Abstract
The present study is designed to investigate the anti-oral cancer properties of Solanum nigrum on oral squamous cell carcinoma. S. nigrum is a Chinese herb used for suppression of various cancers. However, the inhibition of S. nigrum on oral cancer is unclear. Therefore, human oral squamous cancer cells (SCC)-4 were used to evaluate the effect of aqueous extracts of S. nigrum (AESN) on cancer cell proliferation, cell cycle, mitochondrial function and apoptosis. The SCC-4 cells were treated by AESN to evaluate the inhibition of cell proliferation and mitochondrial function in vitro. Our results suggested that AESN markedly increased reactive oxygen species production. AESN also promoted caspase-9 and caspase-3 activation and subsequent triggering of the mitochondrial apoptotic pathway. The inhibition of glucose uptake was alleviated mediated by a dose-dependent manner in SCC-4 cells with AESN treatment for 24 h, resulting in mitochondrial fission. These results suggested that AESN has potential to be used as a functional food in adjuvant chemotherapy for treating human oral cancer by suppression of mitochondrial function.
Keywords: Oral squamous cell carcinoma, Solanum nigrum, Mitochondrial function, Apoptosis, Mitochondrial fission
Graphical abstract
1. Introduction
Oral cancer is the fifth most common neoplasm worldwide, accounting for more than 500,000 cases annually.1 In Taiwan it has the fastest-rising incidence and mortality rate of any cancer and is the sixth most common cause of cancer death, being more prevalent in males than in females. Tobacco and alcohol consumption have been reported to be the major factors in the development of oral cancer.2 Diets low in carotenoids and vitamin A, poor oral hygiene and indoor air pollution are also recognized as factors in oral cancer.3, 4 However, betel quid chewing is one of the most important causes of oral cancer in Taiwan, with high mortality and poor prognosis. Therefore, in an effort to improve patient survival and quality of life, new therapeutic approaches focusing on the molecular target and mechanism that mediate tumor cell growth or cell death has gained much attention.
Oxidative damage to cellular macromolecules can arise through the overproduction of reactive oxygen species (ROS) and faulty antioxidant and/or DNA repair mechanisms that result in cancer.5 Chronic inflammation can lead to the production of chemical intermediates such as nitrogen oxide, which in turn can mediate DNA damage and block the DNA repair system.6, 7, 8 Chemoprevention has evolved as a novel approach to control the incidence of oral cancer. Therefore, it is important to establish chemoprevention in an experimental animal tumor model that mimics specific characteristics of human oral squamous cell carcinoma. Chemoprevention by dietary agents has evolved as an effective strategy to control the incidence of oral cancer. The present study was designed to evaluate the inhibitory effects of the water extract from Solanum nigrum (AESN) on squamous cancer cells-4 (SCC-4).
2. Materials and methods
2.1. Sample isolation
The S. nigrum was collected in Tainan market (Taiwan) on January. The leaf of S. nigrum (1 kg) was extracted with water (10 L) three times at room temperature. After evaporating the solvents under vacuum at 40 °C and frozen-dried, a residue powder was obtained (179.4 g).
2.2. Cell culture
Human SCC-4 cell lines were obtained from the Bioresource Collection and Research Center (Hsinchu, Taiwan). SCC-4 cells were maintained in DMEM/Ham's F-12 (1:1 v/v) medium supplemented with 100 mL/L FBS, 1.5 g/L sodium bicarbonate, 400 ng/mL hydrocortisone and 10 mL/L antibiotic solution. Cells were incubated in 5% CO2 and 95% humidified atmosphere at 37 °C.
2.3. Cell viability
The cell-killing effect of AESN against oral cancer cells was measured using the crystal violet staining assay. Cells were seed on 24-well plates (3 × 104 cells per well) and treated with various concentrations of AESN for 24 and 48 h, respectively. The medium was then removed, washed with phosphate buffered saline (PBS) and stained with 2 g/L crystal violet in 100 mL/L phosphate-buffered formaldehyde for 20 min before being washed with water. The crystal violet bound to the cells was dissolved in 20 g/L SDS solution and its absorbance at 600 nm was measured.
2.4. Apoptosis analysis
For apoptosis detection, floating cells in the medium and adherent cells were collected after 24 h of AESN treatment. Cells were harvested, washed in ice-cold PBS and resuspended in 200 μL of binding buffer before being incubated in 5 μL of Annexin V-fluorescein isothiocyanate (FITC) (BD Biosciences) solution and 5 μL of propidium iodide (PI) at room temperature for 15 min in the dark. Then 300 μL of binding buffer was added. Cells were analyzed by flow cytometry. Untreated cells were used as the control for double staining.
2.5. Assay for caspase-3
After treatment of AESNfor 12 h, SCC-4 cells were treated with anti-caspase-3 and caspase-9 antibody with fluorescent dyes for 30 min. After PBS wish, cells were analyzed by flow cytometry. Untreated cells were used as the control for double staining.
2.6. Assay for oxidative stress
The level of oxidative stress was monitored by the measurement of ROS. Collected cells were suspended in 500 μL of PBS and mixed with 10 μM (final concentration) of dichloro-dihydro-fluorescein diacetate (DCFH-DA) to incubate for 20 min at 37 °C. The cells were washed thrice with phosphate-buffered saline (PBS) to remove redundant DCFH-DA. The cell pellet was mixed with 500 μL of PBS, and the ROS level was assayed by flow cytometry (Becton-Dickinson, San Jose, CA).
2.7. Measurement of glucose uptake
SCC-4 cells was treated with 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-d-glucose (2-NBDG) for 30 min after ethanolic extract of dandelion induction for 24 h. Ability of glucose uptake in SCC-4 oral cancer cells was assayed by flow cytometry (Becton-Dickinson, San Jose, CA).
2.8. Western blot
Cells were lysed in ice-cold lysis buffer containing 20 mM Tris–HCl (pH 7.4), 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), 2 mM ethylenediaminetetraacetic acid (EDTA), 10 mM NaF, 1 mM phenylmethylsulfonyl fluoride (PMSF), 500 μM sodium vanadate, and 10 μg/mL aprotinin overnight. Then, the cell extract was centrifuged (12,000g for 10 min) to recover the supernatant. The supernatant was taken as the cell extract. The cell protein was resolved on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to polyvinyldiene fluoride (PVDF) membrane. The membranes were blocked with 5% nonfat dry milk solution for 1 h and incubated overnight with primary antibodies for 4 h; subsequently, the membrane was washed 3 times each for 5 min in phosphate-buffered saline with Tween 20 (PBST), shaken in a solution of horseradish peroxidase (HRP)-linked secondary antibody for 1 h, and washed 3 more times each for 5 min in PBST. The expressions of proteins were detected by enhanced chemiluminescent (ECL) reagent (Millipore, Billerica, MA).
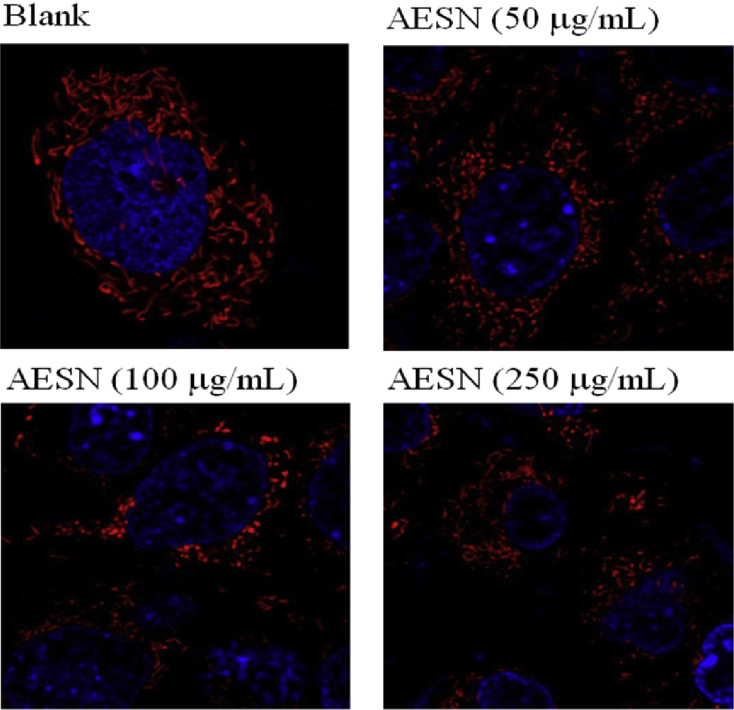
2.9. Mitochondrial fission
Cells were treated by 250 nM of MitoTracker Deep-Red FM (Invitrogen) for 30 min in serum-free culture medium. After wash with PBC twice, nuclei were stained by Hochest 33342 for 10 min. Themitochondrial morphology was observed by confocal microscope.
2.10. Statistical analysis
Experimental results were analyzed in triplicates and expressed as means ± standard deviation (SD). The results were subjected to one-way analysis of variance (ANOVA) and Duncan's multiple range tests and the significance of differences between sample means was calculated. P ≤ 0.05 was considered significant.
3. Results
3.1. Cell viability
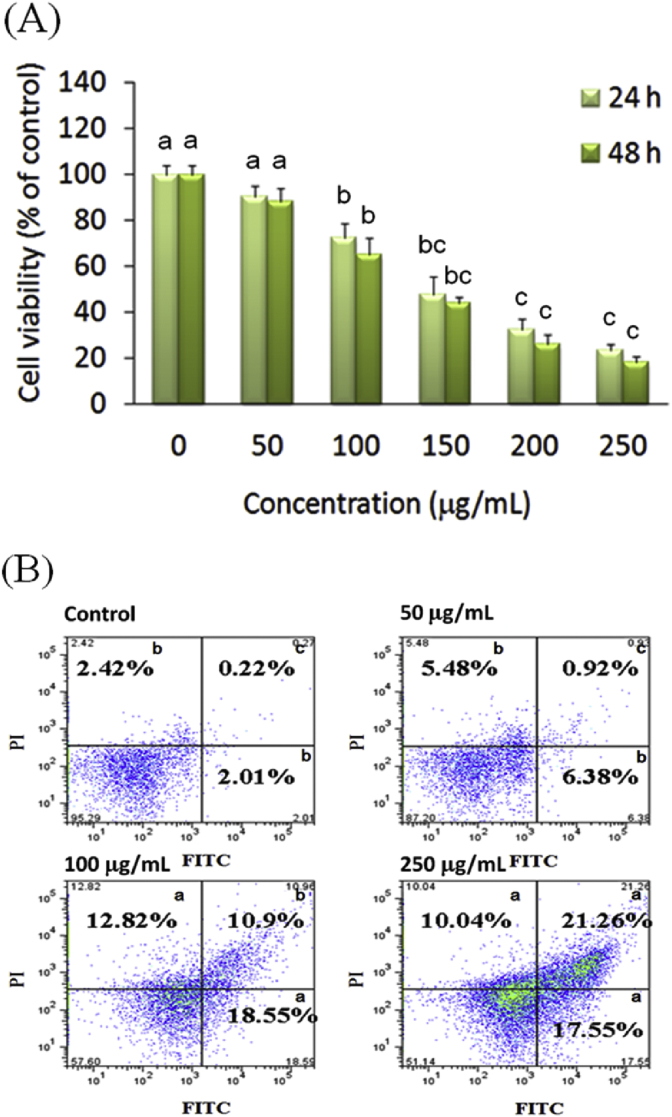
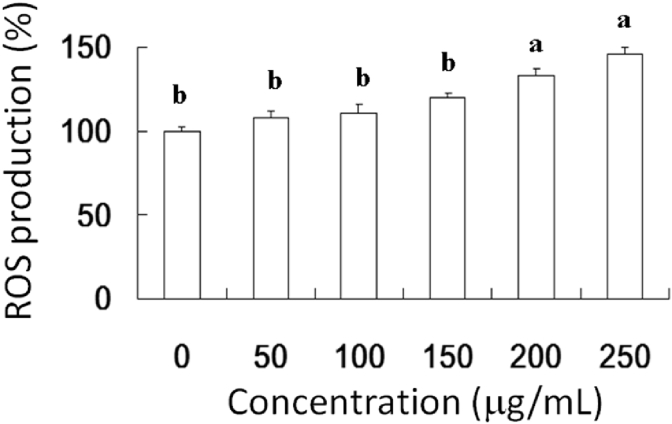
The dose-dependent and time-dependent effects of AESN on the viability of oral cancer SCC-4 cells were examined after 24 h and 48 h treatment, respectively. AESN significantly exerted the inhibitory effect on cell viability of SCC-4 cells after 24 h and 48 h treatment (Fig. 1A). To further elucidate the mechanism of action of AESN on SCC-4 cells, we examined the effect of AESN on apoptosis by performing Annexin V-FITC/PI double staining. This staining method along with flow cytometry enables the quantitative assessment of living (Annexin V-FITC negative/PI negative), early apoptotic (Annexin V-FITC positive/PI negative), late apoptotic/necrotic (Annexin V-FITC positive/PI positive) and dead (Annexin V-FITC negative/PI positive) cells. The effects of a 24 h with AESN treatment on SCC-4 cell apoptosis are shown in Fig. 1B. We found that 50, 100, and 250 μg/mL of AESN treatment markedly elevated apoptotosis and necrosis. Our results suggested that 200 and 250 μg/mL of AESN markedly increased ROS production by 24 h treatment (Fig. 2).
Fig. 1.
(A) Inhibitory effect of AESN on cell viability of SCC-4 after 24 h and 48 h treatment. Data were shown as mean ± SD (n = 3). Significantly difference was shown as various letters (between a, b, bc, c in 24 h or 48 h) (P < 0.05). (B) Induction of cell apoptosis and necrosis by AESN in SCC-4 cells. After 24 h treatment of AESN, the apoptotic event was detected by co-staining with Annexin V and PI using flow cytometry. Untreated cells were used as the control for double staining. a,b,cValues with one different letter superscript are significantly different from each other (P < 0.05).
Fig. 2.
Induction of oxidative stress by AESN in SCC-4 cells. ROS level of SCC-4 cells treated with AESN for 24 h was measured by flow cytometry. Data were shown as mean ± SD (n = 3). a,bValues with one different letter superscript are significantly different from each other (P < 0.05).
3.2. Inhibition of glucose uptake and mitochondrial activity
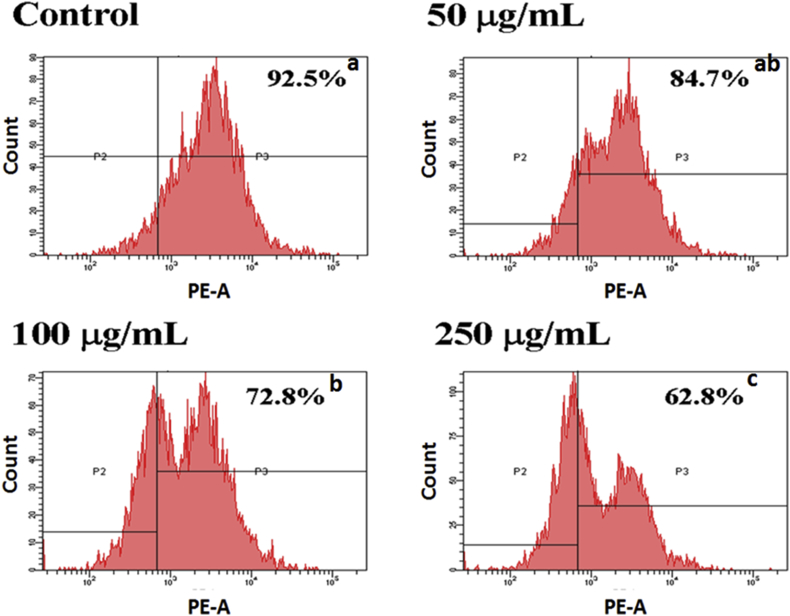
During cell proliferation, glucose metabolism and ATP production both play important roles for providing cell growth. However, if absorption of glucose was inhibited in turn cell growth was suppressed. We investigated that AESN affected the glucose (2-NBDG) uptake. The inhibition of glucose uptake was alleviated mediated by a dose-dependent manner in SCC-4 cells with AESN treatment for 24 h were 92.5% (control), 84,7% (AESN 50 μg/mL), 72.8% (100 μg/mL), and 62.8% (250 μg/mL), respectively (Fig. 3). Given that mitochondrial morphology affects energy imbalance and is continuously changed through fusion and fission events, a tight coordination between mitochondrial dynamics and interorganelle interactions is crucial. Mitochondrial fission results in an impaired insulin-dependent glucose uptake.9 As shown in Fig. 4, the mitochondrial morphology was clearly changed by AESN treatment, mitochondrial fission was observed in AESN treated groups.
Fig. 3.
Inhibition of glucose uptake in SCC-4 cells treated by AESN induction for 24 h. The glucose uptake was assayed by flow cytometry. a,b,cValues with one different letter superscript are significantly different from each other (P < 0.05).
Fig. 4.
The mitochondrial morphology of SCC-4 cells treated by AESN was observed by MitoTracker-Deep Red stain with confocal microscopy.
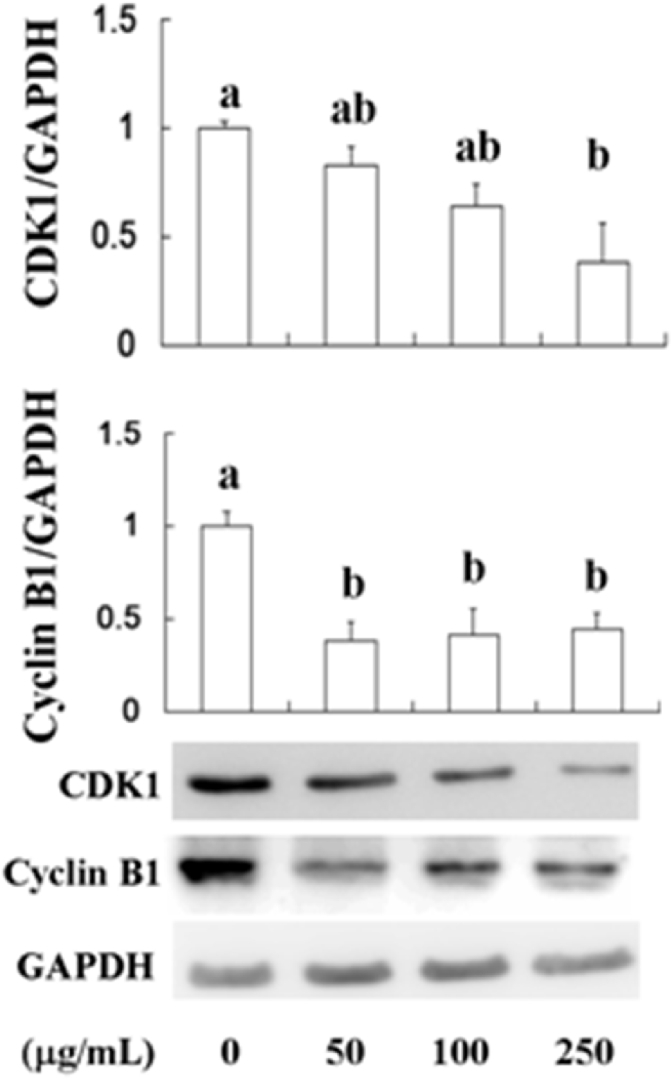
3.3. Cell cycle arrest
Cancer is one of the hallmarks of cancer is the lack of regulation in the cell cycle. The role of cyclin B1 is to transition the cell from G2 to M phase but becomes unregulated in cancer cells where overexpression of cyclin B1 can lead to uncontrolled cell growth by binding to its partner cyclin-dependent kinases (CDKs).10 Binding of CDKs can lead to phosphorylation of other substrates at inappropriate time and unregulated proliferation. CDK1 is a highly conserved protein that functions as a serine/threonine kinase, and is a key player in cell cycle regulation. CDK1 is regulated by its binding with its cyclin partners. AESN has found to inhibit cell viability and induce apoptosis. These effects were also associated with the repression of CDK1 and cyclin B1 levels in SCC-4, resulting in cell proliferation inhibition. We found that CDK1 and cyclin B1 both were suppressed by AESN (50, 100, and 250 μg/mL) treatment (Fig. 5).
Fig. 5.
Suppressions of CDK1 and cyclin B1 by AESN. CDK1 and cyclin B1 protein levels were detected by Western blot after treating with AESN in SCC-4 cells. Data were shown as mean ± SD (n = 3). a,b,cValues with one different letter superscript are significantly different from each other (P < 0.05).
4. Discussion
Several lines of evidence demonstrate that excessive oxidative stress caused by ROS and reactive nitrogen species (RNS) can promote disease progression via the oxidation of biomolecules such as DNA, lipids, and proteins.11, 12 High levels of ROS in the mitochondria can result in free radicals attacking membrane phospholipids, which precede mitochondrial membrane depolarization. Mitochondrial depolarization, which is considered an irreversible step in apoptosis, triggers a cascade of caspases.13 In the present study, the 250 μg/mL AESN treatment efficiently resulted in ROS synthesis (Fig. 2). The enhancement of ROS production led to increased apoptosis events (Fig. 1).
Cyclin binding alters access to the active site of CDK1, allowing for CDK1 activity; furthermore, cyclins impart specificity to CDK1 activity. At least some cyclins contain a hydrophobic patch that may directly interact with substrates, conferring target specificity.14 Cyclin B1 is a regulatory protein involved in mitosis, which complexes with CDK1 to form the maturation-promoting factor. Two alternative transcripts have been found, a constitutively expressed transcript and a cell cycle-regulated transcript that is expressed predominantly during G2/M phase of the cell cycle. The different transcripts result from the use of alternate transcription initiation sites.15 Cyclin B1 contributes to the switch-like all or none behavior of the cell in deciding to commit to mitosis. Cyclin B1-CDK1 is involved in the early events of mitosis, such as chromosome condensation, nuclear envelope breakdown, and spindle pole assembly. A suppressed Cyclin B1 and CDK1 expression was shown after AESN treatment, which infers that AESN might target G2/M phase and then mediates cell cycle progression and apoptosis events. In recent years, natural food products have received increasing attention because of their potential roles in the prevention and/or intervention of cancers, including lung cancer. Oxidative damage to cellular macromolecules can arise through the overproduction of ROS and faulty antioxidant and/or DNA repair mechanisms that result in cancer.16 Phytochemicals offer significant protective benefits against oxidative damage.17 The antiproliferative activities of polyphenols such as delphinidin, cyanidin, peonidin, petunidin, and malvidin have been previously reported.17 AESN, as shown in the present study, suppressed cell viability (Fig. 1) and induced apoptosis in the SCC-4 cancer cells via increased ROS expression, enhanced release of cytochrome c, and induction of caspase-3, caspase-8, and caspase-9 expression and enhanced p53 expression.
Many biologic markers provide information on differentiation, proliferation and prognosis. In addition, some phytochemicals arrest the cell cycle in cancer cells by altering signal transduction pathways and via the induction of apoptosis through the generation of ROS; ensuing cell death is accompanied by the activation of certain stress kinases.18 Different caspases mediate two apoptotic signaling pathways. Initiation of the Fas signaling pathway by either Fas/death receptors or tumor necrosis factor (TNF)-receptor causes recruitment of Fas-associated protein with death domain (FADD) via interactions between the death domains of Fas and FADD. The binding of procaspase-8 to the Fas/FADD complex, in turn activating caspase-3 and inducing apoptosis, activates caspase-8. The second apoptotic pathway is regulated by the mitochondrial release of cytochrome c, resulting in the activation of caspase-9 and caspase-3.19 We found that the elevation of caspase-3 was induced by AESN treatment in SCC-4 oral cells.
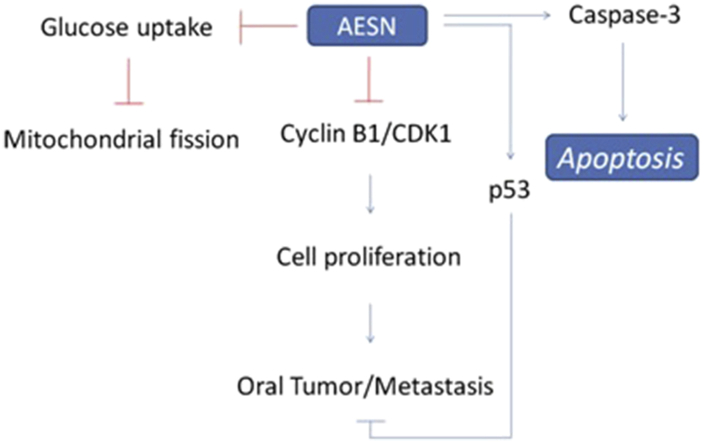
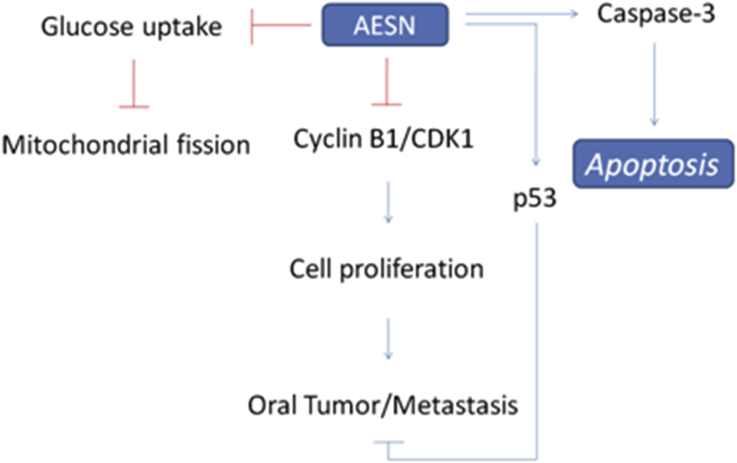
Our recent researches have reported that S. nigrum showed anti-cancer activity for hepatocellular carcinoma cells,20 human ovarian carcinoma cells,21 human colorectal carcinoma cells,22 and human endometrial carcinoma cells.23 We found that AESN exerts a chemoprevention activity in the inhibition of mitochondrial function (such as mitochondrial fusion), which is possible for oral cancer prevention in this study. DMBA model has been reported to as model of oral tumor to evaluate the chemoprotection and regulation of mitochondrial fusion.24, 25, 26 We also found that AESN may serve as a possible functional food in the development of human oral cancer adjuvant chemotherapy (Fig. 6).
Fig. 6.
The potential mechanism of AESN on oral cancer.
Conflict of interest
The author(s) declare(s) that there is no conflict of interests regarding the publication of this paper.
Funding
This research work and subsidiary spending were supported by the Shin Kong Wu Ho-Su Memorial Hospital (SKH-TMU-104-09) (Taiwan, ROC).
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Parkin D.M., Bray F., Ferlay J., Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Blot W.J., McLaughlin J.K., Winn D.M. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–3287. [PubMed] [Google Scholar]
- 3.McLaughlin J.K., Gridley G., Block G. Dietary factors in oral and pharyngeal cancer. J Nat Cancer Ins. 1988;80:1237–1243. doi: 10.1093/jnci/80.15.1237. [DOI] [PubMed] [Google Scholar]
- 4.Pintos J., Franco E.L., Kowalski L.P., Oliveira B.V., Curado M.P. Use of wood stoves and risk of cancers of the upper aero-digestive tract: a case-control study. Int J Epidemiol. 1998;27:936–940. doi: 10.1093/ije/27.6.936. [DOI] [PubMed] [Google Scholar]
- 5.Klaunig J.E., Kamendulis L.M., Hocevar B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 2010;38:96–109. doi: 10.1177/0192623309356453. [DOI] [PubMed] [Google Scholar]
- 6.Wink D.A., Vodovotz Y., Laval J., Laval F., Dewhirst M.W., Mitchell J.B. The multifaceted roles of nitric oxide in cancer. Carcinogenesis. 1998;19:711–721. doi: 10.1093/carcin/19.5.711. [DOI] [PubMed] [Google Scholar]
- 7.Marnett L.J., DuBois R.N. COX-2: a target for colon cancer prevention. Ann Rev Pharmacol Toxicol. 2002;42:55–80. doi: 10.1146/annurev.pharmtox.42.082301.164620. [DOI] [PubMed] [Google Scholar]
- 8.Pace E., Siena L., Ferraro M. Role of prostaglandin E2 in the invasiveness, growth and protection of cancer cells in malignant pleuritis. Eur J Cancer. 2006;42:2382–2389. doi: 10.1016/j.ejca.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Hsu W.H., Lee B.H., Pan T.M. Leptin-induced mitochondrial fusion mediates hepatic lipid accumulation. Int J Obes. 2015;39:1750–1756. doi: 10.1038/ijo.2015.120. [DOI] [PubMed] [Google Scholar]
- 10.Cheng H.C., Chien H., Liao C.H., Yang Y.Y., Huang S.Y. Carotenoids suppress proliferating cell nuclear antigen and cyclin D1 expression in oral carcinogenic models. J Nutr Biochem. 2007;18:667–675. doi: 10.1016/j.jnutbio.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Burke A., Fitzgerald G.A. Oxidative stress and smoking-induced vascular injury. Prog Cardiovasc Dis. 2003;46:79–90. doi: 10.1016/s0033-0620(03)00076-8. [DOI] [PubMed] [Google Scholar]
- 12.Nakao L.S., Iwai L.K., Kalil J., Augusto O. Radical production from free and peptide-bound methionine sulfoxide oxidation by peroxynitrite and hydrogen peroxide/iron (II) FEBS Lett. 2003;547:87–91. doi: 10.1016/s0014-5793(03)00674-4. [DOI] [PubMed] [Google Scholar]
- 13.Stohs S.J., Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 14.Brown N.R., Noble M.E., Endicott J.A., Johnson L.N. The structural basis for specificity of substrate and recruitment peptides for cyclin-dependent kinase. Nat Cell Biol. 1999;1:438–443. doi: 10.1038/15674. [DOI] [PubMed] [Google Scholar]
- 15.Yuan J., Kramer A., Matthess Y. Stable gene silencing of cyclin B1 in tumor cells increases susceptibility to taxol and leads to growth arrest in vivo. Oncogene. 2006;25:1753–1762. doi: 10.1038/sj.onc.1209202. [DOI] [PubMed] [Google Scholar]
- 16.Pietenpol J.A., Stewart Z.A. Cell cycle checkpoint signaling: cell cycle arrest versus apoptosis. Toxicology. 2002;181/182:475–481. doi: 10.1016/s0300-483x(02)00460-2. [DOI] [PubMed] [Google Scholar]
- 17.Wattenberg L.W. An overview of chemoprevention: current status and future prospects. Proc Soc Exp Biol Med. 1997;216:133–141. doi: 10.3181/00379727-216-44163. [DOI] [PubMed] [Google Scholar]
- 18.Umansky V., Ushmorov A., Ratter F. Nitric oxide-mediated apoptosis in human breast cancer cells requires changes in mitochondrial functions and is independent of CD95 (APO-1/Fas) Int J Oncol. 2000;16:109–117. doi: 10.3892/ijo.16.1.109. [DOI] [PubMed] [Google Scholar]
- 19.Hsu W.H., Lee B.H., Pan T.M. Red mold dioscorea-induced G2/M arrest and apoptosis in human oral cancer cells. J Sci Food Agric. 2010;90:2709–2715. doi: 10.1002/jsfa.4144. [DOI] [PubMed] [Google Scholar]
- 20.Wang C.K., Lin Y.F., Tai C.J. Integrated treatment of aqueous extract of Solanum nigrum-potentiated cisplatin- and doxorubicin-induced cytotoxicity in human hepatocellular carcinoma cells. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/675270. Article ID 675270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C.W., Chen C.L., Wang C.K. Cisplatin-, doxorubicin-, and docetaxel-induced cell death promoted by the aqueous extract of Solanum nigrum in human ovarian carcinoma cells. Integr Cancer Ther. 2015;14:546–555. doi: 10.1177/1534735415588826. [DOI] [PubMed] [Google Scholar]
- 22.Tai C.J., Wang C.K., Chang Y.J., Lin C.S., Tai C.J. Aqueous extract of Solanum nigrum leaf activates autophagic cell death and enhances docetaxel-induced cytotoxicity in human endometrial carcinoma cells. Evid Based Complement Alternat Med. 2012;2012 doi: 10.1155/2012/859185. Article ID 859185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tai C.J., Wang C.K., Tai C.J. Aqueous extract of Solanum nigrum leaves induces autophage and enhances cytotoxicity of cisplatin, doxoorubicin, docetaxel, and 5-flurouracil in human colorectal carcinoma cells. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/514719. Article ID 514719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu W.H., Lee B.H., Pan T.M. Protection of Monascus-fermented dioscorea against DMBA-induced oral injury in hamster by anti-inflammatory and antioxidative potentials. J Agric Food Chem. 2010;58:6715–6720. doi: 10.1021/jf100889w. [DOI] [PubMed] [Google Scholar]
- 25.Hsu W.H., Lee B.H., Pan T.M. Effects of red mold dioscorea on oral carcinogenesis in DMBA-induced hamster animal model. Food Chem Toxicol. 2011;49:1292–1297. doi: 10.1016/j.fct.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Huang H.C., Syu K.Y., Lin J.K. Chemical composition of Solanum nigrum Linn extract and induction of autophage by leaf water extract and its major flavonoids in AU565 breast cancer cells. J Agric Food Chem. 2010;58:8699–8708. doi: 10.1021/jf101003v. [DOI] [PubMed] [Google Scholar]