Abstract
Rickettsia amblyommatis, formerly named Rickettsia amblyommii and ‘Candidatus Rickettsia amblyommii’ is an intracellular bacterium belonging to the spotted fever group Rickettsia. It is highly prevalent in Amblyomma americanum and in other Amblyomma spp. throughout the Western Hemisphere. R. amblyommatis has been cultivated in chicken fibroblast, primary embryonated chicken eggs, Vero cells and arthropod-derived cells. Because of the affinity of rickettsiae to invade vascular endothelial cells, we tried to isolate R. amblyommatis from a nymph of Amblyomma cajennense s.l. collected in Saltillo (Coahulia, Mexico) using human umbilical vein endothelial cells (HUVEC). One tick half was analysed by ompA PCR and was found to be positive for R. amblyommatis. The other half was selected for in vitro culture of Rickettsia spp. It was triturated in 1 mL of endothelial cell growth medium with 1% antibiotic–antimycotic solution, and the homogenate was inoculated into a HUVEC line. Culture was maintained at 33°C in endothelial cell growth medium plus 2 mM l-glutamine and 2% fetal calf serum, with 5% CO2. The medium was changed weekly. Culture was checked by Gimenez stain for Rickettsia-like intracellular organisms. After 48 days of incubation, Rickettsia-like organisms were observed in HUVEC. PCR assays and sequencing of ompA gene in the culture suspension showed 100% identity with R. amblyommatis. This isolate was successfully established in HUVEC, and it has been deposited in the collection of the Center of Rickettsioses and Arthropod-Borne Diseases, Infectious Diseases Department, Hospital San Pedro–Center of Biomedical Research from La Rioja, Logroño, Spain. The HUVEC line is a useful tool for the isolation of R. amblyommatis.
Keywords: Amblyomma cajennense, Candidatus Rickettsia amblyommii, HUVEC line, Rickettsia amblyommatis
Introduction
Rickettsia amblyommatis is an intracellular bacterium belonging to the spotted fever group Rickettsia. It was isolated from an Amblyomma americanum adult tick collected from vegetation in the US state of Tennessee in 1973 and was designated as strain WB-8-2 T [1], [2]. In 1995, Stothard [3] characterized that strain and a new one also detected in A. americanum (strain MO 85-1084) by molecular tools. The rrs sequence was similar to others in the spotted fever group Rickettsia. Nevertheless, analysis of the 17 kDa gene indicated that WB-8-2 T and MO 85-1084 were different from other known species of the genus [3]. From 1995 to 2016, it was named as Rickettsia amblyommii and ‘Candidatus Rickettsia amblyommii’ in the scientific literature, although these names have never been validated. In 2016, Karpathy et al. proposed the novel species name R. amblyommatis, which confirmed to the rules of the International Code of Nomenclature of Prokaryotes [4]. This bacterium is highly prevalent in A. americanum, and it has been also detected in other Amblyomma species throughout the Western Hemisphere as Amblyomma maculatum in the United States [5], and Amblyomma auricularium, Amblyomma cajennense, Amblyomma coelebs, Amblyomma geayi, Amblyomma humerale, Amblyomma longirostre, Amblyomma mixtum, Amblyomma neummanni, Amblyomma hadanii, Amblyomma oblongoguttatum, Amblyomma ovale, Amblyomma sculptum and Amblyomma tonellidae in Central and South America [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]. Nowadays, several of these Amblyomma species are within A. cajennense s.l., because this taxon has been recently reassessed, including A. cajennense sensu stricto, A. mixtum, A. sculptum, Amblyomma interandinum, A. tonellidae and Amblyomma patinoi [20].
R. amblyommatis has never been confirmed as a human pathogen, although some serologic evidence suggests that humans develop an immune response to this organism and it may be associated with disease manifestations in some patients [21], [22]. It has been demonstrated that an isolate from a Costa Rican strain of ‘Ca. R. amblyommii’ causes fever and pathologic signs of disease in guinea pigs [23].
To date, R. amblyommatis has been cultivated in chicken fibroblast, primary embryonated chicken eggs, Vero cells, the mosquito cell Sua5B and the tick cells ISE6 and AAE2 [6], [24], [25].
In an attempt to prove the usefulness of human umbilical vein endothelial cells (HUVEC) for the isolation and maintenance of Rickettsia spp., we tried to isolate R. amblyommatis from a nymph of A. cajennense s.l. collected in Saltillo (Coahulia, Mexico).
Materials and methods
A nymph of A. cajennense s.l. collected in Saltillo in June 2014 was sent to the Center of Rickettsiosis and Arthropod-Borne Diseases (Infectious Diseases Department, Hospital San Pedro–Center of Biomedical Research from La Rioja, Logroño, Spain). The tick was genetically identified using PCR assays targeting the mitochondrial 12S rRNA and 16S rRNA fragment genes [26], [27]. The obtained sequences showed the highest identities (99.7% and 99%, respectively) with A. cajennense s.l. sequences from GenBank (accession no. JX987841 and KX544819). Moreover, the 16S rRNA nucleotide sequence also showed the same identity with the sequence from a tick specimen classified as A. mixtum (GenBank accession no. KT820359). The arthropod was surface sterilized by immersion in 1% benzalkonium chloride for 5 minutes and 70% ethanol for 1 minute, and rinsed twice with sterile distilled water [28]. One tick half analyzed by ompA PCR was found to be positive for R. amblyommatis [29], [30]. The other half was selected for in vitro culture of Rickettsia spp. It was triturated in 1 mL of endothelial cell growth medium (Sigma-Aldrich) with 1% antibiotic–antimycotic solution (Gibco), and the homogenate was inoculated into a HUVEC line. Culture was maintained at 33°C in endothelial cell growth medium plus 2 mM l-glutamine and 2% fetal calf serum, with 5% CO2 atmosphere. For the first 3 days, 100 U/mL penicillin and 100 μg/mL streptomycin were also added. The medium was changed weekly, and culture by Gimenez stained to check for Rickettsia-like intracellular organisms. When the staining method was positive, ompA PCR and sequencing was used to confirm the Rickettsia species in the cells. Two negative controls, one that used water instead of template DNA and the other that used template DNA but no primers, as well as a positive control of Rickettsia slovaca strain S14ab DNA (from the collection of the Center of Rickettsiosis and Arthropod-Borne Diseases), were included in all PCR assays. Passages onto fresh, uninfected cells were performed, and aliquots of infected subcultures were also tested by PCR.
Results
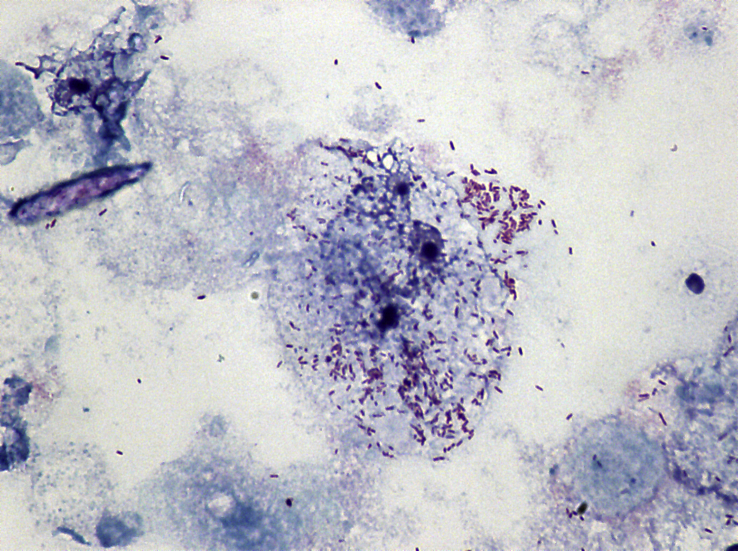
After 48 days of incubation, intracellular Rickettsia-like organisms were observed in HUVEC using Gimenez stain (Fig. 1). PCR assays and sequencing of the ompA gene in culture suspension showed 100% identity with R. amblyommatis (GenBank accession no. CP003334). The bacteria were taken through three subcultures in HUVEC, and the ompA sequence obtained by PCR carried out at passage 6 was identical to that of the original isolate. This isolate was successfully established in HUVEC, and it has been deposited in the collection of the Center of Rickettsiosis and Arthropod-Borne Diseases (R. amblyommatis strain 4Me).
Fig. 1.
Gimenez-stained cytocentrifuge smear (100X magnification) showing infection of human umbilical vein endothelial cell with Rickettsia amblyommatis at day 48 after inoculation with Amblyomma cajennense tick homogenate.
Discussion
Our results correspond to the first isolation of R. amblyommatis from an infected A. cajennense s.l. tick in the HUVEC line.
HUVEC comprise the same ontogenetic type of cells which rickettsiae parasitize in vivo. Consequently, these cells are widely used as a model system for studying rickettsia–host cell interactions in vitro [31], [32], [33], [34], [35], [36], but they have been little used for isolating rickettsia species [37].
R. amblyommatis had been previously cultivated in chicken fibroblast, primary embryonated chicken eggs, Vero cells and the arthropod-derived lines ISE6, AAE2 and Sua5B [6], [24], [25]. The mosquito cell line Sua 5B has been used to isolate R. amblyommatis from wild specimens of A. americanum. Infection was stable in the cells for over 40 passages with no decrease in the cell infection rate, which shows this mosquito cell can be highly effective for isolating and cultivating Rickettsia from ticks [24], and it is known that tick cell lines are effective for the isolation of Rickettsia spp. [25], [38], [39], [40], [41], [42]. Nevertheless, the isolation of R. amblyommatis in an endothelial cell line gives us a new tool for the isolation of rickettsia because this cell line has shown a high permissiveness to infection with this intracellular bacterium; it has also shown advantages over other cell lines using standard, commercially available media.
R. amblyommatis has never been directly detected in human clinical samples, although there has been serologic evidence in the United States that this rickettsial agent could cause spotted fever illness [21], [22]. In addition, R. amblyommatis was detected in a tick that subsequently caused rash at the bite site in a patient without other symptoms [43].
The nymph of A. cajennense s.l. infected with R. amblyommatis was collected in Saltillo, a region located in the northern Mexico on the border with the US state of Texas. In Mexico, R. amblyommatis has been detected in A. mixtum (A. cajennense s.l.) detached from people [19].
R. amblyommatis may also play a role in the ecology and epidemiology of other pathogenic spotted fever group rickettsiae because A. americanum is a potential vector of at least two confirmed rickettsial pathogens, Rickettsia rickettsii and Rickettsia parkeri, and it is possible that the observed high rates of R. amblyommatis infection could inhibit the transovarial transmission of these pathogenic rickettsiae [44]. Rocky Mountain spotted fever (RMSF) is an emerging public health concern in the United States and near the US–Mexico border, a site that recently saw several fatal cases of RMSF. In all cases, infection was caused by R. rickettsii [45]. Nevertheless, there have been suspected cases of RMSF where the causative agent, R. rickettsii, was not identified in the local tick population. In these areas, patients with clinical signs of RMSF had low or no detectable antibodies to R. rickettsii, resulting in an inability to confirm a diagnosis. On the other hand, there are seroepidemiologic studies that indicate that humans are being exposed to R. amblyommatis, and this species might be responsible for cases classified as RMSF [21], [46].
There are cases of RMSF that correspond to the geographic range of A. americanum. In these areas, it has been suggested that reports of RMSF are more likely due to other Rickettsia spp. [47], [48].
Because R. amblyommatis is suspected to be a human pathogen, the availability of cell lines of proven effectiveness in the isolation of this microorganism allows us to characterize this bacterium. The development of culture systems for the growth of Rickettsia is critical to the genetic and antigenic evaluation of pathogenic and nonpathogenic species.
Acknowledgements
We are grateful to A. Díaz Castaño, Centro Hospitalario La Concepción, Saltillo, Coahuila, Mexico, for providing ticks. We would like to acknowledge the financial support of ‘Fondo Europeo de Desarrollo Regional’.
Conflict of interest
None declared.
References
- 1.Burgdorfer W., Cooney J.C., Thomas L.A. Zoonotic potential (Rocky Mountain spotted fever and tularemia) in the Tennessee Valley region. II. Prevalence of Rickettsia rickettsi and Francisella tularensis in mammals and ticks from land between the Lakes. Am J Trop Med Hyg. 1974;23:109–117. doi: 10.4269/ajtmh.1974.23.109. [DOI] [PubMed] [Google Scholar]
- 2.Burgdorfer W., Hayes S.F., Thomas L.A. A new spotted fever group rickettsia from the Lone Star tick, Amblyomma americanum. In: Burgdorfer W., Anacker R.L., editors. Rickettsiae and rickettsial diseases. Academic Press; New York: 1981. pp. 595–602. [Google Scholar]
- 3.Stothard D.R. The Ohio State University; Columbus: 1995. The evolutionary history of the genus Rickettsia as inferred from 16S and 23S rRNA genes and the 17 kDa cell surface antigen gene. Unpublished doctoral dissertation. [Google Scholar]
- 4.Karpathy S.E., Slater K.S., Goldsmith C.S., Nicholson W.L., Paddock C.D. Rickettsia amblyommatis sp. nov., a spotted fever group Rickettsia associated with multiple species of Amblyomma ticks in North, Central and South America. Int J Syst Evol Microbiol. 2016;66:5236–5243. doi: 10.1099/ijsem.0.001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trout R., Steelman C.D., Szalanski A.L., Williamson P.C. Rickettsiae in Gulf coast ticks, Arkansas, USA. Emerg Infect Dis. 2010;16:830–832. doi: 10.3201/eid1605.091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labruna M.B., Whitworth T., Bouyer D.H., McBride J., Camargo L.M., Camargo E.P. Rickettsia bellii and Rickettsia amblyommii in Amblyomma ticks from the state of Rondônia, Western Amazon, Brazil. J Med Entomol. 2004;41:1073–1081. doi: 10.1603/0022-2585-41.6.1073. [DOI] [PubMed] [Google Scholar]
- 7.Labruna M.B., McBride J.W., Bouyer D.H., Camargo L.M., Camargo E.P., Walker D.H. Molecular evidence for a spotted fever group Rickettsia species in the tick Amblyomma longirostre in Brazil. J Med Entomol. 2004;41:533–537. doi: 10.1603/0022-2585-41.3.533. [DOI] [PubMed] [Google Scholar]
- 8.Labruna M.B., Pacheco R.C., Nava S., Brandão P.E., Richtzenhain L.J., Guglielmone A.A. Infection by Rickettsia bellii and Candidatus ‘Rickettsia amblyommii’ in Amblyomma neumanni ticks from Argentina. Microb Ecol. 2007;54:126–133. doi: 10.1007/s00248-006-9180-3. [DOI] [PubMed] [Google Scholar]
- 9.Parola P., Matsumoto K., Socolovschi C., Parzy D., Raoult D. A tick-borne rickettsia of the spotted-fever group, similar to Rickettsia amblyommii, in French Guyana. Ann Trop Med Parasitol. 2007;101:185–188. doi: 10.1179/136485907154557. [DOI] [PubMed] [Google Scholar]
- 10.Hun L., Troyo A., Taylor L., Barbieri A.M., Labruna M.B. First report of the isolation and molecular characterization of Rickettsia amblyommii and Rickettsia felis in Central America. Vector Borne Zoonotic Dis. 2011;11:1395–1397. doi: 10.1089/vbz.2011.0641. [DOI] [PubMed] [Google Scholar]
- 11.Ogrzewalska M., Uezu A., Labruna M.B. Ticks (Acari: Ixodidae) infesting wild birds in the Atlantic forest in northeastern Brazil, with notes on rickettsial infection in ticks. Parasitol Res. 2011;108:665–670. doi: 10.1007/s00436-010-2111-8. [DOI] [PubMed] [Google Scholar]
- 12.Saraiva D.G., Nieri-Bastos F.A., Horta M.C., Soares H.S., Nicola P.A., Pereira L.C. Rickettsia amblyommii infecting Amblyomma auricularium ticks in Pernambuco, northeastern Brazil: isolation, transovarial transmission, and transstadial perpetuation. Vector Borne Zoonotic Dis. 2013;13:615–618. doi: 10.1089/vbz.2012.1223. [DOI] [PubMed] [Google Scholar]
- 13.Alves A.S., Melo A.L., Amorim M.V., Borges A.M., Gaíva E., Silva L. Seroprevalence of Rickettsia spp. in equids and molecular detection of ‘Candidatus Rickettsia amblyommii’ in Amblyomma cajennense sensu lato ticks from the Pantanal region of Mato Grosso, Brazil. J Med Entomol. 2014;51:1242–1247. doi: 10.1603/ME14042. [DOI] [PubMed] [Google Scholar]
- 14.Castro A.M., Garcia G.G., Dzul-Rosado K., Aguilar A., Castillo J., Gabster A. Questing Amblyomma mixtum and Haemaphysalis juxtakochi (Acari: Ixodidae) infected with Candidatus ‘Rickettsia amblyommii’ from the natural environment in Panama Canal Basin, Panama. Trop Med Health. 2015;43:217–222. doi: 10.2149/tmh.2015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soares H.S., Barbieri A.R., Martins T.F., Minervino A.H., de Lima J.T., Marcili A. Ticks and rickettsial infection in the wildlife of two regions of the Brazilian Amazon. Exp Appl Acarol. 2015;65:125–140. doi: 10.1007/s10493-014-9851-6. [DOI] [PubMed] [Google Scholar]
- 16.Tarragona E.L., Cicuttin G.L., Mangold A.J., Mastropaolo M., Nazarena De Salvo M., Nava S. Rickettsia infection in Amblyomma tonelliae, a tick species from the Amblyomma cajennense complex. Ticks Tick Borne Dis. 2015;6:173–177. doi: 10.1016/j.ttbdis.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Faccini-Martínez Á.A., Ramírez-Hernández A., Forero-Becerra E., Cortés-Vecino J.A., Escandón P., Rodas J.D. Molecular evidence of different Rickettsia species in Villeta, Colombia. Vector Borne Zoonotic Dis. 2016;16:85–87. doi: 10.1089/vbz.2015.1841. [DOI] [PubMed] [Google Scholar]
- 18.Mastropaolo M., Tarragona E.L., Silaghi C., Pfister K., Thiel C., Nava S. High prevalence of ‘Candidatus Rickettsia amblyommii’ in Amblyomma ticks from a spotted fever endemic region in North Argentina. Comp Immunol Microbiol Infect Dis. 2016;46:73–76. doi: 10.1016/j.cimid.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Sánchez-Montes S., Ríos-Muñoz C.A., Espinosa-Martínez D.V., Guzmán-Cornejo C., Berzunza-Cruz M., Becker I. First report of ‘Candidatus Rickettsia amblyommii’ in west coast of Mexico. Ticks Tick Borne Dis. 2016;7:1139–1145. doi: 10.1016/j.ttbdis.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Nava S., Beati L., Labruna M.B., Cáceres A.G., Mangold A.J., Guglielmone A.A. Reassessment of the taxonomic status of Amblyomma cajennense (Fabricius, 1787) with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n. sp. and Amblyomma patinoi n. sp., and reinstatement of Amblyomma mixtum, and Amblyomma sculptum (Ixodida: Ixodidae) Ticks Tick Borne Dis. 2014;5:252–276. doi: 10.1016/j.ttbdis.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Apperson C.S., Engber B., Nicholson W.L., Mead D.G., Engel J., Yabsley M.J. Tick-borne diseases in North Carolina: is ‘Rickettsia amblyommii’ a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 2008;8:597–606. doi: 10.1089/vbz.2007.0271. [DOI] [PubMed] [Google Scholar]
- 22.Delisle J., Mendell N.L., Stull-Lane A., Bloch K.C., Bouyer D.H., Moncayo A.C. Human infections by multiple spotted fever group rickettsiae in Tennessee. Am J Trop Med Hyg. 2016;94:1212–1217. doi: 10.4269/ajtmh.15-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivas J.J., Moreira-Soto A., Alvarado G., Taylor L., Calderón-Arguedas O., Hun L. Pathogenic potential of a Costa Rican strain of ‘Candidatus Rickettsia amblyommii’ in Guinea pigs (Cavia porcellus) and protective immunity against Rickettsia rickettsii. Ticks Tick Borne Dis. 2015;6:805–811. doi: 10.1016/j.ttbdis.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X., Ren X., Norris D.E., Rasgon J.L. Distribution and infection frequency of ‘Candidatus Rickettsia amblyommii’ in Maryland populations of the lone star tick (Amblyomma americanum) and culture in an Anopheles gambiae mosquito cell line. Ticks Tick Borne Dis. 2012;3:38–42. doi: 10.1016/j.ttbdis.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sayler K.A., Wamsley H.L., Pate M., Barbet A.F., Alleman A.R. Cultivation of Rickettsia amblyommii in tick cells, prevalence in Florida lone star ticks (Amblyomma americanum) Parasit Vectors. 2014;7:270. doi: 10.1186/1756-3305-7-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black W.C., Piesman J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc Natl Acad Sci U S A. 1994;91:10034–10038. doi: 10.1073/pnas.91.21.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beati L., Keirans J.E. Analysis of the systematic relationships among ticks of the genera Rhipicephalus and Boophilus (Acari: Ixodidae) based on mitochondrial 12S ribosomal DNA gene sequences and morphological characters. J Parasitol. 2001;87:32–48. doi: 10.1645/0022-3395(2001)087[0032:AOTSRA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Bell-Sakyi L. Continuous cell lines from the tick Hyalomma anatolicum anatolicum. J Parasitol. 1991;77:1006–1008. [PubMed] [Google Scholar]
- 29.Regnery R.L., Spruill C.L., Plikaytis B.D. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oteo J.A., Portillo A., Blanco J.R., Ibarra V., Santibáñez S. Rickettsia africae infection. Three cases confirmed by PCR. Med Clin (Barc) 2004;122:786–788. doi: 10.1016/s0025-7753(04)74386-9. [DOI] [PubMed] [Google Scholar]
- 31.Hong J.E., Santucci L.A., Tian X., Silverman D.J. Superoxide dismutase–dependent, catalase-sensitive peroxides in human endothelial cells infected by Rickettsia rickettsii. Infect Immun. 1998;66:1293–1298. doi: 10.1128/iai.66.4.1293-1298.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng H.M., Walker D.H. Mechanisms of intracellular killing of Rickettsia conorii in infected human endothelial cells, hepatocytes, and macrophages. Infect Immun. 2000;68:6729–6736. doi: 10.1128/iai.68.12.6729-6736.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eremeeva M.E., Dasch G.A., Silverman D.J. Quantitative analyses of variations in the injury of endothelial cells elicited by 11 isolates of Rickettsia rickettsii. Clin Diagn Lab Immunol. 2001;8:788–796. doi: 10.1128/CDLI.8.4.788-796.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Astrup E., Lekva T., Davì G., Otterdal K., Santilli F., Oie E. A complex interaction between Rickettsia conorii and Dickkopf-1—potential role in immune evasion mechanisms in endothelial cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0043638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong B., Lee Y.S., Lee I., Shelite T.R., Kunkeaw N., Xu G. Compartmentalized, functional role of angiogenin during spotted fever group rickettsia-induced endothelial barrier dysfunction: evidence of possible mediation by host tRNA-derived small noncoding RNAs. BMC Infect Dis. 2013;13:285. doi: 10.1186/1471-2334-13-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y., Valbuena G., Walker D.H., Gazi M., Hidalgo M., DeSousa R. Endothelial cell proteomic response to Rickettsia conorii infection reveals activation of the Janus kinase (JAK)-signal transducer and activator of transcription (STAT)-inferferon stimulated gene (ISG) 15 pathway and reprogramming plasma membrane integrin/cadherin signaling. Mol Cell Proteomics. 2016;15:289–304. doi: 10.1074/mcp.M115.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dawson J.E., Candal F.J., George V.G., Ades E.W. Human endothelial cells as an alternative to DH82 cells for isolation of Ehrlichia chaffeensis, E. canis, and Rickettsia Rickettsii. Pathobiology. 1993;61:293–296. doi: 10.1159/000163808. [DOI] [PubMed] [Google Scholar]
- 38.Alberdi M.P., Nijhof A.M., Jongejan F., Bell-Sakyi L. Tick cell culture isolation and growth of Rickettsia raoultii from Dutch Dermacentor reticulatus ticks. Ticks Tick Borne Dis. 2012;3:349–354. doi: 10.1016/j.ttbdis.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simser J.A., Palmer A.T., Munderloh U.G., Kurtti T.J. Isolation of a spotted fever group rickettsia, Rickettsia peacockii, in a Rocky Mountain wood tick, Dermacentor andersoni, cell line Jason A. Appl Environ Microbiol. 2001;67:546–552. doi: 10.1128/AEM.67.2.546-552.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pornwiroon W., Pourciau S.S., Foil L.D., Macaluso K.R. Rickettsia felis from cat fleas: isolation and culture in a tick-derived cell line. Appl Environ Microbiol. 2006;72:5589–5595. doi: 10.1128/AEM.00532-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferrari F.A.G., Goddard J., Moraru G.M., Smith W.E.C., Varela-Stokes A.S. Isolation of ‘Candidatus Rickettsia andeanae’ (Rickettsiales: Rickettsiaceae) in embryonic cells of naturally infected Amblyomma maculatum (Ixodida: Ixodidae) J Med Entomol. 2013;50:1118–1125. doi: 10.1603/me13010. [DOI] [PubMed] [Google Scholar]
- 42.Kurtti T.J., Felsheim R.F., Burkhardt N.Y., Oliver J.D., Heu C.C., Munderloh U.G. Rickettsia buchneri sp. nov., a rickettsial endosymbiont of the blacklegged tick Ixodes scapularis. Int J Syst Evol Microbiol. 2015;65:965–970. doi: 10.1099/ijs.0.000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Billeter S.A., Blanton H.L., Little S.E., Levy M.G., Breitschwerdt E.B. Detection of Rickettsia amblyommii in association with a tick bite rash. Vector Borne Zoonotic Dis. 2007;7:607–610. doi: 10.1089/vbz.2007.0121. [DOI] [PubMed] [Google Scholar]
- 44.Macaluso K.R., Sonenshine D.E., Ceraul S.M., Azad A.F. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J Med Entomol. 2002;39:809–813. doi: 10.1603/0022-2585-39.6.809. [DOI] [PubMed] [Google Scholar]
- 45.Drexler N.A., Yaglom H., Casal M., Fierro M., Kriner P., Murphy B. Fatal Rocky Mountain spotted fever along the United States–Mexico Border, 2013–2016. Emerg Infect Dis. 2017;23:1621–1626. doi: 10.3201/eid2310.170309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaughn M.F., Delisle J., Johnson J., Daves G., Williams C., Reber J. Seroepidemiologic study of human infections with spotted fever group Rickettsiae in North Carolina. J Clin Microbiol. 2014;52:3960–3966. doi: 10.1128/JCM.01733-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paddock C.D., Finley R.W., Wright C.S., Robinson H.N., Schrodt B.J., Lane C.C. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis. 2008;47:1188–1196. doi: 10.1086/592254. [DOI] [PubMed] [Google Scholar]
- 48.McQuiston J.H., Zemtsova G., Perniciaro J., Hutson M., Singleton J., Nicholson W.L. Afebrile spotted fever group Rickettsia infection after a bite from a Dermacentor variabilis tick infected with Rickettsia montanensis. Vector Borne Zoonotic Dis. 2012;12:1059–1061. doi: 10.1089/vbz.2012.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]