Abstract
Purpose
To investigate whether treatment with xanthohumol (XN), the principal prenylated chalconoid from Humulus lupulus (hops), is protective in a mouse model of light-induced retinal degeneration (LIRD).
Methods
Mice (129S2/SvPasCrl) were intraperitoneally injected with vehicle or XN prior to toxic light exposure and every 3 days thereafter. Retinal function was assessed by electroretinograms at 1, 2, and 4 weeks following toxic light exposure. Visual acuity was tested by optokinetic tracking 1 week and 4 weeks after toxic light exposure. Retina sections were stained with hematoxylin and eosin for morphologic analysis or by TUNEL. Redox potentials were assessed in retinal tissue by measuring levels of cysteine (CYS), cystine (CYSS), glutathione (GSH), and glutathione disulfide (GSSG) using HPLC with fluorescence detection.
Results
Toxic light significantly suppressed retinal function and visual acuity, severely disrupted the photoreceptor cell layer, and significantly decreased the number of nuclei and increased the accumulation of TUNEL-labeled cells in the outer nuclear layer. These effects were prevented by XN treatment. Treatment with XN also maintained GSSG and CYSS redox potentials and the total CYS pool in retinas of mice undergoing toxic light exposure.
Conclusions
XN treatment partially preserved visual acuity and retinal function in the LIRD mouse. Preservation of retinal CYS and of GSSG and CYSS redox potentials may indicate that XN treatment induces an increased antioxidant response, but further experiments are needed to verify this potential mechanism. To our knowledge, this is the first study to report protective effects of XN in a model of retinal degeneration.
Keywords: retinal degeneration, xanthohumol, flavonoid
Hops flowers of the Humulus lupulus plant are an ingredient used in beer making to add aroma and bitter flavor.1 The prenylated chalconoid xanthohumol (XN) is mainly secreted as a part of the hop resin but is also found in the trichomes of young leaves; it exists ubiquitously throughout the hops plant at 0.1% to 1% dry weight.2 As extracted from the hops plant, XN is protective in a range of disease models (see Ref. 2 for review). Since chalconoids are open-chain precursors of flavonoids and isoflavonoids, they can have multiple mechanisms of action, possibly directly or after derivatization,3 and thus their use could result in a wide range of biological actions in the retina.
Treatment with XN suppressed cerebral neuronal apoptosis, restricted focal cerebral ischemia, and maintained neurobehavioral scores in rats following middle cerebral artery occlusion (MCAO)-induced focal cerebral ischemia.4 Yen et al.4 concluded that XN treatment protected cerebral neurons by maintaining cellular redox potential by preventing inflammatory responses that inactivate antioxidative enzymes such as glutathione peroxidase and catalase. XN is also protective in other cell types. Yang et al.5 report that XN treatment mitigated hepatitis C virus–induced liver hepatocyte damage and prevented apoptosis by reducing oxidative stress, inflammation, and lipid peroxidation.
Similar to these models, oxidative stress and apoptosis are observed with the retinal functional and morphologic loss that occurs in several models of retinal degeneration and in retinal degenerative diseases.6–8 For instance, AMD may in part result from oxidative stress combined with an impaired cysteine/cystine (CYS/CYSS) redox potential (but not necessarily glutathione/glutathione disulfide [GSH/GSSG] redox potential).9–12 Thus, we hypothesized that treatment with XN would protect against retinal degeneration, possibly by maintaining redox potentials and preventing oxidative damage and retinal cell apoptosis. To test this, we treated mice undergoing light-induced retinal degeneration (LIRD) with intraperitoneal (IP) injections of XN or vehicle and compared the resulting effects on vision, retinal health, and redox potentials. Here we report that treatment with XN protected against LIRD functional and morphologic loss, prevented cell death, and maintained redox potential in the retina.
Materials and Methods
Animals
All mouse handling procedures and care were approved by the Emory Institutional Animal Care and Use Committee and followed the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Adult (3 months old) 129S2/SvPasCrl (129SV) male mice were obtained from Charles River Laboratory (Wilmington, MA, USA) and were housed under a 12:12-hour light-dark cycle (7 AM on and 7 PM off). During the light cycle, light levels measured at the bottom of mouse cages ranged from 5 to 45 lux. Mice had access to standard mouse chow (Lab Diet 5001; LabDiet, Inc., St. Louis, MO, USA) ad libitum and weighed 28 to 32 g throughout the study; XN treatment did not significantly alter weight (data not shown). Mice were euthanized with compressed CO2 for all experiments.
Drug Administration
A stock solution of 141.11 μM XN (CAS: 6754-58-1; Sigma Life Science, St. Louis, MO, USA) was prepared on the first injection day of each experiment by dissolving XN in a vehicle of Cremophor (CAS: 61791-12-6; Sigma Life Science), ethanol, and normal saline in a 1:1:4 ratio.4 XN and vehicle solutions were made fresh at the beginning of each experiment and stored in darkness at 4°C when not directly in use. 129SV mice (n = 5 per group) received IP injections of either vehicle or XN (0.4 or 0.8 mg/kg dose; specified by experiment as reported in Results) using an injection volume of 10 μL of solution per gram of mouse body weight in accordance with in vivo rodent experiments of Yen et al.4 Injections were administered the day before toxic light exposure, a second time 1 hour before light exposure the following day, and every 3 days afterward for the duration of the experiment. This dosing regimen was previously established during in vivo testing of another candidate neuroprotectant.13,14
Light Damage
Atropine eye drops (1%) (NDC: 17478-215-02; Akorn, Inc., Lake Forest, IL, USA) were administered at 1 hour and again at 30 minutes prior to toxic light exposure. For experimental light exposure, animals were individually housed in white polypropylene cages for 2 to 6 hours with food and water ad libitum while exposed to standard (50 lux) or toxic (50,000 lux) levels of light from a white light-emitting diode light source (Fancier Studio, Hayward, CA, USA).15 After this exposure, animals were returned to home cages under normal lighting conditions for the remainder of the experiment.
Electroretinograms (ERGs)
The complete ERG protocol was detailed previously.13 Briefly, mice were dark-adapted overnight. In preparation for ERGs, mice were anesthetized with IP injections of ketamine (100 mg/mL: AmTech Group, Inc., Tempe, AZ, USA) and AnaSed xylazine (20 mg/mL; Santa Cruz Animal Health, Dallas, TX, USA).13 Proparacaine (1%; Akorn, Inc.) and tropicamide (1%; Akorn, Inc.) eye drops were administered to reduce eye sensitivity and dilate pupils. Once anesthetized, mice were placed on a heating pad inside a Faraday cage in front of a desktop Ganzfeld stimulator (UTAS-3000; LKC Technologies, Gaithersburg, MD, USA). A DTL fiber active electrode was placed on top of each cornea. A drop of Refresh Tears (Allergan, Dublin, Ireland) was added to each eye to maintain conductivity with the electrode fibers. The reference electrode was a 1-cm needle inserted into the cheek, and the ground electrode was placed in the tail. ERGs were recorded for the scotopic condition (−3.0 to 2.1 log cd-s/m2 and increasing flash stimulus intervals from 2 to 70 seconds). Mice recovered from anesthesia individually in cages placed partly on top of heated water pads. ERGs were performed 1 week after light damage and again at 2 and 4 weeks following light exposure.
Optokinetic Tracking (OKT)
Visual acuity as a function of spatial frequency threshold assessment of mice was measured by a trained observer/operator inducing and recording visual, reflexive, tracking behavior (OKT) of individual mice using a virtual optomotor system (OptoMotry; Cerebral Mechanics, Inc., Lethbridge, Alberta, Canada). Briefly, a mouse was placed on a platform inside a chamber with four walls comprised of computer monitors displaying vertical dark and white lines in motion. The perceived visual environment was that of being inside a revolving cylinder of vertical stripes. The mouse moved its head left or right (depending on the direction the lines were spinning) in order to visually follow the movement of the lines in a reflexive tracking motion.16,17 The spatial frequency in cycles per degree (i.e., the thickness of the dark lines) was progressively narrowed in a staircase pattern by the trained observer/operator until the mouse no longer made detectable head-tracking movements. The highest spatial frequency threshold at which tracking motions were still present was recorded as the visual acuity capability of the mouse. OKT assessments were conducted under photopic conditions and at 100% line contrast 1 week and 4 weeks following toxic light exposure.16,18
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling
Independent experiments were conducted to determine the effect of XN treatment on LIRD TUNEL-positive cells using the same light damage conditions described above. Mice were injected with 0.4 mg/kg XN or vehicle once on the day before and once on the day of toxic light exposure. Mice were euthanized at 0, 6, or 24 hours following light exposure, and eyes were fixed in buffered zinc formalin (Z-fix; Anatech, Inc., Battle Creek, MI, USA) for 17 hours (n = 4 per time point). Eyes were dehydrated, embedded in paraffin, and sectioned through the sagittal plane on a microtome at 5-μm increments. Sections containing the optic nerve and center of the cornea were selected for staining to ensure that consistent regions were examined between animals. Slides were deparaffinized in Coplin jars with 100 mL of xylene for 5, 3, and 2 minutes, respectively. Slides were then rehydrated in a series of 100-mL ethanol solutions for 3 minutes each: 100%, 90%, 80%, 70%, 60%, 50%. Slides were immersed in PBS for 5 minutes each. After dehydration, a TUNEL assay was performed on the sections according to the protocol for TUNEL (DeadEnd Fluorometric TUNEL Kit; Promega, Fitchburg, WI, USA). Stained sections were imaged using fluorescent microscopy, and TUNEL-positive cells in the outer nuclear layer (ONL) were manually counted by 636.5 × 636.5-μm fields of view located immediately adjacent to the optic nerve head for each retina using software (Adobe Photoshop Creative Suite 4; Adobe Systems, Inc., San Jose, CA, USA).13
Tissue Collection and Morphology
Mice were euthanized at designated intervals following LIRD. For experiments in which mice were treated with XN over the course of 4 weeks, retinas were collected from the left eye and flash-frozen at −80°C. Right eyes were fixed in 4% paraformaldehyde overnight and washed with PBS the following morning. Eyes were dehydrated and paraffin-embedded as described above. Eyes were then sectioned at width of 5 μm and stained with hematoxylin and eosin (H&E).19 All sections were imaged with a bright-field microscope (Leica DM LB; Leica, Buffalo Grove, IL, USA) at 20× power.19
ONL nuclei were counted within 100-μm-wide segments spaced at 250, 750, 1250, 1750, and 2250 μm from the optic nerve head in both the dorsal and ventral directions. Mean counts of n = 4 to 5 retinas per group were plotted as a spidergram diagram.
Assessing Redox Potential and CYS Pool in Retina HPLC With Fluorescence Detection
In a separate experiment, mice injected with 0.8 mg/kg XN the day before and the morning of toxic light exposure were euthanized after 2 and 4 hours of exposure (n = 3). Retinas were collected immediately following death and were flash-frozen at −80°C. Retinas were used for mechanistic analysis via HPLC measuring reduced GSH/GSSG, CYS/CYSS concentrations. GSH, GSSG, CYS, and CYSS levels can indicate a system's ability to mediate oxidative stress. An equal volume of 10% perchloric acid and preservation solution containing iodacetic acid (6.7 μM) and boric acid (0.1 M) with 5 μM γ-glutamyl-glutamate as an internal standard were added to the aforementioned tissues as previously described in Yeligar et al.20 A bicinchoninic acid assay (Thermo Scientific, Rockford, IL, USA) was used to normalize protein levels.21 GSH, GSSG, CYS, and CYSS levels in the retina were measured by HPLC with fluorescence detection after samples were derivatized with dansyl chloride.22 The redox potentials for the GSH/GSSG and CYS/CYSS thiol pairs were calculated using the Nernst equation: Eh = E0 + (RT/2F)ln([GSSG]/[GSH]2) and Eh = E0 + (RT/2F)ln([CYSS]/[CYS]2) for each respective thiol pair. E0 is the standard potential for the respective redox couple, R is the gas constant, T is absolute temperature, and F is the Faraday constant. A more negative Eh indicates a lower level of oxidation of the GSH/GSSG or CYS/CYSS thiol pair.23
Statistical Analyses
Graphs and analyses were conducted using statistical software (Prism 7; GraphPad Software, Inc., La Jolla, CA, USA). One-way and 2-way repeated measures ANOVAs and Student's t-tests were performed for ERG, OKT, HPLC, and morphometric data. For all analyses, results were considered statistically significant if P < 0.05. All graphs display data as mean ± SEM. The stated n is the number of animals used in each group.
Results
XN Treatment Preserved Retinal Function Following Exposure to Toxic Light
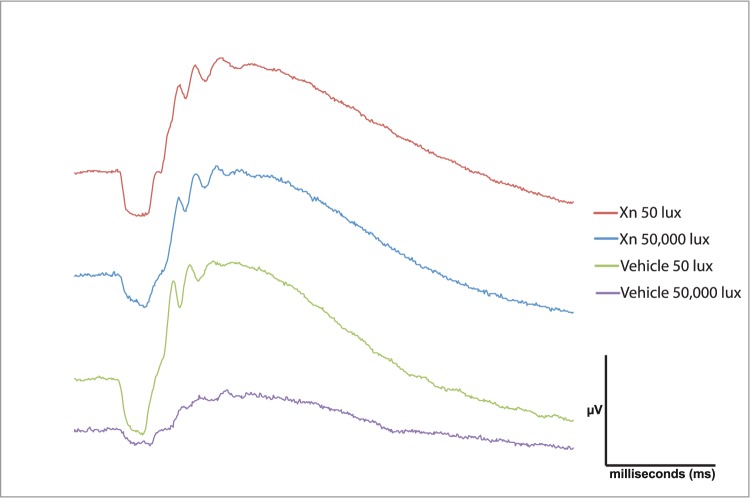
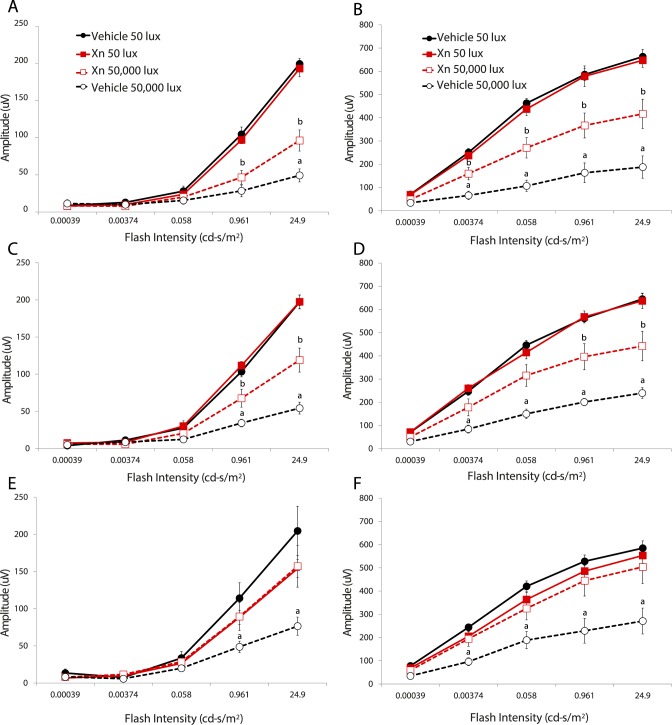
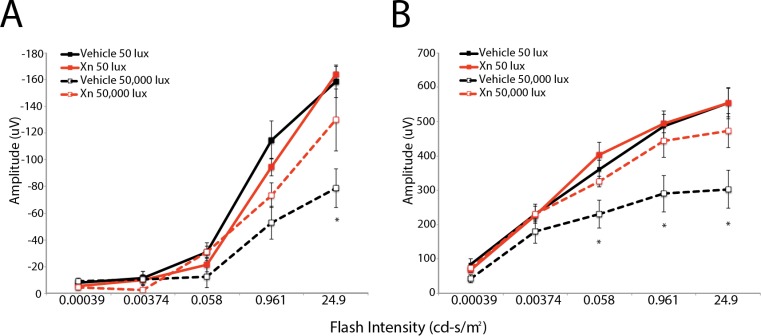
Representative traces of ERG waveforms show preservation of a-waves and b-waves in mice treated with XN and exposed to 50,000-lux light for 6 hours compared to vehicle-treated mice (Fig. 1). Vehicle-treated mice exhibited a significant decrease in a- and b-wave amplitudes at 1, 2, and 4 weeks following exposure to 50,000-lux light (Fig. 2), similar to previous reports.24,25 Systemic injections of XN (0.4 mg/kg) markedly prevented this loss of function, as demonstrated by between 30% and 90% preservation of a- and b-wave amplitudes over all three time points (P < 0.05, Fig. 2). XN (0.8 mg/kg) treatment showed similar preservation of both a- and b-wave amplitudes measured at 2 weeks following toxic light exposure (Fig. 3). Amplitudes of XN-treated mice did not decline even as late as 4 weeks after toxic light exposure, suggesting that treatment sustained preservation over this period (Fig. 2). It is noted that at 4 weeks following exposure to toxic light XN-treated mice exposed to 50-lux light exhibited somewhat diminished a-wave amplitudes (Fig. 2), though this was not statistically significantly different from the vehicle-treated 50-lux light group.
Figure 1.
XN (0.4 mg/kg) treatment preserves a- and b-wave amplitudes 4 weeks following toxic light exposure. Representative ERG waveforms of one eye of a single mouse from each treatment group. Mice treated with vehicle and exposed to 50,000 lux for 6 hours show the most diminished a- and b-wave amplitudes. Treatment with XN protected against the loss of a- and b-wave amplitudes. The ERG flash intensity was 24.9 cd-s/m2 and was the same for all four mice.
Figure 2.
XN (0.4 mg/kg) treatment preserves mouse visual function for several weeks following toxic light exposure. Scotopic ERG a-wave (A, C, E) and b-wave (B, D, F) amplitudes from 129SV mice at 1 (top), 2 (middle), and 4 weeks (bottom) after light exposure. Mice injected with vehicle and exposed to 50,000-lux light for 6 hours (open black circles, black dashed lines) exhibited the most severe decreases in a- and b-wave amplitude, whereas mice given XN and subjected to toxic light (open red squares, red dashed lines) exhibited partial a-wave and b-wave preservation as compared to vehicle and XN-treated mice exposed to 50 lux (filled black and red squares, respectively). For each treatment group n = 5,a,b P < 0.05 versus all other groups.
Figure 3.
XN (0.8 mg/kg) treatment preserves mouse retinal function following toxic light exposure. Scotopic ERG a-wave (A) and b-wave (B) amplitudes measured at 2 weeks following toxic light exposure. Mice injected with vehicle and exposed to 50,000-lux light for 6 hours exhibit the greatest reduction in a- and b-wave amplitudes. Mice treated with XN and exposed to toxic light show moderate a-wave preservation, while b-waves are more preserved. For each group n = 3, *P < 0.05 versus all other groups.
XN Treatment Protected Visual Acuity Following Exposure to Toxic Light
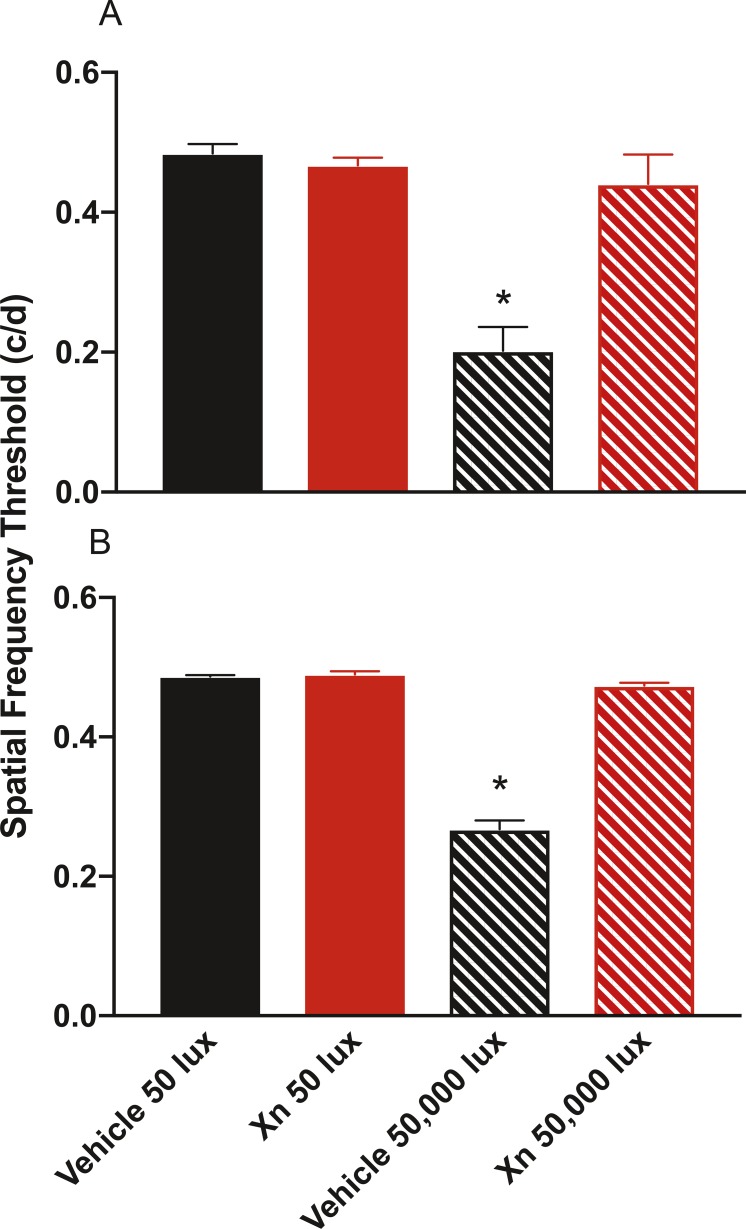
Treatment with XN preserved visual acuity in mice exposed to toxic bright light by about 50% (P < 0.0003). Optokinetic tracking (OKT) was used to measure the spatial threshold as a metric of visual acuity of mice 1 and 4 weeks after experimental light exposure. Mice exposed to 50,000-lux light for 6 hours treated with vehicle showed a significant loss in visual acuity (about a 60% reduction) as compared to those mice treated with vehicle and exposed to 50 lux. Treatment with XN prevented this loss of visual acuity in mice exposed to 50,000 lux, as seen in Figure 4.
Figure 4.
XN treatment provides complete protection of spatial frequency threshold as measured by OKT at 1 and 4 weeks after light exposure. One week after light damage (A), vehicle-treated mice exposed to 50,000 lux for 6 hours (black striped bar) exhibited visual acuities that were significantly lower than mice exposed to 50 lux treated with either XN or vehicle (all dim groups). XN-treated mice exposed to 50,000-lux light for 6 hours (red striped bar), however, exhibited full preservation of visual acuity as compared to mice treated with XN or vehicle exposed to 50 lux (solid red and black bar, respectively). These data were consistent when measured at 4 weeks following toxic light exposure (B). For XN-treated mice exposed to 50 lux, n = 3; for all other treatment groups n = 5 (*P < 0.05 vs. other groups).
XN Treatment Preserved Retinal Morphology Following Exposure to Toxic Light
Microscopy of H&E-stained sections of eyes harvested 4 weeks after toxic light exposure shows that exposure caused a marked degradation of morphology in the outer retina (Fig. 5). Photoreceptor cell inner segments and outer segments and most of the nuclei of the ONL were eliminated across the span of the retina (Fig. 5). Treatment with XN prevented much of this degeneration (Fig. 5). Compared to mice exposed to 50 lux, mice exposed to 50,000-lux light for 6 hours had loss of nuclei across the ONL on the sagittal plane (Fig. 6). This loss was prevented in mice treated with XN (Fig. 6).
Figure 5.
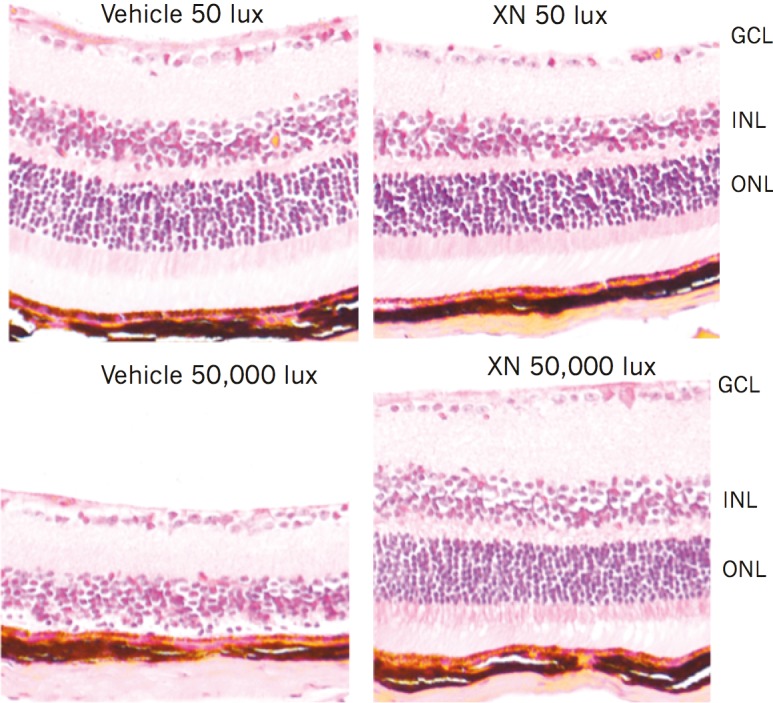
XN (0.4 mg/kg) treatment preserves retinal cell layers 4 weeks following light damage. Paraffin-embedded retinal sections (5 μm) stained with H&E. Ganglion cell layer (GCL), inner nuclear layer (INL), and ONL are labeled. Sections are average representations of mice in each treatment group. Vehicle-treated mice exposed to 50,000 lux showed near complete loss of photoreceptor cell and ONLs. XN-treated mice exposed to 50,000 lux showed preservation of both photoreceptor cell layer and ONL.
Figure 6.
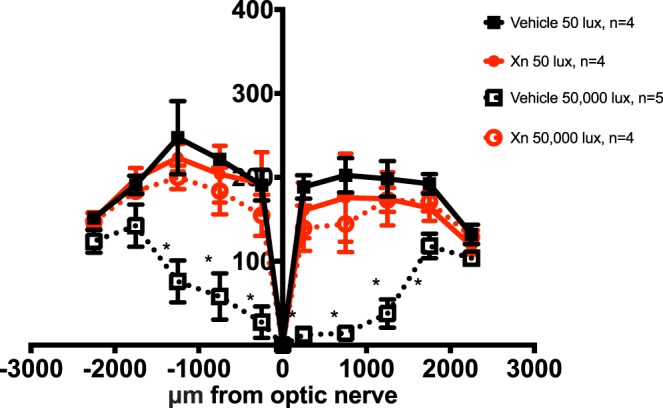
XN (0.4 mg/kg) preserves nuclei of the retinal ONL in mice exposed to 50,000 lux. Nuclei were counted in discrete regions of retinal sections starting at 250 μm from the optic nerve head and extending every 500 μm outward along both the dorsal (positive values on abscissa) and ventral periphery (negative numbers on abscissa). Vehicle-treated mice exposed to 50,000 lux showed significant loss of nuclei close to the optic nerve head. XN-treated mice exposed to bright light do not differ significantly from vehicle-treated mice exposed to dim light. XN-treated mice exposed to 50,000 lux showed preservation of these nuclei throughout the length of the retina (n = 5 for vehicle-treated mice exposed to 50,000 lux, and n = 4 for all other groups. Means are ±SEM. Two-way ANOVA multiple comparisons test; *P < 0.05).
XN Treatment Delayed and Diminished Retinal Cell Death Following Exposure to Toxic Light
Representative paraffin-embedded retinal sections from vehicle-treated mice euthanized at 0, 6, and 24 hours following toxic light exposure show that immediately following light exposure (0 hour time point), the number of TUNEL-positive cells in ocular sections from mice exposed to toxic light and treated with vehicle or XN were statistically indistinguishable, with an average of 30 ± 11 (SEM) cells per retina. TUNEL-positive cell numbers in vehicle-treated mice increased to 360 ± 38 cells 6 hours following exposure and further increased to 489 ± 23 cells 24 hours following exposure (Fig. 7). Treatment with XN significantly prevented this increase in TUNEL-positive cell number (P < 0.001 compared to vehicle-treated group at 6 hours and P < 0.0001 compared to vehicle-treated group at 24 hours). In XN-treated mice, at 6 hours following exposure, TUNEL-positive ONL counts increased to 173 ± 36, but at 24 hours counts increased to only 173 ± 24, indicating no additional increase between 6 and 24 hours post exposure, as was observed in the vehicle-injected condition (Fig. 7).
Figure 7.
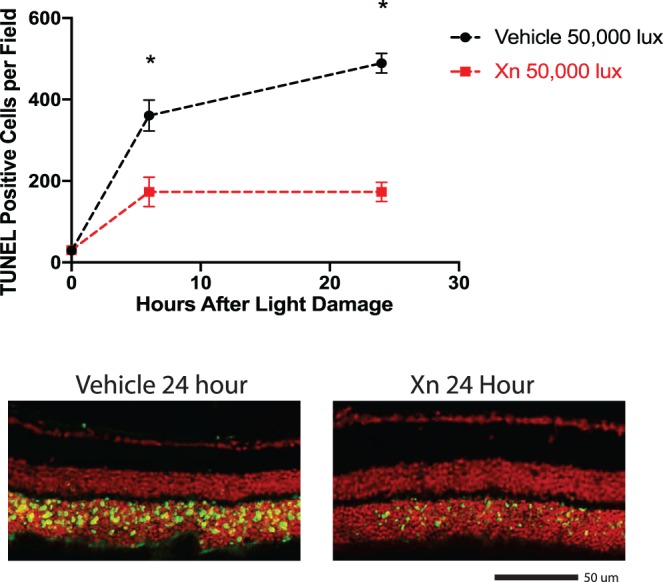
XN treatment decreases the number of TUNEL-positive cells following toxic light exposure. Groups of XN- and vehicle-treated mice received toxic light exposure and were euthanized immediately, 6 hours, or 24 hours after exposure. Retinas were collected, sectioned, and used for a TUNEL assay. XN-treated mice exhibited significantly fewer TUNEL-positive cells compared to the vehicle-treated group at both 6 hours and 24 hours post-toxic light exposure (*P < 0.0001 vs. other groups; Student's t-test, error bars represent SEM). Lower panels are representative images of retinal sections used in quantification.
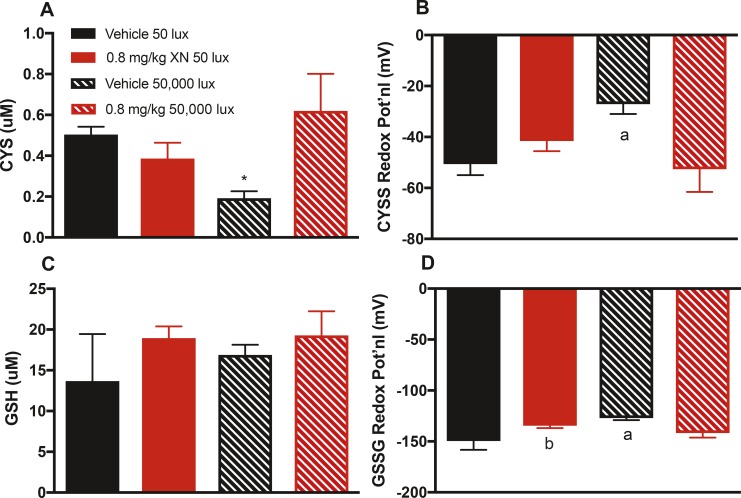
Effect of XN Treatment on Retina Redox Potential and Total CYS and GSH Pools
Retinal levels of CYS, CYSS, GSH, and GSSG were measured to gauge the capacity for retinal tissue to respond to oxidative stress–associated toxic light exposure.10,26 CYS levels were decreased in vehicle-treated mice exposed to 50,000-lux light for 2 hours compared to vehicle-treated mice exposed to 50 lux. Treatment with XN prevented this loss (Fig. 8A). Retinal CYSS redox potential was diminished in vehicle-treated mice exposed to 50,000-lux light compared to mice exposed to 50-lux light. Treatment with XN prevented this loss (Fig. 8B). Retinal GSSG redox potential was diminished in vehicle-treated mice exposed to 50,000-lux light compared to mice exposed to 50-lux light. Treatment with XN prevented this loss (Fig. 8D). The total GSH pool did not differ across treatment groups (Fig. 8C), suggesting a decoupling between the GSH/GSSG and CYS/CYSS recycling processes, as commonly found in other redox systems,10,26 including in retinal degenerative disease models and patients.9–12
Figure 8.
XN treatment maintains retinal CYS level and CYSS and GSSG redox potentials during toxic light exposure. (A) Mice exposed to 50,000-lux light for 2 hours and treated with vehicle showed significantly lower total retinal CYS compared to that of mice exposed to 50-lux light or mice treated with XN (*P < 0.05 vs. other groups). Here and in other panels, solid black bar represents vehicle-treated mice exposed to 50-lux light; solid red bar represents XN-treated mice exposed to 50-lux light; striped black bar represents vehicle-treated mice exposed to 50,000-lux light; striped red bar represents XN-treated mice exposed to 50,000-lux light. (B) Mice treated with vehicle and exposed to 50,000-lux light have significantly reduced CYSS redox potential compared to all other groups (aP < 0.01 vs. other groups). (C) GSH levels do not significantly differ among treatment groups. (D) Redox potential of GSSG was significantly reduced in vehicle-treated mice exposed to 50,000 lux compared to all other groups (aP < 0.05 vs. vehicle 50 lux and 0.8 mg/kg XN 50,000 lux; bP < 0.05 vs. vehicle 50 lux). Treatment with XN at 0.8 mg/kg restores redox potentials of GSSG and CYSS. Error bars represent the SEM and n = 3 per group for all panels).
Discussion
Our data indicate that systemic injections of XN protected photoreceptor cells from death in a mouse model of LIRD, possibly by maintaining retinal redox potential and diminishing oxidative stress and damage. XN treatment preserved retinal and visual function as assessed by ERG and OKT, respectively. Treatment prevented loss of nuclei and significantly delayed and reduced the accumulation of TUNEL-positive cells in the ONL. XN treatment also maintained retinal CYS pools and CYSS and GSSG redox potentials.
The functional and morphologic protective effects are in agreement with similar outcomes from a rat stroke model in which rats undergoing cerebral ischemia following MCAO had brain function and structure maintained following systemic XN treatment.4 The current protective finding also is in agreement with studies showing that polyphenols, flavonoids, and chalconoids are protective in several light damage models of retinal degeneration.27–32
In the rat stroke model study4 and in studies of hepatocyte injury,5 it was concluded that XN treatment was protective via mechanisms that maintained redox potentials and reduced oxidative stress, inflammation, and lipid peroxidation. Similarly, and as hypothesized based on data from AMD patients and retinal culture models,9–12 we found that XN treatment prevented changes to retinal CYS, CYSS, and GSSG levels and redox potentials, but not GSH levels.
GSH/GSSG and CYS/CYSS redox potentials can give insight into the antioxidant capabilities of XN-treated animals because the relationship between the two sets of redox potentials can provide a rational basis to distinguish general contributors of pro-oxidant events and antioxidant defenses to oxidative stress.10,22 A more negative redox potential is an indication that GSSG is more likely to be regenerated into GSH. This would indicate that the system favors the regeneration of GSH, thus increasing its radical scavenging capabilities. CYS is more reactive than GSH; oxidants will preferentially oxidize CYS so that the redox balance can be preserved by the supply of GSH located in the tissue.10 While there is no significant difference seen in GSH levels between treatment groups in our current experiments, the GSH/GSSG and CYS/CYSS recycling pathways are known to act independently of one another.10 Oxidative stress caused by toxic light exposure may alter CYS levels at a greater rate than GSH levels, as suggested by data from AMD patients and models.9–12
Based on the mechanism by which bright-light exposure is thought to induce retinal degeneration, it may be that XN protects against LIRD by reducing oxidative stress in photoreceptor cells. Upon exposure to bright light, rapid retinol recycling occurs within the photoreceptors at a level well above normal.33 Such processes create an environment that produces many oxidative free radicals.33 Prolonged exposure to these oxidative radicals can impair mitochondrial respiration/metabolism33 and lead to apoptosis of photoreceptor cells, resulting in decreased retinal and visual function.34 While the TUNEL assay is able to label double-strand DNA breaks associated with cell death possibly resulting from oxidative stress, there is debate as to whether or not the TUNEL assay labels only apoptotic cells.35,36 It is clear that mice exposed to toxic light in this study had increased levels of cell death in their retina compared to dim-light exposed animals. Further studies would be needed to determine whether this cell death is specifically apoptotic. Regardless, a potential mechanism of action is that XN can act as an antioxidant, operating through the nuclear factor erythroid 2-related factor 2 (Nrf2) and antioxidant response element (ARE) signaling pathways (though many other potential mechanisms are possible25). This mechanism of action for XN would be similar to another study that investigated the mechanism of protection of PC12 neuronal cells with XN.37 Yao et al.38 showed that XN displayed moderate free radical scavenging capabilities but upregulated many cytoprotective genes, for example Nrf2, and their gene products such as glutathione.38
XN has the potential to be used in a therapeutic clinical environment as it has the ability to cross the blood–brain barrier39 and thus possibly cross the blood–retina barrier. Our current data indirectly support this, since IP injections of XN had effects in the retina. However, we did not demonstrate the transport of XN into the neural retina, which awaits future pharmacokinetic and pharmacodynamic studies beyond the intended scope of this project. Several pharmacokinetic and pharmacodynamic studies in rats and humans indicate that neuroprotective plasma concentrations cannot be achieved by beer consumption.4,40,41 Further studies with XN are warranted to examine its potential protective capabilities as well as a deeper investigative study examining the mechanism of protection.
Acknowledgments
The authors thank the Biomarker Core of Emory University led by Lou Ann S. Brown, PhD, for conducting measurements of cysteine and glutathione metabolites by HPLC.
Supported by Grants NIH R01EY014026, R01EY021592, R01EY016470, T32EY07092, P30EY006360, VA RR&D C1924P I21RX001924, VA RR&D C9246C (Atlanta Veterans Administration Center of Excellence in Vision and Neurocognitive Rehabilitation), and Abraham J. & Phyllis Katz Foundation, as well as by an unrestricted grant to the Department of Ophthalmology at Emory University from Research to Prevent Blindness, Inc.
Disclosure: N.F. Henneman, None; S.L. Foster, None; M.A. Chrenek, None; J.T. Sellers, None; C.B. Wright, None; R.H. Schmidt, None; J.M. Nickerson, None; J.H. Boatright, None
References
- 1. Dresel M, Dunkel A, Hofmann T. . Sensomics analysis of key bitter compounds in the hard resin of hops (Humulus lupulus L.) and their contribution to the bitter profile of Pilsner-type beer. J Agric Food Chem. 2015; 63: 3402– 3418. [DOI] [PubMed] [Google Scholar]
- 2. Liu M, Hansen PE, Wang G,et al. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus). Molecules. 2015; 20: 754– 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gomes MN, Muratov EN, Pereira M,et al. Chalcone derivatives: promising starting points for drug design. Molecules. 2017; 22: 1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yen TL, Hsu CK, Lu WJ,et al. Neuroprotective effects of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus), in ischemic stroke of rats. J Agric Food Chem. 2012; 60: 1937– 1944. [DOI] [PubMed] [Google Scholar]
- 5. Yang M, Li N, Li F,et al. Xanthohumol, a main prenylated chalcone from hops, reduces liver damage and modulates oxidative reaction and apoptosis in hepatitis C virus infected Tupaia belangeri. Int Immunopharmacol. 2013; 16: 466– 474. [DOI] [PubMed] [Google Scholar]
- 6. Faghiri Z, Bazan NG. . PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp Eye Res. 2010; 90: 718– 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gelfand BD, Ambati J. . A revised hemodynamic theory of age-related macular degeneration. Trends Mol Med. 2016; 22: 656– 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shaw PX, Stiles T, Douglas C,et al. Oxidative stress, innate immunity, and age-related macular degeneration. AIMS Mol Sci. 2016; 3: 196– 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiang S, Moriarty-Craige SE, Orr M, Cai J, Sternberg P Jr, Jones DP. . Oxidant-induced apoptosis in human retinal pigment epithelial cells: dependence on extracellular redox state. Invest Ophthalmol Vis Sci. 2005; 46: 1054– 1061. [DOI] [PubMed] [Google Scholar]
- 10. Jones DP, Mody VC Jr, Carlson JL, Lynn MJ, Sternberg P Jr.. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002; 33: 1290– 1300. [DOI] [PubMed] [Google Scholar]
- 11. Moriarty-Craige SE, Adkison J, Lynn M,et al. Antioxidant supplements prevent oxidation of cysteine/cystine redox in patients with age-related macular degeneration. Am J Ophthalmol. 2005; 140: 1020– 1026. [DOI] [PubMed] [Google Scholar]
- 12. Moriarty-Craige SE, Ha KN, Sternberg P Jr, et al. Effects of long-term zinc supplementation on plasma thiol metabolites and redox status in patients with age-related macular degeneration. Am J Ophthalmol. 2007; 143: 206– 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boatright JH, Moring AG, McElroy C,et al. Tool from ancient pharmacopoeia prevents vision loss. Mol Vis. 2006; 12: 1706– 1714. [PubMed] [Google Scholar]
- 14. Phillips MJ, Walker TA, Choi HY,et al. Tauroursodeoxycholic acid preservation of photoreceptor structure and function in the rd10 mouse through postnatal day 30. Invest Ophthalmol Vis Sci. 2008; 49: 2148– 2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chrenek MA, Liao TL, Sellers JT, Boatright JH, Nickerson JM. . An inexpensive LED light box for light damage in rodents. Invest Ophthalmol Vis Sci. 2012; 53: 2559– 2559. [Google Scholar]
- 16. Prusky GT, Alam NM, Beekman S, Douglas RM. . Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004; 45: 4611– 4616. [DOI] [PubMed] [Google Scholar]
- 17. Douglas RM, Alam NM, Silver BD, McGill TJ, Tschetter WW, Prusky GT. . Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis Neurosci. 2005; 22: 677– 684. [DOI] [PubMed] [Google Scholar]
- 18. Wright CB, Chrenek MA, Feng W,et al. The Rpe65 rd12 allele exerts a semidominant negative effect on vision in mice. Invest Ophthalmol Vis Sci. 2014; 55: 2500– 2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lawson EC, Han MK, Sellers JT,et al. Aerobic exercise protects retinal function and structure from light-induced retinal degeneration. J Neurosc. 2014; 34: 2406– 2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yeligar SM, Harris FL, Hart CM, Brown LA. . Glutathione attenuates ethanol-induced alveolar macrophage oxidative stress and dysfunction by downregulating NADPH oxidases. Am J Physiol Lung Cell Mol Physiol. 2014; 306: L429– L441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown LA, Ping XD, Harris FL, Gauthier TW. . Glutathione availability modulates alveolar macrophage function in the chronic ethanol-fed rat. Am J Physiol Lung Cell Mol Physiol. 2007; 292: L824– L832. [DOI] [PubMed] [Google Scholar]
- 22. Jones DP, Carlson JL, Samiec PS,et al. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta. 1998; 275: 175– 184. [DOI] [PubMed] [Google Scholar]
- 23. Kirlin WG, Cai J, Thompson SA, Diaz D, Kavanagh TJ, Jones DP. . Glutathione redox potential in response to differentiation and enzyme inducers. Free Radic Biol Med. 1999; 27: 1208– 1218. [DOI] [PubMed] [Google Scholar]
- 24. Noell WK, Walker VS, Kang BS, Berman S. . Retinal damage by light in rats. Invest Ophthalmol. 1966; 5: 450– 473. [PubMed] [Google Scholar]
- 25. Organisciak DT, Vaughan DK. . Retinal light damage: mechanisms and protection. Prog Retin Eye Res. 2010; 29: 113– 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grunwell JR, Gillespie SE, Ward JM,et al. Comparison of glutathione, cysteine, and their redox potentials in the plasma of critically ill and healthy children. Front Pediatr. 2015; 3: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ogawa K, Kuse Y, Tsuruma K, Kobayashi S, Shimazawa M, Hara H. . Protective effects of bilberry and lingonberry extracts against blue light-emitting diode light-induced retinal photoreceptor cell damage in vitro. BMC Complement Altern Med. 2014; 14: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huynh TP, Mann SN, Mandal NA. . Botanical compounds: effects on major eye diseases. Evid Based Complement Alternat Med. 2013; 2013: 549174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao Z, Sun T, Jiang Y,et al. Photooxidative damage in retinal pigment epithelial cells via GRP78 and the protective role of grape skin polyphenols. Food Chem Toxicol. 2014; 74: 216– 224. [DOI] [PubMed] [Google Scholar]
- 30. Wang Y, Zhao L, Lu F,et al. Retinoprotective effects of bilberry anthocyanins via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms in a visible light-induced retinal degeneration model in pigmented rabbits. Molecules. 2015; 20: 22395– 22410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Costa BL, Fawcett R, Li GY, Safa R, Osborne NN. . Orally administered epigallocatechin gallate attenuates light-induced photoreceptor damage. Brain Res Bull. 2008; 76: 412– 423. [DOI] [PubMed] [Google Scholar]
- 32. Wang Y, Zhao L, Huo Y,et al. Protective effect of proanthocyanidins from sea buckthorn (Hippophae rhamnoides L.) seed against visible light-induced retinal degeneration in vivo. Nutrients. 2016; 8:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen Y, Perusek L, Maeda A. . Autophagy in light-induced retinal damage. Exp Eye Res. 2016; 144: 64– 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maeda A, Maeda T, Golczak M,et al. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J Biolog Chem. 2009; 284: 15173– 15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R. . In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology. 1995; 21: 1465– 1468. [DOI] [PubMed] [Google Scholar]
- 36. Negoescu A, Lorimier P, Labat-Moleur F,et al. In situ apoptotic cell labeling by the TUNEL method: improvement and evaluation on cell preparations. J Histochem Cytochem. 1996; 44: 959– 968. [DOI] [PubMed] [Google Scholar]
- 37. Yao J, Zhang B, Ge C, Peng S, Fang J. . Xanthohumol, a polyphenol chalcone present in hops, activating Nrf2 enzymes to confer protection against oxidative damage in PC12 cells. J Agric Food Chem. 2015; 63: 1521– 1531. [DOI] [PubMed] [Google Scholar]
- 38. Yao J, Zhang B, Ge C, Peng S, Fang J. . Xanthohumol, a polyphenol chalcone present in hops, activating Nrf2 enzymes to confer protection against oxidative damage in PC12 cells. J Agric Food Chem. 2015; 63: 1521– 1531. [DOI] [PubMed] [Google Scholar]
- 39. Zamzow DR, Elias V, Legette LL, Choi J, Stevens JF, Magnusson KR. . Xanthohumol improved cognitive flexibility in young mice. Behav Brain Res. 2014; 275: 1– 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Legette L, Karnpracha C, Reed RL,et al. Human pharmacokinetics of xanthohumol, an antihyperglycemic flavonoid from hops. Mol Nutr Food Res. 2014; 58: 248– 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Legette L, Ma L, Reed RL,et al. Pharmacokinetics of xanthohumol and metabolites in rats after oral and intravenous administration. Mol Nutr Food Res. 2012; 56: 466– 474. [DOI] [PMC free article] [PubMed] [Google Scholar]