Abstract
The present study was aimed to evaluate advanced glycation end products (AGEs) inhibitory activity of alcohol and hydro-alcohol extract (DAE and DHE) of Dillenia indica L. (Family: Dilleniaceae) and its potential in treatment of diabetic nephropathy by targeting markers of oxidative stress. D. indica was evaluated for its in vitro inhibitory activity against formation of AGEs by using bovine serum albumin. Diabetes was induced in male Wistar rats by streptozotocin (65 mg/kg i.p.) 15 min after nicotinamide (230 mg/kg, i.p.) administration. Diabetic rats were treated with different doses of extracts (100, 200 and 400 mg/kg) to analyze their nephroprotective effect. Tissue antioxidant enzymes level was measured along with the formation of AGEs in kidney to assess the effect of D. indica in ameliorating oxidative stress. D. indica showed significant inhibition of AGEs formation in vitro. D. indica produced significant attenuation in the glycemic status, renal parameter, lipid profile and level of antioxidant enzymes proving efficacy in diabetic nephropathy. Moreover, D. indica produced significant reduction in the formation of AGEs in kidneys. The present study concludes that D. indica as a possible therapeutic agent against diabetic nephropathy.
Keywords: Advanced glycation end products, Betulinic acid, Hyperglycemia, Oxidative stress
Graphical abstract
1. Introduction
Chronic hyperglycemia is the main culprit for the development of diabetic complications like diabetic nephropathy, diabetic neuropathy, diabetic retinopathy etc.1 Among these, diabetic nephropathy (DN) is the major cause of morbidity and mortality due to end stage renal disease.2 DN is precipitated due to an array of factors including aldose reductase activation, elevated hexosamine shunt, activation of protein kinase C, impaired insulin/C peptide action, and formation of advanced glycation end products (AGEs) which modulate various biochemical pathways to orchestrate auto-oxidative glycosylation and polyol pathways leading to structural and functional aberration of nephrons.3, 4 Sasai et al (2012) have found that AGEs can induce the expression of both plasminogen activator inhibitor-1 and tissue transglutaminase mRNA in tubular epithelial cells. Both gene products play an important role in the regulation of extracellular matrix protein degradations. They concluded that AGEs are powerful stimulants of renal epithelial cells, which stimulate the cells to recruit fibrosis-exacerbating macrophages.5 AGEs have been implicated in the pathogenesis of DN, which is indicated by the fact that the AGEs inhibitors such as aminoguanidine, pyridoxamine, and ALT-946 ameliorated renal damage in diabetic patients.6 It has been reported that hyperglycemia enhances the level of reactive oxygen species, which then facilitates the formation of AGEs.7 At the same time, the interactions of AGEs with their receptors alter intracellular signaling and enhance the release of pro-inflammatory cytokines and free radicals that contribute toward the pathology of diabetic complications.8, 9, 10 This mutual interaction leads to the thickening of the basement membrane, altered filtration, and, ultimately, loss of glomerular function.11 Therefore, drugs that either inhibit the formation of AGE or break AGE-induced cross-links have been shown to be renoprotective in experimental models of DN. The recent strategy for alleviating the oxidative damage in diabetes mellitus is based on supplementation with certain dietary antioxidants such as vitamins E and C and flavonoids.12
Dillenia indica L. (Dilleniaceae), commonly known as Elephant apple has been used traditionally to cure various ailments. Traditionally the juices of leaves, bark and leaves are mixed and given orally for the treatment of cancer and diarrhea in the tribal areas of Mizoram, India.13 The leaves and bark are used as a laxative and astringent. The leaves extract has also shown antioxidant activity in vitro.14 Chemical investigation of the plant evolved pentacyclic triterpene lactone,15 dihydro-isorhamnetin, dillenetin,16 lupeol, betulinaldehyde, betulinic acid and Stigmasterol.17 Kaur et al, 2016 isolated a new chromane (3,5,7-trihydroxy-2-(4-hydroxybenzyl)-chroman-4-one) moiety from alcoholic extract of D. indica and evaluated its antioxidant and antidiabetic potential.18 D. indica is a medicinal plant used for treating diabetes mellitus and related symptoms.19 The present study aims to explore beneficial effects of alcohol and hydro-alcohol extract of D. indica in DN along with the examination of AGEs expression in kidneys. Also keeping in mind the importance of betulinic acid, its quantification in the plant extract has also been carried out using HPLC, as betulinic acid has manifested large number of biological activities including anti-inflammatory,20 anti-leukemic activities, antioxidant and antidiabetic activity etc.21
2. Material and methods
2.1. Animals
Adult male Wistar rats (250–300 g) were obtained from NIPER, Mohali. They were housed in standard environmental conditions maintained at 23 ± 2 °C with 12 h light-dark cycle. Animals were fed with standard rodent diet and water ad libitum. Experimental protocol was approved by Institutional Animal Ethical Committee (IAEC) and the experiments were performed according to the guidelines of CPCSEA (MMCP/IAEC/13/11).
2.2. Preparation of extract
D. indica leaves were procured from Kurukshetra University, Kurukshetra in the month of March, 2014 and authenticated by Dr. Sunita Garg, NISCAIR New Delhi. A voucher specimen (NISCAIR/RHMD/Consult/2013/2295/75) was deposited in the herbarium of NISCAIR, New Delhi for future reference. Botanical name of the plant was verified from published literature and database.22 500 g of powdered dried leaves were subjected to Soxhlet extraction sequentially using petroleum ether 60–80 °C, chloroform, ethanol and hydro-alcohol (40 %) in a ratio of (1:8 w/v)23. The extractive solution was filtered through filter paper, dried under vacuum yielding 17.67 % of alcohol (DAE) and 9.43 % hydro-alcohol extract (DHE). Extracts were redissolved in water for further experimentation.
2.3. Chemicals
Streptozotocin (STZ) was purchased from Sigma-Aldrich, Milwaukee, USA and Nicotinamide from Finar India Ltd. Betulinic acid, standard for HPLC, was purchased from Sigma–Aldrich, Milwaukee, USA. All the other chemicals used were of analytical grade. Serum insulin level was determined using Insulin ELISA kit (Diametra, Milano, Italy). Diagnostic kits for the biochemical estimation were obtained from Reckon Diagnostics, India.
2.4. Phytochemical screening
DAE and DHE extracts were subjected to phytochemical screening for the identification of chemical constituents, including alkaloids, carbohydrates, fixed oils and fats, terpenoids, phenols, tannins, glycosides, saponins, proteins, amino-acids and Flavonoids.24, 25, 26
2.5. Quantitative analysis
2.5.1. Determination of total flavonoids
Leaf powder (10 g) was extracted with 100 mL of 80% aq. methanol three times at room temperature. Extract was filtered through Whatman filter paper no. 42. The filtrate was then evaporated to dryness and weighed to a constant weight.27
2.5.2. Determination of total saponins
20 g of powdered leaves were taken in conical flask and 100 mL of 20% aqueous ethanol was added. The mixture was heated over a hot water bath for 4 h with continuous stirring at about 55 °C. The mixture was filtered and the residue re-extracted with another 200 mL of 20% ethanol. The combined extracts were reduced to 40 mL over water bath at about 90 °C. The concentrate was transferred into a 250 mL separating funnel and 20 mL of diethyl ether was added and shaken vigorously. The aqueous layer was recovered while the ether layer was discarded. The purification process was repeated. Then 60 mL n-butanol was added. The combined n-butanol extracts were washed twice with 10 mL of 5% aqueous sodium chloride. The remaining solution was heated in a water bath. After evaporation the sample was dried in the oven to a constant weight, the saponins content was calculated as percentage.28
2.5.3. Terpenoid extraction and TLC analysis
Powdered leaves (50 g) of D. indica were extracted with methanol and water (4:1) at room temperature for 24 h. The extract was filtered through Whatman filter paper No. 1 and then reduced to 1/10th the volume at 40 °C. Filtrate was then acidified with 2 M sulphuric acid (pH 0.89) followed by extraction with chloroform (three times the volume). The mixture was stirred and allowed to stand in a separating funnel. The non-aqueous layer was separated and evaporated till dryness. The dried extract contained chemical constituents like terpenoids which were further analyzed using thin layer chromatography (TLC).
TLC was performed according to the method described by Harborne using glass plates (20 × 20 cm) coated (0.5 mm) with silica gel. Dried plates were used for applying the sample of each extract dissolved in methanol (approximately 5 mg/mL). TLC plates were then developed in 100% chloroform. The spots were visualized using concentrated sulphuric acid as a spray reagent followed by heating of plates at 100 °C for 10 min. The spots were identified based on the color, produced on reacting with a spray reagent.25
2.6. Quantification of betulinic acid by using HPLC
Betulinic acid was quantified in DAE and DHE by high-performance liquid chromatography (HPLC) analysis using a Waters 2795 HPLC system with a UV detector. Precisely weighed sample (DAE and DHE; 99.63 mg) were extracted with 100 ml methanol in an ultrasonic bath and filtered through a 0.45 μm filter. 1 ml of the filtrate was further diluted up to 25 ml (0.04 mg/ml, w/v). An aliquot of 20 μl of each sample was injected onto the HPLC column. An Hypersil ODS C18 column (250 × 4.6-mm internal diameter; 5-μM particle-size) was employed (Merck, Germany), at ambient temperature (20 °C) and UV-detector were adjusted to 210 nm. Elution was carried out with acetonitrile: water (9:1) pH 3.0 (with phosphoric acid) at a flow-rate of 1ml/min and the eluate was monitored at 210 nm.21 The calculated concentration of betulinic acid was expressed in mg/g.
2.7. In- vitro anti-glycation activity
In vitro antiglycation activity of DAE and DHE was examined by testing their ability to inhibit the fluorescence of BSA in accordance with a previous method.29 The reaction mixture of BSA (10 mg/mL), 1.1 M fructose in 0.1 M phosphate buffered-saline (PBS), pH 7.4 containing 0.02% sodium azide with or without extract (DAE and DHE; dissolved in PBS; 50–500 μg/mL) was incubated in darkness at 37 °C for 1, 2, 3, and 4 weeks. AGE formation was measured by fluorescent intensity at an excitation wavelength 355 nm and emission wavelength 460 nm. Aminoguanidine (AG) was used as a positive control for this study. The percentage inhibition of AGE formation was determined by following formula:
2.8. Diabetic nephropathy
A single dose of 65 mg/kg STZ prepared in freshly prepared in 0.1 M citrate buffer (pH 4.5) was used to induce diabetes after 15 min of nicotinamide injection (230 mg/kg, i.p.).30 Nicotinamide was prepared in normal saline. Diabetes was confirmed 72 h after STZ injection, the blood samples were collected via retro-orbital plexus under ether anesthesia and fasting serum glucose was estimated using GOD-POD diagnostic kit. The rats having serum glucose levels more than 250 mg/dL were selected for the study. Different doses of the DAE and DHE (100, 200 and 400 mg/kg) were selected on the basis of oral acute toxicity studies reported in the literature and also followed the criteria given by Butterveck and Nahrstedt, 2012,31 not to test the extracts at doses that are not likely to have practical utility.21, 31, 32 As diabetic nephropathy symptoms typically develop after 30–45 days.33 At the end of 30 days the levels of serum urea, uric acid, creatinine and BUN were significantly high suggesting development of diabetic nephropathy.
Animals were divided into nine groups and each group consists of six rats.
Group 1: normal control
Group 2: DN control
Group 3: DN + 100 mg/kg DAE
Group 4: DN + 200 mg/kg DAE
Group 5: DN + 400 mg/kg DAE
Group 6: DN + 100 mg/kg DHE
Group 7: DN + 200 mg/kg DHE
Group 8: DN + 400 mg/kg DHE
Group 9: DN + 10 mg/kg glimepride
Rats were fasted overnight and blood samples were collected from retro-orbital plexus under ether anesthesia. Biochemical estimation was done by measuring blood glucose level, lipid profile (total cholesterol, triglyceride, HDL, LDL, VLDL levels), uric acid, urea, BUN and creatinine levels by using commercially available kits of Reckon Diagnostics Pvt. Ltd. Serum insulin level was determined using Insulin ELISA kit (Diametra, Milano, Italy). Biochemical estimation was carried out at an interval of 15 days on 30th, 45th, 60th and 75th day of study. Body weight was measured before the induction of diabetes and then at an interval of 15 days during the treatment period (30th to 75th day). Randomization of animals was carried out at the start of treatment i.e., 30th day. Animals were sacrificed by cervical dislocation at the end of the study and liver, kidney and pancreas were obtained and stored at −70 °C for biochemical studies.
2.9. Biochemical analysis
Tissue (kidney, pancreas and liver) homogenate was prepared in 0.1 M Tris–HCl buffer (pH 7.4) to estimate thiobarbituric acid reactive substances (TBARS)34 and level of antioxidant enzymes, viz. superoxide dismutase (SOD) and reduced glutathione (GSH).35
2.10. AGEs estimation in kidneys
AGEs levels in the kidneys were determined by a method as previously described by Sensi et al (1996).36 Briefly, kidneys were homogenized in 2 mL of 0.25 M sucrose followed by centrifugation at 900 g at 5 °C and the supernatant was separated. The pellet was re suspended in 2 mL sucrose solution and centrifuged and the supernatant obtained was mixed with the previous one. The proteins present were precipitated by adding equal volume of trichloroacetic acid (TCA). Following centrifugation at 4 °C with 900 g, the protein pellet obtained was mixed with 1 mL methanol twice to remove the lipid fraction. The insoluble protein, after washing with 10% cooled TCA was centrifuged and the residue was solubilized in 1 mL of 1 N NaOH and the protein concentration was estimated by measuring the absorbance at 280 nm against BSA standard curve. The AGEs content was then measured fluorometrically with an emission at 440 nm and excitation at 370 nm, and the results were expressed as relative fluorescence units (RFU)/mg protein.
2.11. Histopathology
Liver, kidney and pancreatic tissues were obtained from the sacrificed animals and fixed in 10% neutral buffered formalin solution, dehydrated in ethanol and embedded in paraffin. Sections of 5 μm thickness were prepared using a rotary microtome and stained with hematoxylin and eosin (H & E) dye for microscopic observations.
2.12. Statistical analysis
Statistical analysis was performed using Graphpad Prism 6. Values were expressed as mean ± SEM and one-way analysis of variance (ANOVA) was used for statistical analysis. ANOVA was followed by Tukey's as post hoc multiple comparison test. The results were considered significant if p ≤ 0.05.
3. Results
3.1. Phytochemical screening
Preliminary phytochemical screening of DAE showed the presence of carbohydrates, flavonoids, tannins and terpenoids; and carbohydrates, polyphenolic compounds, flavonoids, saponins, tannins and terpenoids were found to be present in DHE. Results obtained are in accordance with the literature evidencing the presence of potential terpenoidal and flavonoidal moieties in the extract. Presence of compounds of these potential categories may be attribute to synergistic pattern of augmentation of the effect against the progression of DN. Considering the results of qualitative analysis of DAE and DHE, the extracts were further quantified for the presence of these potential constituents. Furthermore, an important constituent, Betulinic acid, was quantified using HPLC.
3.2. Quantitative analysis of DAE and DHA
The quantity of flavonoids, saponins and terpenoids in DAE was found in the following order 4.0% > 1.4% > 0.05% of powdered leaves respectively and in that of DHE was found to be 4.8% > 1.6% > 0.04% respectively.
3.3. Quantitative analysis of betulinic acid
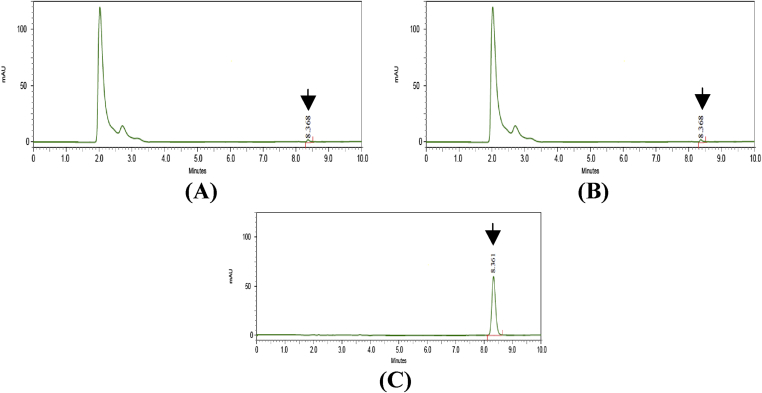
The chromatogram obtained for DAE and DHE (Fig. 1) showed a separate distinct peak for betulinic acid at RT 8.368 min when compared with RT of standard betulinic acid at 8.361 min (Fig. 1). The concentration of betulinic acid was found to be 32.23 and 35.78 mg/gm in DAE and DHE respectively.
Fig. 1.
HPLC chromatogram for DAE (A), DHE (B) standard Betulinic acid (C). Arrow indicates peak for betulinic acid.
3.4. In-vitro anti-glycation activity
In the present study, the formation of AGEs was monitored weekly by measuring fluorescence intensity of the BSA-fructose solutions for 4 weeks. A significant inhibition of AGEs formation (93.37%) was observed in fructose-induced glycated BSA plus aminoguanidine (500 μg/mL). At 4th week of incubation, the percentage inhibitions of AGEs formation by DAE (50–500 μg/mL) was 38.07% to 95.33%, respectively and for DHE was found to be 38.66 % to 95.80 % respectively.
3.5. Effect of DAE and DHE on body weight, food and water intake
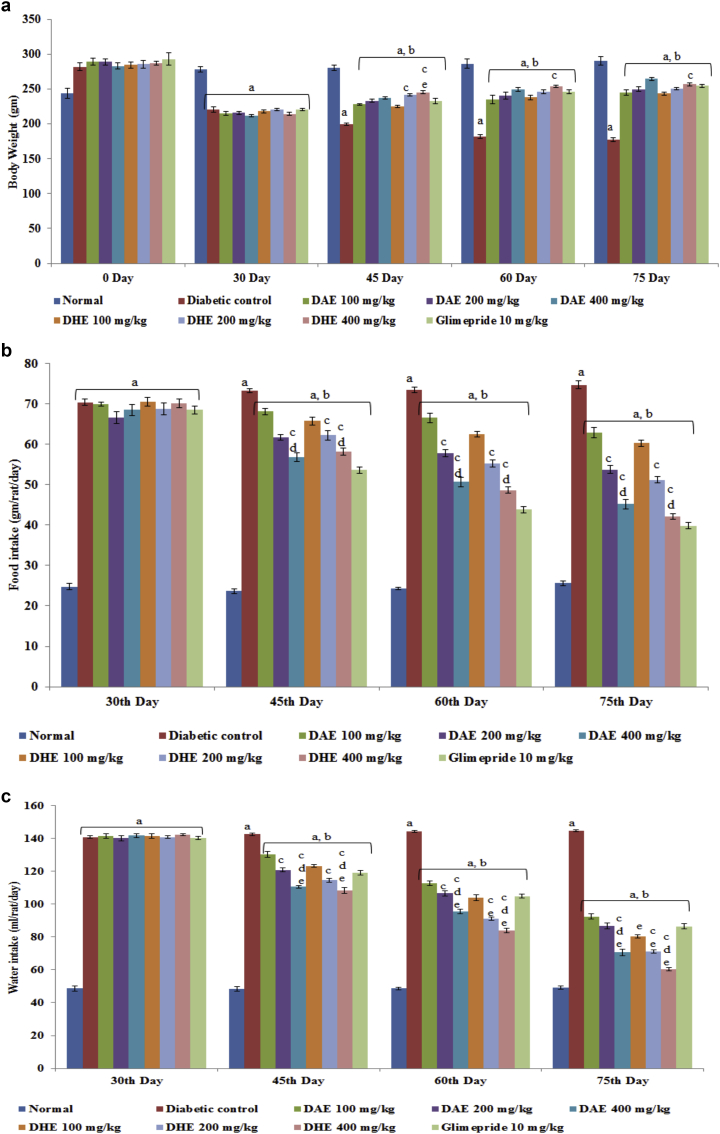
Before the induction of diabetes by intraperitoneal injection of STZ, there was no significant difference in the body weight of normal control rats (244.17 ± 7.49 g) and diabetic control rats (268.00 ± 2.08 g). After 30 days of induction, significant decrease in body weight of diabetic control rats (220.67 ± 3.98) was observed compared to normal rats (278.83 ± 3.79 g). Administration of DAE and DHE (100, 200 and 400 mg/kg) in experimental rats improved the weight loss as compared to diabetic control rats (Fig. 2a). Glimepride also inhibited the weight loss in rats. On 75th day, 400 mg/kg DAE showed significant increase in body weight compared to 100 mg/kg of DAE. Oral administration of DAE and DHE effectively reversed the elevated food and water intake in diabetic rats (Fig. 2b and c).
Fig. 2.
. a: Effect of DAE and DHE on body weight (g). Values are represented as Mean ± SEM (n = 6). Data was analyzed by using One-way ANOVA followed by Tukey's multiple test; a vs control; b vs Diabetic control; cD. indica extract 100 mg/kg; e vs Glimepride 10 mg/kg. p < 0.05. b: Effect of DAE and DHE on food intake (gm/rat/day). Values are represented as Mean ± SEM (n = 6). Data was analyzed by using One-way ANOVA followed by Tukey's multiple test; a vs control; b vs Diabetic control; cD. indica extract 100 mg/kg; e vs Glimepride 10 mg/kg. p < 0.05. c: Effect of DAE and DHE on water intake (ml/rat/day). Values are represented as Mean ± SEM (n = 6). Data was analyzed by using One-way ANOVA followed by Tukey's multiple test; a vs control; b vs Diabetic control; cD. indica extract 100 mg/kg; e vs Glimepride 10 mg/kg. p < 0.05.
3.6. Effect of DAE and DHE on fasting blood glucose level
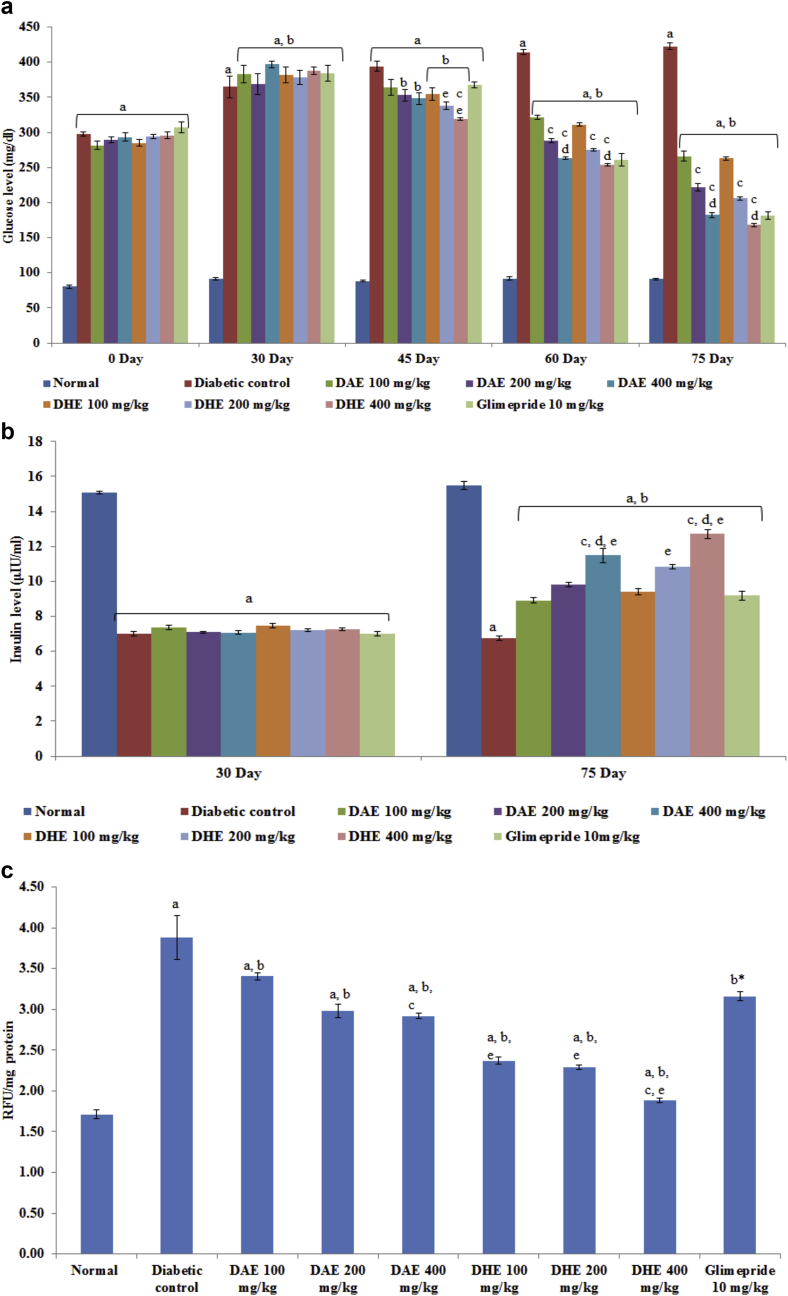
Administration of different doses of extracts was started after 30 days of STZ administration and continued for up to 75th day. After 30 days of STZ injection, significant increase in fasting blood glucose level was observed in diabetic control rats (364.67 ± 15.85 mg/dL) in comparison to normal control group (91.59 ± 1.89 mg/dL) (Fig. 3a). Chronic treatment with different doses of DAE and DHE (100, 200 and 400 mg/kg) for 45 days, significantly attenuated the elevated fasting blood glucose level (266.17 ± 7.07, 221.83 ± 5.70, 182.17 ± 3.59 mg/dL; 262.83 ± 2.10, 205.83 ± 2.08, 168.17 ± 2.54 mg/dL respectively; p < 0.001). Glimepride treated group also resulted in significant attenuation of fasting blood glucose level (181.83 ± 5.40 mg/dL; p < 0.001) as compared to diabetic control group.
Fig. 3.
a: Effect of DAE and DHE on blood glucose level (mg/dl). Values are represented as Mean ± SEM (n = 6). Data was analyzed by using One-way ANOVA followed by Tukey's multiple test; a vs control; b vs Diabetic control; cD. indica extract 100 mg/kg; d vs D. indica extract 200 mg/kg, e vs Glimepride 10 mg/kg. p < 0.05. b: Effect of DAE and DHE on serum insulin level (μIU/ml). Values are represented as Mean ± SEM (n = 6). Data was analyzed by using One-way ANOVA followed by Tukey's multiple test; a vs control; b vs Diabetic control; cD. indica extract 100 mg/kg; d vs D. indica extract 200 mg/kg, e vs Glimepride 10 mg/kg. p < 0.05. c: Effect of DAE and DHE on AGEs in kidney (RFU/mg protein). Values are represented as Mean ± SEM (n = 6). Data was analyzed by using One-way ANOVA followed by Tukey's multiple test; a vs control; b vs Diabetic control; c vs D. indica extract 100 mg/kg; d vs D. indica extract 200 mg/kg; e vs Glimepride 10 mg/kg. p < 0.05.
3.7. Effect of DAE and DHE on fasting insulin level
A significant decrease in fasting insulin level (7.00 ± 0.12 μU/mL) was observed in STZ-treated diabetic rats in comparison to normal control rats (15.07 ± 0.08 μU/mL). Administration of 100, 200 and 400 mg/kg of DAE and DHE for 45 days produced significant increase in serum insulin level [DAE (8.92 ± 0.15, 9.83 ± 0.13, 11.48 ± 0.39 μU/mL respectively); DHE (9.39 ± 0.18, 10.82 ± 0.12, 12.72 ± 0.26 μU/mL respectively)] (Fig. 3b).
3.8. Effect of DAE and DHE on kidney index, serum urea, uric acid, creatinine and BUN
In our experiments, elevated level of biomarkers i.e., kidney index (kidney weight to body weight ratio) serum urea, uric acid, creatinine and BUN indicated the development of DN. Kidney index was found to be significantly higher in diabetic control rats (0.90 ± 0.014 %) in comparison to normal control rats (0.44 ± 0.011 %). Groups treated with 100, 200 and 400 mg/kg DAE (0.62 ± 0.006, 0.58 ± 0.005, 0.52 ± 0.007 respectively) and DHE (0.60 ± 0.013, 0.53 ± 0.008, 0.50 ± 0.005 respectively) showed attenuation in kidney index in comparison to diabetic control group. When compared with diabetic control group, administration of different doses of DAE and DHE (100, 200 and 400 mg/kg) significantly decreased the elevated level of serum urea, uric acid, creatinine and BUN (Table 1). Moreover, attenuating effect was found to increase with dose and 400 mg of DAE and DHE produced maximum decrease in the level of biomarkers. Glimepride (10 mg/kg) also significantly attenuated the elevated level of these parameters.
Table 1.
Effect of DAE and DHE on renal function estimation in diabetic-nephropathy Wistar rats.
| Parameters → |
Urea (mg/dl) |
Uric Acid (mg/dl) |
Creatinine (mg/dl) |
BUN (mg/dl) |
||||
|---|---|---|---|---|---|---|---|---|
| Groups ↓ | 30th day | 75th day | 30th day | 75th day | 30th day | 75th day | 30th day | 75th day |
| Normal | 34.33 ± 0.67 | 35.83 ± 0.95 | 5.06 ± 0.17 | 5.07 ± 0.13 | 0.70 ± 0.02 | 0.70 ± 0.01 | 16.03 ± 0.31 | 16.73 ± 0.44 |
| Diabetic control | 79.33 ± 1.23 a | 89.33 ± 1.41 a | 12.75 ± 0.79 a | 15.10 ± 0.38 a | 2.93 ± 0.06 a | 3.92 ± 0.03 a | 37.05 ± 0.58 a | 41.72 ± 0.66 a |
| DAE 100 mg/kg | 83.67 ± 1.03 a | 62.00 ± 1.07 a, b | 12.87 ± 0.55 a | 8.65 ± 0.15 a, b | 3.89 ± 0.08 a, b | 2.06 ± 0.05 a, b | 39.07 ± 0.48 a | 28.95 ± 0.50 a, b |
| DAE 200 mg/kg | 84.67 ± 1.03 a | 52.83 ± 0.91a, b, c | 13.06 ± 0.60 a | 7.91 ± 0.19 a, b | 4.02 ± 0.07 a, b | 1.72 ± 0.05 a, b | 39.54 ± 0.48 a | 24.67 ± 0.43a, b, c |
| DAE 400 mg/kg | 83.17 ± 1.43 a | 48.00 ± 1.04a,b,c, d, e | 12.54 ± 0.20 a | 7.25 ± 0.15a, b, c | 4.13 ± 0.04 a, b | 1.44 ± 0.04a,b, c, d | 38.84 ± 0.67 a | 22.42 ± 0.48a,b,c, d, e |
| DHE 100 mg/kg | 83.00 ± 0.97 a | 51.50 ± 0.56 a, b | 12.85 ± 0.52 a | 8.53 ± 0.33 a, b | 3.94 ± 0.07 a, b | 2.06 ± 0.07 a, b | 38.76 ± 0.45 a | 24.05 ± 0.26 a, b |
| DHE 200 mg/kg | 85.50 ± 1.06 a | 47.50 ± 0.89a, b, e | 13.09 ± 0.57 a | 7.74 ± 0.25 a, b | 4.02 ± 0.05 a, b | 1.73 ± 0.06a, b, c | 39.93 ± 0.50 a | 22.18 ± 0.41a, b, c |
| DHE 400 mg/kg | 84.00 ± 1.35 a | 42.00 ± 1.19a,b,c, d, e | 12.55 ± 0.24 a | 7.11 ± 0.10a, b, c | 4.07 ± 0.04 a, b | 1.28 ± 0.04a,b,c, d, e | 39.23 ± 0.63 a | 19.61 ± 0.55a,b,c, d, e |
| Glimepride 10 mg/kg | 83.83 ± 1.17 a | 55.50 ± 1.26 a, b | 12.42 ± 0.44 a | 7.67 ± 0.18 a, b | 4.07 ± 0.05 a | 1.56 ± 0.07 a, b | 39.15 ± 0.55 a | 25.92 ± 0.59 a, b |
Each group (n = 6) represents Mean ± SEM. Data was analyzed by using One-way ANOVA followed by Tukey's multiple test (p < 0.05); a vs control, b vs Diabetic control, c vs D. indica extract 100 mg/kg, d vs D. indica extract 200 mg/kg, e vs glimepride 10 mg/kg.
3.9. Effect of DAE and DHE on serum lipid profile
STZ-injection produced dyslipidemia in the form of elevated serum TC, TG level, LDL, VLDL and reduced HDL level in diabetic control group compared to normal control rats. Administration of 100, 200 and 400 mg/kg of DAE and DHE produced significant attenuation of TC with the dose. Similarly, elevated TG levels were also found to be attenuated (123.00 ± 1.27, 115.83 ± 1.17, 106.33 ± 1.21 mg/dL and 118.17 ± 1.23, 111.83 ± 0.79 and 93.67 ± 1.85 mg/dL respectively for DAE and DHE) after administration of 100, 200, 400 mg/kg DAE and DHE respectively as compared to diabetic rats. Moreover, significant reduction in levels of LDL and VLDL was observed for different doses of DAE, DHE (100, 200 and 400 mg/kg). Significant decrease in level of serum HDL-c was observed in diabetic control rats in comparison to normal control group. Treatment with DAE, DHE (100, 200 and 400 mg/kg) increased the level of HDL-c with the dose (Table 2).
Table 2.
Effect of DAE and DHE on Lipid Profile in diabetic-nephropathy Wistar rats.
| Parameters → |
TC (mg/dl) |
TG (mg/dl) |
LDL (mg/dl) |
VLDL (mg/dl) |
HDL (mg/dl) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Groups ↓ | 30th day | 75th day | 30th day | 75th day | 30th day | 75th day | 30th day | 75th day | 30th day | 75th day |
| Normal | 104.83 ± 3.52 | 104.33 ± 2.04 | 77.11 ± 2.40 | 71.67 ± 1.41 | 34.90 ± 3.91 | 34.50 ± 1.64 | 15.43 ± 0.48 | 14.33 ± 0.28 | 54.50 ± 1.12 | 55.50 ± 0.96 |
| Diabetic control | 255.50 ± 2.72 a | 295.33 ± 2.10 a | 168.00 ± 2.14 a | 202.50 ± 1.61 a | 192.90 ± 2.94 a | 229.33 ± 2.21 a | 33.60 ± 0.43 a | 40.50 ± 0.32 a | 29.00 ± 0.97a | 25.50 ± 0.77 a |
| DAE 100 mg/kg | 270.67 ± 1.53 a | 193.83 ± 1.91 a, b | 160.00 ± 2.71 a | 123.00 ± 1.27 a, b | 211.83 ± 1.36 a | 133.90 ± 1.61 a, b | 32.00 ± 0.54 a | 24.60 ± 0.25 a, b | 26.83 ± 0.80 a | 35.33 ± 0.56 a, b |
| DAE 200 mg/kg | 242.50 ± 3.48 a | 154.33 ± 2.44a, b, c | 158.00 ± 2.59 a | 115.83 ± 1.17a, b, c | 184.73 ± 3.29 a | 109.33 ± 3.06a, b, c | 31.60 ± 0.52 a | 23.17 ± 0.28a, b, c | 26.17 ± 1.02 a | 38.33 ± 0.85 a, b |
| DAE 400 mg/kg | 248.83 ± 3.69 a | 154.33 ± 2.33a,b, c, d | 157.83 ± 1.23 a | 106.33 ± 1.21a,b, c, d | 190.77 ± 4.03 a | 93.40 ± 2.21a,b, c, d | 31.57 ± 0.25 a | 21.27 ± 0.24a,b, c, d | 26.50 ± 0.77 a | 39.67 ± 0.67a,b, c, e |
| DHE 100 mg/kg | 280.67 ± 2.24 a | 187.33 ± 2.12 a, b | 161.67 ± 2.57 a | 118.17 ± 1.23 a, b | 220.33 ± 2.53 a, b | 125.27 ± 2.20 a, b | 32.33 ± 0.51 a | 24.07 ± 0.27 a, b | 28.00 ± 0.69 a | 38.00 ± 0.69 a, b |
| DHE 200 mg/kg | 268.83 ± 3.78 a | 167.17 ± 1.91a, b, c | 159.33 ± 2.24 a | 111.83 ± 0.80 a, b | 209.80 ± 4.26 a | 104.67 ± 1.96a, b, c | 31.87 ± 0.45 a | 23.17 ± 0.28a, b, c | 27.17 ± 0.98 a | 39.33 ± 1.06 a, b |
| DHE 400 mg/kg | 275.17 ± 2.65 a | 146.50 ± 1.79a,b, c, d | 160.00 ± 0.73 a | 93.67 ± 1.85a,b,c, d, e | 216.17 ± 3.00 a | 82.27 ± 1.47a,b,c, d, e | 32.00 ± 0.15 a | 20.40 ± 0.21a,b, c, d | 27.00 ± 0.82 a | 43.83 ± 0.71a,b,c, d, e |
| Glimepride 10 mg/kg | 251.50 ± 2.63 a | 151.83 ± 2.19 a, b | 160.83 ± 1.43 a | 103.83 ± 1.80 a, b | 190.83 ± 2.99 a | 94.90 ± 1.59 a, b | 32.17 ± 0.29 a | 20.77 ± 036 a, b | 28.50 ± 0.77 a | 36.17 ± 0.80 a, b |
Each group (n = 6) represents Mean ± SEM. Data was analyzed by using One-way ANOVA followed by Tukey's multiple test (p < 0.05); a vs control, b vs Diabetic control, c vs D. indica extract 100 mg/kg, d vs D. indica extract 200 mg/kg, e vs glimepride 10 mg/kg.
3.10. Effect of DAE and DHE on antioxidant enzymes and TBARS
Intraperitoneal administration of STZ resulted in significant decrease in level of antioxidant enzymes (SOD and GSH) of pancreas, kidney and liver of diabetic rats. Treatment with 100, 200 and 400 mg/kg of DAE and DHE significantly increased the level of antioxidant enzymes viz. SOD and GSH compared to diabetic rats. Level of TBARS was found to be elevated in diabetic rats as compared to normal control group whereas administration of DAE and DHE significantly reduced the level of TBARS in comparison to diabetic rats (Table 3).
Table 3.
Effect of DAE and DHE on level of antioxidant enzymes and lipid peroxidation (TBARS) in diabetic-nephropathy Wistar rats.
| Parameters → |
SOD (U/mg protein) |
GSH (μM/mg protein) |
TBARS (nmol/mg protein) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Groups ↓ | Kidney | Pancreas | Liver | Kidney | Pancreas | Liver | Kidney | Pancreas | Liver |
| Normal | 3.87 ± 0.08 | 3.77 ± 0.15 | 4.22 ± 0.06 | 70.94 ± 0.472 | 67.62 ± 0.285 | 76.22 ± 0.477 | 0.55 ± 0.02 | 0.49 ± 0.01 | 0.65 ± 0.01 |
| Diabetic control | 1.24 ± 0.03a | 1.24 ± 0.03 a | 1.16 ± 0.04 a | 35.27 ± 0.438 a | 40.40 ± 0.438a | 41.37 ± 0.336 a | 2.27 ± 0.03 a | 2.25 ± 0.03 a | 2.69 ± 0.02 a |
| DAE 100 mg/kg | 1.82 ± 0.02 a, b | 1.60 ± 0.02 a | 1.29 ± 0.01 a | 40.91 ± 0.968 a, b | 42.05 ± 0.645a | 45.70 ± 0.499 a, b | 2.07 ± 0.02 a, b | 1.97 ± 0.02 a, b | 2.49 ± 0.02 a, b |
| DAE 200 mg/kg | 2.31 ± 0.03 a, b, c | 2.50 ± 0.06 a, b, c | 2.25 ± 0.04 a, b, c | 53.08 ± 1.411 a, b, c | 52.14 ± 0.970 a, b, c | 51.86 ± 0.689 a, b, c | 1.93 ± 0.01 a, b, c | 1.51 ± 0.03 a, b, c | 2.10 ± 0.02 a, b, c |
| DAE 400 mg/kg | 3.53 ± 0.02a, b, c, d | 3.52 ± 0.05a, b, c, d | 3.32 ± 0.03a, b, c, d | 59.51 ± 0.563a, b, c, d | 58.93 ± 0.881a, b, c, d | 65.63 ± 0.727a,b, c, d, e | 1.60 ± 0.02a, b, c, d | 1.16 ± 0.02a, b, c, d | 1.66 ± 0.03a, b, c, d |
| DHE 100 mg/kg | 1.99 ± 0.01 a, b | 1.88 ± 0.02 a | 1.59 ± 0.04 a | 40.91 ± 0.968 a, b | 42.06 ± 0.646 a, b | 45.70 ± 0.499a | 1.97 ± 0.03 a, b | 1.77 ± 0.02 a, b | 2.22 ± 0.02 a, b |
| DHE 200 mg/kg | 2.54 ± 0.03 a, b, c | 2.63 ± 0.04 a, b, c | 2.53 ± 0.05 a, b, c | 53.08 ± 1.411 a, b, c | 52.15 ± 0.970 a, b, c | 50.20 ± 2.042 a, b, c | 1.73 ± 0.01 a, b, c | 1.31 ± 0.03 a, b, c | 1.90 ± 0.02 a, b, c |
| DHE 400 mg/kg | 3.73 ± 0.02a, b, c, d | 3.69 ± 0.03a, b, c, d | 3.61 ± 0.03a, b, c, d | 59.51 ± 0.563a, b, c, d | 58.94 ± 0.881a, b, c, d | 65.63 ± 0.727a,b, c, d, e | 1.40 ± 0.02a,b, c, d, e | 1.03 ± 0.01a, b, c, d | 1.36 ± 0.03a,b, c, d, e |
| Glimepride 10 mg/kg | 3.41 ± 0.03 a, b | 3.49 ± 0.04 a, b | 3.55 ± 0.04 a, b | 60.51 ± 0.488 a, b | 57.78 ± 0.605 a, b | 61.59 ± 0.553 a, b | 1.69 ± 0.03 a, b | 1.05 ± 0.03 a, b | 1.54 ± 0.03 a, b |
Each group (n = 6) represents Mean ± SEM. Data was analyzed by using One-way ANOVA followed by Tukey's multiple test (p < 0.05); a vs control, b vs Diabetic control, c vs D. indica extract 100 mg/kg, d vs D. indica extract 200 mg/kg, e vs glimepride 10 mg/kg.
3.11. Effect of DAE and DHE on AGEs in kidneys
Present study showed strong inhibitory activity of DAE and DHE against AGEs formation in vitro in comparison to aminoguanidine. Therefore, in vivo examination of AGEs formation in renal tissue was carried out. Induction of diabetes in rats led to a significant increase in kidney AGEs level when compared to normal animals. Administration of DAE, DHE (100, 200 and 400 mg/kg) and glimepride significantly reduced the AGEs levels in kidneys when compared to diabetic control group (Fig. 3c).
3.12. Histopathology
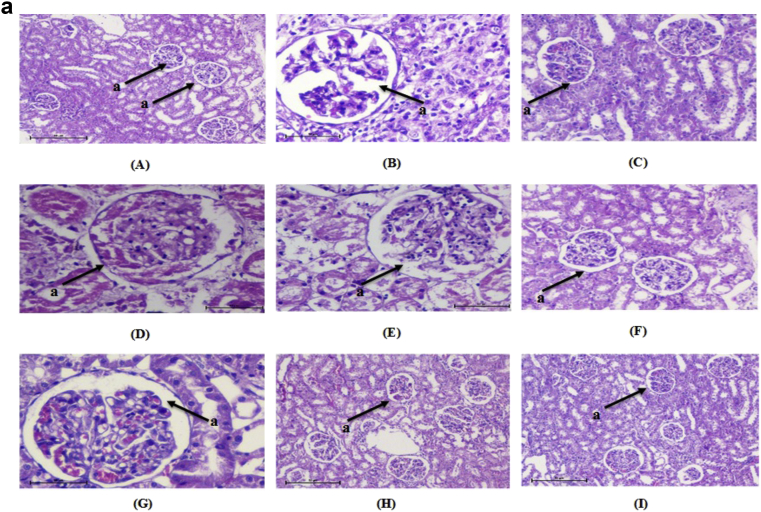
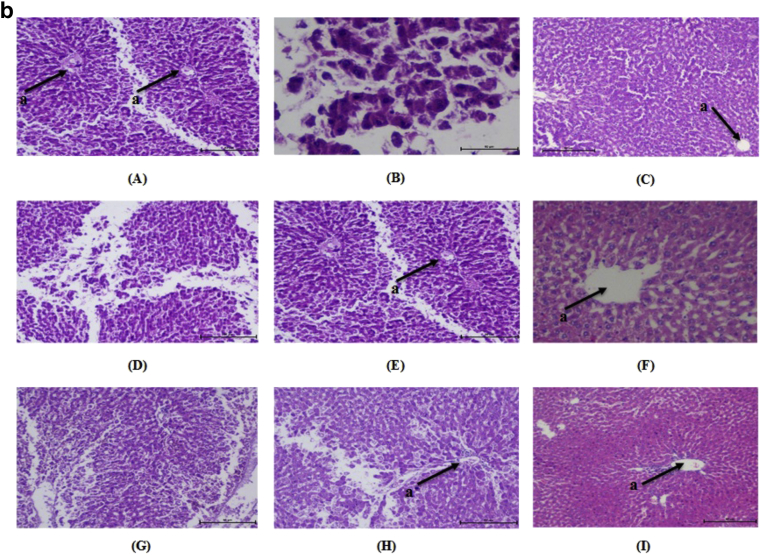
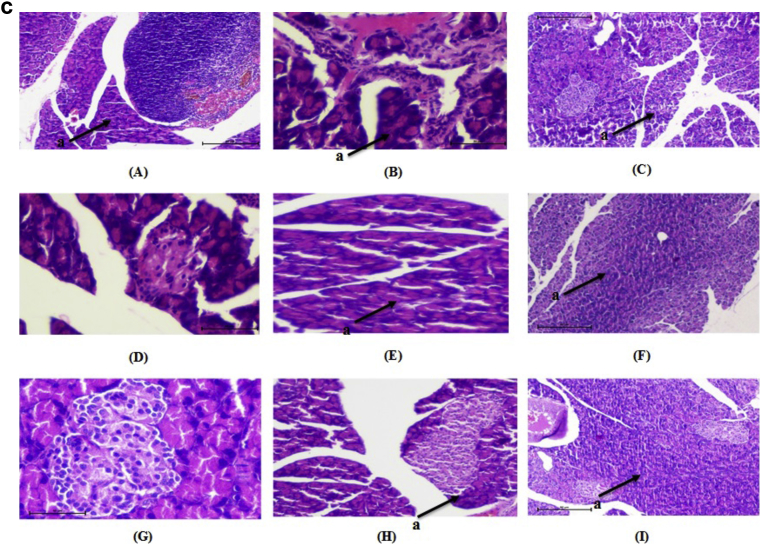
Kidney of normal control animals showed normal renal parenchyma with renal glomeruli as a glomerulus and Bowman's capsule and surrounded by proximal and distal tubules. Kidney of diabetic rats showed mesangial expansion and thickening of glomerular capillaries. Medullary tubules show vacuolation and dilation of the descending thin loop. Atrophy of glomeruli was seen in STZ induced diabetic rats. Administration of DAE, DHE and glimepride to the experimental animals significantly reduced mesangial expansion, membrane thickness and atrophy (Fig. 4a). Liver of normal rats, normal central vein with radiating sinusoid cords were present. There was no sinusoid congestion; swelling and necrotic cells. Diabetic rats demonstrated perivenular inflammatory collection and hyperplasia of kupffer cell with condensed nuclei and fatty infiltration. These pathological changes were reversed by the administration of DAE and DHE to experimental animals (Fig. 4b). Pancreatic cells of normal control group showed normal architecture with normal acini and islets cells with no signs of edema and inflammation. In diabetic rats, inflammation, disorganization of the islets and steatosis were observed. Cell infiltration was seen in the acinar cells along with necrosis and shrinkage of islet cells. These changes were ameliorated in DAE and DHE administered diabetic rats (Fig. 4c).
Fig. 4.
a: Histopathological changes in Kidney of normal and treated rats (H & E 10×). “a” shows the structure of glomerulus. (A) Normal, (B) diabetic nephropathy control, (C) Glimepride 10 mg/kg, (D) DAE 100 mg/kg treated, (E) DAE 200 mg/kg, (F) DAE 400 mg/kg treated, (G), DHE 100 mg/kg treated, (H) DHE 200 mg/kg treated and (I) DHE 400 mg/kg treated. b: Histopathological changes in Liver of normal and treated rats (H & E 10×). “a” shows the structure of central vein in liver. (A) Normal, (B) diabetic nephropathy control, (C) Glimepride 10 mg/kg, (D) DAE 100 mg/kg treated, (E) DAE 200 mg/kg, (F) DAE 400 mg/kg treated, (G), DHE 100 mg/kg treated, (H) DHE 200 mg/kg treated and (I) DHE 400 mg/kg treated. c: Histopathological changes in pancreatic islet of normal and treated rats (H & E 10×). “a” shows the structure of β-cells. (A) Normal, (B) diabetic nephropathy control, (C) Glimepride 10 mg/kg, (D) DAE 100 mg/kg treated, (E) DAE 200 mg/kg, (F) DAE 400 mg/kg treated, (G), DHE 100 mg/kg treated, (H) DHE 200 mg/kg treated and (I) DHE 400 mg/kg treated.
4. Discussion
DN is one of the major complications of diabetes mellitus and a leading cause of end-stage renal failure in the world today. About 15–25 % of type 1 diabetes patients and 30–40% of patients with type 2 diabetes suffer from diabetic nephropathy.37 In spite of the availability of therapeutic agents which retard the progression of diabetic nephropathy, there has been renewed interest in the use of herbal medicines in order to prevent the genesis of this complication.38 In our present investigation, we have evaluated the protective effects of DAE and DHE on STZ-induced diabetic nephropathy in rats.
STZ is a sensitive inducer of the organ lesions in rodents. It destroys pancreatic β-cells inducing hyperglycemia and renal injury. Thus it proves to be an efficient animal model to authenticate the therapeutic effect of a natural phytomedicine on renal damage related to diabetes in vivo.39 STZ injected rats exhibit clinic-pathological features including biochemical, oxidative and metabolic changes which also presented in human. Nicotinamide decreases the severity of streptozotocin-induced diabetes in animals due to its antioxidant effect.40 Therefore, STZ-nicotinamide induced type II diabetes model was selected for the study. STZ does not possess nephrotoxic potential and all changes in the kidney function after STZ administration in rats can thus be attributed to altered metabolism in diabetes.41 The present study aims to evaluate chronic complication of diabetes i.e. diabetic nephropathy using STZ-nicotinamide model for type 2 diabetes.
Studies have shown that, significant decrease in the levels of fasting blood glucose might be explained by the stimulation of the residual pancreatic mechanism, the regeneration or protection of pancreatic cells that were partially destroyed by STZ, the potentiation of the insulin secretion from protected β-cells of the islets of Langerhans and probably by the increase in the peripheral utilization of glucose.42 Authors have proposed several mechanisms of action for plants containing polyphenolic compound including, protection of the pancreatic β-cells from oxidative damage, increased insulin secretion, increased sensitivity of peripheral tissues in response to insulin and reduced gastrointestinal glucose absorption.43, 44 In diabetic individuals, lack of insulin leads to various clinical signs and symptoms, including chronic hyperglycemia, elevated blood glucose level in urine, constant thirst (polydipsia), increased urination (polyuria), weight loss, and severe starvation (polyphagia).45 Insulin deficiency causes hyperglycemia and when the blood glucose level is higher than the renal filtration threshold, there is the presence of glucose in urine, as well as increased excreted urine volume due to osmotic imbalance.45 Insulin is a hormone that facilitates the glucose transport into muscle cells and adipocytes, increases the synthesis and storage of cellular proteins, muscle glycogen and triacylglycerides in adipocytes, and decrease protein catabolism.46 Lack of insulin causes intense catabolism process of structural proteins and β-oxidation of fatty acids to form sub-products to gluconeogenesis, the loss or breakdown of these structural proteins directly reflect in reduced body weight,47, 48, 49 this process can be observed in diabetic control animals. Treatment for 45 days with DAE and DHE (400 mg/kg) significantly reduced hyperglycemia, hypoinsulinemia and polydipsia, polyphagia and loss of body mass.
During the course of diabetes, excessive formation of AGEs under hyperglycemic condition plays a major role in the pathogenesis of DN.50 The formation of AGEs in renal tissue plays a crucial role in the development of diabetic nephropathy. The irreversible formation of AGEs affects proteins and lipids thus causing damage to the blood vessels and kidneys.51 AGEs are found in almost all tissues examined from STZ-induced diabetic rats. Moreover, kidneys are more susceptible to AGE formation than other tissues.52 AGEs accumulation is the only mechanism that leads to structural aberrations in kidneys of DN animals.11, 50, 53 Inhibition of the formation of AGEs is a potentially therapeutic avenue in DN. The present study demonstrates that DAE and DHE exhibits potential inhibitory effect against the formation of AGEs in vitro. Moreover, increased level of AGE was found in the serum of STZ-diabetic rats whereas treatment with DAE and DHE lowered the elevated AGE levels. It was discovered the flavonoids could reduce serum AGEs, proteinuria, and systolic blood pressure, decrease the receptor of AGEs and connective tissue growth factor expression as well as oxidative stress in the kidney, while increasing renal antioxidant enzyme activity.54, 55 Presence of flavonoid content in the extracts of D. indica, could be the possible reason for the attenuation of formation of AGEs and oxidative stress. Thus DAE and DHE exhibited the potential to protect the kidney by decreasing the formation of AGE in the circulation of the STZ-diabetic rats.
Oxidative stress ensues due to the hyperactivity of polyol pathway induced by hyperglycemia and acts as major promoter of diabetic complications including DN.56 It is well accepted that, the high oxidative stress in diabetics considerably contributes to the complication of this disease57 and excessive production of free radicals is an observed phenomenon in association with diabetic complications. Various metabolic pathways like AGE pathway, polyol pathway, PKC pathway and hexosamine pathway directly or indirectly alter the redox capacity of the cell and increase the production of ROS. STZ exerts its diabetogenic action via increased production of ROS and inhibition of antioxidative enzymes (SOD and GSH) that scavenge ROS.58 Increased concentration of lipid peroxidation (LPO) markers viz., TBARS were observed in kidney, liver and pancreas of experimental animals. This increased level of lipid peroxidation could be associated to increase in free radicals' generation in diabetes caused primarily due to high blood glucose levels, which upon autoxidation generates free radicals.59 The great antioxidant potential of medicinal plants especially those with large content of polyphenolic compounds has been proven, these antioxidant plants has gained an important role in the treatment for diseases that present high production of free radicals, especially metabolic and genetic disorders related diseases as diabetes, dyslipidemia and cancer. Among the antioxidant phenolic compounds, we can highlight, phenolic acids, flavonoids, tannins, coumarins and carotenoids.60, 61, 62, 63 Flavonoids are the reputed compounds known for their high antioxidant capacity. Flavonoids reduce oxidative stress via inhibition of free radical generation, reducing the degradation of GSH or either increasing GSH formation and detoxify intermediary oxygen reactive products of LPO.64
In the present study, phytochemical screening revealed that flavonoids, tannins, triterpenoid, phenolic compounds and saponins are present in DAE and DHE. Content of these secondary metabolites in D. indica were found to in following order flavonoids > saponins > terpenoids according to the results of quantitative analysis. Presence of compounds of these potential categories may be attribute to synergistic pattern of augmentation between the most active compounds and other compounds in the extracts to produce effect against the progression of DN. Triterpenoids are known to stimulate insulin release from pancreas and reduces oxidative stress whereas phenolic compounds are potent radical scavengers which increase the levels of antioxidant enzymes and reduce lipid peroxidation65, 66 Thus, the ameliorating effect of D. indica against oxidative stress can be attributed to the presence of these secondary metabolites. Quantification of DAE and DHE using HPLC showed the presence of Betulinic acid (pentacyclic lupine type triterpenoid) in a good amount as compared to one quantified by Kumar et al, 2010.21 Betulinic acid has anti-leukemic, anti-inflammatory properties and also reduced blood glucose and lipid level in obese male swiss mice thereby improving lipid homeostasis.67 A recent study by Silva et al, 201668 suggested that betulinic acid has potential effects in the treatment of type 2 diabetes mellitus mainly by reducing the absorption and production of glucose and it also increases insulin sensitivity. Besides these betulinic acid has protective effect against oxidative stress, thus preventing the co-morbidities related to diabetes. In the present study, betulinic acid and other triterpenoids present in the plant might be helpful in the amelioration of chronic blood glucose level and its related consequences.
DN is known by not only dysfunctional glucose metabolism but also dyslipidemia, especially in type 2 diabetes. Lipid abnormalities including increased level of TC, TG, LDL, VLDL and decreased HDL-c level predisposes diabetic patient to atherosclerosis and other cardiovascular complications like coronary heart disease.69 Hyperglycemia, atherosclerosis and dyslipidemia are major causes of cardiovascular diseases and are linked to have interrelation with free radical generation.70 Similarly, in the present study, a positive correlation between hyperglycemia and dyslipidemia was observed. Serum TC, TG LDL and VLDL levels were found to be significantly elevated whereas HDL levels decreased. This dyslipidemia occurs due to increased breakdown of lipid and free fatty acids from peripheral stores.71 Moreover, hyperlipidemia with elevated levels of triglycerides, and free fatty acids results in oxidative stress and inflammation and may independently potentiate the adverse effects of hyperglycemia.72 Administration of DAE and DHE significantly reduced TC, TG, LDL and VLDL levels. Moreover, DAE and DHE effectively increased the level of serum HDL-c.
DN is one of the most serious complications of diabetes and it is characterized by elevated the level of serum creatinine, blood urea nitrogen, creatinine clearance as well as kidney hypertrophy.73 Hyperglycemia could enhance glomerulosclerosis and accelerate the progress of DN. These changes are a result of abnormal glucose regulation, hemodynamic changes within the kidney and increased oxidative stress.74 Free radicals attack important macromolecules leading to cell damage and homeostatic disruption, evidently leading to the degenerative diseases that afflict humanity.75 DN is characterized by a series of renal structure abnormality including basement membrane thickening, mesangial expansion, glomerulosclerosis and tubulointerstitial fibrosis.76 These changes are attributed to persistent hyperglycemia and increased levels of BUN, creatinine, urea and uric acid.73 Treatment of diabetic rats with DAE and DHE effectively reduced the levels of urea, uric acid, creatinine and BUN indicating their increased clearance from kidney. The whole kidney weight of diabetic animals was found to be increased when compared with normal control animals. This may be due to enlargement of lining cells of tubules, fatty infiltration, large area of hemorrhage and lymphocyte infiltration in STZ-induced diabetic rats. In our present study, oral administration of DAE and DHE significantly reduced the kidney weight/body weight ratio (kidney index) to near normal value.
5. Conclusion
Anti-hyperglycemic effect of medium and high dose of DAE and DHE reversed the renal lesions and oxidant/antioxidant status. The results further confirmed that DAE and DHE have an intrinsic ability to inhibit AGE's formation in addition to its antioxidant and anti-hyperglycemic activity. It can be concluded that by attenuating hyperglycemia, oxidative stress and markers of DN, DAE and DHE has renoprotective activity in STZ induced diabetic nephropathy in rats. Therefore, our results provide further insight into therapeutic strategies for diabetic nephropathy.
Conflict of interest
The author has no conflict of interest.
Acknowledgement
Financial assistance (F. No. SB/FT/LS-359/2012) from Department of Science and Technology,New Delhi,Government of India is highly acknowledged.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Kaur N., Kishore L., Singh R. Attenuating diabetes: what really works? Curr Diabetes Rev. 2016;12:259–278. doi: 10.2174/1573399811666150826115410. [DOI] [PubMed] [Google Scholar]
- 2.Pan D., Zhang D., Wu J. A novel proteoglycan from Ganoderma lucidum fruiting bodies protects kidney function and ameliorates diabetic nephropathy via its antioxidant activity in C57BL/6 db/db mice. Food Chem Toxicol. 2014;63:111–118. doi: 10.1016/j.fct.2013.10.046. [DOI] [PubMed] [Google Scholar]
- 3.Greene D.A., Lattimer S.A., Sima A.A. Sorbitol, phosphoinositides and sodium– potassium-ATPase in the pathogenesis of diabetic complications. N Engl J Med. 1987;316:599–606. doi: 10.1056/NEJM198703053161007. [DOI] [PubMed] [Google Scholar]
- 4.Kishore L., Kaur N., Singh R. Distinct biomarkers for early diagnosis of diabetic nephropathy. Curr Diabetes Rev. 2016 Dec 7 doi: 10.2174/1573399812666161207123007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Sasai Y., Iwakawa K., Yanagida K. Advanced glycation endproducts stimulate renal epithelial cells to release chemokines that recruit macrophages, leading to renal fibrosis. Biosci Biotechnol Biochem. 2012;76:1741–1745. doi: 10.1271/bbb.120347. [DOI] [PubMed] [Google Scholar]
- 6.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 7.Kaur N., Kishore L., Singh R. Diabetic autonomic neuropathy: pathogenesis to pharmacological management. J Diabetes Metab. 2014;5:402. [Google Scholar]
- 8.Ahmed N. Advanced glycation endproducts – role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67(1):3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Wu D., Wen W., Qi C. Ameliorative effect of berberine on renal damage in rats with diabetes induced by high-fat diet and streptozotocin. Phytomedicine. 2012;19(8–9):712–718. doi: 10.1016/j.phymed.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Kaur N., Kishore L., Singh R. Therapeutic effect of Linum usitatissimum L. in STZ-nicotinamide induced diabetic nephropathy via inhibition of AGE’s and oxidative stress. J Food Sci Technol. 2017;54:408–421. doi: 10.1007/s13197-016-2477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kishore L., Kaur N., Singh R. Renoprotective effect of Bacopa monnieri via inhibition of advanced glycation end products and oxidative stress in STZ-nicotinamide-induced diabetic nephropathy. Ren Fail. 2016;38:1528–1544. doi: 10.1080/0886022X.2016.1227920. [DOI] [PubMed] [Google Scholar]
- 12.Raafat K., Samy W. Amelioration of diabetes and painful diabetic neuropathy by Punica granatum L. Extract and its spray dried biopolymeric dispersions. Evid Based Complement Altern Med. 2014;2014:12. doi: 10.1155/2014/180495. Article ID 180495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma H.K., Chhangte L., Dolui A.K. Traditional medicinal plants in Mizoram, India. Fitoterapia. 2001;72:146–161. doi: 10.1016/s0367-326x(00)00278-1. [DOI] [PubMed] [Google Scholar]
- 14.Kirtikar K.R., Basu B.D. 2nd edition. Volume 1. Oriental Enterprises; Dehardun: 2003. pp. 75–77. (Indian Medicinal Plants). [Google Scholar]
- 15.Banerji N., Majumdar P., Dutta N.I. A new pentacyclic triterpene lactone from Dillenia indica. Phytochemistry. 1975;14:1447–1448. [Google Scholar]
- 16.Haque M.E., Islam M.N., Hossain M., Mohamad A.U., Karim M.F., Rahman M.A. Antimicrobial and cytotoxic activities of Dillenia indica. Dhaka Univ J Pharm Sci. 2008;7:103–105. [Google Scholar]
- 17.Parvin M.N., Rahman M.S., Islam M.S., Rashid M.A. Chemical and biological investigations of Dillenia indica Linn. Bangladesh J Pharmacol. 2009;4:122–125. [Google Scholar]
- 18.Kaur N., Kishore L., Singh R. Antidiabetic effect of new chromane isolated from Dillenia indica L. leaves in streptozotocin induced diabetic rats. J Func Foods. 2016;22:547–555. [Google Scholar]
- 19.Tarak D., Namsa N.D., Tangjang S. An inventory of the ethnobotanicals used as anti-diabetic by a rural community of Dhemaji district of Assam, Northeast India. J Ethnopharmacol. 2011;138:345–350. doi: 10.1016/j.jep.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Recio M.C., Giner R.M., Manez S. Investigations on the steroidal anti-inflammatory activity of triterpenoids from Diospyros leucomelas. Planta Med. 1995;61:9–12. doi: 10.1055/s-2006-957988. [DOI] [PubMed] [Google Scholar]
- 21.Kumar D., Mallick S., Vedasiromoni J.R., Pal B.C. Anti-leukemic activity of Dillenia indica L. fruit extract and quantification of betulinic acid by HPLC. Phytomedicine. 2010;17(6):431–435. doi: 10.1016/j.phymed.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 22.The Plant List. 2015. www.theplantlist.org.
- 23.de Oliveira Helison Carvalho, e Souza Belmira Silva Farias, dos Santos Igor Victor Ferreira, Resque Rafael Lima, Keita Hady, Fernandes Caio Pinho. Hypoglycemic effect of formulation containing hydroethanolic extract of Calophyllum brasiliense in diabetic rats induced by streptozotocin. Rev Bras Farmacogn. 2016;26:634–639. [Google Scholar]
- 24.Trease G.E., Evans W.C. 12th ed. Balliere Tindall; London: 1989. Text Book of Pharmacognosy. [Google Scholar]
- 25.Harbourne J.B. Chapman and Hall; London: 1998. Phytochemical Methods – A Guide to Modern Techniques of Plant Analysis. [Google Scholar]
- 26.Kaur N., Kishore L. Antioxidant activity of methanolic extract of Phaseolus trilobus root powder. IJPPS. 2012;4:271–275. [Google Scholar]
- 27.Boham B.A., Kocipal- Abyazan R. Flavonoids and condensed tannins from leaves of Hawaiian vaccinium vaticulatum and V. calycinum. Pac Sci. 1974;48:458–463. [Google Scholar]
- 28.Obadoni B.O., Ochuko P.O. Phytochemical studies and comparative efficacy of crude extracts of some homeostatic plants in Edo and Delta States of Nigeria. Glob J Pure Appl Sci. 2001;8:203–208. [Google Scholar]
- 29.Matsuda H., Wang T., Managi H., Yoshikawa M. Structural requirements of flavonoids for inhibition of protein glycation and radical scavenging activities. Bioorg Med Chem. 2003;11:5317–5323. doi: 10.1016/j.bmc.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 30.Bhateja P.K., Singh R. Antidiabetic activity of Acacia tortilis (Forsk.) Hayne ssp. raddiana polysaccharide on streptozotocin-nicotinamide induced diabetic rats. Biomed Res Int. 2014;2014:9. doi: 10.1155/2014/572013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butterweck V., Nahrstedt A. What is the best strategy for preclinical testing of botanicals? A critical perspective. Planta Medica. 2012;78:747–754. doi: 10.1055/s-0031-1298434. [DOI] [PubMed] [Google Scholar]
- 32.Yeshwante S.B., Juvekar A.R., Nagmoti D.M., Wankhede S. In vivo analgesic activity of methanolic extract of Dillenia indica (L) leaves. Pharmacologyonline. 2011;3:1084–1096. [Google Scholar]
- 33.Putt D.A., Zhong Q., Lash L.H. Adaptive changes in renal mitochondrial redox status in diabetic nephropathy. Toxicol Appl Pharmacol. 2012;258(2):188–198. doi: 10.1016/j.taap.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 34.Ohkawa H., Ohishi N., Yagi K. Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 35.Owen O.G. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 36.Sensi M., Pricci F., Pugliese G. Role of advanced glycation end-products (AGE) in late diabetic complications. Diabetes Res Clin Pract. 1996;28:9–17. doi: 10.1016/0168-8227(94)01061-4. [DOI] [PubMed] [Google Scholar]
- 37.Wang G.G., Lu X.H., Li W., Zhao X., Zhang C. Protective effects of luteolin on diabetic nephropathy in STZ-induced diabetic rats. Evid Based Complement Altern Med. 2011;2011:1–7. doi: 10.1155/2011/323171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh R., Kishore L., Kaur N. Diabetic peripheral neuropathy: current perspective and future directions. Pharmacol Res. 2014;80:21–35. doi: 10.1016/j.phrs.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Mao Z.M., Shen S.M., Wan Y.G. Huangkui capsule attenuates renal fibrosis in diabetic nephropathy rats through regulating oxidative stress and p38MAPK/Akt pathways, compared to α-lipoic acid. J Ethnopharmacol. 2015;173:256–265. doi: 10.1016/j.jep.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 40.Yamada K., Nonaka K., Hanafusa T., Miyaki T., Toyoshima H., Tarvis S. Preventive and therapeutic effects of large dose nicotinamide on diabetes associated with insulinitis. Diabetes. 1982;31(9):749–753. doi: 10.2337/diab.31.9.749. [DOI] [PubMed] [Google Scholar]
- 41.Kishore L., Kaur N., Singh R. Bacosine isolated from aerial parts of Bacopa monnieri improves the neuronal dysfunction in Streptozotocin-induced diabetic neuropathy. J Func Foods. 2017;34:237–247. [Google Scholar]
- 42.Aybar M.A., Sanchez Riera A., Grau A., Sanchez S.S. Hypoglycemic effect of the water extract of Smallantus sonchifolium (yacon) leaves in normal and diabetic rats. J Ethnopharmacol. 2001;74:125–132. doi: 10.1016/s0378-8741(00)00351-2. [DOI] [PubMed] [Google Scholar]
- 43.Sezik E., Aslan M., Yesilada E., Ito S. Hypoglycemic activity of Gentiana olivieri and isolation of the active constituent through bioassay-directed fractionation techniques. Life Sci. 2005;76:1223–1238. doi: 10.1016/j.lfs.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 44.Panda S., Kar A. Apigenin (4’,5,7-trihydroxyflavone) regulates hyperglycemia, thyroid dysfunction and lipid peroxidation in alloxan induced diabetic mice. J Pharm Pharmacol. 2007;59:1543–1548. doi: 10.1211/jpp.59.11.0012. [DOI] [PubMed] [Google Scholar]
- 45.Mahendran G., Manoj M., Murugesh E. In vivo anti-diabetic, antioxidant and molecular docking studies of 1,2,8-trihydroxy-6-methoxy xanthone and 1,2-dihydroxy-6-methoxyxanthone-8-O-d-xylopyranosyl isolated from Swertia corymbosa. Phytomedicine. 2014;21:1237–1248. doi: 10.1016/j.phymed.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 46.May M.E., Buse M.G. Effects of branched-chain amino acids on protein turnover. Diabetes Metab Rev. 1989;5:227–245. doi: 10.1002/dmr.5610050303. [DOI] [PubMed] [Google Scholar]
- 47.Ramesh B., Pugalendi K.V. Antihyperglycemic effect of umbelliferone in streptozotocin-diabetic rats. J Med Food. 2006;9:562–566. doi: 10.1089/jmf.2006.9.562. [DOI] [PubMed] [Google Scholar]
- 48.Zafar M., Naqvi S.N. Effects of STZ-induced diabetes on the relative weights of kidney, liver and pancreas in albino rats: a comparative study. Int J Morphol. 2010;28:135–142. [Google Scholar]
- 49.Cheng D., Liang B., Li Y. Antihyperglycemic effect of Ginkgo biloba extract in streptozotocin-induced diabetes in rats. Biomed Res Int. 2013;2013:1–7. doi: 10.1155/2013/162724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forbes J.M., Thallas V., Thomas M.C. The breakdown of preexisting advanced glycation end products is associated with reduced renal fibrosis in experimental diabetes. FASEB J. 2003;17:1762–1764. doi: 10.1096/fj.02-1102fje. [DOI] [PubMed] [Google Scholar]
- 51.Sohn E., Kim J., Kim C. The extract of Litsea japonica reduced the development of diabetic nephropathy via the inhibition of advanced glycation end products accumulation in db/db mice. Evid Based Complement Altern Med. 2013;2013:1–9. doi: 10.1155/2013/769416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niedowicz D.M., Daleke D.L. The role of oxidative stress in diabetic complications. Cell Biochem Biophys. 2005;43:289–330. doi: 10.1385/CBB:43:2:289. [DOI] [PubMed] [Google Scholar]
- 53.Kishore L., Kaur N., Singh R. Nephroprotective effect of Paeonia emodi via inhibition of advanced glycation end products and oxidative stress in streptozotocin–nicotinamide induced diabetic nephropathy. J Food Drug Anal. 2016 doi: 10.1016/j.jfda.2016.08.009. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X., Xiao Y., Gao H. Grape seed proanthocyanidins ameliorate diabetic nephropathy via modulation of levels of AGE, RAGE and CTGF. Nephron Exp Nephrol. 2009;111:e31–41. doi: 10.1159/000191103. [DOI] [PubMed] [Google Scholar]
- 55.Mansouri E., Panahi M., Ghaffari M.A., Ghorbani A. Effects of grape seed proanthocyanidin extract on oxidative stress induced by diabetes in rat kidney. Iran Biomed J. 2011;15:100–106. [PMC free article] [PubMed] [Google Scholar]
- 56.Dunlop M. Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney Int. 2000;77:S3–S12. doi: 10.1046/j.1523-1755.2000.07702.x. [DOI] [PubMed] [Google Scholar]
- 57.Baynes J.W., Thorpe S.R. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 58.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–546. [PubMed] [Google Scholar]
- 59.Li Y., Nishimura T., Teruya K. Protective mechanism of reduced water against alloxan-induced pancreatic beta-cell damage: scavenging effect against reactive oxygen species. Cytotechnology. 2002;40:139–149. doi: 10.1023/A:1023936421448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marles R.J., Farnsworth N.R. Antidiabetic plants and their active constituents. Phytomedicine. 1995;2:137–189. doi: 10.1016/S0944-7113(11)80059-0. [DOI] [PubMed] [Google Scholar]
- 61.Perez R.M., Zavala G.M.A., Perez S.G., Perez C.G. Antidiabetic effect of com-pounds isolated from plants. Phytomedicine. 1998;5:55–75. doi: 10.1016/S0944-7113(98)80060-3. [DOI] [PubMed] [Google Scholar]
- 62.Ojewole J.A.O. Hypoglycemic effect of Clausena anisata (Willd) Hookmethanolic root extract in rats. J. Ethnopharmacol. 2002;81:231–237. doi: 10.1016/s0378-8741(02)00085-5. [DOI] [PubMed] [Google Scholar]
- 63.Aslan M., Orhan N., Orhan D.D., Ergun F. Hypoglycemic activity and antioxi-dant potential of some medicinal plants traditionally used in Turkey for diabetes. J Ethnopharmacol. 2010;128:384–389. doi: 10.1016/j.jep.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 64.Singh R., Kaur N., Kishore L., Gupta G.K. Management of diabetic complications: a chemical constituents based approach. J Ethnopharmacol. 2013;150:51–70. doi: 10.1016/j.jep.2013.08.051. [DOI] [PubMed] [Google Scholar]
- 65.Dewanjee S., Das A.K., Sahu R., Gangopadhyay M. Antidiabetic activity of Diospyros peregrina fruit: effect on hyperglycemia, hyperlipidemia and augmented oxidative stress in experimental type 2 diabetes. Food Chem Toxicol. 2009;47:2679–2685. doi: 10.1016/j.fct.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 66.Afolayan A.J., Sunmonu T.O. Artemisia afra Jacq. Ameliorates oxidative stress in the pancreas of streptozotocin-induced diabetic wistar rats. Biosci Biotech Biochem. 2011;75:2083–2086. doi: 10.1271/bbb.100792. [DOI] [PubMed] [Google Scholar]
- 67.De Melo C.L., Queiroz M.G.R., Filho A.C.V.A. Betulinic acid, a natural pentacyclic triterpenoid, prevents abdominal fat accumulation in mice fed a high-fat diet. J Agric Food Chem. 2009;57:8776–8781. doi: 10.1021/jf900768w. [DOI] [PubMed] [Google Scholar]
- 68.Silva F.S., Oliveria P.J., Durate M.F. Oleanolic, Ursolic and Betulinic acids as food supplements or pharmaceutical agents for type 2 diabetes: promise or illusion? J Agric Food Chem. 2016;64:2991–3008. doi: 10.1021/acs.jafc.5b06021. [DOI] [PubMed] [Google Scholar]
- 69.Thomas S., Karalliedde J. Diabetic nephropathy. Medicine. 2015;43:20–25. [Google Scholar]
- 70.Singh R., Devi S., Gollen R. Role of free radical in atherosclerosis, diabetes and dyslipidaemia: larger-than-life. Diabetes Metab Res Rev. 2015;31:113–126. doi: 10.1002/dmrr.2558. [DOI] [PubMed] [Google Scholar]
- 71.Maria E.L., Dennis J.M., Stocker R. Actions of “antioxidants”in the protection against atherosclerosis. Free Radic Biol Med. 2012;53:863–884. doi: 10.1016/j.freeradbiomed.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 72.O'Keefe J.H., Bell D.S.H. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism): is a cardiovascular risk factor. Am J Cardiol. 2007;100:899–904. doi: 10.1016/j.amjcard.2007.03.107. [DOI] [PubMed] [Google Scholar]
- 73.Cowie C.C., Port F.K., Wolfe R.A., Savage P.J., Moll P.P., Hawthorne V.M. Disparities in incidence of diabetic end-stage renal disease according to race and type of diabetes. N Engl J Med. 1989;321:1074–1079. doi: 10.1056/NEJM198910193211603. [DOI] [PubMed] [Google Scholar]
- 74.Aurell M., Bjorck S. Determination of progressive renal disease in diabetes mellitus. Kidney Int. 1992;41:38–42. [PubMed] [Google Scholar]
- 75.Tomás-Zapico C., Coto-Montes A. Melatonin as antioxidant under pathological processes. Recent Pat Endocr Metab Immune Drug Discov. 2007;1:63–82. [Google Scholar]
- 76.Abe H., Matsubara T., Arai H., Doi T. Role of Smad1 in diabetic nephropathy: molecular mechanisms and implications as a diagnostic marker. Histol Histopathol. 2011;26:531–541. doi: 10.14670/HH-26.531. [DOI] [PubMed] [Google Scholar]