Abstract
Engineered liver systems come in a variety of platform models, from 2-dimensional cocultures of primary human hepatocytes and stem cell–derived progeny, to 3-dimensional organoids and humanized mice. Because of the species-specificity of many human hepatropic pathogens, these engineered systems have been essential tools for biologic discovery and therapeutic agent development in the context of liver-dependent infectious diseases. Although improvement of existing models is always beneficial, and the addition of a robust immune component is a particular need, at present, considerable progress has been made using this combination of research platforms. We highlight advances in the study of hepatitis B and C viruses and malaria-causing Plasmodium falciparum and Plasmodium vivax parasites, and underscore the importance of pairing the most appropriate model system and readout modality with the particular experimental question at hand, without always requiring a platform that recapitulates human physiology in its entirety.
Keywords: Liver, Liver Models, 3D, in vitro, in vivo, Hepatotropic, Pathogen, HBV, HCV, Malaria, Falciparum, Vivax
Abbreviations used in this paper: 2D, 2-dimensional; 3D, 3-dimensional; EBOV, Ebola virus; HBV, hepatitis B virus; HBC, hepatitis C virus; HLC, hepatocyte-like cells; iHLC, induced pluripotent stem cell–derived hepatocyte-like cells; LASV, Lassa virus; MPCC, micropatterned coculture system; PCR, polymerase chain reaction; SACC, self-assembling coculture
Summary.
This article discusses existing 2-dimensional and 3-dimensional liver models and their applications toward studying hepatotropic pathogens. The importance of selecting the most appropriate models and readout modalities to answer specific scientific questions is emphasized.
The liver is the largest internal organ in the body, and performs vital and diverse functions in metabolism of carbohydrates, proteins, and lipids; bioproduct synthesis; immunologic processes; and detoxification. Although many viruses, parasites, and bacteria specifically target the cells of the liver, the liver is also exposed to blood-borne pathogens that circulate systemically, or that are derived from the gut,1 because of its location at the convergence of the hepatic artery and portal vein.
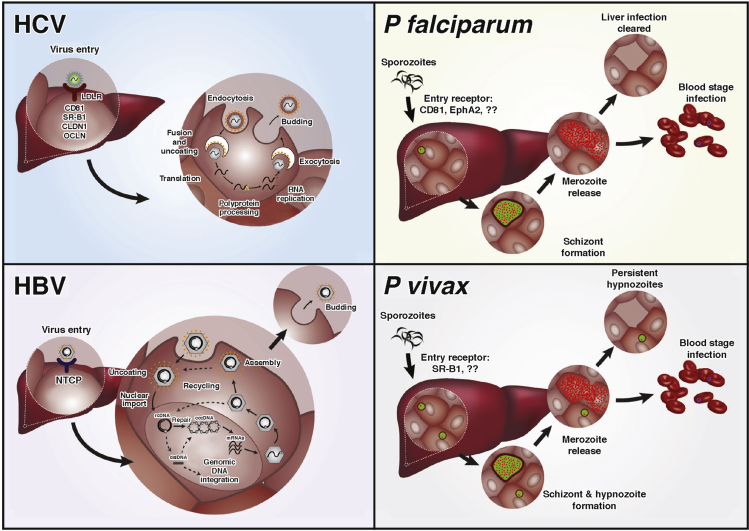
Most liver pathogens specifically target the most abundant cell type in the liver, the hepatocyte, for completion of their life cycles or developmental stages. These include hepatitis viruses and Plasmodium protozoan parasites (Figure 1), which together account for an enormous burden on human health. Hepatitis B virus (HBV) and hepatitis C virus (HCV) infect the livers of more than 350 million people worldwide, and are the main causes for chronic liver diseases, such as liver cirrhosis and hepatocellular carcinoma.2 Plasmodium parasites, which cause malaria, result in more than 200 million infections annually3 and require asymptomatic development in the liver before initiating fevers associated with blood stage infection. Other hepatropic pathogens, including several viruses and bacteria that cause systemic infection, can also target the liver and cause severe liver damage (Table 1).
Figure 1.
Life cycles of 4 major human hepatotropic pathogens. HCV is a single-stranded, positive-sense RNA virus that belongs to the Flaviviridae family. Initial viral attachment to the hepatocyte membrane is mediated through glycosaminoglycans and the LDL receptor. Interactions with other host factors CD81, scavenger receptor class B member 1 (SRB1), claudin 1 (CLDN1), occludin (OCLDN), and possibly other molecules, such as CLDN9, CLDN6, EphA2, and epidermal growth factor receptor, are required for cell entry. Clathrin-mediated endocytosis of the virus is followed by fusion of the viral and endosomal membranes, resulting in the release of nucleocapsid into the cytoplasm. Positive-strand genomic RNA is released into the cytosol on uncoating of the viral nucleocapsid, which initiates synthesis of the HCV polyprotein. Host cell lipid synthesis pathways are tightly linked to the later stages of assembly and virus release. HBV is a DNA virus that belongs to the family Hepadnaviridae. HBV enters the hepatocyte via the sodium/bile acid cotransporter NTCP.23 After uncoating, the partially relaxed double-stranded circular viral DNA (rcDNA) is directed to the nucleus where viral DNA lesions are repaired by the host machinery, converting into covalently closed circular DNA (cccDNA), which serves as a template for viral RNA production. Five transcripts are made that encode envelope, core and X antigens, viral polymerase, and pregenomic RNA (pgRNA). pgRNA can be reverse transcribed into rcDNA, which is assembled with the viral capsids and released from the host cell. During reverse transcription of pgRNA double-stranded linear (dsl) DNA can be formed and are capable of integration into human chromosomes. Plasmodium falciparum and Plasmodium vivax are apicomplexan parasites. Plasmodium sporozoites are deposited into the human skin via bite of an infected Anopheles mosquito and travel to the liver where they invade hepatocytes. CD8138, 148 and EphA2106 for P falciparum, and more recently SR-B1134 for P vivax, have been implicated as required entry factors. On invasion of hepatocytes, parasites differentiate and divide by schizogony to form thousands of progeny, merozoites, which are released into the bloodstream where they can cyclically invade red blood cells, initiating the blood stage of the disease. P vivax has an additional, unique aspect of its liver development where a subset of the parasites, called hypnozoites, remain dormant and can reactivate weeks to years after the initial infection to reinitiate disease.
Table 1.
Summary of Liver Models Applied to Human Hepatotropic Pathogens
| Plasmodium falciparum | Plasmodium vivax | HBV | HCV | Other hepatotropic pathogens | ||
|---|---|---|---|---|---|---|
| 2D cultures | Cancer cell lines | HepG2-A16104, 105 HC04106 |
HepG2-A1620, 21 HC04107 |
Rat Q7108 HepaRG109 Hep3B110 HuS-E/2111 HLCZ01112 HepG2-NTCP22, 23 Huh7-NTCP22 |
HLCZ01112 Huh7/Huh7.5 113, 114, 115 Huh6116 Hep3B117 JHH-4118 HepG2+IMY-N9119 LH86120 |
Brucella: HepG2121 Listeria: HepG2122, 123 HEV: PLC/PRF/5, A549124, 125, 126 HEV: HepaRG, PICM-19127 DenV: Huh7,31 HepG1,128 HepG2129 YF: HepG2,130 PH5CH8131 EBOV: Huh7,27 HepG228 LASV: Huh729 HCMV: HepG230 |
| Primary cells | Adult PHH132, 133, 134 | Adult PHH134 | Adult PHH135 Fetal PHH136 HepCHLine-7137 |
Adult PHH138 | HCMV: adult PHH4 DenV: adult PHH128 |
|
| iPS cells | iHLC50 | iHLC50 | iHLC39 iPS-HPCs139 iPS-Heps139 |
iPS-iHLC41, 47, 48, 49 | ||
| Co-cultures | MPCC38 | MPCC | Adult PHH + mouse fibroblasts39 Fetal PHH + nonparenchymal cells35 |
Adult PHH + mouse fibroblasts40 | ||
| 3D cultures | Cocultures | Huh7.5-NTCP + LSEC62 PHH + BAECs66 |
Rotating wall vessel53 3D radial flow bioreactor51 Hollow fiber system52 Polyethylene glycol hydrogel54 Gelatin polymer55 Alginate beads58 Matrigel60 Huh7 spheroids57 spheroids59 |
Entamoeba: Huh7 + LSEC62, 63 HEV: Rotating wall vessel140 |
||
| Animal models | Rodent models | Alb-UPA/SCID70, 71 FRG74 TK-NOG76 DRAG91 Ectopic artificial livers81 |
FRG75 | NOD/SCID141 Trimera142 uPA/RAG2143 |
Alb-uPA/SCID77, 144 FRG72 TK-NOG73 AFC8-Hu HSC/Hep92, 145 Genetically humanized mice80 |
HDV: NOD/SCID141 HCMV: HuNSG146 HCMV: Alb-uPA/SCID147 |
2D, 2-dimensional; 3D, 3-dimensional; BAEC, bovine aortic endothelial cells; DenV, dengue virus; HBV, hepatitis B virus; HCMV, human cytomegalovirus; HCV, hepatitis C virus; HDV, hepatitis D virus; HLC, hepatocyte-like cells; iHLC, induced pluripotent stem cells–derived hepatocyte-like cells; HPC, hepatic progenitor like cells; iPS, induced pluripotent stem; LASV, Lassa virus; LSEC, liver sinusoidal endothelial cells; MPCC, micropatterned coculture system; NTCP, liver bile acid transporter; PHH, primary human hepatocyte.
In addition to hepatocytes, the liver is also populated by other cell types, such as Kupffer cells, liver sinusoidal endothelial cells, cholangiocytes, and stellate cells, some of which can be targeted by pathogens. For example, human cytomegalovirus can infect bile duct epithelia cells and stromal cells,4 whereas dengue virus can replicate in Kupffer cells5 and hepatocytes.6 Aside from being a site for massive pathogen amplification, the liver also hosts such pathogens as Entamoeba hystolytica, a protozoan parasite that travels from the gut via portal vein, invades the liver parenchyma, and remains extracellularly, forming amoebic liver abscesses.
To study the mechanisms of pathogen-host cell interactions and to develop novel therapeutics against liver pathogens, robust model systems that can faithfully replicate human hepatotropic infections are needed. Human hepatoma-derived cell lines have been widely used to study the biology of hepatotropic pathogens and to test candidate drugs and vaccines. However, because of their uncontrolled proliferation, abnormal liver-specific functions,7, 8 or the stringent host dependence of some human hepatotropic pathogens, immortalized cell lines do not always fully recapitulate the entire pathogen life cycle. Animal models are also not alternatives because many human-tropic species can only infect hepatocytes of human origin. Human hepatocytes are thus considered the gold standard cell type to study the biology of human hepatotropic pathogens and pathogen-host interactions, yet conservation of the polarized morphology and functions of hepatocytes ex vivo is challenging.
To develop systems that closely recapitulate human liver biology and support hepatotropic infections, tissue engineering tools have been applied to create 2-dimensional (2D), 3-dimensional (3D), and humanized mouse systems by using a combination of cell lines, primary human hepatocytes, or stem cell–derived cells with various extracellular matrix manipulations (Table 1). The available systems are capable of modeling some, but not all, aspects of the shared pathogen-host interaction, thus researchers should carefully select a model that is best suited to the specific question being investigated. In this review, we summarize key aspects of each platform, their advantages and disadvantages, and discuss biologic insights gained using models of liver infections, with a focus on HBV and HCV viruses and the major species of human malaria parasites, Plasmodium falciparum and Plasmodium vivax (Figure 1). For more technical details on the assembly of various engineered liver model systems we recommend a collection of recent review articles.9, 10, 11, 12
2-Dimensional Monolayer Cell Culture Systems
Despite their regenerative potential in the human body, isolated human primary hepatocytes are difficult to maintain in vitro, requiring adherence to extracellular matrices to survive for more than a few hours.13 Even in adherent formats, fully confluent monolayers of primary hepatocytes are only appropriate for short-term studies because of a rapid decline in hepatic functions after 48 hours.14, 15 Hepatocytes cultured between collagen layers16 retain metabolic stability over longer periods of time; however, the batch-to-batch variation in collagen, combined with limitations in drug access caused by collagen’s barrier effect, limit their use. Ongoing work is focused on immortalizing mature hepatocytes to address cell source limitations.17, 18 In one example, transduction of viral genes E6 and E7 led to expansion of primary hepatocytes.19 In these cells, infectivity of cell culture–derived HCV was similar to that observed in Huh7 hepatoma cells 9 days postinfection. However, it should be noted that although such approaches provide the ability to capture patient-to-patient variability in response to pathogen infection, these cells have not yet been evaluated for long-term culture and hold the risk of oncogenic transformation.
For infections lasting a few days, monolayers of human hepatoma-derived cell lines represent a renewable alternative to primary hepatocytes that is relatively simple to maintain and amplify, and amenable to scale up for drug screening. Moreover, stable transfected lines can be produced because these are dividing cells. Historically, hepatoma cells have been invaluable tools for expanding knowledge of hepatic-amplified pathogens and understanding of host-pathogen interactions. For example, in vitro infection of CD81-deficient HepG2-A16 cells with different human Plasmodium parasites (P vivax and P falciparum)20, 21 provided insight into species-specific host entry factors required to establish malaria infection. Likewise, comparison of differentiated and naive HepaRG cells confirmed the liver bile acid transporter (NTCP) as the entry receptor used by HBV and hepatitis D virus to infect human hepatocytes.22, 23 Notably, stable transfected hepatoma cell lines remain the primary source for generation of infectious virion stocks of HCV24 and HBV,25, 26 among others. Human hepatoma cell lines have also proven to be useful for studying other hepatotropic viruses and bacteria that infect the human body systemically, namely Ebola (EBOV),27, 28 Lassa (LASV),29 human cytomegalovirus,30 and dengue31 viruses (Table 1).
2-Dimensional Coculture Systems
Not all aspects of host-pathogen interactions can be recapitulated in immortalized cell lines. In one example, certain clinical isolates of HCV cannot robustly infect hepatoma cell lines and require primary human hepatocytes for propagation.32 Furthermore, for liver pathogens, such as P vivax, where dormant liver forms can remain in culture for weeks, long-term culture is essential but cannot be maintained in cell lines because the infected cells continue to proliferate and might detach from culture. HCV, HBV, and human Plasmodium species have been studied in primary human hepatocyte monocultures (Table 1); however, the short lifetime of the cells in culture presents a hurdle for any scientific inquiries that require longer-term analyses. To overcome these problems, tissue-engineering tools have been applied to drive immortalized cell lines toward more polarized or differentiated states, and to maintain primary human hepatocyte functions, such as biosynthesis (often tracked by measuring albumin production) and metabolism (cytochrome P-450 [CYP] enzyme activities) for a longer period. Cell-cell interactions are pivotal to the function of many organ systems, including the liver. Cocultivation of hepatocytes with nonparenchymal cells has been shown to preserve hepatocyte phenotype because of heterotypic interactions between cell types, and via homotypic interactions between parenchymal cells.33, 34 In one example, Zhou et al35 created a 2D coculture system where irregular patches of human fetal hepatocytes were surrounded by nonparenchymal cells. In this format, hepatocyte hallmarks were maintained for 72 days, and the authors observed successful HBV infection, albeit requiring high viral titers.35 More recently, a random, self-assembling coculture (SACC) of primary human hepatocytes and mouse fibroblasts was shown to be susceptible to very high HBV genome copies derived from cell culture or purified from patient plasma. Infection in this system was maintained for more than 30 days and, in a proof-of-concept experiment, the authors demonstrated that the system could potentially be used for testing anti-HBV drugs.36
The Bhatia laboratory previously developed a micropatterned coculture system (MPCC), in which hepatocytes are positioned in “islands” via photolithographic patterning of collagen, and are subsequently surrounded by mouse embryonic fibroblasts.37 In this configuration, MPCCs were shown to exhibit induced and stable hepatocyte phenotypes for 4–6 weeks, and remain permissive to P falciparum,38 P vivax,38 HBV,39 and HCV40, 41 infections. During HBV and HCV infection of MPCCs, persistent viral infection was maintained for nearly 3 weeks, offering the potential for testing antiviral therapeutics.39, 40 Comparison of MPCC infections versus other culture platforms, including random cocultures similar to the SACC method, using the same source of human primary hepatocytes and stromal cells, revealed more robust infection in the MPCC cultures, which could be further enhanced by blocking the hepatocyte innate immune response.39, 40 Testing of several antivirals and antibodies against HCV, including those under preclinical development, in the MPCC system revealed that the system closely mimicked in vivo antiviral responses.40
In addition, MPCCs support full developmental liver stages of both P falciparum and P vivax, with the release of merozoites from hepatocytes and their subsequent infection of overlaid human red blood cells.42 For P vivax, hypnozoites are observed in these cultures for up to 21 days, enabling potential reactivation studies in an in vitro format (Gural et al, unpublished data, 2018). Hepatocytes cultured in MPCCs exhibit human-specific hepatic drug metabolism and have been shown to be more predictive of drug metabolites and drug-induced liver injury than previous in vitro liver platforms.37, 43 Human hepatic drug metabolism is especially important within the context of P vivax, because primaquine, the only clinically available antihypnozoite drug, requires bioactivation by the liver. In the MPCC model, this phenomenon has been used to observe an IC50 shift in parasite killing in response to primaquine in donors with differential enzymatic activity (Gural et al, unpublished data, 2018).
In a recent study, metabolic clearance functions of a collection of monoculture and coculture platforms were interrogated across a diverse set of enzyme markers, including 5 major cytochrome P-450 enzymes. Specifically, this study focused on SACC and MPCC cultures at 1-week postseeding and found higher activity in the MPCCs across all enzymes monitored, with most pronounced difference being in the CYP2D6 enzyme. With respect to their use in long-term studies, the MPCC platform has been demonstrated to maintain hepatic-specific function for more than 4 weeks, whereas function in SACCs has not been studied beyond 3 weeks, to date. Finally, although the MPCC system uses a single source of primary hepatocytes per assay, SACCs pool cells from multiple human hepatocyte donors, thus preventing interrogation of donor-specific properties, such as CYP2D6 activity, which is important within the context of required drug bioactivation, as described previously.
Stem Cell–Derived 2-Dimensional Culture Systems
The platforms described in the previous section depend on access to sources of adult, primary human hepatocytes. Human embryonic stem cells and induced pluripotent stem cells offer a renewable and alternative source of these essential cells. Numerous studies have differentiated human embryonic stem cells and induced pluripotent stem cells to yield hepatocyte-like cells (HLCs) that exhibit phenotypic and functional traits resembling those of fetal liver cells.44, 45 These HLCs offer a wide array of traceable genetic backgrounds, and the potential to be generated in a patient-specific manner, enabling investigation of host genetics within the context of host-pathogen interactions, and may also be used in target identification for drug development. Notably, use of these cells necessitates proactive genetic characterization to screen for any aberrations that might occur during the proliferation and differentiation processes.46
Several groups have demonstrated that induced pluripotent stem cells–derived HLCs (iHLCs) express HCV and HBV entry receptors, and support productive viral infection with HCV or HBV particles,41, 47, 48, 49 Viral load in iHLCs is higher than in hepatoma cell lines,47 and antiviral innate immune response is readily detectable on HCV or HBV infection, with increased expression of various type I interferon-stimulated genes similar to that observed in primary human hepatocytes in MPCCs.39, 41 Furthermore, infected iHLCs can be used for drug screening purposes because they respond to anti-HCV and -HBV drugs.39, 41, 47
During the differentiation process, cultures of pluripotent stem cells that achieve the definitive endoderm stage are not yet permissive to HCV infection, whereas more mature hepatic progenitor cells support the entire viral life cycle. Interestingly, Wu et al48 characterized this transition from resistance to susceptibility by showing that cells become permissive with induction of liver-specific microRNA-122 and other host factors including epidermal growth factor receptor and EphA2, and a down-regulation of some interferon-stimulated genes. iHLC infection of P falciparum and P vivax revealed that permissiveness of iHLCs began at the hepatoblast stage. More importantly, it was shown that in this system, iHLCs could be chemically matured to enable primaquine bioactivation, opening the door to potential antimalarial drug testing applications.50 Together, these studies demonstrate that the use of patient-derived iHLCs may enable personalized in vitro culture models for hepatotropic infections in the future.
3-Dimensional Cell Culture Systems
Although 2D systems are easy to create and enable straightforward microscopic monitoring of infection, culture conditions in these systems lack the complexity and architecture of the liver microenvironment, which thus prohibit interrogation of the functional role of the extracellular matrix, and/or the impact of different cell types on liver infections. In light of these limitations, several 3D-engineered liver models have been created to recapitulate certain aspects of liver function that cannot be monitored in 2D. Early attempts to model HCV infection in 3D included the use of a 3D radial-flow bioreactor to grow a human hepatoma cell line.51 The bioreactor is a vertically extended cylindrical matrix with porous bead microcarriers, providing sufficient oxygen and nutrient supply to cells. Huh7 cells grown in the bioreactor maintain a polarized state, and can support productive HCV infection for months.51
Similarly, hollow fiber systems52 and 3D rotating wall vessels53 are used to model HCV infection in 3D. These systems, however, require a large quantity of cells, and are challenging to scale up. Alternative 3D cultures of hepatoma cell lines using polyethylene glycol–based hydrogels,54 thermoreversible gelatin polymers,55, 56, 57 alginate,58 galactosylated cellulosic sponges,59 Matrigel,60, 61 and collagen62 have been developed and shown to be permissive to HCV or HBV infections. In these systems, cell lines were shown to maintain more differentiated states, exhibit better hepatic function,58 and differential gene expression profiles,55, 56, 60, 62 as compared with their 2D counterparts. Moreover, several groups demonstrated that 3D liver spheroids could be manufactured at specific sizes and easily scaled up.
Another example where the recreation of a 3D liver-like microenvironment allows the study of early hepatic infection is the migration of E hystolytica through human liver sinusoidal endothelial cells and Huh7 cell layers.62, 63 The layers of human cells are separated by a collagen scaffold, enabling imaging of cell barrier crossing and 3D migration of the parasites toward hepatocytes, which recapitulates initial invasion of the liver and eventually leads to parenchyma destruction and abscess formation.
To further mimic shear stress, blood flow, and the extracellular environment within a tissue, several liver-on-a-chip models have recently been created64, 65, 66, 67 and hold great potential for modeling liver-specific pathogens. As an added benefit, different cell types within the liver or from other organs can be assembled on the same chip, making it possible to study the effects of different cell types on the progress of infection. Alternatively, decellularized pig and human livers have been repopulated with human cells to create 3D structures with physiologically relevant architectures.68, 69 Although not many groups have directly used these more complex models to study hepatotropic infections, we believe that these techniques can potentially offer novel insights into host-pathogen interactions. Selection of the most appropriate model system is guided by the combination of the specific question under interrogation, and the desired method for tracking infection responses under those circumstances.
Humanized Mouse Models
In particular infection studies, it is necessary to consider organ-organ interactions, not to mention more complex 3D architecture than what has been achieved thus far in vitro; however, conventional mouse models cannot be used for studying pathogens that have strict hepatotropism. To overcome this barrier, mouse models with “humanized” livers, or that bear ectopic human liver structures, have been developed. The strategy used entails induction of liver injury with physical, chemical, or surgical insults, followed by transplantation or implantation of primary human hepatocytes. One added advantage of these systems over other 3D liver models is the possibility of incorporating human immune cells in the mice, which may have an impact on pathogen development, clearance, and drug responses.
The first model used to monitor HCV and P falciparum,70, 71 infections in vivo was the Alb-uPA/SCID chimeric mouse where human hepatocytes were transplanted into urokinase-type plasminogen activator-transgenic SCID mice, which undergo liver failure. Subsequently, FNRG/FRG (human hepatocytes transplanted into Fah[-/-], Rag2[-/-], and Il2rγ[-/-] mice with or without a NOD background), and TK-NOG (human hepatocytes transplanted into herpes simplex virus type-1 thymidine kinase mice) chimeric mouse models were validated for HCV,72, 73 HBV,72, 73 P falciparum,74 P vivax,75 and Plasmodium ovale76 infections. Interestingly, Calattini et al72 showed that HCV particles produced in the FRG model have different biophysical properties and receptor usage compared with cell culture–derived virions, suggesting fundamental differences between in vitro and in vivo pathogenesis of HCV infection.
Humanized mouse models can also be used for drug screening purposes, although they are better served as a bridge between in vitro models and human trials, because their maintenance and cost are bottlenecks. Mailly et al77 demonstrated in Alb-uPA/SCID mice that antibodies targeting HCV entry factor Claudin-1 can act as antiviral therapy, as shown by inhibition of HCV entry, cell-cell transmission, and virus-induced signaling, leading to a clearance of persistent HCV infection. The FRG mouse model was used to test a canonical drug for P vivax, primaquine, in early treatment mode (causal prophylaxis), and reported clearance of hypnozoites.75
Another caveat of xenotransplantation mice models is the difficulty to create and maintain them.78, 79 To overcome this issue, Dorner et al80 created a genetically humanized mouse expressing human factors. Transgenic mice expressing human entry receptors (CD81 and occludin) crossed with an immune-deficient inbred mouse supported HCV infection in murine hepatocytes. As a more affordable alternative to the available humanized mouse models, Ng et al81 created microporous polyethylene glycol cryogels implanted into mice as ectopic livers, without the need for injuring the mouse liver, offering the added advantage that it can be rapidly generated for immediate use. More recently, human tissue seeds composed of primary human hepatocytes, human endothelial cells, and fibroblasts in degradable hydrogels were implanted into FNRG mice where the host-derived regenerative stimuli favored organized expansion of the grafts. The resulting graft contained bile duct–like structures and a vascular network, supported by the presence of red blood cells in the seeds.82 The potential ability to connect these ectopic grafts to the vascular network of the animal raises the prospect of studying local and systemic effects of hepatotropic pathogens in the future.
Readout Modalities of Infection
An important determinant when choosing engineered systems for studying hepatotropic pathogens is the readout modality used to assess infection properties. Although 2D systems generally offer a variety of simple and scalable readouts, 3D systems and animal models require more complex and laborious methods to monitor infection processes. Readouts can be terminal, such as polymerase chain reaction (PCR) or cell fixation followed by immunohistochemistry, or real-time, such as genetic modification of the pathogen of interest for live imaging, or detection of secreted molecules. Next we briefly describe the assays typically used for the main hepatic-amplified pathogens (HBV, HCV, and Plasmodium) in the different systems. The selection of each assay and model system may vary based on the experimental question at hand.
PCR-based assays and fixed sample imaging are both commonly used terminal assays for quantification of total pathogen burden. PCR is highly sensitive, but cannot determine the number or type of cells infected, and so is suboptimal for questions regarding the heterogeneity of host cell susceptibility. HBV infection is classically analyzed by PCR quantification of viral DNA, cccDNA, and pgRNA, which are indicative of active viral replication in infected cells. Similarly, HCV and Plasmodium infections can be monitored by detection of viral RNA or Plasmodium 18S rRNA, respectively.50, 74, 75 In contrast, imaging of fixed samples provides structural information about the pathogen and quantification of disease load. For example, the distribution of viruses and parasites inside hepatocytes and across the tissue system can be visualized by immunostaining of pathogen-specific antibodies.10, 38, 50, 74, 75 As an alternative method, Ramanan et al83 demonstrated that single-molecule fluorescence in situ hybridization can be used to detect viral RNA levels in fixed samples by labeling both strands of HCV viral RNA with high specificity and sensitivity. This single cell phenotypic analysis enables studies of subpopulation infection patterns and kinetics. Together, these methods offer a wealth of information about the pathogen’s infection level, and are compatible with the 2D and 3D models described previously, although in the case of the latter these methods are more laborious because they require sectioning of the liver constructs or humanized livers.
Live imaging offers the advantage of dynamic monitoring of infection in real-time. Because of the tissue scattering effect, live fluorescence imaging is feasible in 2D systems, but becomes more challenging in 3D or animal models. Fluorescent protein-based reporter lines have been created for HCV and P falciparum by inserting the reporter gene into the pathogen’s genome, allowing visualization in live cells.84, 85 One observed caveat for the genetically modified HCV virus was attenuated RNA replication compared with wild-type virus.86 To overcome this bottleneck, a cell-based reporter system has been developed to distinguish between HCV infected and uninfected cells. In this system, protease activity of the HCV nonstructural protein 3-4A in infected cells causes a mitochondrial-anchored fluorescence reporter to be shuttled into the nucleus, allowing monitoring of viral replication in real time.87 For pathogens like P vivax where no transgenic lines exist, live imaging of parasite development has been challenging, thus many questions remain regarding the processes involved in reactivation of hypnozoites, development of schizonts, and release of merosomes from the liver. Although 2D systems are amenable to live fluorescent imaging, bioluminescent live imaging is a better option for monitoring HCV86 and P falciparum88 infections in 3D and animal models.
An alternative readout modality that enables kinetic monitoring of the infection process without the need to perturb infected cells is detection of released molecules or pathogen progeny into the environment. On completing the viral replication cycle, for example, HBV- and HCV-infected cells release newly synthesized viral particles, which can be quantified without the need for terminating the experiment. For example, during HBV infection, surface and envelope antigens can be detected by enzyme-linked immunosorbent assay in culture supernatants or animal sera. For Plasmodium parasites, propagation of the parasite beyond the liver stage offers a unique readout modality.89, 90 Quantification of infected blood can serve as a proxy for final liver stage burden. Parasites that have egressed out of hepatocytes go on to infect red blood cells, if accessible to them. In the case of humanized mouse models, FNRG mice must be injected with human erythrocytes immediately before parasite egress from the liver, whereas the TK-NOG model can sustain >80% human erythrocyte chimerism for up to 5 weeks after a single engraftment.76 To date, the only model where the entire P falciparum life cycle could be maintained without the need for exogenous human erythrocyte injection is the DRAG model,91 where HLA class II molecules are expressed in NRG mice to favor human hematopoietic stem cell engraftment. In vitro overlay of MPCCs with erythrocytes or reticulocytes also results in blood stage infection for both P falciparum38 and P vivax (Gural et al, unpublished data, 2018).
Conclusions and Future Work
Because liver-targeting pathogens are diverse, often with narrow tropism, models that recapitulate authentic host-pathogen interactions are critical for the development of liver-acting therapeutic interventions. With the landscape of available models, and the evolution of new platforms that continue to be developed to support infection by a diverse repertoire of hepatotropic pathogens, it remains essential that researchers match their selected model and readout pairing to the specific question at hand.
The model systems and readout modalities described in this review form a list of combinations that can be used to interrogate different aspects of pathogen development. For example, 2D cultures are easier to setup and offer the added benefit of portability, especially for such pathogens as P vivax, which lack laboratory-adapted strains and thus require on-site experimentation in endemic areas. 2D systems are also more amenable to high throughput drug screening than their 3D counterparts. The biologic complexity that human hepatoma cell lines lack can be overcome by use of primary human hepatocytes, which more closely resemble a functional human liver. Furthermore, donor variability of human hepatocytes can be used as a tool for interrogating donor-specific biology, such as host factors that determine invasion and development, and pathogen response to bioactivation-requiring drugs. Stem cell–derived systems offer a renewable alternative and provide the benefit of fine-tuning specific aspects of host factors. However, it should be noted that these cultures require significant culture expertise, and may not represent a fully differentiated adult hepatocyte phenotype. In comparison with 2D models, 3D models enable the study of more complex microenvironments on pathogen development, and can be adapted for use in perfused organ-organ “on chip” models, which may be of particular value for hepatropic pathogens that impact multiple tissues (eg, malaria, LASV, EBOV). Finally, humanized mouse models offer a multiorgan system to study aspects of pathogen development and clearance mechanisms that require a more complex microenvironment. During the course of liver infection, the invading pathogen encounters several liver-resident and/or recruited immune cells. However, except for few humanized animals that incorporate both human hepatocytes and immune cells,92 most of the models described here lack this aspect of the liver environment, although several are amenable to the addition of immune cells. In one example, Kupffer cells were successfully added to the MPCC platform, but this configuration has yet to be probed in the context of infection.93 Addition of other key innate effector cells, such as complement, neutrophils, and natural killer cells, and antibodies could enable the study of immunologic responses against human pathogens in vitro.
Novel readout modalities that can monitor live cultures would be a valuable addition to the existing repertoire and further unleash the power of engineered liver platforms. Efforts should focus on improving genetic engineering tools to modify the genomes of HBV and P vivax, for which no reporter lines are available. For HBV, reporter viruses have not been successfully created because of the compact nature of the viral genome. For P vivax, lack of a continuous blood culture makes genetic modification of the parasite impossible. Furthermore, discovery of new secreted biomarkers could aid in kinetic tracking of infection. An example could be the adaptation of a protease detection system, such as those already developed for cancer94, 95, 96, 97 and HCV87 monitoring. This would be especially useful within the context of Plasmodium infections, because no secreted biomarkers have been described to date. It is known that malaria parasites encode a serine protease, which can be found in the serum of malaria-infected individuals and is hypothesized to play a role in parasite egress out of infected hepatocytes.98 Such an approach could allow live tracking of not only laboratory strains but also clinical isolates of both P falciparum and P vivax. Lastly, with the advent of high-throughput sequencing techniques and single-cell analysis modalities, such as laser capture microdissection,70, 99 Drop-seq,100 and Seq-well,101 one can expect a more thorough understanding of the biology of both the pathogen and its host with single-cell resolution.
In recent years, exciting clinical progress has been made in the study of hepatotropic pathogens, notably with the cure of HCV,102, 103 and licensing of the first malaria vaccine, RTS,S. Nevertheless, infectious liver diseases continue to prevail and pose a great global health burden, particularly given that the access to medical interventions is not universal. Although tissue engineering has contributed greatly to the current available knowledge of hepatotropic pathogens, we envision a continued effort to develop better liver models. This goal is of importance not only for the major hepatotropic pathogens discussed in this review but also for pathogens that have exclusively been studied in human hepatoma cell lines, such as EBOV or LASV. These hemorrhagic fever viruses are prime examples of zoonotic viruses that accidentally infect humans, causing severe disease and high mortality, yet they remain understudied. The recent EBOV outbreak led to growing public concern regarding the devastating risks such pathogens pose to human health, and demand on the scientific community to develop better prevention and treatment options. Building on the knowledge of HCV, HBV, and Plasmodium, we anticipate that adaptation of the described engineered models may significantly advance the understanding of the pathogenesis of these new global threats.
Acknowledgments
All authors wrote the manuscript.
Footnotes
Conflicts of interest The following author discloses the following: Sangeeta N. Bhatia is a cofounder of Ascendance, which commercially manufactures and distributes micropatterned co-cultures. The remaining authors disclose no conflicts.
References
- 1.Jenne C.N., Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996–1006. doi: 10.1038/ni.2691. [DOI] [PubMed] [Google Scholar]
- 2.Protzer U., Maini M.K., Knolle P.A. Living in the liver: hepatic infections. Nat Rev Immunol. 2012;12:201–213. doi: 10.1038/nri3169. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . WHO; Geneva: 2017. World malaria report 2016. [Google Scholar]
- 4.Sinzger C., Bissinger L., Viebahn R., Oettle H., Radke C., Schmidt C., Jahn G. Hepatocytes are permissive for human cytomegalovirus infection in human liver cell culture and in vivo. J Infect Dis. 1999;180:976–986. doi: 10.1086/315032. [DOI] [PubMed] [Google Scholar]
- 5.Huerre M.R., Lan N.T., Marianneau P., Hue N.B., Khun H., Hung N.T., Khen N.T., Drouet M.T., Huong V.T., Ha D.Q., Buisson Y., Deubel V. Liver histopathology and biological correlates in five cases of fatal dengue fever in Vietnamese children. Virchows Arch. 2001;438:107–115. doi: 10.1007/s004280000329. [DOI] [PubMed] [Google Scholar]
- 6.Seneviratne S.L., Malavige G.N., de Silva H.J. Pathogenesis of liver involvement during dengue viral infections. Trans R Soc Trop Med Hyg. 2006;100:608–614. doi: 10.1016/j.trstmh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Wilkening S., Stahl F., Bader A. Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metab Dispos. 2003;31:1035–1042. doi: 10.1124/dmd.31.8.1035. [DOI] [PubMed] [Google Scholar]
- 8.Lecluyse E.L., Witek R.P., Andersen M.E., Powers M J. Organotypic liver culture models: meeting current challenges in toxicity testing. Critical Reviews in Toxicology. 2012;42:501–548. doi: 10.3109/10408444.2012.682115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatia S.N., Underhill G.H., Zaret K.S., Fox I.J. Cell and tissue engineering for liver disease. Sci Transl Med. 2014;6:245sr2. doi: 10.1126/scitranslmed.3005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.March S., Ramanan V., Trehan K., Ng S., Galstian A., Gural N., Scull M.A., Shlomai A., Mota M.M., Fleming H.E., Khetani S.R., Rice C.M., Bhatia S.N. Micropatterned coculture of primary human hepatocytes and supportive cells for the study of hepatotropic pathogens. Nat Protoc. 2015;10:2027–2053. doi: 10.1038/nprot.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramanan V., Scull M.A., Sheahan T.P., Rice C.M., Bhatia S.N. New methods in tissue engineering: improved models for viral infection. Annu Rev Virol. 2014;1:475–499. doi: 10.1146/annurev-virology-031413-085437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ware B.R., Khetani S.R. Engineered liver platforms for different phases of drug development. Trends Biotechnol. 2017;35:172–183. doi: 10.1016/j.tibtech.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godoy P., Hewitt N.J., Albrecht U., Andersen M.E., Ansari N., Bhattacharya S., Bode J.G., Bolleyn J., Borner C., Böttger J., Braeuning A., Budinsky R.A., Burkhardt B., Cameron N.R., Camussi G., Cho C.-S., Choi Y.-J., Craig Rowlands J., Dahmen U., Damm G., Dirsch O., Donato M.T., Dong J., Dooley S., Drasdo D., Eakins R., Ferreira K.S., Fonsato V., Fraczek J., Gebhardt R., Gibson A., Glanemann M., Goldring C.E.P., Gómez-Lechón M.J., Groothuis G.M.M., Gustavsson L., Guyot C., Hallifax D., Hammad S., Hayward A., Häussinger D., Hellerbrand C., Hewitt P., Hoehme S., Holzhütter H.-G., Houston J.B., Hrach J., Ito K., Jaeschke H., Keitel V., Kelm J.M., Kevin Park B., Kordes C., Kullak-Ublick G.A., LeCluyse E.L., Lu P., Luebke-Wheeler J., Lutz A., Maltman D.J., Matz-Soja M., McMullen P., Merfort I., Messner S., Meyer C., Mwinyi J., Naisbitt D.J., Nussler A.K., Olinga P., Pampaloni F., Pi J., Pluta L., Przyborski S.A., Ramachandran A., Rogiers V., Rowe C., Schelcher C., Schmich K., Schwarz M., Singh B., Stelzer E.H.K., Stieger B., Stöber R., Sugiyama Y., Tetta C., Thasler W.E., Vanhaecke T., Vinken M., Weiss T.S., Widera A., Woods C.G., Xu J.J., Yarborough K.M., Hengstler J.G. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol. 2013;87:1315–1530. doi: 10.1007/s00204-013-1078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatia S.N., Balis U.J., Yarmush M.L., Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 15.Bhatia S.N., Balis U.J., Yarmush M.L., Toner M. Probing heterotypic cell interactions: hepatocyte function in microfabricated co-cultures. J Biomater Sci Polym Ed. 1998;9:1137–1160. doi: 10.1163/156856298x00695. [DOI] [PubMed] [Google Scholar]
- 16.Dunn J.C., Yarmush M.L., Koebe H.G., Tompkins R.G. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 1989;3:174–177. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- 17.Schippers I.J., Moshage H., Roelofsen H., Müller M., Heymans H.S., Ruiters M., Kuipers F. Immortalized human hepatocytes as a tool for the study of hepatocytic (de-)differentiation. Cell Biol Toxicol. 1997;13:375–386. doi: 10.1023/a:1007404028681. [DOI] [PubMed] [Google Scholar]
- 18.Tsuruga Y., Kiyono T., Matsushita M., Takahashi T., Kasai H., Matsumoto S., Todo S. Establishment of immortalized human hepatocytes by introduction of HPV16 E6/E7 and hTERT as cell sources for liver cell-based therapy. Cell Transplant. 2008;17:1083–1094. [PubMed] [Google Scholar]
- 19.Levy G., Bomze D., Heinz S., Ramachandran S.D., Noerenberg A., Cohen M., Shibolet O., Sklan E., Braspenning J., Nahmias Y. Long-term culture and expansion of primary human hepatocytes. Nat Biotechnol. 2015;33:1264–1271. doi: 10.1038/nbt.3377. [DOI] [PubMed] [Google Scholar]
- 20.Hollingdale M.R., Collins W.E., Campbell C., Schwartz A.L. In vitro culture of two populations (dividing and nondividing) of exoerythrocytic parasites of Plasmodium vivax. Am J Trop Med Hyg. 1985;34:216–222. doi: 10.4269/ajtmh.1985.34.216. [DOI] [PubMed] [Google Scholar]
- 21.Hollingdale M.R., Collins W.E., Campbell C.C. In vitro culture of exoerythrocytic parasites of the North Korean strain of Plasmodium vivax in hepatoma cells. Am J Trop Med Hyg. 1986;35:275–276. doi: 10.4269/ajtmh.1986.35.275. [DOI] [PubMed] [Google Scholar]
- 22.Ni Y., Lempp F.A., Mehrle S., Nkongolo S., Kaufman C., Fälth M., Stindt J., Königer C., Nassal M., Kubitz R., Sültmann H., Urban S. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology. 2014;146:1070–1083. doi: 10.1053/j.gastro.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 23.Yan H., Zhong G., Xu G., He W., Jing Z., Gao Z., Huang Y., Qi Y., Peng B., Wang H., Fu L., Song M., Chen P., Gao W., Ren B., Sun Y., Cai T., Feng X., Sui J., Li W. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 2012. 2012;1:1–28. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinmann E, Pietschmann T. Hepatitis C virus: from molecular virology to antiviral therapy. 2013;369:17–49.
- 25.Ladner S.K., Otto M.J., Barker C.S., Zaifert K., Wang G.H., Guo J.T., Seeger C., King R.W. Inducible expression of human hepatitis B virus (HBV) in stably transfected hepatoblastoma cells: a novel system for screening potential inhibitors of HBV replication. Antimicrob Agents Chemother. 1997;41:1715–1720. doi: 10.1128/aac.41.8.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sells M., Chen M.L., Acs G. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci U S A. 1987;84:1005–1009. doi: 10.1073/pnas.84.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holzer M., Krahling V., Amman F., Barth E., Bernhart S.H., Carmelo V.A., Collatz M., Doose G., Eggenhofer F., Ewald J., Fallmann J., Feldhahn L.M., Fricke M., Gebauer J., Gruber A.J., Hufsky F., Indrischek H., Kanton S., Linde J., Mostajo N., Ochsenreiter R., Riege K., Rivarola-Duarte L., Sahyoun A.H., Saunders S.J., Seemann S.E., Tanzer A., Vogel B., Wehner S., Wolfinger M.T., Backofen R., Gorodkin J., Grosse I., Hofacker I., Hoffmann S., Kaleta C., Stadler P.F., Becker S., Marz M. Differential transcriptional responses to Ebola and Marburg virus infection in bat and human cells. Sci Rep. 2016;6:34589. doi: 10.1038/srep34589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johansen L.M., Dewald L.E., Shoemaker C.J., Hoffstrom B.G., Lear-rooney C.M., Stossel A., Nelson E., Delos S.E., Simmons J., Grenier J.M., Pierce L.T., Pajouhesh H., Lehár J., Hensley L.E., Glass P.J., White J.M., Olinger G.G. A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity. Sci Transl Med. 2015;7:1–14. doi: 10.1126/scitranslmed.aaa5597. [DOI] [PubMed] [Google Scholar]
- 29.Asper M., Sternsdorf T., Hass M., Rhode A., Schmitz H., Drosten C., Gu S. Inhibition of different Lassa virus strains by alpha and gamma interferons and comparison with a less pathogenic arenavirus inhibition of different Lassa virus strains by alpha and gamma interferons and comparison with a less pathogenic arenavirus. J Virol. 2004;78:3162–3169. doi: 10.1128/JVI.78.6.3162-3169.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lepiller Q., Abbas W., Kumar A., Tripathy M.K., Herbein G. HCMV activates the IL-6-JAK-STAT3 axis in HepG2 cells and primary human hepatocytes. PLoS One. 2013;8:e59591. doi: 10.1371/journal.pone.0059591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilgard P. Heparan Sulfate proteoglycans initiate dengue virus infection of hepatocytes. Hepatology. 2000;32:1069–1077. doi: 10.1053/jhep.2000.18713. [DOI] [PubMed] [Google Scholar]
- 32.Gottwein J.M., Bukh J. Cutting the gordian knot-development and biological relevance of hepatitis C virus cell culture systems. Adv Virus Res. 2008;71:51–133. doi: 10.1016/S0065-3527(08)00002-X. [DOI] [PubMed] [Google Scholar]
- 33.Guguen-Guillouzo C., Clément B., Baffet G., Beaumont C., Morel-Chany E., Glaise D., Guillouzo A. Maintenance and reversibility of active albumin secretion by adult rat hepatocytes co-cultured with another liver epithelial cell type. Exp Cell Res. 1983;143:47–54. doi: 10.1016/0014-4827(83)90107-6. [DOI] [PubMed] [Google Scholar]
- 34.Bhatia S.N., Balis U.J., Yarmush M.L., Toner M. Microfabrication of hepatocyte/fibroblast co-cultures: role of homotypic cell interactions. Biotechnol Prog. 1998;14:378–387. doi: 10.1021/bp980036j. [DOI] [PubMed] [Google Scholar]
- 35.Zhou M., Zhao F., Li J., Cheng Z., Tian X., Zhi X., Huang Y., Hu K. Long-term maintenance of human fetal hepatocytes and prolonged susceptibility to HBV infection by co-culture with non-parenchymal cells. J Virol Methods. 2014;195:185–193. doi: 10.1016/j.jviromet.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Winer B.Y., Huang T.S., Pludwinski E., Heller B., Wojcik F., Lipkowitz G.E., Parekh A., Cho C., Shrirao A., Muir T.W., Novik E., Ploss A. Long-term hepatitis B infection in a scalable hepatic co-culture system. Nat Commun. 2017;8:125. doi: 10.1038/s41467-017-00200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khetani S.R., Bhatia S.N. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2008;26:120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 38.March S., Ng S., Velmurugan S., Galstian A., Shan J., Logan D.J., Carpenter A.E., Thomas D., Sim B.K.L., Mota M.M., Hoffman S.L., Bhatia S.N. A microscale human liver platform that supports the hepatic stages of Plasmodium falciparum and vivax. Cell Host Microbe. 2013;14:104–115. doi: 10.1016/j.chom.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shlomai A., Schwartz R.E., Ramanan V., Bhatta A., de Jong Y.P., Bhatia S.N., Rice C.M. Modeling host interactions with hepatitis B virus using primary and induced pluripotent stem cell-derived hepatocellular systems. Proc Natl Acad Sci. 2014;111:12193–12198. doi: 10.1073/pnas.1412631111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ploss A., Khetani S.R., Jones C.T., Syder A.J., Trehan K., Gaysinskaya V.A., Mu K., Ritola K., Rice C.M., Bhatia S.N. Persistent hepatitis C virus infection in microscale primary human hepatocyte cultures. Proc Natl Acad Sci. 2010;107:3141–3145. doi: 10.1073/pnas.0915130107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz R.E., Trehan K., Andrus L., Sheahan T.P., Ploss A., Duncan S.A., Rice C.M., Bhatia S N. Modeling hepatitis C virus infection using human induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2012;109:2544–2548. doi: 10.1073/pnas.1121400109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.March S., Ng S., Velmurugan S., Galstian A., Shan J., Logan D.J., Carpenter A.E., Thomas D., Sim B.K.L., Mota M.M., Hoffman S.L. Resource a microscale human liver platform that supports the hepatic stages of Plasmodium falciparum and vivax. Cell Host Microbe. 2013;14:104–115. doi: 10.1016/j.chom.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones C.N., Tuleuova N., Lee Y.J., Ramanculov E., Reddi A.H., Zern M.A., Revzin A. Cultivating hepatocytes on printed arrays of HGF and BMP7 to characterize protective effects of these growth factors during in vitro alcohol injury. Biomaterials. 2010;31:5936–5944. doi: 10.1016/j.biomaterials.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz R.E., Fleming H.E., Khetani S.R., Bhatia S.N. Pluripotent stem cell-derived hepatocyte-like cells. Biotechnol Adv. 2014;32:504–513. doi: 10.1016/j.biotechadv.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huch M., Gehart H., van Boxtel R., Hamer K., Blokzijl F., Verstegen M.M.A., Ellis E., van Wenum M., Fuchs S.A., de Ligt J., van de Wetering M., Sasaki N., Boers S.J., Kemperman H., de Jonge J., Ijzermans J.N.M., Nieuwenhuis E.E.S., Hoekstra R., Strom S., Vries R.R.G., van der Laan L.J.W., Cuppen E., Clevers H. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. doi: 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peterson S.E., Loring J.F. Genomic instability in pluripotent stem cells: implications for clinical applications. J Biol Chem. 2014;289:4578–4584. doi: 10.1074/jbc.R113.516419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sa-ngiamsuntorn K., Wongkajornsilp A., Phanthong P., Borwornpinyo S., Kitiyanant N., Chantratita W., Hongeng S. A robust model of natural hepatitis C infection using hepatocyte-like cells derived from human induced pluripotent stem cells as a long-term host. Virol J. 2016;13:59. doi: 10.1186/s12985-016-0519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu X., Robotham J.M., Lee E., Dalton S., Kneteman N.M., Gilbert D.M., Tang H. Productive hepatitis C virus infection of stem cell-derived hepatocytes reveals a critical transition to viral permissiveness during differentiation. PLoS Pathog. 2012;8:e1002617. doi: 10.1371/journal.ppat.1002617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshida T., Takayama K., Kondoh M., Sakurai F., Tani H., Sakamoto N., Matsuura Y., Mizuguchi H., Yagi K. Use of human hepatocyte-like cells derived from induced pluripotent stem cells as a model for hepatocytes in hepatitis C virus infection. Biochem Biophys Res Commun. 2011;416:119–124. doi: 10.1016/j.bbrc.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 50.Ng S., Schwartz R.E., March S., Galstian A., Gural N., Shan J., Prabhu M., Mota M.M., Bhatia S.N. Human iPSC-derived hepatocyte-like cells support plasmodium liver-stage infection in vitro. Stem Cell Reports. 2015;4:348–359. doi: 10.1016/j.stemcr.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aizaki H., Nagamori S., Matsuda M., Kawakami H., Hashimoto O., Ishiko H., Kawada M., Matsuura T., Hasumura S., Matsuura Y., Suzuki T., Miyamura T. Production and release of infectious hepatitis C virus from human liver cell cultures in the three-dimensional radial-flow bioreactor. Virology. 2003;314:16–25. doi: 10.1016/s0042-6822(03)00383-0. [DOI] [PubMed] [Google Scholar]
- 52.Mizumoto H., Ishihara K., Nakazawa K., Ijima H., Funatsu K., Kajiwara T. A new culture technique for hepatocyte organoid formation and long-term maintenance of liver-specific functions. Tissue Eng Part C Methods. 2008;14:167–175. doi: 10.1089/ten.tec.2007.0373. [DOI] [PubMed] [Google Scholar]
- 53.Sainz B., TenCate V., Uprichard S.L. Three-dimensional Huh7 cell culture system for the study of hepatitis C virus infection. Virol J. 2009;6:103. doi: 10.1186/1743-422X-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cho N.-J., Elazar M., Xiong A., Lee W., Chiao E., Baker J., Frank C.W., Glenn J.S. Viral infection of human progenitor and liver-derived cells encapsulated in three-dimensional PEG-based hydrogel. Biomed Mater. 2009;4:11001. doi: 10.1088/1748-6041/25/1/011001. [DOI] [PubMed] [Google Scholar]
- 55.Aly H.H., Shimotohno K., Hijikata M. 3D cultured immortalized human hepatocytes useful to develop drugs for blood-borne HCV. Biochem Biophys Res Commun. 2009;379:330–334. doi: 10.1016/j.bbrc.2008.12.054. [DOI] [PubMed] [Google Scholar]
- 56.Rajalakshmy A., Malathi J., Madhavan H., Samuel J. Mebiolgel, a thermoreversible polymer as a scaffold for three dimensional culture of Huh7 cell line with improved hepatocyte differentiation marker expression and HCV replication. Indian J Med Microbiol. 2015;33:554. doi: 10.4103/0255-0857.167330. [DOI] [PubMed] [Google Scholar]
- 57.Murakami K., Ishii K., Ishihara Y., Yoshizaki S., Tanaka K., Gotoh Y., Aizaki H., Kohara M., Yoshioka H., Mori Y., Manabe N., Shoji I., Sata T., Bartenschlager R., Matsuura Y., Miyamura T., Suzuki T. Production of infectious hepatitis C virus particles in three-dimensional cultures of the cell line carrying the genome-length dicistronic viral RNA of genotype 1b. Virology. 2006;351:381–392. doi: 10.1016/j.virol.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 58.Tran N.M., Dufresne M., Duverlie G., Castelain S., Défarge C., Paullier P., Legallais C. An appropriate selection of a 3D alginate culture model for hepatic Huh-7 cell line encapsulation intended for viral studies. Tissue Eng Part A. 2013;19:103–113. doi: 10.1089/ten.TEA.2012.0139. [DOI] [PubMed] [Google Scholar]
- 59.Ananthanarayanan A., Nugraha B., Triyatni M., Hart S., Sankuratri S., Yu H. Scalable spheroid model of human hepatocytes for hepatitis C infection and replication. Mol Pharm. 2014;11:2106–2114. doi: 10.1021/mp500063y. [DOI] [PubMed] [Google Scholar]
- 60.Molina-Jimenez F., Benedicto I., Dao Thi V.L., Gondar V., Lavillette D., Marin J.J., Briz O., Moreno-Otero R., Aldabe R., Baumert T.F., Cosset F.L., Lopez-Cabrera M., Majano P.L. Matrigel-embedded 3D culture of Huh-7 cells as a hepatocyte-like polarized system to study hepatitis C virus cycle. Virology. 2012;425:31–39. doi: 10.1016/j.virol.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 61.Liu S., Chen R., Hagedorn C.H. Direct visualization of hepatitis C virus-infected Huh7.5 cells with a high titre of infectious chimeric JFH1-EGFP reporter virus in three-dimensional Matrigel cell cultures. J Gen Virol. 2014;95:423–433. doi: 10.1099/vir.0.055772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petropolis D.B., Faust D.M., Tolle M., Rivière L., Valentin T., Neuveut C., Hernandez-Cuevas N., Dufour A., Olivo-Marin J.C., Guillen N. Human liver infection in a dish: Easy-to-build 3D liver models for studying microbial infection. PLoS One. 2016;11:1–16. doi: 10.1371/journal.pone.0148667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petropolis D.B., Faust D.M., Deep Jhingan G., Guillen N. A new human 3D-liver model unravels the role of galectins in liver infection by the parasite Entamoeba histolytica. PLoS Pathog. 2014;10:9. doi: 10.1371/journal.ppat.1004381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schepers A., Li C., Chhabra A., Seney B.T., Bhatia S. Engineering a perfusable 3D human liver platform from iPS cells. Lab Chip. 2016;16:2644–2653. doi: 10.1039/c6lc00598e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang Y.B., Duchemin N., Rawat S., Lamontagne J., Bouchard M., Noh M. Human liver on a chip: engineering liver sinusoid on a chip for hepatitis B virus replication study. Microchips. 2015;8:756–758. [Google Scholar]
- 66.Kang Y., Rawat S., Duchemin N., Bouchard M., Noh M. Human liver sinusoid on a chip for hepatitis B virus replication study. Micromachines. 2017;8:27. [Google Scholar]
- 67.Ma C., Zhao L., Zhou E.M., Xu J., Shen S., Wang J. On-chip construction of liver lobule-like microtissue and its application for adverse drug reaction assay. Anal Chem. 2016;88:1719–1727. doi: 10.1021/acs.analchem.5b03869. [DOI] [PubMed] [Google Scholar]
- 68.Baptista P.M., Siddiqui M.M., Lozier G., Rodriguez S.R., Atala A., Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53:604–617. doi: 10.1002/hep.24067. [DOI] [PubMed] [Google Scholar]
- 69.Mazza G., Rombouts K., Rennie Hall A., Urbani L., Vinh Luong T., Al-Akkad W., Longato L., Brown D., Maghsoudlou P., Dhillon A.P., Fuller B., Davidson B., Moore K., Dhar D., De Coppi P., Malago M., Pinzani M. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci Rep. 2015;5:13079. doi: 10.1038/srep13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sacci J.B., Alam U., Douglas D., Lewis J., Tyrrell D.L.J., Azad A.F., Kneteman N.M. Plasmodium falciparum infection and exoerythrocytic development in mice with chimeric human livers. Int J Parasitol. 2006;36:353–360. doi: 10.1016/j.ijpara.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 71.Morosan S., Hez-Deroubaix S., Lunel F., Renia L., Giannini C., Rooijen N.V., Battaglia S., Blanc C., Eling W., Sauerwein R., Hannoun L., Belghiti J., Brechot C., Kremsdorf D., Druilhe P. Liver-stage development of Plasmodium falciparum, in a humanized mouse model. J Infect Dis. 2006;193:996–1004. doi: 10.1086/500840. [DOI] [PubMed] [Google Scholar]
- 72.Calattini S., Fusil F., Mancip J., Dao Thi V.L., Granier C., Gadot N., Scoazec J.Y., Zeisel M.B., Baumert T.F., Lavillette D., Dreux M., Cosset F.L. Functional and biochemical characterization of hepatitis C virus (HCV) particles produced in a humanized liver mouse model. J Biol Chem. 2015;290:23173–23187. doi: 10.1074/jbc.M115.662999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kosaka K., Hiraga N., Imamura M., Yoshimi S., Murakami E., Nakahara T., Honda Y., Ono A., Kawaoka T., Tsuge M., Abe H., Hayes C.N., Miki D., Aikata H., Ochi H., Ishida Y., Tateno C., Yoshizato K., Sasaki T., Chayama K. A novel TK-NOG based humanized mouse model for the study of HBV and HCV infections. Biochem Biophys Res Commun. 2013;441:230–235. doi: 10.1016/j.bbrc.2013.10.040. [DOI] [PubMed] [Google Scholar]
- 74.Vaughan A.M., Mikolajczak S.A., Wilson E.M., Grompe M., Kaushansky A., Camargo N., Bial J., Ploss A., Kappe S.H.I. Complete Plasmodium falciparum liver-stage development in liver-chimeric mice. J Clin Invest. 2012;122:3618–3628. doi: 10.1172/JCI62684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mikolajczak S.A., Vaughan A.M., Kangwanrangsan N., Roobsoong W., Fishbaugher M., Yimamnuaychok N., Rezakhani N., Lakshmanan V., Singh N., Kaushansky A., Camargo N., Baldwin M., Lindner S.E., Adams J.H., Sattabongkot J., Kappe S.H.I. Plasmodium vivax liver stage development and hypnozoite persistence in human liver-chimeric mice. Cell Host Microbe. 2015;17:526–535. doi: 10.1016/j.chom.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soulard V., Bosson-Vanga H., Lorthiois A., Roucher C., Franetich J.-F., Zanghi G., Bordessoulles M., Tefit M., Thellier M., Morosan S., Le Naour G., Capron F., Suemizu H., Snounou G., Moreno-Sabater A., Mazier D. Plasmodium falciparum full life cycle and Plasmodium ovale liver stages in humanized mice. Nat Commun. 2015;6:7690. doi: 10.1038/ncomms8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mailly L., Xiao F., Lupberger J., Wilson G.K., Aubert P., Duong F.H.T., Calabrese D., Leboeuf C., Fofana I., Thumann C., Bandiera S., Lütgehetmann M., Volz T., Davis C., Harris H.J., Mee C.J., Girardi E., Chane-Woon-Ming B., Ericsson M., Fletcher N., Bartenschlager R., Pessaux P., Vercauteren K., Meuleman P., Villa P., Kaderali L., Pfeffer S., Heim M.H., Neunlist M., Zeisel M.B., Dandri M., McKeating J.A., Robinet E., Baumert T.F. Clearance of persistent hepatitis C virus infection in humanized mice using a claudin-1-targeting monoclonal antibody. Nat Biotechnol. 2015;33:549–554. doi: 10.1038/nbt.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Legrand N., Ploss A., Balling R., Becker P.D., Borsotti C., Brezillon N., Debarry J., de Jong Y., Deng H., Di Santo J.P., Eisenbarth S., Eynon E., Flavell R.A., Guzman C.A., Huntington N.D., Kremsdorf D., Manns M.P., Manz M.G., Mention J.-J., Ott M., Rathinam C., Rice C.M., Rongvaux A., Stevens S., Spits H., Strick-Marchand H., Takizawa H., van Lent A.U., Wang C., Weijer K., Willinger T., Ziegler P. Humanized mice for modeling human infectious disease: challenges, progress, and outlook. Cell Host Microbe. 2009;6:5–9. doi: 10.1016/j.chom.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Jong Y.P., Rice C.M., Ploss A. New horizons for studying human hepatotropic infections. J Clin Invest. 2010;120:650–653. doi: 10.1172/JCI42338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dorner M., Horwitz J.A., Robbins J.B., Barry W.T., Feng Q., Mu K., Jones C.T., Schoggins J.W., Catanese M.T., Burton D.R., Law M., Rice C.M., Ploss A. A genetically humanized mouse model for hepatitis C virus infection. Nature. 2011;474:208–211. doi: 10.1038/nature10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ng S., March S., Galstian A., Gural N., Stevens K.R., Mota M.M., Bhatia S.N. Towards a humanized mouse model of liver stage malaria using ectopic artificial livers. Sci Rep. 2017;7:45424. doi: 10.1038/srep45424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stevens K.R., Scull M.A., Ramanan V., Fortin C.L., Chaturvedi R.R., Knouse K.A., Xiao J.W., Fung C., Mirabella T., Chen A.X., McCue M.G., Yang M.T., Fleming H.E., Chung K., de Jong Y.P., Chen C.S., Rice C.M., Bhatia S.N. In situ expansion of engineered human liver tissue in a mouse model of chronic liver disease. Sci Transl Med. 2017;9:399. doi: 10.1126/scitranslmed.aah5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramanan V., Trehan K., Ong M.L., Luna J.M., Hoffmann H.H., Espiritu C., Sheahan T.P., Chandrasekar H., Schwartz R.E., Christine K.S., Rice C.M., van Oudenaarden A., Bhatia S.N. Viral genome imaging of hepatitis C virus to probe heterogeneous viral infection and responses to antiviral therapies. Virology. 2016;494:236–247. doi: 10.1016/j.virol.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moradpour D., Evans M.J., Gosert R., Yuan Z., Blum H.E., Goff S.P., Brett D., Rice C.M., Lindenbach B.D. Insertion of green fluorescent protein into nonstructural protein 5A allows direct visualization of functional hepatitis C virus replication complexes. Func J Virol. 2004;78:7400–7409. doi: 10.1128/JVI.78.14.7400-7409.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Talman A.M., Blagborough A.M., Sinden R.E. A Plasmodium falciparum strain expressing GFP throughout the parasite’s life-cycle. PLoS One. 2010;5:e9156. doi: 10.1371/journal.pone.0009156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koutsoudakis G., Kaul A., Steinmann E., Kallis S., Lohmann V., Pietschmann T., Bartenschlager R. Characterization of the early steps of hepatitis C virus infection by using luciferase reporter viruses. J Virol. 2006;80:5308–5320. doi: 10.1128/JVI.02460-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jones C.T., Catanese M.T., Law L.M.J., Khetani S.R., Syder A.J., Ploss A., Oh T.S., Schoggins J.W., MacDonald M.R., Bhatia S.N., Rice C.M. Real-time imaging of hepatitis C virus infection using a fluorescent cell-based reporter system. Nat Biotechnol. 2010;28:167–171. doi: 10.1038/nbt.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vaughan A.M., Mikolajczak S.A., Camargo N., Lakshmanan V., Kennedy M., Lindner S.E., Miller J.L., Hume J.C.C.C., Kappe S.J., II A transgenic Plasmodium falciparum NF54 strain that expresses GFP-luciferase throughout the parasite life cycle. Mol Biochem Parasitol. 2012;186:143–147. doi: 10.1016/j.molbiopara.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 89.Ruivo M.T.G., Vera I.M., Sales-Dias J., Meireles P., Gural N., Bhatia S.N., Mota M.M., Mancio-Silva L. Host AMPK is a modulator of Plasmodium liver infection. Cell Rep. 2016;16:1–7. doi: 10.1016/j.celrep.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McCall M.B.B., Wammes L.J., Langenberg M.C.C., van Gemert G.-J., Walk J., Hermsen C.C., Graumans W., Koelewijn R., Franetich J.-F., Chishimba S., Gerdsen M., Lorthiois A., van de Vegte M., Mazier D., Bijker E.M., van Hellemond J.J., van Genderen P.J.J., Sauerwein R.W. Infectivity of Plasmodium falciparum sporozoites determines emerging parasitemia in infected volunteers. Sci Transl Med. 2017;9:2490. doi: 10.1126/scitranslmed.aag2490. [DOI] [PubMed] [Google Scholar]
- 91.Wijayalath W., Majji S., Villasante E.F., Brumeanu T.D., Richie T.L., Casares S. Humanized HLA-DR4.RagKO.IL2RγcKO.NOD (DRAG) mice sustain the complex vertebrate life cycle of Plasmodium falciparum malaria. Malar J. 2014;13:386. doi: 10.1186/1475-2875-13-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Washburn M.L., Bility M.T., Zhang L., Kovalev G.I., Buntzman A., Frelinger J.A., Barry W., Ploss A., Rice C.M., Su L. A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology. 2011;140:1334–1344. doi: 10.1053/j.gastro.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nguyen T.V., Ukairo O., Khetani S.R., McVay M., Kanchagar C., Seghezzi W., Ayanoglu G., Irrechukwu O., Evers R. Establishment of a hepatocyte-Kupffer cell coculture model for assessment of proinflammatory cytokine effects on metabolizing enzymes and drug transporters. Drug Metab Dispos. 2015;43:774–785. doi: 10.1124/dmd.114.061317. [DOI] [PubMed] [Google Scholar]
- 94.Kwong G.A., von Maltzahn G., Murugappan G., Abudayyeh O., Mo S., Papayannopoulos I.A., Sverdlov D.Y., Liu S.B., Warren A.D., Popov Y., Schuppan D., Bhatia S.N. Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease. Nat Biotechnol. 2012;31:63–70. doi: 10.1038/nbt.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grimm J., Kirsch D.G., Windsor S.D., Kim C.F.B., Santiago P.M., Ntziachristos V., Jacks T., Weissleder R. Use of gene expression profiling to direct in vivo molecular imaging of lung cancer. Proc Natl Acad Sci U S A. 2005;102:14404–14409. doi: 10.1073/pnas.0503920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weissleder R., Tung C.-H., Mahmood U., Bogdanov A. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 97.Weissleder R. Molecular imaging in cancer. Science. 2006;312:1168–1171. doi: 10.1126/science.1125949. [DOI] [PubMed] [Google Scholar]
- 98.Tawk L., Lacroix C., Gueirard P., Kent R., Gorgette O., Thiberge S., Mercereau-Puijalon O., Menard R., Barale J.-C. A key role for Plasmodium subtilisin-like SUB1 protease in egress of malaria parasites from host hepatocytes. J Biol Chem. 2013;288:33336–33346. doi: 10.1074/jbc.M113.513234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cubi R., Vembar S.S., Biton A., Franetich J.-F., Bordessoulles M., Sossau D., Zanghi G., Bosson-Vanga H., Benard M., Moreno A., Dereuddre-Bosquet N., Le Grand R., Scherf A., Mazier D. Laser capture microdissection enables transcriptomic analysis of dividing and quiescent liver stages of Plasmodium relapsing species. Cell Microbiol. 2017;19:e12735. doi: 10.1111/cmi.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Macosko E.Z., Basu A., Satija R., Nemesh J., Shekhar K., Goldman M., Tirosh I., Bialas A.R., Kamitaki N., Martersteck E.M., Trombetta J.J., Weitz D.A., Sanes J.R., Shalek A.K., Regev A., McCarroll S.A. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161:1202–1214. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gierahn T.M., Wadsworth M.H., Hughes T.K., Bryson B.D., Butler A., Satija R., Fortune S., Love J.C., Shalek A.K. Seq-Well: portable, low-cost RNA sequencing of single cells at high throughput. Nat Methods. 2017;14:395–398. doi: 10.1038/nmeth.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Afdhal N., Reddy K.R., Nelson D.R., Lawitz E., Gordon S.C., Schiff E., Nahass R., Ghalib R., Gitlin N., Herring R., Lalezari J., Younes Z.H., Pockros P.J., Di Bisceglie A.M., Arora S., Subramanian G.M., Zhu Y., Dvory-Sobol H., Yang J.C., Pang P.S., Symonds W.T., McHutchison J.G., Muir A.J., Sulkowski M., Kwo P. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection protocol. N Engl J Med. 2014;370:1483–1493. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 103.Kowdley K.V., Gordon S.C., Reddy K.R., Rossaro L., Bernstein D.E., Lawitz E., Shiffman M.L., Schiff E., Ghalib R., Ryan M., Rustgi V., Chojkier M., Herring R., Di Bisceglie A.M., Pockros P.J., Subramanian G.M., An D., Svarovskaia E., Hyland R.H., Pang P.S., Symonds W.T., McHutchison J.G., Muir A.J., Pound D., Fried M.W., ION-3 Investigators Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 104.Chattopadhyay R., de la Vega P., Paik S.H., Murata Y., Ferguson E.W., Richie T.L., Ooi G.T. Early transcriptional responses of HepG2-A16 liver cells to infection by Plasmodium falciparum sporozoites. J Biol Chem. 2011;286:26396–26405. doi: 10.1074/jbc.M111.240879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hollingdale M.R., Nardin E.H., Tharavanij S., Schwartz A.L., Nussenzweig R.S. Inhibition of entry of Plasmodium falciparum and P. vivax sporozoites into cultured cells; an in vitro assay of protective antibodies. J Immunol. 1984;132:909–913. [PubMed] [Google Scholar]
- 106.Kaushansky A., Douglass A.N., Arang N., Vigdorovich V., Dambrauskas N., Kain H.S., Austin L.S., Sather D.N., Kappe S.H.I. Malaria parasites target the hepatocyte receptor EphA2 for successful host infection. Science. 2015;350:1089–1092. doi: 10.1126/science.aad3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sattabongkot J., Yimamnuaychoke N., Leelaudomlipi S., Rasameesoraj M., Jenwithisuk R., Coleman R.E., Udomsangpetch R., Cui L., Brewer T.G. Establishment of a human hepatocyte line that supports in vitro development of the exo-erythrocytic stages of the malaria parasites Plasmodium falciparum and P. vivax. Am J Trop Med Hyg. 2006;74:708–715. [PubMed] [Google Scholar]
- 108.Shih C., Li L.-S., Roychoudhury S., Hot M.H. In vitro propagation of human hepatitis B virus in a rat hepatoma cell line (Morris hepatoma/reverse transcription/Dane partides/animal model) Med Sci. 1989;86:6323–6327. doi: 10.1073/pnas.86.16.6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gripon P., Rumin S., Urban S., Le Seyec J., Glaise D., Cannie I., Guyomard C., Lucas J., Trepo C., Guguen-Guillouzo C. Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci U S A. 2002;99:15655–15660. doi: 10.1073/pnas.232137699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lin S.-J., Shu P.-Y., Chang C., Ng A.-K., Hu C.-P. IL-4 suppresses the expression and the replication of hepatitis B virus in the hepatocellular carcinoma cell line Hep3B. J Immunol. 2003;171:4708–4716. doi: 10.4049/jimmunol.171.9.4708. [DOI] [PubMed] [Google Scholar]
- 111.Huang H.-C., Chen C.C., Chang W.-C., Tao M.-H., Huang C. Entry of hepatitis B virus into immortalized human primary hepatocytes by clathrin-dependent endocytosis. J Virol. 2012;86:9443–9453. doi: 10.1128/JVI.00873-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang D., Zuo C., Wang X., Meng X., Xue B., Liu N., Yu R., Qin Y., Gao Y., Wang Q., Hu J., Wang L., Zhou Z., Liu B., Tan D., Guan Y., Zhu H. Complete replication of hepatitis B virus and hepatitis C virus in a newly developed hepatoma cell line. Proc Natl Acad Sci U S A. 2014;111:E1264–E1273. doi: 10.1073/pnas.1320071111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Blight K.J., Mckeating L.A., Rice C.M. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J Virol. 2002;76:13001–13014. doi: 10.1128/JVI.76.24.13001-13014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Murray E.M., Grobler J.A., Markel E.J., Pagnoni M.F., Paonessa G., Simon A.J., Flores O.A. Persistent replication of hepatitis C virus replicons expressing the -lactamase reporter in subpopulations of highly permissive Huh7 cells. J Virol. 2003;77:2928–2935. doi: 10.1128/JVI.77.5.2928-2935.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lindenbach B.D. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 116.Windisch M., Frese M., Kaul A., Trippler M., Lohmann V., Bartenschlager R. Dissecting the interferon-induced inhibition of hepatitis C virus replication by using a novel host cell line. J Virol. 2005;79:13778–13793. doi: 10.1128/JVI.79.21.13778-13793.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kambara H., Fukuhara T., Shiokawa M., Ono C., Ohara Y., Kamitani W., Matsuura Y. Establishment of a novel permissive cell line for the propagation of hepatitis C virus by expression of microRNA miR122. J Virol. 2012;86:1382–1393. doi: 10.1128/JVI.06242-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shiokawa M., Fukuhara T., Ono C., Yamamoto S., Okamoto T., Watanabe N., Wakita T., Matsuura Y. Novel permissive cell lines for complete propagation of hepatitis C virus. J Virol. 2014;88:5578–5594. doi: 10.1128/JVI.03839-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Date T., Kato T., Miyamoto M., Zhao Z., Yasui K., Mizokami M., Wakita T. Genotype 2a hepatitis C virus subgenomic replicon can replicate in HepG2 and IMY-N9 cells. J Biol Chem. 2004;279:22371–22376. doi: 10.1074/jbc.M311120200. [DOI] [PubMed] [Google Scholar]
- 120.Zhu H., Dong H., Eksioglu E., Hemming A., Cao M., Crawford J.M., Nelson D.R., Liu C. Hepatitis C virus triggers apoptosis of a newly developed hepatoma cell line through antiviral defense system. Gastroenterology. 2007;133:1649–1659. doi: 10.1053/j.gastro.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 121.Delpino M.V., Barrionuevo P., Scian R., Fossati C.A., Baldi P.C. Brucella-infected hepatocytes mediate potentially tissue-damaging immune responses. J Hepatol. 2010;53:145–154. doi: 10.1016/j.jhep.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 122.Dramsi S., Biswas I., Cossart P. Entry of Listeria monocytogenes into hepatocytes requires expression of InIB. a surface protein of the internalin multigene family. 1995;16:251–261. doi: 10.1111/j.1365-2958.1995.tb02297.x. [DOI] [PubMed] [Google Scholar]
- 123.Wilson-kubalek E.M., Rodney K., Vadia S., Arnett E., Seveau S. The pore-forming toxin listeriolysin O mediates a novel entry pathway of L. monocytogenes into human hepatocytes. 2011;7:11. doi: 10.1371/journal.ppat.1002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tanaka T., Takahashi M., Kusano E., Okamoto H. Development and evaluation of an efficient cell-culture system for hepatitis E virus. J Gen Virol. 2007;88:903–911. doi: 10.1099/vir.0.82535-0. [DOI] [PubMed] [Google Scholar]
- 125.Tanaka T., Takahashi M., Takahashi H., Ichiyama K., Hoshino Y., Nagashima S., Mizuo H., Okamoto H. Development and characterization of a genotype 4 hepatitis E virus cell culture system using a HE-JF5/15F strain recovered from a fulminant hepatitis patient. J Clin Microbiol. 2009;47:1906–1910. doi: 10.1128/JCM.00629-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qi Y., Zhang F., Zhang L., Harrison T.J., Huang W., Zhao C., Kong W., Jiang C., Wang Y. Hepatitis E virus produced from cell culture has a lipid envelope. PLoS One. 2015;10:1–15. doi: 10.1371/journal.pone.0132503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rogee S., Talbot N., Caperna T., Bouquet J., Barnaud E., Pavio N. New models of hepatitis E virus replication in human and porcine hepatocyte cell lines. J Gen Virol. 2013;94:549–558. doi: 10.1099/vir.0.049858-0. [DOI] [PubMed] [Google Scholar]
- 128.Basu A., Jain P., Sarkar P., Gangodkar S., Deshpande D., Ganti K., Shetty S., Ghosh K. Dengue virus infection of SK Hep1 cells: inhibition of in vitro angiogenesis and altered cytomorphology by expressed viral envelope glycoprotein. FEMS Immunol Med Microbiol. 2011;62:140–147. doi: 10.1111/j.1574-695X.2011.00794.x. [DOI] [PubMed] [Google Scholar]
- 129.Thepparit C., Phoolcharoen W., Suksanpaisan L., Smith D.R. Internalization and propagation of the dengue virus in human hepatoma (HepG2) cells. Intervirology. 2004;47:78–86. doi: 10.1159/000077830. [DOI] [PubMed] [Google Scholar]
- 130.Lefeuvre A., Contamin H., Decelle T., Fournier C., Lang J., Deubel V., Marianneau P. Host-cell interaction of attenuated and wild-type strains of yellow fever virus can be differentiated at early stages of hepatocyte infection. Microbes Infect. 2006;8:1530–1538. doi: 10.1016/j.micinf.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 131.Woodson S.E., Holbrook M.R. Infection of hepatocytes with 17-D vaccine-strain yellow fever virus induces a strong pro-inflammatory host response. J Gen Virol. 2011;92:2262–2271. doi: 10.1099/vir.0.031617-0. [DOI] [PubMed] [Google Scholar]
- 132.Silvie O., Charrin S., Billard M., Franetich J.-F., Clark K.-L., van Gemert G.-J., Sauerwein R.W., Dautry F., Boucheix C., Mazier D., Rubinstein E. Cholesterol contributes to the organization of tetraspanin-enriched microdomains and to CD81-dependent infection by malaria sporozoites. J Cell Sci. 2006;119:1992–2002. doi: 10.1242/jcs.02911. [DOI] [PubMed] [Google Scholar]
- 133.Yalaoui S., Huby T., Franetich J.-F., Gego A., Rametti A., Moreau M., Collet X., Siau A., van Gemert G.-J., Sauerwein R.W., Luty A.J.F., Vaillant J.-C., Hannoun L., Chapman J., Mazier D., Froissard P. Scavenger receptor BI boosts hepatocyte permissiveness to Plasmodium infection. Cell Host Microbe. 2008;4:283–292. doi: 10.1016/j.chom.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 134.Manzoni G., Marinach C., Topçu S., Briquet S., Grand M., Tolle M., Gransagne M., Lescar J., Andolina C., Franetich J.-F., Zeisel M.B., Huby T., Rubinstein E., Snounou G., Mazier D., Nosten F., Baumert T.F., Silvie O. Plasmodium P36 determines host cell receptor usage during sporozoite invasion. Elife. 2017:6. doi: 10.7554/eLife.25903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Galle P.R., Hagelstein J., Kommerell B., Volkmann M., Schranz P., Zentgraf H. In vitro experimental infection of primary human hepatocytes with hepatitis B virus. Gastroenterology. 1994;106:664–673. doi: 10.1016/0016-5085(94)90700-5. [DOI] [PubMed] [Google Scholar]
- 136.Zhou M., Huang Y., Cheng Z., Zhao F., Li J., Zhi X., Tian X., Sun W., Hu K. Revival, characterization, and hepatitis B virus infection of cryopreserved human fetal hepatocytes. J Virol Methods. 2014;207:29–37. doi: 10.1016/j.jviromet.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 137.Sai L.T., Yao Y.-Y., Guan Y.-Y., Shao L.-H., Ma R.-P., Ma L.-X. Hepatitis B virus infection and replication in a new cell culture system established by fusing HepG2 cells with primary human hepatocytes. J Microbiol Immunol Infect. 2016;49:471–476. doi: 10.1016/j.jmii.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 138.Fournier C., Sureau C., Coste J., Ducos J., Pageaux G., Larrey D., Domergue J., Maurel P. In vitro infection of adult normal human hepatocytes in primary culture by hepatitis C virus. J Gen Virol. 1998;79:2367–2374. doi: 10.1099/0022-1317-79-10-2367. [DOI] [PubMed] [Google Scholar]
- 139.Kaneko S., Kakinuma S., Asahina Y., Kamiya A., Miyoshi M., Tsunoda T., Nitta S., Asano Y., Nagata H., Otani S., Kawai-Kitahata F., Murakawa M., Itsui Y., Nakagawa M., Azuma S., Nakauchi H., Nishitsuji H., Ujino S., Shimotohno K., Iwamoto M., Watashi K., Wakita T., Watanabe M. Human induced pluripotent stem cell-derived hepatic cell lines as a new model for host interaction with hepatitis B virus. Sci Rep. 2016;6:29358. doi: 10.1038/srep29358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Berto A., Van der Poel W.H.M., Hakze-van der Honing R., Martelli F., La Ragione R.M., Inglese N., Collins J., Grierson S., Johne R., Reetz J., Dastjerdi A., Banks M. Replication of hepatitis E virus in three-dimensional cell culture. J Virol Methods. 2013;187:327–332. doi: 10.1016/j.jviromet.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 141.Ohashi K., Marion P.L., Nakai H., Meuse L., Cullen J.M., Bordier B.B., Schwall R., Greenberg H.B., Glenn J.S., Kay M. Sustained survival of human hepatocytes in mice: a model for in vivo infection with human hepatitis B and hepatitis delta viruses. Nat Med. 2000;6:327–331. doi: 10.1038/73187. [DOI] [PubMed] [Google Scholar]
- 142.Ilan E., Burakova T., Dagan S., Nussaum O., Lubin I., Eren R., Ben-Moshe O., Arazi J., Berr S., Neville L., Yuen L., Mansour T.S., Gillard J., Eid A., Jurim O., Shouval D., Reisner Y., Galun E. The hepatitis B virus-trimera mouse: a model for human HBV infection and evaluation of anti-HBV therapeutic agents. Hepatology. 1999;29:553–562. doi: 10.1002/hep.510290228. [DOI] [PubMed] [Google Scholar]
- 143.Dandri M. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology. 2001;33:981–988. doi: 10.1053/jhep.2001.23314. [DOI] [PubMed] [Google Scholar]
- 144.Mercer D.F., Schiller D.E., Elliott J.F., Douglas D.N., Hao C., Rinfret A., Addison W.R., Fischer K.P., Churchill T., Lakey J.R., Tyrrell D.L., Kneteman N.M. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927–933. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- 145.Bility M.T., Zhang L., Washburn M.L., Curtis T.A., Kovalev G.I., Su L. Generation of a humanized mouse model with both human immune system and liver cells to model hepatitis C virus infection and liver immunopathogenesis. Nat Protoc. 2012;7:1608–1617. doi: 10.1038/nprot.2012.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Crawford L.B., Tempel R., Streblow D.N., Kreklywich C., Smith P., Picker L.J., Nelson J.A., Caposio P. Human cytomegalovirus induces cellular and humoral virus-specific immune responses in humanized BLT mice. Sci Rep. 2017;7:937. doi: 10.1038/s41598-017-01051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kawahara T., Lisboa L.F., Cader S., Douglas D.N., Nourbakhsh M., Pu C.H., Lewis J.T., Churchill T.A., Humar A., Kneteman N.M. Human cytomegalovirus infection in humanized liver chimeric mice. Hepatol Res. 2013;43:679–684. doi: 10.1111/j.1872-034X.2012.01116.x. [DOI] [PubMed] [Google Scholar]
- 148.Silvie O., Rubinstein E., Franetich J.-F., Prenant M., Belnoue E., Rénia L., Hannoun L., Eling W., Levy S., Boucheix C., Mazier D. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat Med. 2002;9:93–96. doi: 10.1038/nm808. [DOI] [PubMed] [Google Scholar]