Abstract
Background
The global increase in acceptance and use of herbal remedies in recent times is still accompanied with poor knowledge of their potential adverse effects and the toxicological implications of their use are underestimated.
Methods
Bon-santé Cleanser® (BSC), a polyherbal containing Anogeissus leiocarpus, Terminalia ivorensis, Massularia acuminate and Macuna pruriens, is an “energizer and hormone booster”. We assessed the effect of BSC on reproductive function after administration for 60 days in male Wistar rats. Rats (150–300 g) were assigned into four groups of 8/group. Control received distilled water (10 ml/kg) while other groups received BSC 250, 500 and 1000 mg/kg/day p.o. respectively. Animals were euthanized by cervical dislocation and samples collected for analysis.
Results
BSC (250 mg/kg) elevated (p < 0.05) follicle stimulating hormone and luteinizing hormone levels respectively. BSC decreased sperm motility and the live-dead ratio at 1000 mg/kg and reduced reproductive hormone at 500 mg/kg and 1000 mg/kg respectively. BSC at 500 mg/kg increased (p < 0.05, F = 3.18–13.21) testicular reduced glutathione level (50.3%) and catalase (43.7%) but not activities of superoxide dismutase, glutathione S-transferase, and malondialdehyde level. Further, BSC influenced Mg, Zn, Cu, P, Mn, Ni and Fe levels (p < 0.05). BSC (1000 mg/kg) decreased testis weight (p < 0.05) and induced mild inflammation characterized by atrophic tubules.
Conclusion
Overall, our data suggest BSC at low doses may increase reproductive hormones regulated by FSH and LH as observed in this study. However, BSC administration should be done with caution as it may induce reproductive toxicity in large doses.
Keywords: Bon-santé Cleanser® capsule, Male reproductive function, Biological elements, Herbal capsule, Toxicology
Graphical abstract
Safety evaluation of Bon-santé cleanser® polyherbal in male Wistar rats: Further investigations on androgenic and toxicological profile.
1. Introduction
The consumer use of herbal products is a serious and growing public health problem despite the relative lack of credible scientific evidence that supports their therapeutic efficacy. The World Health Organization (WHO) has placed interest and commemorates the judicious applications of alternative and complementary medicines that might have passed or become justified for their various indications.1 Similarly, other food and drug regulatory agencies have embraced this suggestion and ordered such for commercialization. This explains an increasing demand for pharmacologic screening and increases discussion on the safety assessment of herbal remedies. There has been convergent reasoning to provide powerful methodologies for proving efficacy, ensuring quality, standardizing good manufacturing practices, testing for safety, and conducting pharmacovigilance surveillance for adverse effects of polyherbal formulations similar to conventional drugs. This encourages such campaign for a holistic approach of the synergistic or antagonistic effects of several available polyherbal preparations.2, 3 More so, since every polyherbal possesses inherent toxicity,4 the lack of a stringent and harmonized quality control and effective monitoring system predispose herbal medications to contamination or adulteration that could prove harmful to humans. Thus, consumers who have the erroneous belief that herbal products are scientifically proven to be effective or are safe because the products are natural may be at serious risks. Agents such as pesticides, toxic herbs, heavy metals and conventional drugs are commonly encountered toxicological concerns in herbal preparations.5 Suggestions are that some biological trace elements are capable of modifying the molecular structures of subcellular constituents and membranes6, 7 or play biochemical roles that reflect their involvement in a large number of enzymes.8 This is supported by the determination of trace elements in medicinal materials9, 10 with evidence of their involvement on the reproductive systems.11, 12, 13 This is particularly true for polyherbal medicines in which constituents have a long traditional use but without ever having been submitted to formal tests of safety compared to conventional medicines.14 Contrastingly, consumers have the erroneous belief that herbal products are safe because they are natural and or synonymous with their orthodox counterparts.15, 16 In addition, there are speculations that agricultural practices, industrial emissions being accounted as indirect contributors16 aside some drugs17 to influence their potentials. In this respect, regulatory authorities including scientific community usually scrutinize their existence.1
Bon-santé Cleanser® (BSC), a polyherbal formula, is manufactured by Dabiron Natural Life Care, Nigeria. BSC polyherbal remedy compositions include Massularia acuminate (G. Don.) Bullock ex Hoyle, Macuna pruriens (L.,) DC, Anogeissus leiocarpus (DC.), and Terminalia ivorensis (A. Chev.) formulated into capsule. Interestingly, both folkloric medicine uses and several proposed properties of the potentials of the extracts of the aforementioned medicinal plantsincluding their phytoconstituents have been verified scientifically in rodents.18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 For instance, M. acuminate (G. Don.) Bullock ex Hoyle, a member of the family Rubiaceae, is used as a chewing stick and aphrodisiac by the Yorubas in southwest Nigeria and has been found to contain a thiophenolic glycoside.19, 20 It is widely used in Ayurvedic system of medicine to treat various ailments including infertility, gingivitis amongst others.19, 29 More so, preliminary studies in rats suggest that this herb can increase testosterone and sexuality.18, 20 M. pruriens (L.) DC is a family of fabaceae and forms one of the most important medicinal plants used to treat many ailments. Folklore explores and benefited from its behavioural and aphrodisiac properties.21 Phytochemical constituents present include alkaloids, anthraquinones, saponins, phenolics, flavonoids, glycosides and tannins according to Yakubu et al20 and Oriola et al19 In addition, some authors have demonstrated the presence of flavones and 3,4-dihydroxyphenylalanine (l-dopa).4, 30 A. leiocarpus and T. ivorensis A. Chev., both combretaceae are used for treating worms and protozoan diseases in animals.1, 31 Also, their bark can be used as a chewing stick and their extracts have shown antibacterial, diarrhea, febrifuges, pain and aphrodisiac properties.23, 25 The A. leiocarpus stem barks contain tannins, astringents, castalagin31 and flavogallonic acid dilactone28, 31 while glycosides, saponins, steroids tannins, astringents were found in T. ivorensis.27 Thus, the androgenic, antipyretic, analgesic, antioxidant and anti-inflammatory potentials of these medicinal plants have been documented.18, 22, 23, 24, 25, 26, 27, 28, 29 However, no study has yet justified the reproductive effects of the combination of these medicinal plants as found in BSC or its wholistic toxicological effects.
Recently, we assessed the safety of this preparation since it is being sold in public places and the manufacturers claimed they have many customers.32 Further, the National Agency for Food and Drug Administration and Control enlists BSC among herbal products in Nigeria. Although, we found that BSC was relatively safe with mild alteration in the liver and heart architectures in rats,32 but its acclaimed indications, however, cannot be juxtaposed on this fact, since no scientific investigations on efficacy have been carried out.
The present study, therefore, assessed the acclaimed hormone boosting effects of Bon-santé Cleanser® polyherbal capsule and reproductive toxicity potentials after a sixty-day sub-chronic administration in male Wistar rats. Also, we determined the influence on the levels of biological trace elements and commented on the quality assurance.
2. Materials and methods
2.1. Drugs and chemicals
The study was carried out in the Department of Pharmacology, University of Lagos, Lagos Nigeria. Bon-santé Cleanser® capsule was obtained from Dabiron Natural Life Care, Nigeria. Thiobarbituric acid (TBA), Ellman's reagent (DTNB) and 1-Chloro-2,4,-dinitrobenzene (CDBN) from Sigma (USA) were purchased from Sigma Chemical Company (USA). Reduced glutathione (GSH), Metaphosphoric acid and Trichloroacetic acid (TCA) were purchased from J.I. Baker (USA). Bovine serum albumin fraction V (BSA) was purchased from SRL, India. Rat Follicle Stimulating Hormone (FSH) (Cat. No.: Rshakrfs-010R) and Luteinizing Hormone (LH) ELISA (Rshakrlh-010SR) kits were purchased from (Biovendor, Shibayagi Co., Ltd. (Japan). RAT Testosterone (RTC001R) ELISA was obtained from Biovendor, Laboratorni, medicina a.s Karasek (Czech Republic). Sodium hydroxide was obtained from MERCK (Germany). All other chemicals and reagents used were of analytical grades. Atomic UV/Visible Spectrophotometer obtained from JENWAY, Bibby Scientific (Model 7300 and 7305) (USA).
2.2. Extraction and preparation of the final polyherbal formulation
BSC was obtained directly from the Dabiron Natural Life Care in Nigeria. It was assigned Batch number 002 and listed with number A7-5321L by the National Agency for Food and Drug Administration and Control (NAFDAC). BSC contains A. leiocarpus (DC., family Combretaceae) Guill & Perr., T. ivorensis (A. Chev., family Combretaceae), M. acuminate (G.Don, family Rubiaceae) Bullock ex Hoyle and Macuna pruriens (L., family leguminosae) DC.in the ratio 4:2:1:1 respectively. The extraction and formulation procedures complied with the regulatory manual of NAFDAC. In this context, a single capsule of BSC (total content 442 mg) was prepared as previously reported32 and administered via oral gavage according to standard toxicological guidelines.
2.3. Animals
Albino rats of the Wistar strain weighing between 150 and 300 g were purchased from the animal house of the Redeemers University, Ogun State, Nigeria. The rats were housed under controlled conditions in the experimental animal handling facility of the College of Medicine, University of Lagos, Nigeria. The experimental animal room had a 12 h light/12 h dark schedule and maintained at a temperature of 22 ± 3 °C throughout the study. Animals were fed with commercially available rat pelleted diet (Ladoke Akintola Growers Mash, Nigeria) and were allowed access to water ad libitum throughout the period of the experiment. The experimental protocols were approved by the Institutional Animal Care and Use Committee, Department of Pharmacology, Therapeutic and Toxicology, College of Medicine, University of Lagos. Animals were certified fit for the experiment by the Institution's Animal Health Officers before the commencement of the study. Beddings were changed on alternate days and the animals were sacrificed in a humane manner at the end of the experiment by cervical dislocation. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the U. S. National Institutes of Health (NIH Publication No. 85-23, revised 1996)” for studies involving experimental animals and the procedures as documented by Kilkenny et al33 for reporting animal research.
2.4. Experimental design and necropsy
Thirty-Two (32) adult male Wistar rats weighing between 150-300 g were divided into 4 groups of 8 rats per group. Group I, the control group, received 10 ml/kg of distilled water daily. BSC was administered to the experimental groups (groups II–IV) at 250 mg/kg, 500 mg/kg and 1000 mg/kg respectively. Both distilled water and BSC were given daily for sixty days via oral gavage. Rats were weighed weekly throughout the course of the experiment. Twenty-four hours after last administration, blood samples were obtained by ocular puncture into either lithium heparin or ethylene diaminetetraacetic acid (EDTA) bottles and animals were subsequently sacrificed by cervical dislocation. The anticoagulated blood samples were centrifuged at 4200 rpm for 5 min to separate the plasma from which all biochemical assays were carried out. The male reproductive system (testis, epididymis) were all harvested, weighed and homogenized in four volumes of buffer solution (0.1M, pH 7.4). A portion of each organ was taken out for histology. The remaining was weighed and homogenized for biochemical assays.
2.5. Analysis of sperm characteristics and morphology
The testes from each rat were carefully exposed and removed along with its adjoining epididymis. The slides on which the sperm cells were counted were heated to 37 °C until the time of the analysis. The analysis was carried out at room temperature using one epididymis of each rat. The left testis was separated from the epididymis and the caudal epididymal tissue was removed and placed in a petri dish containing 1 mL normal saline solution. An incision of about 1 mm was made in the caudal epididymis to liberate its spermatozoa into the saline solution. Progressive sperm motility, sperm count, and sperm viability were then examined under the microscope attached to a Celestron® Digital Microscope Imager (Torrance, CA 90503) and viewed under X40 objective according to the method described by Raji et al34 and modified by Kale and Awodele.32 Epididymal sperm motility was assessed by calculating motile spermatozoa per unit area and was expressed as percentage motility. Epididymal sperm count was done using the improved Neubauer hemocytometer and expressed as million/ml of suspension. The sperm viability was also determined using Eosin/Nigrosin stain. The motile (live) sperm cells were unstained while the non-motile (dead) sperms absorbed the stain. The stained and unstained sperm cells were counted and an average value for each was recorded from which percentage viability was calculated. Sperm morphology was evaluated by staining the sperm smears on microscope slides with two drops of Walls and Ewa stain after they were air-dried. The slides were examined under the microscope under oil immersion with X 100 objectives.
2.6. Assessment of reproductive hormone
Serum concentrations of male reproductive hormones were measured using micro plate enzyme-linked immunosorbent assay (ELISA) and expressed as Units/Litre. Direct immune-enzymatic determination of the Luteinizing (LH) and Follicle Stimulating Hormones (FSH) was carried out by the method of Odell et al35 while testosterone level was determined following the principle according to Joshi et al36 as described in manufacturer's manual.
2.7. Rat FSH and LH ELISA
Briefly, in rat FSH or LH ELISA Kit, biotin-conjugated anti-FSH/anti-LH and standard or samples were incubated in monoclonal anti-FSH antibody-coated wells. After 15 h incubation and washing, HRP (horse radish peroxidase)-conjugated avidin was added, and incubated for 30 min. After washing, HRP-complex remaining in wells was reacted with a chromogenic substrate (TMB) for 20 min, and the reaction was stopped by addition of acidic solution, and the absorbance of a yellow product was measured spectrophotometrically at 450 nm. The absorbance is nearly proportional to FSH or LH concentration. FSH or LH concentrations in the unknown samples were then extrapolated given their respected standard curve.35
2.8. Rat testosterone ELISA
A 10 μl of each of sample with new disposable tips into appropriate wells was dispensed in a 100 μl of incubation Buffer into each well. Added was a 50 μl enzyme Conjugate into each well which was incubated for 60 min at room temperature on a microplate mixer. This was discarded and the well rinsed 4 times with diluted washing solution (300 μl per well). Then 200 μl was added of substrate solution to each well and incubated standing for 30 min in the dark. The reaction was stopped by adding 50 μl of stop solution to each well and the absorbance determined for each well at 450 nm.36
2.9. Total protein determination and evaluation of oxidant/anti-oxidant status
Total serum protein (TP) concentration was determined according to the principles based on the Biuret reaction.37 The method of Beutler et al38 was followed in estimating the activity of reduced glutathione (GSH). Glutathione S-transferase (GST) activity was determined according to the method described by Habig et al39 The level of superoxide dismutase (SOD) levels was determined by the method of Misra and Fridovich.40 Catalase activity was determined according to the method of Sinha.41 Lipid peroxidation activity was determined by measuring the formation of thiobarbituric acid reactive substances (TBARS) according to the method of Varshney and Kale.42
2.10. Determination of trace elements levels
Biological trace elements in crude BSC sample and in rat serum were determined using Atomic Absorption Spectrophotometer (MODEL AA-400, PERKIEMER). A capsule of BSC was dissolved in 100 ml deionised water and filtered with glass wool to obtain solution of crude sample. Analyses of trace elements followed the methods of Sprague and Slavin43 with guidelines with the standard produces of the Perkin-Elmer Corp.
2.11. Histological assessment
Briefly, samples from rat testis were trimmed to about 5 mm thickness, fixed in 10% formol saline and then transferred to graded concentrations of alcohol for two hours. Infiltration was carried out twice by passing each tissue through molten paraffin wax in an oven at a temperature of 30 °C for one and a half hours each. The tissues embedded in molten paraffin wax were later placed on a wooden block and trimmed to size. Serial sections (10 μ thick) were made using a rotatory microtome, floated in a warm water bath at a temperature of 30–40 °C and placed on slides. Sections were then stained, mounted on slides using dimethyl paraffinate xylene (DPX) as a mounting agent and viewed under a light microscope.
2.12. Statistical analysis
Data presented are mean ± standard error of the mean (SEM) and were analyzed using Statistical Package for Social Sciences (SPSS, version 20) software for windows (SPSS, Inc., Chicago, Illinois, USA). Differences between groups were determined by one-way analysis of variance, and posthoc testing was performed for intergroup comparisons using the least significant difference (LSD).44 Figures were obtained using GraphPad Prism 6. A P-value <0.05 was considered significant.
3. Results
3.1. Sperm characteristics and morphology
Table 1 shows the result of BSC on sperm characteristics and morphology in normal and BSC-treated rats. BSC demonstrated elevated (p > 0.05, F = 2.27) sperm count in the treated animals by 32.6%, 12.1%, and 13.2% respectively when compared with control group at the doses administered. Although, BSC (250 mg/kg and 500 mg/kg) increased sperm motility non-significantly (p > 0.05, F = 3.38), the highest dose (1000 mg/kg) however, significantly (p < 0.05, F = 3.38–6.07) reduced sperm motility as well as live-dead ratio when compared with control group. In addition, BSC did not significantly alter sperm morphology as the percentage of abnormal sperm in the treated rats was not significantly different from those of control.
Table 1.
Effect of sub-chronic administration of Bon-santé Cleanser® polyherbal formula on sperm characteristics.
| Treatment | SC × 106a | Motilitya | L/D ratio | Abnormalitya |
|---|---|---|---|---|
| Control (DW, 10 ml/kg) | 64.50 ± 9.43 | 72.75 ± 6.29 | 82.75 ± 4.51 | 83.00 ± 2.35 |
| BSC (250 mg/kg) | 85.50 ± 8.29∗ (−32.6) |
76.25 ± 8.85 (−4.8) |
83.00 ± 7.78 (−0.3) |
78.50 ± 10.25 (5.4) |
| BSC (500 mg/kg) | 72.25 ± 7.21 (−12.1) |
72.25 ± 5.27 (0.7) |
77.25 ± 5.85 (6.6) |
84.00 ± 3.48 (−1.2) |
| BSC (1000 mg/kg) | 73.00 ± 8.19 (−13.2) |
42.33 ± 2.04∗ (41.8) |
40.67 ± 3.95∗ (50.9) |
65.66 ± 6.23 (20.9) |
Data are expressed in percentage as mean ± SEM. “a” in superscript indicates %. ∗p < 0.05 when compared with control group. Values in parenthesis represent % change; (−) increase; (+) decrease. DW: Distilled water; BSC: Bon-santé Cleanser®. SC: Sperm count; L/D: Sperm Live/Dead ratio.
3.2. Reproductive hormone levels
Serum follicle stimulating hormone (FSH) and luteinizing hormone (LH) were significantly elevated (p < 0.05, F = 2.84–8.22) by 243.0% and 616% in the BSC-treated rats at 250 mg/kg when compared with the control group as indicated in Table 2. While 500 mg/kg did not alter FSH, it increased (p < 0.05, F = 8.22) LH by 136%. Also, BSC (1000 mg/kg) elevated FSH and LH by 31.3% and 48% (p > 0.05, F = 2.84–8.22) respectively. Both 500 mg/kg and 1000 mg/kg doses of BSC increased (p > 0.05, F = 8.22) the LH levels when compared with control group. Similarly, the levels of testosterone were not significantly different from those of control animals for BSC 250 mg/kg. However, testosterone levels decreased (p < 0.05, F = 1.65) in rats that were administered BSC 500 mg/kg and 1000 mg/kg.
Table 2.
Serum levels of reproductive hormone following sub-chronic administration of Bon-santé Cleanser® polyherbal formula.
| Treatment | FSH (units/L) | LH (units/L) | TST (units/L) |
|---|---|---|---|
| Control (DW, 10 ml/kg) | 0.16 ± 0.01 | 0.25 ± 0.06 | 2.49 ± 0.14 |
| BSC (250 mg/kg) | 0.55 ± 0.07∗ (−243.0) |
1.79 ± 0.38∗ (−616.0) |
2.39 ± 0.26 (4.01) |
| BSC (500 mg/kg) | 0.11 ± 0.09 (31.3) |
0.59 ± 0.59∗ (−136.0) |
0.54 ± 0.54∗ (78.3) |
| BSC (1000 mg/kg) | 0.21 ± 0.04 (−31.3) |
0.37 ± 0.04 (−48.0) |
0.22 ± 0.07∗ (91.2) |
Data are expressed as mean ± SEM. ∗p < 0.05 when compared with control group. Values in parenthesis represent % change; (−) increase; (+) decrease. BSC= Bon-santé Cleanser®. DW: Distilled water; FSH: Follicle Stimulating Hormone; LH: Luteinizing Hormone; TST: Testosterone, TSW: Testis weight.
3.3. Tissue total protein levels and oxidant/antioxidant status
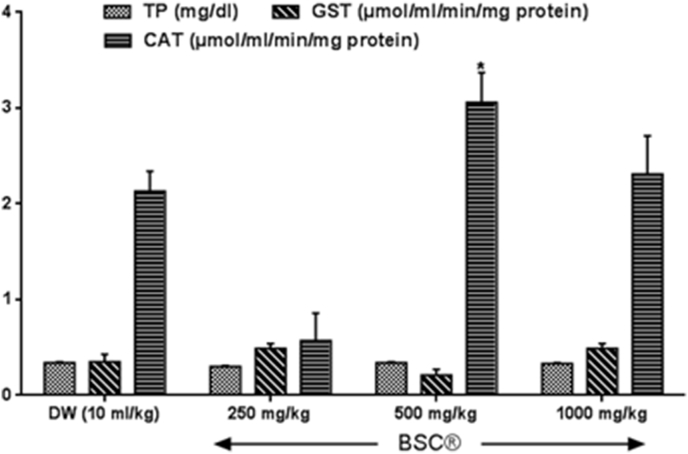
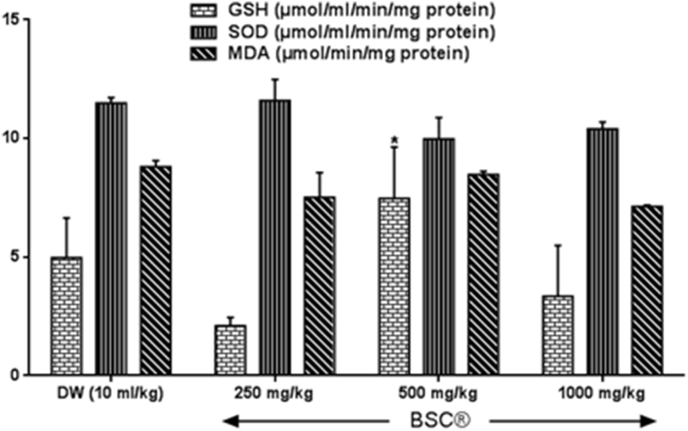
Fig. 1, Fig. 2 show effect of BSC on oxidant/antioxidant status of rat testis. BSC did alter total protein levels as observed in this study, although, BSC at 250 mg/kg showed slight significant reduction by 11% (p > 0.05, F = 5.18) in the TTP levels in the testis when compared with the control group as presented in Fig. 3. Testicular reduced glutathione (GSH) and glutathione S-transferase (GST) activities, superoxide dismutase (SOD) and catalase (CAT) levels were modulated by in BSC-treated rats when compared with control. While BSC at 250 mg/kg causes testicular GSH activity and CAT levels to decreased (p < 0.05, F = 2.16–4.83) by 57.7% and 72.3%. BSC only increase (p < 0.05) GSH and CAT at dose 500 mg/kg in the testis by 50.3% and 43.7% (p < 0.05, F = 3.18–13.21) respectively. Further, malondialdehyde activity (an important by-product and useful index of lipid peroxidation) at the doses administered was reduced in the BSC-treated rats, although, this was not significantly (p > 0.05, F = 8.37) different from control distilled water group.
Fig. 1.
Effect of subchronic administration of Bon-santé cleanser® polyherbal formula on testicular total protein levels and antioxidant activity. Data are expressed as mean ± SEM. *p < 0.05 when compared with control distilled water group. TP: Total protein; CAT: Catalase; GST: Glutathione-S-transferase; DW: Distilled water; BSC: Bon-santé cleanser®.
Fig. 2.
Effect of subchronic administration of Bon-santé cleanser® polyherbal formula on testicular antioxidant activity and lipid peroxidation. Data are expressed as mean ± SEM. *p < 0.05 when compared with control group. Superoxide dismutase; GSH: Reduced glutathione; MDA = Malondialdehyde; DW = Distilled water; BSC = Bon-santé cleanser®.
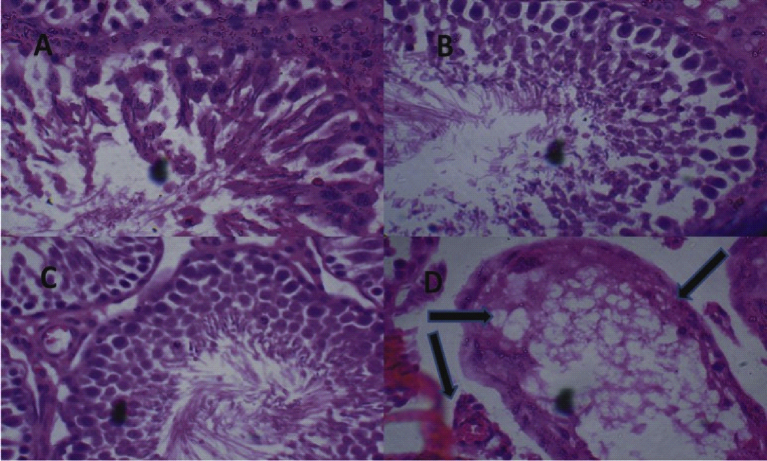
Fig. 3.
(A) Control Group (Distilled Water, 10 ml/kg) shows normal testis. (B) BSC (250 mg/kg) shows normal testis. (C) BSC (500 mg/kg) shows normal testis (D) BSC (1000 mg/kg) shows the presence of atrophic seminiferous tubules shrunken in size and devoid of lining spermatogenic series cells and spermatozoa (see arrow). (H & E, mag. x 400). (BSC = Bon-sante Cleanser®).
3.4. Body and testis weight
The result for change in body and testis weights is presented in Table 3. BSC at 250 mg/kg and 500 mg/kg did not significantly (p > 0.05, F = 2.52) change weight of testis in male rats when compared with those of control animals (distilled water, 10 ml/kg). However, BSC at 1000 mg/kg and 500 mg/kg significantly increased (p < 0.05, F = 2.52–7.64) testis weight and body weight respectively in the treated animals when compared with control group.
Table 3.
Testis and body weights following sub-chronic administration of Bon-santé Cleanser®polyherbal formula.
| Treatment | TSW (g/kg) weight | % Body weight |
|---|---|---|
| Control (DW, 10 ml/kg) | 6.3 ± 0.9 | −3.56 |
| BSC (250 mg/kg) | 6.5 ± 0.9 (−3.17) |
−10.38 |
| BSC (500 mg/kg) | 6.4 ± 0.7 (−1.59) |
−14.0∗ |
| BSC (1000 mg/kg) | 3.8 ± 0.7∗ (39.68) |
−9.09 |
Data are expressed as mean ± SEM. n = 8; ∗p < 0.05 when compared with control group. Values in parenthesis represent % change; (−) increase; (+) decrease. BSC: Bon-santé Cleanser®. TSW: Testis weight.
3.5. Biological trace elements levels
The results presented in Table 4 show the effect of BSC on biological trace elements. BSC (250, 500 and 1000 mg/kg) significantly decreases (p < 0.05, F = 1.46–11.24) Mg and Zn levels respectively. Also, BSC (250 and 1000 mg/kg) increase (p < 0.05, F = 3.07–5.46) Ni and P levels as well. BSC increased (p < 0.05, F = 2.51) Cu levels at 250 mg/kg and 500 mg/kg doses. BSC at 250 mg/kg elevated Mn (p < 0.05, F = 1.46) levels in the rats. However, Fe levels slightly decreased (p < 0.05, F = 3.28) at 500 mg/kg and 1000 mg/kg in the BSC-treated rats when compared with control distilled water group.
Table 4.
Effect of sub-chronic administration of Bon-santé Cleanser® polyherbal formula on serum trace elements levels.
| Elements | BSC STD | Control (DW, 10 ml/kg) |
BSC (250 mg/kg) |
BSC (500 mg/kg) |
BSC (1000 mg/kg) |
|---|---|---|---|---|---|
| Mg | 2.89 ± 0.04 | 2.69 ± 0.006 | 1.03 ± 0.001# | 1.67 ± 0.003# | 3.4 ± 0.001# |
| Zn | 3.70 ± 0.001 | 4.06 ± 0.03 | 3.62 ± 0.01# | 3.84 ± 0.002# | 3.92 ± 0.003∗ |
| P | 8.13 ± 0.21 | 9.00 ± 0.01 | 9.68 ± 0.14# | 9.01 ± 0.001 | 9.61 ± 0.001# |
| Ni | 0.20 ± 0.01 | 0.42 ± 0.01 | 0.23 ± 0.01# | 0.43 ± 0.001 | 0.33 ± 0.001# |
| Mn | 0.32 ± 0.009 | 0.24 ± 0.001 | 0.27 ± 0.014∗ | 0.24 ± 0.001 | 0.23 ± 0.001 |
| Cu | 0.10 ± 0.001 | 0.32 ± 0.003 | 0.38 ± 0.002# | 0.39 ± 0.023# | 0.29 ± 0.004# |
| Fe | 3.15 ± 0.03 | 3.21 ± 0.001 | 3.25 ± 0.001 | 3.13 ± 0.026∗ | 2.19 ± 0.002# |
Data are expressed as mean ± SEM; n = 8; units: mg/L; ∗p < 0.05 or #p < 0.001 when compared with control. BSC: Bon-santé Cleanser®; DW: Distilled water; STD: Standard; Mg: Magnesium; Zn: Zinc; P: Phosphorus; Ni: Nickel; Mn: Manganese; Cu: Copper; Fe: Iron.
4. Discussion
Our previous study on BSC showed the polyherbal remedy to be relatively safe with mild alteration in the liver and heart architectures in rats.32 Several other reports exist in the literature on issues relating to quality as well as unexpected adverse reactions or toxicity associated with the use of herbal remedies.45, 46, 47, 48, 49 Public acceptance and use of many herbal products including BSC continue to soar without sufficient data (if any for many herbs) on their toxicity profile or clinical efficacy to guide consumers on the rational use of such products. The uninformed belief or exaggerated confidence placed on the safety and efficacy of these herbal medicinal products over conventional or orthodox medications in many quarters calls for concern and thorough screening of these products in order to promote public health and safety.47, 48, 49 More so, since every polyherbal possesses inherent toxicity, the lack of a stringent and harmonized quality control and effective monitoring system predispose herbal medications to contamination or adulteration that could prove harmful to humans.50, 51 Therefore, the present study which provides data on some safety aspects of the polyherbal formula, Bon-santé Cleanser®, is an effort geared towards making useful information based on scientific investigation available in order to promote the safe and rational use of the product.
The ethnobotanical uses of the various medicinal plants compounded into BSC capsule have been verified either as individual agent or concoction19, 20, 29 and additional aphrodisiac activities of other extracts have been reported in experimental models.52 In rationalizing the efforts of Food and Drug Administration Agency, in our laboratory, we took special interest to investigating the safety of herbal products in rodents.32, 53 Although, sub-chronic administration (daily treatment for 60 days) of BSC in this study did not produce any significant change in sperm count and morphology at all dose levels when compared with control, the highest dose (1000 mg/kg) administered, however, significantly reduced sperm motility and live-dead ratio in the rats (Table 1). This latter effect (reduction in sperm motility and live-dead ratio) coupled with its ability to also induce mild inflammation characterized by atrophic tubules in the testis at this dose to suggest that this polyherbal formula may be capable of reducing fertility and ultimately exert testicular damage. In addition, the significant decrease in the weight of the testis relative to body weight produced by BSC (1000 m/kg) gives credence to this speculation and strongly supports the possibility of this polyherbal formula in inducing testicular injury and male reproductive toxicity.
Data from our study also suggest that at lower dose, BSC may be able to enhance levels of hormone under the regulatory control of the anterior pituitary hormones, FSH and LH (Table 2). This somewhat gives credence to the acclaimed “hormone boosting” benefits of this polyherbal formula in folk medicine. BSC significantly elevated serum levels of FSH and LH at the lowest dose (250 mg/kg) administered in this study. Higher doses (500 and 1000 mg/kg) of the polyherbal formula, however, did not exert any significantly different change in the level of this hormones when compared with the control rats. The decrease in the level of FSH and LH observed at the higher doses of BSC when compared with the lower dose (250 mg/kg) may be related to negative feedback effect on the anterior pituitary. The ability of BSC to enhance serum level of FSH and LH may be of particular benefit in conditions associated with a deficiency in levels and/or actions of reproductive or endocrine hormones that are regulated by these anterior pituitary hormones. There have been reports that LH could demonstrate some exaggerated effects with FSH thereby stimulating follicular growth and ovulation.54, 55 Also, studies of positive stimulation by FSH and LH, which agreed with local paracrine signalling, have been reported.54, 56 This may, in part help maintains oestrogen secretion by the dominant follicle and thus, may provide such suitability for BSC to also be investigated in female species.
Our result also revealed that serum testosterone concentration did not change significantly in the rats following sub-chronic administration of BSC. Although 500 and 1000 mg/kg doses of BSC reduced serum testosterone concentration by 78.3% and 91.2% respectively when compared with the control rats, the reduction was not statistically significant. Considering the large percentage reduction in the level of this major male androgenic hormone, it will not be out of place to watch out for adverse effects related to impairment in testosterone production or release in cases of long-term or chronic use of this polyherbal remedy.
Since many natural products particularly those of plant origin have demonstrated therapeutic value in many diseases with documented evidence of involvement of direct or indirect antioxidant mechanisms,57, 58, 59 we decided to assess the influence of sub-chronic administration of BSC on testicular oxidant/antioxidant balance in the rats (Fig. 1, Fig. 2). Although, we did not investigate the direct antioxidant potential of BSC by inducing conditions of oxidative stress, its potential to interfere with testicular antioxidant defense system or promote the generation of free radicals and/or reactive intermediates via enhancement of lipid peroxidation was evaluated. Results from our study indicate that treatment with BSC (250–1000 mg/kg) did not produce any significant change or disturbance in testicular antioxidant defense as the activities of superoxide dismutase, catalase, glutathione-S-transferase as well as reduced glutathione level in the treated rats were not significantly different from those of the control rats. Similarly, malondialdehyde (an important by-product and useful index of lipid peroxidation) level in the BSC-treated rats was also not significantly different from control. While several authors have demonstrated similar observation with many established antioxidant herbal or medicinal plants in normal animals or animals without oxidative stress-mediated pathologies,60, 61, 62 further studies will be required to establish the direct antioxidant potential or otherwise of BSC in conditions of oxidative tissue/organ dysfunction or damage.
Many formulated functional foods or food supplements are now enriched with essential trace elements like copper, iodine, iron or zinc. This has left an impressive progress towards rationalizing policies with respect to the trace-element content of foods and chemicals for special human applications. Evidence abounds of the involvement of biological trace elements in reproductive toxicology.63, 64, 65, 66, 67 It is now understood that advances in knowledge of the importance and roles of trace elements are essential for health and well-being of humans and animals. The serum biological elements measured in both control and BSC treated groups were not above the in vitro BSC standard alone (Table 4). We observed that BSC administration has such potential to alter biological trace element levels particularly Fe, Zn and P as observed in our results levels. Mn level was only elevated by the lowest dose used in this study in contrast to Mg. Zinc is a potent regulator of programmed cell death in animals. While certain cell-type-specific concentrations of intracellular free zinc are required to protect cells from death, zinc depletion may commit cells to death in diverse systems. Zinc is also involved in the synthesis of DNA and RNA and has been shown to cause impaired male fertility by reducing sperm motility, morphological abnormalities and reduced spermatogenesis in zinc deficient rats.68 The simultaneous decreased in Mg and Zn levels produced by BSC in this study could trigger reproductive cells apoptosis and may cause dysfunction to reproductive organs. The increase in phosphorus by BSC polyherbal formula may either be through mobilization of phosphorus stored in bone or by other means. Although, a sub-chronic administration as observed in our present study could provide an overview on potential toxicity; however, the limitation of our study lies in the duration since a chronic toxicity evaluation of BSC would be necessary in order to comment on the long-term outcomes. Also, the manufacturer of BSC claims that herbal remedy possesses “energy boosting” property, we did not investigate the relationship between energy generation and intake of phosphorus in this study. Furthermore, BSC also produced increase in Cu, an essential trace element required by several enzymes responsible for numerous metabolic processes essential for life. These enzymes are indispensible in cellular activity for signal transduction and cell regulation. But, over expression of these biological trace elements could precipitated toxicities including those of the reproductive system and this could result in altered homeostatic processes and reproductive life.
5. Conclusion
In conclusion, data from this study suggest that BSC at low doses (≤250 mg/kg) may play a role in improving reproductive health as revealed by its ability to elevate serum levels of FSH and LH, thus, justifying in part, the acclaimed folkloric benefit as a “hormone booster”. BSC, on the other hand, also showed potential to cause reproductive toxicity as evident by its adverse effects on some sperm parameters and testicular morphology and alter biological trace element levels. While BSC shows potential as an enhancer of levels of reproductive hormones that are regulated by the pituitary hormones, FSH and LH, the observed dose-related adverse effects of this polyherbal formula on male reproductive function calls for its cautious use particularly on chronic administration. Although it may be cumbersome and extensive, we further recommend the elucidation of all the chemical structures of BSC.
Declarations
The research was self-funded by all authors.
Ethics approval and consent to participate
This study was approved by the University of Lagos, Nigeria ethical committee on the use of animals, and conformed to the Guide for the Care and Use of Laboratory Animals published by the U. S. National Institutes of Health (NIH Publication No. 85-23, revised 1996)” for studies involving experimental animals.
Consent for publication
Authors gave their consent to the publication of this manuscript.
Availability of data and materials
The results reported in this study were those directly obtained from this study.
Funding
This research was solely funded by AO, KOE, AOO, EM, SBA, and AOAO.
Authors' contributions
AO and KOE designed and coordinated all laboratory experiments. KOE conducted all experiments. AO, KOE, AOO, EM, SBA, and AOAO conducted statistical analysis, drafted the manuscript and interpreted the results. All authors read and approved the manuscript.
Conflict of interest
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgement
The technical assistance of Mr. M. Chijoke of the Department of Pharmacology, Therapeutics and Toxicology, College of Medicine, University of Lagos, Nigeria and Mr G.G. Daramola of the Department of Chemical Sciences, Redeemers University, Ede, Osun state, Nigeria are gratefully acknowledged. We also acknowledged the manufacturer of Bon-Sante® Cleanser Capsules, Dabiron Natural Life Care Nigeria, for grating the permission to assess this product.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
List of abbreviations
- BSC
Bon-santé cleanser®
- LD50
Median Lethal Dose
- ED50
Median Effective Dose
- FSH
Follicle Stimulating Hormone
- LH
Luteinizing Hormone
References
- 1.Teschke R., Frenzel C., Glass X., Schulze J., Eickhoff A. Herbal hepatotoxicity: a critical review. Br J Clin Pharmacol. 2013;75:630–636. doi: 10.1111/j.1365-2125.2012.04395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo G.A., Liang Q.L., Zhang R.L., Wang Y.M., Liu Q.F., Hu P. Study of chemical matteromics and prescription of traditional Chinese medicine and an analysis of material foundation of compound prescription Qingkailing. World Sci Technol Modern Trad Chin Med Materia Medica. 2006;8:6–15. [Google Scholar]
- 3.Walker A.F. Herbal medicine: the science of the art. Proc Nutr Soc. 2006;65:145–152. doi: 10.1079/pns2006487. [DOI] [PubMed] [Google Scholar]
- 4.Ramya K.B., Thaakur S. Herbs containing L-Dopa: an update. Anc Sci Life. 2007;27:50. [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen P.A., Ernst E. Safety of herbal supplements: a guide for cardiologists. Cardio Ther. 2010;28:246–253. doi: 10.1111/j.1755-5922.2010.00193.x. [DOI] [PubMed] [Google Scholar]
- 6.Truong-Tran A.Q., Carter J., Ruffin R., Zalewski P.D. New insights into the role of zinc in the respiratory epithelium. Immunol Cell Biol. 2001;79:170–177. doi: 10.1046/j.1440-1711.2001.00986.x. [DOI] [PubMed] [Google Scholar]
- 7.Obi E., Akunyili D.N., Ekpo B., Orisakwe O.E. Heavy metal hazards of Nigerian herbal remedies. Sci Total Environ. 2006;369:35–41. doi: 10.1016/j.scitotenv.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 8.Li S.X., Lin L.X., Lin J., Zheng F.Y., Wang Q.X., Weng W. Speciation analysis, bioavailability and risk assessment of trace metals in herbal decoctions using a combined technique of in Vitro digestion and biomembrane filtration as sample pretreatment method. Phytochem Anal. 2010;21:590–596. doi: 10.1002/pca.1239. [DOI] [PubMed] [Google Scholar]
- 9.Arpadjan S., Celik G., Taskesen S., Gucer S. Arsenic, cadmium and lead in medicinal herbs and their fractionation. Food Chem Toxicol. 2008;46:2871–2875. doi: 10.1016/j.fct.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Li C.L., Zhou G.Y., Hu F.Z., Xu W.H., Chen G.C. Determination of trace determination of elements in medicinal materials of cultivated and wild Rhizoma et Radix Notopterygii vegetated in different months by flame atomic absorption spectrometry. Guang pu xue yu guang pu fen xi = Guang pu. 2011;31:1122–1125. [PubMed] [Google Scholar]
- 11.Papanikolaou N.C., Hatzidaki E.G., Belivanis S., Tzanakakis G.N., Tsatsakis A.M. Lead toxicity update. A brief review. Med Sci Monit. 2005;11:329–336. [PubMed] [Google Scholar]
- 12.Helmersson A., von Arnold S., Bozhkov P.V. The level of free intracellular zinc mediates programmed cell death/cell survival decisions in plant embryos. Plant Physiol. 2008;147:1158–1167. doi: 10.1104/pp.108.122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao M.M., Meena A.K., Galib G. Detection of toxic heavy metals and pesticide residue in herbal plants which are commonly used in the herbal formulations. Environ Monit Assess. 2011;181:267–271. doi: 10.1007/s10661-010-1828-2. [DOI] [PubMed] [Google Scholar]
- 14.Kesharwani R., Singh D., Jacob V. Pharmacovigilance: the emerging trend and its future prospects. Glob J Pharm Res. 2013;2:1561–1584. [Google Scholar]
- 15.Calixto J.B. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents) Braz J Med Biol Res. 2000;33:179–189. doi: 10.1590/s0100-879x2000000200004. [DOI] [PubMed] [Google Scholar]
- 16.Street R.A. Heavy metals in medicinal plant products - an African perspective. South Afr J Bot. 2012;82:67–74. [Google Scholar]
- 17.Affum A.O., Shiloh D.O., Adomako D. Monitoring of arsenic levels in some ready-to-use anti-malaria herbal products from drug sales outlets in the Madina area of Accra, Ghana. Food Chem Toxicol. 2013;56:131–135. doi: 10.1016/j.fct.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 18.Yakubu M.T., Akanji M.A., Oladiji A.T., Adesokan A.A. Androgenic potentials of aqueous extract of Massularia acuminata (G. Don) Bullock ex Hoyl. stem in male Wistar rats. J Ethnopharmacol. 2008;118:508–513. doi: 10.1016/j.jep.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Yakubu M.T., Awotunde O.S., Ajiboye T.O., Oladiji A.T., Akanji M.A. Pro-sexual effects of aqueous extracts of Massularia acuminata root in male Wistar rats. Andrologia. 2011;43:334–340. doi: 10.1111/j.1439-0272.2011.01080.x. [DOI] [PubMed] [Google Scholar]
- 20.Oriola A.O., Aladesanmi A.J., Idowu T.O., Akinkunmi E.O., Obuotor E.M., Ogunsina M.O. A new bioactive thiophenolic glycoside from the leaf of Massularia acuminata (g. Don Bullock) ex Hoyle (Rubiaceae) Afr J Trad Compl Alter Med. 2014;11:319–323. doi: 10.4314/ajtcam.v11i2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muthu K., Krishnamoorthy P. Evaluation of androgenic activity of Mucuna pruriens in male rats. Afr J Biotech. 2013;10:15017–15019. [Google Scholar]
- 22.Shukla K.K., Mahdi A.A., Ahmad M.K., Shankhwar S.N., Rajender S., Jaiswar S.P. Mucuna pruriens improves male fertility by its action on the hypothalamus-pituitary-gonadal axis. Fertil Steril. 2009;92:1934–1940. doi: 10.1016/j.fertnstert.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 23.Akanbi O.M., Omonkhua A.A., Cyril-Olutayo C.M., Fasimoye R.Y. The antiplasmodial activity of Anogeissus leiocarpus and its effect on oxidative stress and lipid profile in mice infected with Plasmodium bergheii. Parasitol Resp. 2012;110:219–226. doi: 10.1007/s00436-011-2472-7. [DOI] [PubMed] [Google Scholar]
- 24.Obogwu M.B., Akindele A.J., Adeyemi O.O. Hepatoprotective and in vivo antioxidant activities of the hydroethanolic leaf extract of Mucuna pruriens (Fabaceae) in antitubercular drugs and alcohol models. China J Nat Med. 2014;12:273–283. doi: 10.1016/S1875-5364(14)60054-6. [DOI] [PubMed] [Google Scholar]
- 25.Belemnaba L., Ouedraogo S., Auger C. Endothelium-independent and endothelium-dependent vasorelaxation by a dichloromethane fraction from Anogeissus Leiocarpus (DC) Guill. Et Perr. (Combretaceae): possible involvement of cyclic nucleotide phosphodiesterase inhibition. Afr J Trad Compl Altern Med. 2012;10:173–179. [PMC free article] [PubMed] [Google Scholar]
- 26.Adeoluwa O.A., Aderibigbe A.O., Agu G.O., Adewole F.A., Eduviere A.T. Neurobehavioural and analgesic properties of ethanol bark extract of Terminalia ivorensis A Chev. (Combrataceae) in mice. Drug Res. 2015;65:545–551. doi: 10.1055/s-0034-1394417. [DOI] [PubMed] [Google Scholar]
- 27.Ponou B.K., Teponno R.B., Ricciutelli M. Dimeric antioxidant and cytotoxic triterpenoid saponins from Terminalia ivorensis A. Chev Phytochem. 2010;71:2108–2115. doi: 10.1016/j.phytochem.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 28.Ndjonka D., Abladam E.D., Djafsia B., Ajonina-Ekoti I., Achukwi M.D., Liebau E. Anthelmintic activity of phenolic acids from the axlewood tree Anogeissus leiocarpus on the filarial nematode Onchocerca ochengi and drug-resistant strains of the free-living nematode Caenorhabditis elegans. J Helminth. 2014;88:481–488. doi: 10.1017/S0022149X1300045X. [DOI] [PubMed] [Google Scholar]
- 29.Aladesanmi A.J., Iwalewa E.O., Akinkunmi E.O. Antimicrobial and antioxidant activities of some Nigerian medicinal plants. Afr J Trad Compl Alter Med. 2007;4:173–184. doi: 10.4314/ajtcam.v4i2.31206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwu M.M., Anyanwu B.N. Phytotherapeutic profile of Nigerian herbs. I: anti-inflammatory and anti-arthritic agents. J Ethnopharmacol. 1982;6(3):263–274. doi: 10.1016/0378-8741(82)90049-6. [DOI] [PubMed] [Google Scholar]
- 31.Shuaibu M.N., Pandey K., Wuyep P.A. Castalagin from Anogeissus leiocarpus mediates the killing of Leishmania in vitro. Parasitol Res. 2008;103:1333–1338. doi: 10.1007/s00436-008-1137-7. [DOI] [PubMed] [Google Scholar]
- 32.Kale O.E., Awodele O. Safety evaluation of Bon-santé cleanser® polyherbal in male Wistar rats. BMC Complementary Altern Med. 2016;16:1–12. doi: 10.1186/s12906-016-1188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving Bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raji Y., Akinsomisoye O.S., Salman T.M. Antispermatogenic activities of Morinda lucida extract in male albino rats. Asian J Androl. 2005;7:405–410. doi: 10.1111/j.1745-7262.2005.00051.x. [DOI] [PubMed] [Google Scholar]
- 35.Odell W.D., Ross G.T., Rayford P.L. Radioimmunoassay for luteinizing hormone in human plasma or serum: physiological studies. J Clin Invest. 1967;46:248–255. doi: 10.1172/JCI105527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joshi U.M., Shah H.P., Sudhama S.P. A sensitive and specific enzymeimmunoassay for serum Testosteone. Steroids. 1979;34:35–46. doi: 10.1016/0039-128x(79)90124-7. [DOI] [PubMed] [Google Scholar]
- 37.Gornall A.G., Bardawill C.J., David M.M. Determination of serum proteins by means of the Biuret reaction. J Biol Chem. 1949;177:751. [PubMed] [Google Scholar]
- 38.Beutler E., Duron O., Kelly B.M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 39.Habig W.J., Pabst M.J., Jacoby W.B. Glutathione-S-transferase, the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 40.Misra H.P., Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 41.Sinha A. Colorimetric assay of catalase. Anal Biochem. 1971;47:389–395. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 42.Varshney R., Kale R.K. Effect of calmodulin antagonist on radiation induced lipid peroxidation in microsomes. Int J Radiat Biol. 1990;58(5):733–743. doi: 10.1080/09553009014552121. [DOI] [PubMed] [Google Scholar]
- 43.Sprague S., Slavin W. Determination of iron, copper, and zinc in blood serum by an atomic absorption method requiring only dilution. At Absorpt Newsl. 1965;4:228. [Google Scholar]
- 44.Levine G. Lawrence Erlbaum Associates Inc., Publishers; Hillsdale, NJ: 1991. A Guide to SPSS for Analysis of Variance; pp. 65–67. [Google Scholar]
- 45.Shaw D. Toxicological risks of Chinese herbs. Planta Medica. 2010;76:2012–2018. doi: 10.1055/s-0030-1250533. [DOI] [PubMed] [Google Scholar]
- 46.Awodele O., Oreagba I.A., Odoma S., Teixeira da Silva J.A., Osunkalu V.O. Toxicological evaluation of aqueous extract of Moringa oleifera Lam. (Moringaceae) J Ethnopharmacol. 2012;139:330–336. doi: 10.1016/j.jep.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Byard R.W., Musgrave I. Herbal medicines and forensic investigations. Forensic Sci Med pathology. 2010;6:81–82. doi: 10.1007/s12024-010-9157-x. [DOI] [PubMed] [Google Scholar]
- 48.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilard V., Balayssac S., Tinaugus A., Martins N., Martino R., Malet-Martino M. Detection, identification and quantification by 1H NMR of adulterants in 150 herbal dietary supplements marketed for improving sexual performance. J Pharm Biomed Anal. 2015;102:476–493. doi: 10.1016/j.jpba.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 50.Kleter G.A., Groot M.J., Poelman M., Kok E.J., Marvin H.J.P. Timely awareness and prevention of emerging chemical and biochemical risks in foods: proposal for a strategy based on experience with recent cases. Food Chem Toxicol. 2009;47:992–1008. doi: 10.1016/j.fct.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 51.Cherian K.M., Mulky M.J., Menon K.K.G. Toxicological considerations in the use of consumer products. Def Sci J. 2014;37:143–159. [Google Scholar]
- 52.Sahoo H.B., Nandy S., Senapati A.K., Sarangi S.P., Sahoo S.K. Aphrodisiac activity of polyherbal formulation in experimental models on male rats. Pharmacogn Res. 2014;6:120–126. doi: 10.4103/0974-8490.129029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Afolabi S.O., Akindele A.J., Awodele O., Anunobi C.C., Adeyemi O.O.A. 90 day chronic toxicity study of Nigerian herbal preparation DAS-77 in rats. BMC Complementary Altern Med. 2012;12:1. doi: 10.1186/1472-6882-12-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foecking E.M., McDevitt M.A., Acosta-Martínez M., Horton T.H., Levine J.E. Neuroendocrine consequences of androgen excess in female rodents. Hormones Behav. 2008;53:673–692. doi: 10.1016/j.yhbeh.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roser J.F. Endocrine and paracrine control of sperm production in stallions. Animal Reproduction Sci. 2001;16:139–151. doi: 10.1016/s0378-4320(01)00151-8. [DOI] [PubMed] [Google Scholar]
- 56.Awodele O., Momoh A.A., Awolola N.A., Kale O.E., Okunowo W.O. The combined fixed-dose antituberculous drugs alter some reproductive functions with oxidative stress involvement in wistar rats. Toxicol Rep. 2016;3:620–627. doi: 10.1016/j.toxrep.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Werz O. Inhibition of 5-lipoxygenase product synthesis by natural compounds of plant origin. Planta Med. 2007;73:1331–1357. doi: 10.1055/s-2007-990242. [DOI] [PubMed] [Google Scholar]
- 58.Divya S.P., Wang X., Pratheeshkumar P. Blackberry extract inhibits UVB-induced oxidative damage and inflammation through MAP kinases and NF-κB signaling pathways in SKH-1 mice skin. Toxicol Appl Pharmacol. 2015;284:92–99. doi: 10.1016/j.taap.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silva R.L., Lopes A.H., Francca R.O. The quassinoid isobrucein B reduces inflammatory hyperalgesia and cytokine production by post-transcriptional modulation. J Nat Prod. 2015;78:241–249. doi: 10.1021/np500796f. [DOI] [PubMed] [Google Scholar]
- 60.Ekor M., Farombi O.E., Emerole G.O. Modulation of gentamicin-induced renal dysfunction and injury by the phenolic extract of soybean (Glycine max) Fund Clin Pharmacol. 2006;20:263–271. doi: 10.1111/j.1472-8206.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 61.Roriz C.L., Barros L., Carvalho A.M., Santos-Buelga C., Ferreira I.C. Scientific validation of synergistic antioxidant effects in commercialised mixtures of Cymbopogon citratus and Pterospartum tridentatum or Gomphrena globosa for infusions preparation. Food Chem. 2015;185:16–24. doi: 10.1016/j.foodchem.2015.03.136. [DOI] [PubMed] [Google Scholar]
- 62.Sen S., Chakraborty R., Thangavel G., Logaiyan S. Hepatoprotective and antioxidant activity of Karisalai Karpam, a polyherbal Siddha formulation against acetaminophen-induced hepatic damage in rats. Anc Sci Life. 2015;34:198. doi: 10.4103/0257-7941.160863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DeAna M., Nicholas R.R., Felicia B. Potential of chromium(III) picolinate for reproductive or developmental toxicity following exposure of male CD-1 mice prior to mating. Biol Trace Elem Res. 2011;143:1666–1672. doi: 10.1007/s12011-011-9002-4. [DOI] [PubMed] [Google Scholar]
- 64.Xiao-fei L., Li-ming Z., Ziwei Z., Ning L., Shi-wen X., Hong-jin L. Manganese-induced effects on testicular trace element levels and crucial hormonal parameters of hyline cocks. Biol Trace Elem Res. 2013;151:217–224. doi: 10.1007/s12011-012-9549-8. [DOI] [PubMed] [Google Scholar]
- 65.Kong L., Tang M., Zhang T. Nickel nanoparticles exposure and reproductive toxicity in healthy adult rats. Int J Mol Sci. 2014;15:21253–21269. doi: 10.3390/ijms151121253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roy N.M., DeWolf S., Carneiro B. Evaluation of the developmental toxicity of lead in the Danio rerio body. Aquat Toxicol. 2015;158:138–148. doi: 10.1016/j.aquatox.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 67.Guanchao Z., Lijun W., Zhizhun G. Association of serum heavy metals and, trace element concentrations with reproductive hormone levels and polycystic ovary syndrome in a Chinese population. Biol Trace Elem Res. 2015;167:1–10. doi: 10.1007/s12011-015-0294-7. [DOI] [PubMed] [Google Scholar]
- 68.Wong W.Y., Thomas C.M., Merkus J.M., Zielhuis G.A., Steegers-Theuuissen R.P. Male factor subfertility: possible causes and the impact of nutritional factors. Fertil Steril. 2000;73:435–442. doi: 10.1016/s0015-0282(99)00551-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The results reported in this study were those directly obtained from this study.