Abstract
Introduction
Isoniazid is a drug for treatment of tuberculosis. One of the main side effects of this drug is hepatotoxicity, which is a major cause of treatment interruption in tuberculosis. This study is about the preventive effect of Salep on this side effect of isoniazid.
Materials and methods
This study is an experimental study in which the preventive effect of salep on isoniazid hepatotoxicity is evaluated. In this study 56 rats were randomly placed in 7 eight members groups including: control group, sham, isoniazid and four isoniazid/salep groups. At the end of the study the laboratory criteria and histological features of liver toxicity were compared in different mentioned groups.
Results
Significant lower serum levels of liver enzymes, billirubin, MDA and TOC; and significant higher levels of TAC and total proteins, were revealed in isoniazid/salep group in compare to isoniazid alone group.
In addition, histological studies had not showed liver injury in isoniazid/salep group, while there was significant liver injury in isoniazid alone group.
Conclusions
Orchid extract (salep), probably because of its antioxidant properties, prevent the destructive effects of isoniazid on the liver.
Keywords: Salep, Isoniazid, Liver, Rat
Graphical abstract
1. Introduction
Tuberculosis (TB), is one of the most important communicable diseases in developing countries including Iran. One of the side effects in treatment of this disease [by Isoniazid (INH), Rifampin (RMP) and Pirazinamide (PZA)] is liver toxicity and sometimes this problem may be conduce drug interruption, to save the patient from hepatotoxicity and death. On the other hand drug resistance mycobacterium is a risk which may happen with drug interruption. Prevention of liver toxicity by natural products in these patients seems to be a good strategy. We have already worked on the Salep (a plant belongs to family Orchidacea) for its protective effects on liver.1 The results of that study were hopeful.
In most cases, during detoxification of drugs, microsomes in the liver causes production of toxic and active metabolites that can damage various tissues including the liver itself.2
INH, is used as a drug for treatment of active and latent tuberculosis along with other first line antituberculosis drugs. The most common side effect of this drug is elevation of liver enzymes level in blood which may be conduce to hepatitis that is a dangress complication in the patient.3 Hepatic toxicity caused by isoniazid can appear as cellular necrosis, steatosis (accumulation of fats), or both. Metabolites of this drug also have toxic effects on liver cells.4 Hydrazine is one of the most important metabolites of isoniazid. In animal models, a statistically significant positive correlation has been reported between plasma levels of hydrazine and severity of hepatic cell destruction caused by treatment with isoniazid.5 Moreover, other studies have proved acetyl hydrazine is another destructive agent of hepatic cells when isoniazid is used for treatment.6
Fortunately, antioxidants can prevent the destructive effects of toxins and various drugs on the liver tissue.7, 8, 9 Plants have always been considered one of the main options for treating poisoned livers because they are available as natural sources of antioxidants. Orchid or Dactylorhiza lancibracteata (C. Koch) Renz, formerly named Orchis maculate L. (or Salep in general dialect), belongs to the family Orchidaceae, has various species, and grows almost everywhere in the world. Its root nodules, can be harvested in early summer, and its medicinal properties may last for about two years.10, 11 This plant contains compounds including nitrogenous materials, starch, proteins, sugars, hydroxybenzaldehydes, ferulic acid, quercetin, daucosterol, cirsilineol, steroids, and glucomannan.12, 13 In traditional medicine, orchid is prescribed as ointment for chest pain, and for treating chest and intestinal disorders, tuberculosis, diarrhea, Parkinson's disease, and cancer.14, 15 Evaluation of the extracts of the roots of orchid revealed that there are numerous compounds in it; such as glucomannan and polyphenols, which possess strong antioxidant properties.
In one of our previous studies we showed that this plant has protective effects against liver toxicity overall.1 Therefore, this research was conducted in rats; to study the preventive effects of aqueous extract of this plant against liver toxicity caused by isoniazid.
2. Materials and methods
2.1. Collection and extraction of the salep
The roots of the plant are used for providing the extract.
The plant was obtained from farmlands around Yasouj (a city in the southwest of Iran). At first the roots of the plant washed by water, and then dried in the laboratory environment (away from direct light of sun). Then by electric mill, the dried roots ground into flour. The ethyl alcohol added to the obtained powder in 5 to 1 proportions (5 part alcohol and 1 part powder) and mixed for 24 h to yield a uniform solution. In the next stage, the solution filtered and dried for 48 h to obtain the dried extract without alcohol. At the end the extract was mixed with distilled water in 20, 40 and 80 mg/mL proportions and used for the study.16
2.2. Animals and their classification
First of all: With due attention to the usefulness of the results of this study for humanity specially the patients with tuberculosis the” Animal care committee” and “ Medical ethics committee “ in Jahrom university of medical sciences approved this study. In these committees it is emphasized that the process on animals should be done with the lowest torment for the animal.
In this research, 56 mature Wistar rats with the average weight of 180–200 g were kept in the animal breeding room at Jahrom University of Medical Sciences for one week to become acclimated to the environment. During the entire research, the rats were kept under a photoperiod of 12 h light: 12 h dark at the ambient temperature of 20–25 °C and had free access to food and water. By considering previous research and the pilot project that was carried out, it was decided to use extract of orchid at the prescribed doses of 80,17, 18 160, and 320 mg/kg. The rats were randomly divided into 7 eight-member groups. In control group any additive material (except food and water) was given to rats, but in the sham group 1 ml of distilled water (based on weight of each rat) was injected intraperitoneally, every day for 4 weeks. The isoniazid group received 50 mg/kg of isoniazid through intraperitoneal injection, and the experimental groups 1, 2, 3, and 4 recieved 50 mg/kg of isoniazid together with 40, 80,160, and 320 mg/kg of the aqueous extract of orchid intraperitoneally every day for 4 weeks.
2.3. Blood sampling and biochemical studies
At the end of the research (on day 29), the rats were weighed and anesthetized by cervical dislocation and, blood samples were taken directly from their hearts. The blood samples were centrifuged at 3000 rpm for 15 min and the serums were kept at -20 °C for measuring concentrations of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total protein, albumin, bilirubin,19 malone dialdehyde (MAD), total oxidant capacity (TOC), and total antioxidant capacity (TAC).
ALT and AST were measured by the Deutsche Gesellschaft fur Klinische Chemie (DGKC) phosphate buffer method, ALP by the para-nitrohphenyl phosphate AMP method, and albumin by the bromocresol green method. Total bilirubin was measured by the diazo method using diazotized sulfanilic acid (employing commercial kits manufactured by Zist Shimi Co. in Iran), and total protein by the biuret reaction (end point) method (using commercial kits manufactured by the Pars Azmoon Co. in Iran).20 MAD levels were determined by the ELISA method employing commercial kits manufactured by the Biospes Company in Italy, and TOC and TAC by the ELIZA method using commercial kits made by the LDN Company in Italy.
2.4. Histological studies
After taking blood samples, the rats were dissected and their livers were removed, weighed accurately, and fixed in 10% formalin. Tissue cross sections of the livers were prepared, stained, and studied under microscope.
2.5. Statistical analysis
One-way ANOVA was used to analyze the information, and Duncan's test was employed to determine the differences between the means where there were statistically significant differences between the various groups. Statistical calculations were performed using SPSS 21, and the selected significant level was (p < 0.05).
3. Findings
3.1. Liver enzymes
No significant differences were observed in mean serum levels of liver enzymes between the control and sham groups; but, mean concentrations of AST, ALT, and ALP was higher in isoniazid-alone group compared with control group and sham groups (p < 0.05).
Furthermore, significant low concentrations of ALT, and ALP are seen in isoniazid/salep groups VI and VII (160 and 320 mg/kg) in compared with isoniazide alone group (p < 0.05) (Table 1).
Table 1.
Values of the studied variables in the various groups (Mean levels).
| Parameters | Groups |
||||||
|---|---|---|---|---|---|---|---|
| Control |
Sham |
Isoniazid 50 mg/kg |
Isoniazid + salep extract at 40 mg/kg |
Isoniazid + salep extract at 80 mg/kg |
Isoniazid + salep extract at 160 mg/kg |
Isoniazid + salep extract at 320 mg/kg |
|
| Group I | Group II | Group III | Group IV | Group V | Group VI | Group VII | |
| AST IU/L |
93.37 ± 2.25 | 93.5 ± 2b | 169.6 ± 11.94a | 156.2 ± 9.03a | 137.3 ± 6.84a | 115.8 ± 4.98b | 105.5 ± 4.22b |
| ALT IU/L |
48.5 ± 1.26 | 48.25 ± 1.19b | 75.3 ± 5.75a | 68.25 ± 5.2a | 69 ± 4a | 56.7 ± 3.2b | 53.3 ± 3.9b |
| ALP IU/L |
483.12 ± 26.68 | 489 ± 25.37b | 758.6 ± 47.97a | 738.37 ± 45.3a | 776.7 ± 36.8a | 565.6 ± 56.8a | 487 ± 23.7b |
| Total protein g/dl |
7.2 ± 0.14 | 7.2 ± 0.16b | 4.5 ± 0.14a | 5.2 ± 0.38a | 5.38 ± 0.27a | 7.07 ± 0.34b | 7.37 ± 0.34b |
| Albumin g/dl |
3.5 ± 0.12 | 3.4 ± 0.14b | 1.6 ± 0.05a | 1.98 ± 0.09a | 2.52 ± 0.14a | 2.9 ± 0.16b | 3.2 ± 0.11b |
| Bilirubin mg/dl |
0.92 ± 0.08 | 0.91 ± 0.05b | 2.86 ± 0.10a | 2.7 ± 0.13a | 2.43 ± 0.07a | 1.6 ± 0.06b | 1.08 ± 0.05b |
| TAC U/ml |
1.5 ± 0.04 | 1.55 ± 0.05b | 0.4 ± 0.04a | 0.55 ± 0.05a | 0.83 ± 0.04b | 1.12 ± 0.03b | 1.43 ± 0.07b |
| TOC U/ml |
2.37 ± 0.11 | 2.36 ± 0.12b | 5.1 ± 0.17a | 4.9 ± 0.13a | 4.57 ± 0.13a | 3.8 ± 0.2a | 2.5 ± 0.13b |
| MDA Nmol/L |
0.12 ± 0.007 | 0.13 ± 0.007b | 6.7 ± 0.1a | 6.3 ± 0.21a | 4.4 ± 0.28a | 2.7 ± 0.22a | 0.4 ± 0.03b |
Means are reported as Mean ± SEM.
P < 0.05 was considered as statistically significant.
P < 0.05 – compared with group I (difference is significant).
P > 0.05 – compared with group I (difference is not significant).
3.2. Serum albumin (ALB), total protein (TP), and total bilirubin levels
There was not any significant difference in the serum levels of ALB and TP in control and sham groups.
However, mean serum levels of ALB and TP were significantly low in isoniazid alone group in compare to the control and sham groups (p < 0.05)
Average serum level of ALB in groups that received isoniazid together with 160, or 320 mg/kg of the extract (groups VI and VII) was significantly high in compare to the isoniazid alone group (p < 0.05).
It seems that in isoniazid/salep groups VI and VII (160 and 320 mg/kg dose of the extract), the ALB and TP levels is similar to the control group.
Average serum levels of total bilirubin in groups that received isoniazid together with 160, or 320 mg/kg of the extract were significantly lower in compare to the isoniazid alone group (p < 0.05).
TAC (Total Antioxidant Capacity), TOC (Total Oxidant Capacity), and MAD (Malone Dialdehyde)
No significant differences were observed between the control and sham groups in the mean levels of these variables. There was significant increase in average serum levels of MAD and TOC, in isoniazid alone group, and significant decrease in average serum levels of TAC in compare to the control group (p < 0.05).
Average levels of MDA and TOC in group VII (that were given isoniazid together with 320 mg/kg of the orchid extract) were significantly lower than their levels in isoniazid alone group (group III) (p < 0.05), and there was not significant difference in their levels compared with control group (P > 0.05).
The average TAC level in the groups receiving isoniazid together with 80, 160, or 320 mg/kg of the extract also were significantly high in compared to the isoniazid alone group (p < 0.05) and was similar to groups I and II (p > 0.05).
3.3. Histological studies
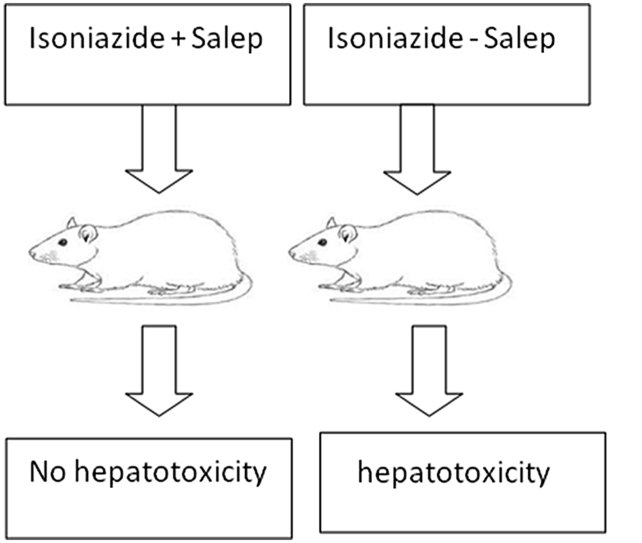
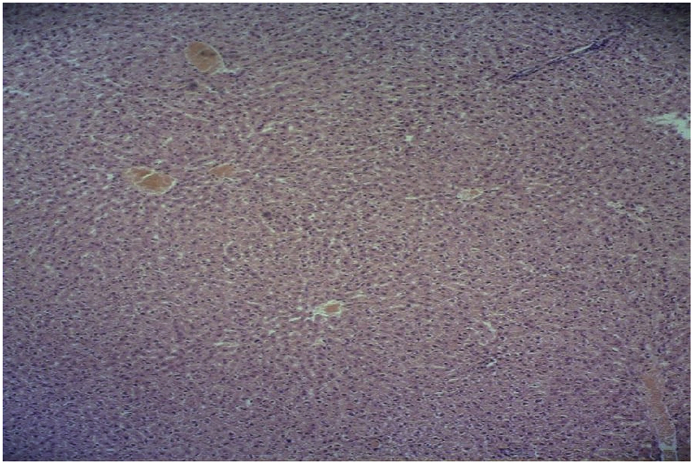
Structure of the liver tissue was healthy and normal in control and sham groups. Tissue studies revealed: normal structure of the lobules, central veins, sinusoids, Kupffer cells, and glycogen distribution; and no lymphocytic infiltration or blood congestion in the vessels of the liver (Fig. 1, Fig. 2).
Fig. 1.
Microscopic view of the liver in the control group: normal structure with no blood congestion or lymphocytic infiltration (Haematoxylin-eosin, at ×100 magnification).
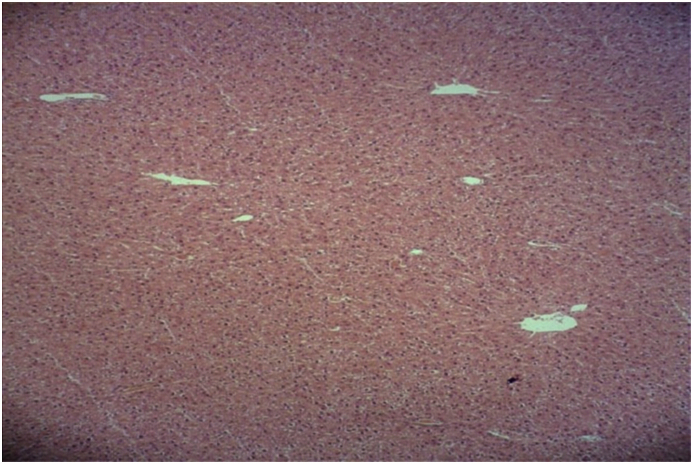
Fig. 2.
Microscopic view of the liver in the sham group: normal structure with no blood congestion in the sinusoids (Haematoxylin-eosin, at ×100 magnification).
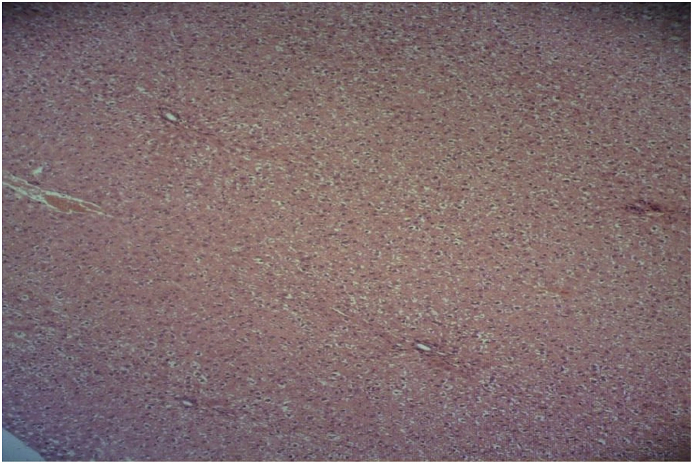
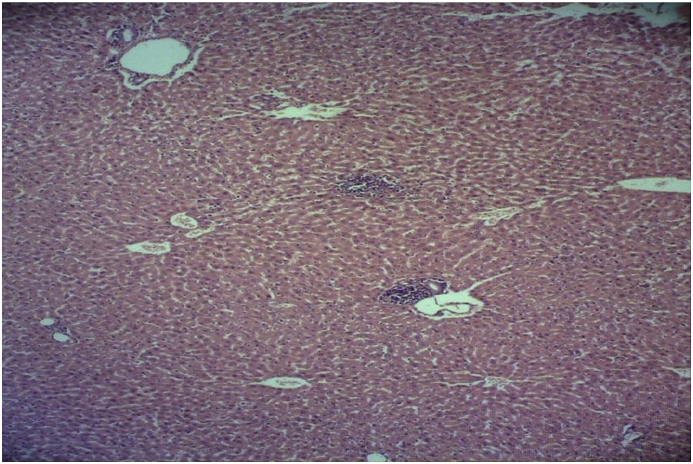
In the isoniazid alone group, hepatocyte necrosis, degenerative changes, scattered proliferation of Kupffer cells, infiltration and increased number of inflammatory cells around the portal space, around the central vein of the lobules, and in the sinusoidal space were revealed. In this group formation of inflammatory and fibrotic bridges between liver lobules were observed too. In addition to these changes, blood congestion in the vessels and in the sinusoids, and slight ballooning of the cells were seen in liver tissue (Fig. 3).
Fig. 3.
Microscopic view of the liver in the isoniazid group (50 mg/kg): hepatocyte necrosis, degenerative changes, infiltration and increased number of inflammatory cells around the portal space (Haematoxylin-eosin, at × 100 magnification).
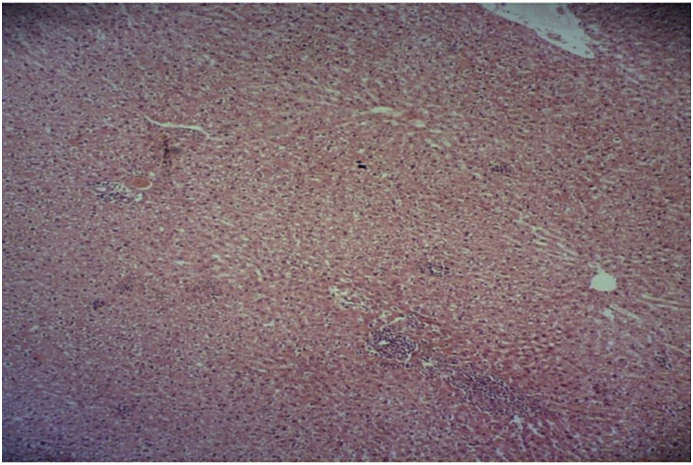
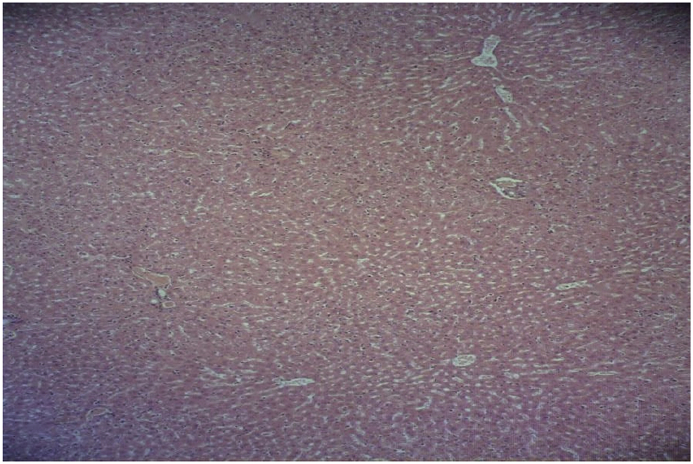
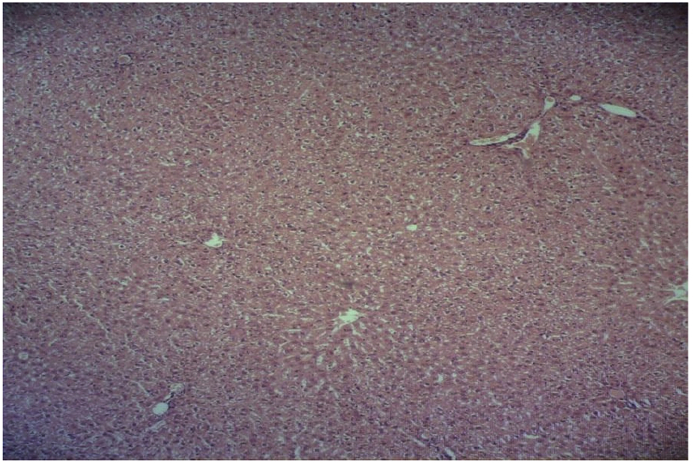
In the isoniazid/salep groups (40, 80, 160, and 320 mg/kg of the orchid extract), the destructive effects of isoniazid decreased in a dose-dependent manner. They declined very slightly, moderately, substantially, and very substantially in the groups receiving the extract at 40, 80, 160, or 320 mg/kg, respectively (Fig. 4, Fig. 5, Fig. 6, Fig. 7).
Fig. 4.
Microscopic view of the liver in the group that received isoniazid together with 40 mg/kg of the extract (Haematoxylin-eosin, at ×100 magnification).
Fig. 5.
Microscopic view of the liver in the group that received isoniazid and the extract at 80 mg/kg (Haematoxylin-eosin, at ×100 magnification).
Fig. 6.
Microscopic view of the liver in the group that was given isoniazid and the extract at 160 mg/kg (Haematoxylin-eosin, at ×100 magnification).
Fig. 7.
Microscopic view of the liver in the group that was given isoniazid and the extract at 320 mg/kg (Haematoxylin-eosin, at ×100 magnification).
4. Discussion
Elevation of liver enzymes (ALT and AST) is well known diagnostic indicators of liver disorder. Liver injury and elevation of liver enzymes is a known side effects of some drugs such as antituberculosis drugs. This complication usually occurs in initial weeks of treatment in these patients. The most important risk factor for hepatotoxicity of tubercilosis drugs is acetylaor phenotype polymorphism; and some other known risk factors such as: old age, malnutrition, alcoholism, HIV infection and hepatitis B and C infections.21
Hepatotoxicity incidence rate is 2.6% with INH and RMP coadministration, 1.1% with RMP alone and 1.6% with INH alone administration.22
In the metabolism circle of INH and RMP, hydrazine is produced which will be oxidized or acetylated and will lead to oxidized monoacetyl hydrazine or acetylated hydrazine respectively. These products both are hepatotoxic and can damage the hepatocytes.23, 24
Damage to liver tissues by isoniazid, will be happen through induction of oxidative stress.25 It should be emphasize that this drug may inhibit mitochondrial cytochrome P450 in the liver. Cytochrome P450 has the role of transferring electrons to the heme section (cofactor) of hemoglobin. Therefore, inhibition of cytochrome P450 causes the production of free radicals in the liver.25
On the other hand; it is showed that, some components such as Polyphenolic compounds and flavonoids (such as quercetin) can protect cells against glutathione depletion through increasing the capacities of antioxidant enzymes (glutathione reductase, glutathione peroxidase, and catalase).26 Antioxidant activities of phenolic compounds are essentially due to their reducing capacity that allows them to act as reducing agents, hydrogen donors, and inactivators of reactive oxygen species.27 Presence of two hydroxyl groups (OH) adjacent to each other increases the reducing capacity and antioxidant activity of quercetin.28 These compounds have protective effects on the liver against injuries resulting from hepatic poisons and free radicals.29, 30
Glucomannan, polyphenols, and ferulic acid, with antioxidant activities, are among the compounds found in salep extract.1 Numerous studies have shown glucomannan can inhibit oxidative stress and effectively reduce levels of ALT and AST.31, 32 In addition, glucomannan can modify and improve the weakness of the antioxidant system in rats that has been on high fat diets. Furthermore, glucomannan increases levels of the antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT), and therefore it can decrease the concentration of MAD in the liver.31, 32
Results of this research indicated intraperitoneal injections of isoniazid at 50 mg/kg for 28 days lead to increased serum levels of ALP, AST, and ALT, and other factors that are markers of liver damage such as MDA, TOC, total bilirubin. Administration of this drug to rats, also redound to reduced TAC, total protein, and serum albumin compared to the control group. Histological studies of this research also showed liver damage by isoniazid. These results are in agreement with those of other studies conducted in this field.33, 34
In the groups receiving isoniazid and various doses of the orchid extract, reductions in serum levels of the liver enzymes and in MDA and TOC, and increases in TAC, total protein, and in serum albumin were observed compared to the isoniazid group. These results show the extract has antioxidant activity and protective effects against the destructive effects of isoniazid. The histological findings of this research also agree with the biochemical findings. It is shown that at higher doses, the extract could prevent liver tissue damage by isoniazid and restore tissue function same as the control group. Results of research carried out by Kargar Jahromi et al. (2015) concerning biochemical factors and liver tissue indicated this extract increased TAC and reduced indices of lipid peroxidation such as MAD and TOC in rats.1
5. Conclusion
It seems antioxidant compounds present in orchid extract stabilize membranes of liver cells through reducing production of free radicals, decreasing release of liver enzymes into blood, and by improving liver protection indices in rats poisoned by isoniazid.
At the end we would like to say that administration of salep (as a complementary drug in TB therapy) may increase the power of the patient against the hepatotoxicity and this drug may decrease the chance of drug interruption.
Conflict of interest
No conflict of interest is in this research for all authors.
Acknowledgements
This study was conducted as part of the findings related to the medical PhD thesis of Mohadeseh Karimi. It performed With IR.JUMS.REC.1394.045 code number under investigation of the committee of ethics in research in Jahrom University of Medical Sciences. We would also like to acknowledge the support and cooperation of research and technology assistant and laboratory staffs of animals' house related to Jahrom University of Medical Sciences, who had extremity of necessary cooperation with us in this work.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Pourahmad M, Kargar Jahromi H, and Kargar Jahromi Z. Protective effect of salep on liver. Hepatitis monthly. 15,(4). [DOI] [PMC free article] [PubMed]
- 2.Lee J., Boyer J.L. Molecular alterations in hepatocyte transport. Semin Liver Dis. 2000;(20):373–384. doi: 10.1055/s-2000-9390. [DOI] [PubMed] [Google Scholar]
- 3.Lei B., Wei C.-J., Tu S.-C. Action mechanism of antitubercular isoniazid activation by Mycobacterium tuberculosis KatG, isolation, and characterization of InhA inhibitor. J Biol Chem. 2000;275(4):2520–2526. doi: 10.1074/jbc.275.4.2520. [DOI] [PubMed] [Google Scholar]
- 4.Karthikeyan S., Krishnmoorthy M.S. Effect of subacute administration of isoniazid and pyridoxine on lipids in plasma, liver and adipose tissues in the rabit. Drug Chem Toxicol. 1991;14:293–303. doi: 10.3109/01480549109002191. [DOI] [PubMed] [Google Scholar]
- 5.Yard A., Mekennis H. Effect of structure on the ability of hydrazin compounds to produce faty livers. J Pharmacol Exp Ther. 1995;(114):391–397. [PubMed] [Google Scholar]
- 6.Woo J., Chan C.H.S., Walubo A., Chan K.K.C. Hydrazine: a possible cause of isonizid – induced hepatic necrosis. J. Med. 1992;(23):51–59. [PubMed] [Google Scholar]
- 7.Ebrahimi S. Sadeghi H., Pourmahmoud A., Askariyan S.H., Askari S., Protective effect of Zizphus Vulgaris extract, on liver toxicity in laboratory rats. Armaghane danesh. 16(2): 172–180.
- 8.Neshat Gharamaleki M., Mohajeri D. The study of the protective effect of black cumin (Nigella sativa linn.) ethanolic extract against rifampin-induced hepatotoxicity in rats. Qom Univ Med Sci J. 2015;8(5):73–84. [Google Scholar]
- 9.Pourahmad M. The protective effect of Aloe vera on paraquat hepatotoxicity. Med Res Health Sci. 2016;5(S):253–258. [Google Scholar]
- 10.Freudenstein J.V., Rasmussen F.N. Sectile pollinia and relationships in theOrchidaceae. Plant Syst Evol. 1997;205(3–4):125–146. [Google Scholar]
- 11.Cozzolino S., Widmer A. Orchid diversity: an evolutionary consequence of deception? Trends Ecol Evol. 2005;20(9):487–494. doi: 10.1016/j.tree.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Lumaga M.R.B., Cozzolino S., Kocyan A. Exine micromorphology of Orchidinae (Orchidoideae, Orchidaceae): phylogenetic constraints or ecological influences? Ann Bot. 2006;98(1):237–244. doi: 10.1093/aob/mcl094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kargar Jahromi H., Jashni H.K., Sameni H., Abedi H.A., Kargar Jahromi Z., Kharameh Z.K. The effect of aqueous extract of Dactylorhiza maculate root on the concentration of hypothalamic-pituitary-thyroid Axis hormones in adult female rats. Int J Med Res Health Sci. 2016;5(5(s)):248–252. [Google Scholar]
- 14.Kaya S., Tekin A.R. The effect of salep content on the rheological characteristics of a typical ice-cream mix. J Food Eng. 2001;47(1):59–62. [Google Scholar]
- 15.Farhoosh R., Riazi A.R. A compositional study on two current types of salep in Iran and their rheological properties as a function of concentration and temperature. Food Hydrocoll. 2007;21(4):660–666. [Google Scholar]
- 16.Faraji Z., Nikzad H., Parivar K., Nikzad M. The effect of aqueous extract of Salep Tubers on the structure of testis and sexual hormones in male mice. J Jahrom Univ Med Sci. 2013;11(1):62. [Google Scholar]
- 17.Kargar Jahromi H., Solhjo K., Solhjo K.A., KargarJahromi Z., Ebrahimian A., Khabbaz K.Z. The effect of aqueous extract of the roots of Salep plants on the serum concentration of FSH and estrogen hormone in female rats. Par J Med Sci. 2015;13(2):39–44. [Google Scholar]
- 18.Kargar Jahromi H., Hajiani M., KargarJahromi Z., Khabbaz Kherameh Z., Dowlatkhah H.R., Mahdiyar M. Investigation of Orchid root aqueous extract treatment on hormone cholecystokinin serum concentration and body weight in male rats. Par J Med Sci. 2015;13(1):15–20. [Google Scholar]
- 19.Mohajeri D., Mesgari Abassi M., Delazar A., Doustar Y., Mousavi G., Amo oughli Tabrizi B. Histopathol study subacute Toxictoxicity of Crocus sativus L (Saffron) stigma total extract on liver and kidney tissues in the rat. Pharm Sci. 2009;15(2):115–124. [Google Scholar]
- 20.Mostafavi-Pour Z., Zal F., Monabati A., Vessal M. Protective effects of a combination of quercetin and vitamin E against cyclosporine A†induced oxidative stress and hepatotoxicity in rats. Hepatol Res. 2008;38(4):385–392. doi: 10.1111/j.1872-034X.2007.00273.x. [DOI] [PubMed] [Google Scholar]
- 21.Yew W.W., Leung C.C. Antituberculosis drugs and hepatotoxicity. Respirology. 2006;11(6):699–707. doi: 10.1111/j.1440-1843.2006.00941.x. [DOI] [PubMed] [Google Scholar]
- 22.Steel M.A., Burk R.F., DesPrez R.M. Toxic hepatitis with isoniazid and rifampin. Chest. 1991;99:467–471. doi: 10.1378/chest.99.2.465. [DOI] [PubMed] [Google Scholar]
- 23.Sodhi C.P., Sodhi C.P., Rana S.V., Mehta S.K., Vaiphei K., Attari S., Mehta S. Study of oxidative-stress in isoniazid-rifampicin induced hepatic injury in young rats. Drug Chem Toxicol. 1997;20(3):255–269. doi: 10.3109/01480549709003881. [DOI] [PubMed] [Google Scholar]
- 24.Askgaard D.S., Wilcke T., Dossing M. Hepatotoxicity caused by the combined action of isoniazid and rifampicin. Thorax. 1995;50(2):213–214. doi: 10.1136/thx.50.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Attri S. Isoniazid- and rifampicin – induced oxidative hepatic injury - protection by N-acetylcysteine. Human Exp Toxicol. 2000;19(9):517–522. doi: 10.1191/096032700674230830. [DOI] [PubMed] [Google Scholar]
- 26.Rice-Evans C.A., Miller N.J., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Rad Biol Med. 1996;20(7):933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 27.Cos P. In vitro antioxidant profile of phenolic acid derivatives. Free Radic Res. 2002;36(6):711–716. doi: 10.1080/10715760290029182. [DOI] [PubMed] [Google Scholar]
- 28.Solhjo K., Kargar Jahromi H., Solhjo K.A., Kargar Jahromi Z., Khabbaz Kherameh Z., Mahdiyar M. The effect of the aqueous extract of Orchid roots on the serum concentration of progesterone and luteinizing hormone in adult female rats. Par J Med Sci. 2015;13(1):21–25. [Google Scholar]
- 29.Areias F.M., Valentao P., Andrade P.B., Ferreres F., Seabra R.M. Phenolic fingerprint of peppermint leaves. Food Chem. 2001;73(3):307–311. [Google Scholar]
- 30.Carreon J.P., Iimenez G.C., Vega J.L. Genotoxic and antigenotoxic properties of Calendula officinalis extracts in rat liver cell cultures treated wiht diethylnitrosamine. Toxicol in vitro. 2002;16:235–258. doi: 10.1016/s0887-2333(02)00005-x. [DOI] [PubMed] [Google Scholar]
- 31.Nurcan D., Keskin E. The effects of aflatoxin and glucomannan on some antioxidants and biochemical parameters in rabbits. Acta Veterinaria. 2008 [Google Scholar]
- 32.Dvorska J.E., Pappas A.C., Karadas F., Speake B.K., Surai P.F. Protective effect of modified glucomannans and organic selenium against antioxidant depletion in the chicken liver due to T-2 toxin-contaminated feed consumption. Comp Biochem Physiol Part C Toxicol Pharmacol. 2007;145(4):582–587. doi: 10.1016/j.cbpc.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Prabakan M., Anandan R., Devaki T. Protective effect of Hemidesmus indicus against rifampicin and isoniazid-induced hepatotoxicity in rats. Fitoterapia. 2000;71(1):55–59. doi: 10.1016/s0367-326x(99)00120-3. [DOI] [PubMed] [Google Scholar]
- 34.Santhosh S., Sini T.K., Anandan R., Mathew P.T. Hepatoprotective activity of chitosan against isoniazid and rifampicin-induced toxicity in experimental rats. Eur J Pharmacol. 2007;572(1):69–73. doi: 10.1016/j.ejphar.2007.05.059. [DOI] [PubMed] [Google Scholar]