M.I., Krepkova L.V., Sherwin C.M. Herbal Medicines: challenges in the Modern World. Part 5. Status and current directions of complementary and alternative herbal medicine worldwide. Expert Rev Clin Pharmacol. 2017;10(3):327–338. doi: 10.1080/17512433.2017.1268917. [DOI] [PubMed] [Google Scholar]
- 24.Alhaider A.A., Korashy H.M., Sayed-Ahmed M.M., Mobark M., Kfoury H., Mansour M.A. Metformin attenuates streptozotocin-induced diabetic nephropathy in rats through modulation of oxidative stress genes expression. Chemico-Biol Interact. 2011;192(3):233–242. doi: 10.1016/j.cbi.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Fu J., Fu J., Yuan J. Anti-diabetic activities of Acanthopanax senticosus polysaccharide (ASP) in combination with metformin. Int J Biol Macromol. 2012;50(3):619–623. doi: 10.1016/j.ijbiomac.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 26.Katakam P.V., Ujhelyi M.R., Hoenig M., Miller A.W. Metformin improves vascular function in insulin-resistant rats. Hypertension. 2000;35(1):108–112. doi: 10.1161/01.hyp.35.1.108. [DOI] [PubMed] [Google Scholar]
- 27.Chakraborty A., Chowdhury S., Bhattacharyya M. Effect of metformin on oxidative stress, nitrosative stress and inflammatory biomarkers in type 2 diabetes patients. Diabetes Res Clin Pract. 2011;93(1):56–62. doi: 10.1016/j.diabres.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 28.Mohamed J., Nafizah A.N., Zariyantey A., Budin S.B. Mechanisms of diabetes-induced liver damage: the role of oxidative stress and inflammation. Sultan Qaboos Univ Med J. 2016;16(2):e132–e141. doi: 10.18295/squmj.2016.16.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Starley B.Q., Calcagno C.J., Harrison S.A. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51(5):1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 30.Tolman K.G., Fonseca V., Dalpiaz A., Tan M.H. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. 2007;30(3):734–743. doi: 10.2337/dc06-1539. [DOI] [PubMed] [Google Scholar]
- 31.Azeemuddin M., Rafiq M., Anturlikar S.D. Extract of a polyherbal formulation ameliorates experimental nonalcoholic steatohepatitis. Journal of Traditional and Complementary Medicine. 2016;6(2):160–167. doi: 10.1016/j.jtcme.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paschos P., Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 2009;13(1):9–19. [PMC free article] [PubMed] [Google Scholar]
- 33.Greenberg A.S., Coleman R.A., Kraemer F.B. The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest. 2011;121(6):2102–2110. doi: 10.1172/JCI46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duarte N., Coelho I.C., Patarrão R.S., Almeida J.I., Penha-Gonçalves C., Macedo M.P. How inflammation impinges on NAFLD: a role for Kupffer cells. Biomed Res Int. 2015;2015 doi: 10.1155/2015/984578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakhuja P. Pathology of alcoholic liver disease, can it be differentiated from nonalcoholic steatohepatitis? World J Gastroenterol WJG. 2014;20(44):16474. doi: 10.3748/wjg.v20.i44.16474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y.A.N., Liu L.E.I., Wang B.I.N., Wang J.U.N., Chen D. Metformin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Biomed Rep. 2013;1(1):57–64. doi: 10.3892/br.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daryoush M., Bahram A.T., Yousef D., Mehrdad N., Brassica rapa L. Extract alleviate early hepatic injury in alloxan-induced diabetic rats. J Med Plants Res. 2011;5(31):6813–6821. [Google Scholar]
- 38.Murali A.R., Carey W.D. Liver test interpretation-approach to the patient with liver disease: a guide to commonly used liver tests. Dis Manag. 2000 [Google Scholar]
- 39.Singh H., Prakash A., Kalia A.N., Majeed A.B.A. Synergistic hepatoprotective potential of ethanolic extract of Solanum xanthocarpum and Juniperus communis against paracetamol and azithromycin induced liver injury in rats. Journal of Traditional and Complementary Medicine. 2016;6(4):370–376. doi: 10.1016/j.jtcme.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng J., Woo S.-L., Hu X. Metformin and metabolic diseases: a focus on hepatic aspects. Front Med. 2015;9(2):173–186. doi: 10.1007/s11684-015-0384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghadge A., Harsulkar A., Karandikar M., Pandit V., Kuvalekar A. Comparative anti-inflammatory and lipid-normalizing effects of metformin and omega-3 fatty acids through modulation of transcription factors in diabetic rats. Genes Nutr. 2016;11(1):10. doi: 10.1186/s12263-016-0518-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collett M.G., Stegelmeier B.L., Tapper B.A. Could nitrile derivatives of turnip (Brassica rapa) glucosinolates be hepato-or cholangiotoxic in cattle? J Agric food Chem. 2014;62(30):7370–7375. doi: 10.1021/jf500526u. [DOI] [PubMed] [Google Scholar]
- 43.Padilla G., Cartea M.E., Velasco P., de Haro A., Ordás A. Variation of glucosinolates in vegetable crops of Brassica rapa. Phytochemistry. 2007;68(4):536–545. doi: 10.1016/j.phytochem.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 44.McDanell R., McLean A., Hanley A., Heaney R., Fenwick G. Chemical and biological properties of indole glucosinolates (glucobrassicins): a review. Food Chem Toxicol. 1988;26(1):59–70. doi: 10.1016/0278-6915(88)90042-7. [DOI] [PubMed] [Google Scholar]