Graphical abstract
Abbreviations: G, glyphosate; GBH, glyphosate-based herbicide; R, Roundup; POEA, polyoxyethylenamines (polyethoxylated tallowamine); QAC, quaternary ammonium compounds
Keywords: Pesticide, Herbicide, Glyphosate, Roundup, POEA, Formulant, Arsenic, Heavy metals
Highlights
-
•
The comparative effects of glyphosate alone and 14 of its formulations were studied.
-
•
Glyphosate was not the major toxic compound in the herbicide formulations.
-
•
Petroleum-based compounds in herbicides were highly more toxic than glyphosate.
-
•
We identified arsenic, chromium, cobalt, lead and nickel in pesticide formulations.
Abstract
The major pesticides of the world are glyphosate-based herbicides (GBH), and their toxicity is highly debated. To understand their mode of action, the comparative herbicidal and toxicological effects of glyphosate (G) alone and 14 of its formulations were studied in this work, as a model for pesticides. GBH are mixtures of water, with commonly 36–48% G claimed as the active principle. As with other pesticides, 10–20% of GBH consist of chemical formulants. We previously identified these by mass spectrometry and found them to be mainly families of petroleum-based oxidized molecules, such as POEA, and other contaminants. We exposed plants and human cells to the components of formulations, both mixed and separately, and measured toxicity and human cellular endocrine disruption below the direct toxicity experimentally measured threshold. G was only slightly toxic on plants at the recommended dilutions in agriculture, in contrast with the general belief. In the short term, the strong herbicidal and toxic properties of its formulations were exerted by the POEA formulant family alone. The toxic effects and endocrine disrupting properties of the formulations were mostly due to the formulants and not to G. In this work, we also identified by mass spectrometry the heavy metals arsenic, chromium, cobalt, lead and nickel, which are known to be toxic and endocrine disruptors, as contaminants in 22 pesticides, including 11 G-based ones. This could also explain some of the adverse effects of the pesticides. In in vivo chronic regulatory experiments that are used to establish the acceptable daily intakes of pesticides, G or other declared active ingredients in pesticides are assessed alone, without the formulants. Considering these new data, this assessment method appears insufficient to ensure safety. These results, taken together, shed a new light on the toxicity of these major herbicides and of pesticides in general.
1. Introduction
Numerous debates have taken place in scientific and regulatory arenas on the toxicity thresholds of pesticides and the levels of these substances permitted by regulators [1]. Among them, the most used around the world are glyphosate (G)-based, and they are also the most spread on edible genetically modified plants rendered tolerant to G [2]. G has recently been the subject of a controversy between agencies such as the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO) and the European Food Safety Agency (EFSA) [3]. The IARC classified G as a probable carcinogen [4], while EFSA did not [5]. According to their detailed reports, this was probably because of the different toxicity profiles of the full formulations and G alone. EFSA is in charge of the assessment of the declared active ingredients of pesticides alone, such as G, which is mainly but not only based on the regulatory studies from the manufacturers. IARC, on the other hand, bases its decisions on epidemiological studies performed after use of the full formulations, among others, as well as animal feeding studies on the formulations and G alone, with the stipulation that all studies considered in the evaluation must be fully available in the public domain.
In order to better understand the mechanisms of action of the pesticide formulations, we tested in this work, as a model, complete GBH formulations on one hand, and their components separately on the other hand, i.e. G and formulants, which are often oxidized petroleum distillates such as families of polyoxyethylenamines (POEA). G is declared to be an active herbicide on plants. In order to calculate the acceptable daily intake (ADI) for regulatory purposes, G alone is tested for toxicity in long-term tests in mammals in vivo. Thus we first tested G alone in plants and human cells at recommended agricultural dilutions. No observable adverse effect was measured. Then we tested the full formulations and some formulants alone in similar dilutions. Both exhibited full herbicidal and cytotoxic activities, without any G.
As endocrine disruption in mammals was proposed for G, a semi-irreversible inhibitor of aromatase [[6], [7]], we also compared the effect on aromatase inhibition below direct toxic levels G, its formulations and formulants. Again, the formulants and formulations demonstrated more endocrine disruptive effects than G.
Finally, we tested if other elements that could participate in toxic or endocrine effects were present in the formulations. Unexpectedly, arsenic (As), cobalt (Co), chromium (Cr), nickel (Ni) and lead (Pb) were present in numerous pesticide formulations, at levels well above admissible ones in water. We discuss why it appears erroneous to calculate the ADIs based on only one chemical of the formulations used in agriculture or gardens.
2. Materials and methods
2.1. Chemicals
GBH formulations and others studied in this work (Table 1) were on the market (approval numbers in parentheses) in France unless otherwise indicated: Bayer GC (Bayer Garden Cambridge UK, 05873567), Clinic EV (Nufarm, 9900039), Glyfos (Cheminova, 9100154), Glyphogan (Adama, 9100537), Kapazin (Arysta, 02.5/12062-2/2010, Hungary), Medallon Premium (Syngenta, 02.5/10506-2/2010, Hungary), Pavaprop-G (Bayer, 9500572), Radical Tech+ (BHS, 2090044), Roundup Bioforce (Monsanto, 9900451), Roundup Classic (Monsanto, 02.5/915/2/2010, Hungary), Roundup Express (Monsanto, 201321), Roundup Grands Travaux plus (GT+, Monsanto, 2020448), Roundup WeatherMax (Monsanto, 27487, Canada) and Total (Sinon Corporation, 02.5/12059-2/2010, Hungary).
Table 1.
Glyphosate-based herbicide (GBH) formulations and others studied in this work.
| Name | Class | dAP | g/L | Formulant | Recommended use | dilution |
|---|---|---|---|---|---|---|
| Bayer GC | G-based herbicides | G (IPA salt) or indicated | 96 | 1–5% POEA | 5.5 L/ha | 4.50% |
| Clinic EV | 360 | 11% POEA | 3 L/ha | 3% | ||
| Glyfos | 360 | 9% POEA | 12 L/ha | 6% | ||
| Glyphogan | 360 | 15.5% POEA | 3 L/ha | 3% | ||
| Kapazin | 360 | C8-10 ethoxylated alcohol (<2 g/L). Triethylene glycol monobutyl ether (<2 g/L) | 5 L/ha | 3% | ||
| Medallon | 360 | 10–20% APG (150 g/L) | 5 L/ha | 3.5% | ||
| Pavaprop-G | 72 | nk | 15 L/ha | 15% | ||
| Radical Tech+ | 151.4 | nk | 1.5 L/ha | 1.50% | ||
| R 3+ | 170 | nk | 14.8 L/ha | 14.80% | ||
| R Bioforce | 360 | nk | 6 L/ha | 6% | ||
| R Classic | 360 | 15.5% POEA | 7 L/ha | 2% | ||
| R Express | 7.2 | nk | 250 L/ha | 100% | ||
| R Ultra | 360 | 16% nk | 3.3 L/ha | 2 % | ||
| R WeatherMax | 540 | Petroleum distillate/Transorb2 | 1.67 L/ha | 1.67% | ||
| R GT+ | 450 | 7.5% ethoxylated etheralkylamine | 5.6 L/ha | 5.60% | ||
| Total | 360 | 58.5% nk | 6 L/ha | 3.5% | ||
| APG | GBH adjuvants | nr | 31% | Alkyl polyglucosides | nr | nr |
| Genamin | nr | 70% | POEA | 5 L/ha | 5% | |
| POEA | nr | >95% | Polyoxyethylenamines | nr | nr | |
| POE-APE | nr | 70% | Polyoxyethylene alkyl ether phosphates | nr | nr | |
| QAC | nr | 30% | Quaternary ammonium compounds | nr | nr | |
| Lonpar | Other herbicides | 2,4-D | 150 | nk | 3 L/ha | 2% |
| Matin | Isoproturon | 500 | nk | 2.4 L/ha | 2.40% | |
| Starane | Fluoroxypyr | 200 | Solvant naphta, alkyl-aryl sulfonates | 1.5 L/ha | 1.50% | |
| Eyetak | Fongicides | Prochloraze | 450 | Solvant naphta, xylene, isobutanol | 1.33 L/ha | 1.33% |
| Folpan | Folpet | 80% | nk | 2 kg/ha | 1.9% | |
| Maronee | Tebuconazole | 250 | N,N-dimethyldecanamide | 1 L/ha | 1% | |
| Opus | Epoxiconazole | 125 | Solvant naphta, ethoxylated fatty alcohol | 1 L/ha | 1% | |
| Pictor | Boscalid | 500 | nk | 0.5/1 kg/ha | 0.5% | |
| Teldor | Fenhexamid | 50% | nk | 1.5 kg/ha | 2% | |
| Polysect | Insecticides | Acetamipride | 5 | 1,2-benzisothiazoline-3-one, ethanol | 10 mL/L | 1% |
| Pyrinex | Chlorpyriphos | 250 | nk | 2 L/ha | 2% |
In GBH, G is present as a salt of isopropyl ammonium (IPA), except in Medallon (di-ammonium salt) and Roundup WeatherMax (potassium salt). dAP declared active principle, G glyphosate, nk not known because undeclared, nr not relevant. The recommended uses and pesticide dilutions according to the manufacturer instructions are indicated.
G (N-phosphonomethyl glycine, G, CAS 1071-83-6) was tested in two forms: G alone (Sigma–Aldrich, Saint Quentin Fallavier, France) or its isopropyl ammonium salt (G-IPA, 386411-94-0, Lamberti, Abizzate, Italy).
Common GBH formulants were: polyoxyethylenamines (POEA) with an average ethoxylation of 15 carbons (POE-15, CAS 61791-26-2, Emulson AG GPE 3SS, Lamberti), and formulated POEA (Genamin T200, Monsanto, 8500170, containing 70% of POE-15); POE-APE, a mixture of alkyl(C8-10) polyoxyethylene ether phosphates (68130-47-2) and polyoxyethylene alkyl ether phosphate (50769-39-6), known as Rolfen Bio (from Lamberti) alkyl polyglucoside (APG, 383178-66-3/110615-47-9, Plantapon LGC, The Soap Kitchen, Torrington, UK); and quaternary ammonium compounds (QAC, 66455-29-6, Emulson AG CB 30, Lamberti).
Three non-GBH (Table 1) were also analyzed: Lonpar (Dow Agrosciences, 8200538), Matin (Tradi Agri, 2020328) and Starane (Dow Agrosciences, 8400600), as well as 6 fungicides: Eyetak (Barclay Chemical, 9400555), Folpan (Makheshiam Agan, 9300143); Maronee (Bayer, 2000420), Opus (BASF, 9200018), Pictor (BASF, 2050075), Teldor (Bayer, 9800244), and 2 insecticides: Polysect ultra SL (Scotts, 2080018) and Pyrinex (Adama, 9900104).
MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) was obtained from Sigma–Aldrich. It was prepared as a 5 mg/mL stock solution in phosphate- buffered saline, filtered through a 0.22 mm filter before use, and diluted to 1 mg/mL in a serum-free medium. 4-androstene-3,17-dione and formestane (4-hydroxyandrost-4-ene-3,17-dione, CGP-32349) were also obtained from DM Labo (Caen, France). [1β-3H] androstenedione (specific activity, 25.3 Ci/mmol; 958.3 GBq/mmol) was purchased from DuPont-New England Nuclear (Les Ulis, France). Ultima-Gold LLT was from Perkin-Elmer.
2.2. Plant treatments and herbicidal observations
Tomato plants (Solanum lycopersicum var. esculentum, 15 cm high on average, 6 leaves) were purchased at Jardiland (Epron, France). They were grown during 3 weeks in 8 × 8 × 8 cm open pots with a 12 h light-dark cycle at room temperature of 18 °C. The soil remained wet, without excessive water. The dilutions of the formulated herbicide (Glyphogan), the formulant (Genamin, 70% POE-15) and G alone were dissolved in plain water. The recommended level of dilution for Glyphogan was 11.25 mL/L i.e. 1.125%). Genamin and G were in the concentration present in the diluted formulation of Glyphogan: 2.5 mL/L and 4.05 g/L respectively. At the beginning of the 4th week, plants were watered and sprayed for 7 days with 80 mL/day of water (control C), G, the formulant Genamin alone, Glyphogan, Roundup GT+ or Roundup WeatherMax diluted similarly. This was reproduced 3 times and outdoors, and in field also on a square meter of grass.
2.3. Human cells and treatments
The human embryonic kidney 293 cell line (HEK 293, ECACC 85120602) was provided by Sigma-Aldrich (Saint-Quentin Fallavier, France). JEG3 cell line (ECACC 92120308) was provided by CERDIC (Sophia-Antipolis, France). Both were validated for toxicity studies of pesticides [8], corresponding to what is observed for fresh tissue or primary cells [7]. These cell lines are even in some instances less sensitive than primary cells [9], and therefore do not overestimate cellular toxicity. Cells were grown with log-tested methods in phenol red-free EMEM (Abcys, Paris, France) containing 2 mM glutamine, 1% non-essential amino acid, 100 U/mL of antibiotics (a mixture of penicillin, streptomycin and fungizone) (Lonza, Saint Beauzire, France), 10 mg/mL of liquid kanamycin (Dominique Dutscher, Brumath, France) and 10% Fetal Bovine Serum (PAA, les Mureaux, France). JEG3 cells were supplemented with 1 mM sodium pyruvate. Cells were grown in this medium at 37 °C (5% CO2, 95% air) during 48 h to 80% confluence, then washed and exposed for 24 h with serum-free EMEM to the formulations of GBH, formulants and G or its salt, diluted in serum-free medium and adjusted to a similar pH. This model has been carefully validated [10] since cytotoxic effects were similar in the presence of serum but delayed by 48 h, and some compounds of the serum may interfere with the test.
2.4. Cytotoxicity measurement
After treatments, succinate dehydrogenase (SD) activity assay (MTT) [11] was applied to HEK293 cells as described previously [12]. The integrity of mitochondrial dehydrogenase enzymes indirectly reflects the cellular mitochondrial respiration. The optical density was measured at 570 nm using a Mithras LB 940 luminometer (Berthold, Thoiry, France).
2.5. Endocrine disruption measurement
Aromatase activity was evaluated in JEG3 according to the tritiated water release assay [13], with a slight modification as previously described [14]. This method, validated for the assessment of endocrine disruption [15] and based on the stereo-specific release of 1b-hydrogen from the androstenedione substrate, was performed as described previously [16]. Formestane, a well-known aromatase inhibitor, was used as a positive control.
2.6. Heavy metals measurements
Analytical measurements were performed in an accredited laboratory following the regulatory standards (reference texts NFEN ISO 17294-1 and 17294-2). Heavy metal concentrations were determined by inductively coupled plasma mass spectrometry (ICP-MS Varian 820-MS from Varian, Australia). For each of the pesticide formulations, the test sample size was 0.5 g out of a 50 mL start sample. Measurements were given by internal and external calibration after correction of the interferences. Limits of quantification were 5 ppb (mg/kg) for Cd 114, Cr 52, Co 59 and total Hg, 8 ppb for the sum of Pb 206, 207 and 208, 10 ppb for Ni 62 and 25 ppb for As 75.
2.7. Statistical analysis
The experiments were repeated at least in triplicate in different weeks in three independent cultures. All data were presented as the means ± standard error of the mean (SEM). In MTT assays, LC50 values were the best-fitted value of a non-linear regression using asymmetric (5-parameters) equation with GraphPad Prism 5 (GraphPad software, La Jolla, USA). In aromatase activity measurements, statistical differences were determined by a non-parametric Wilcoxon (Mann-Whitney) rank-sum test, using GraphPad Prism 5 (GraphPad software, La Jolla, USA).
3. Results
3.1. Glyphosate and formulations on plants
Three main GBH (R1, R2, R3) were tested at recommended agricultural dilutions of 11.25 mL/L (1.1%) for their herbicidal properties over 7 days. Simultaneously and in similar conditions for the first time, plants were also submitted to G and to the main family of formulants POEA alone (F), solubilized in water at levels present in the diluted formulation R1 (Fig. 1).
Fig. 1.
Glyphosate (G) alone does not demonstrate herbicidal activity. Tomato plants were watered and sprayed during 120 h with 80 mL/day of water only (control C), or G alone at the same concentration as in the G-based herbicide R1 (Glyphogan) used at the recommended agricultural dilution of 11.25 mL/L (1.1%), like R2 (Roundup GT+) and R3 (Roundup WeatherMax). The main family of formulants (F, Genamin, 70% POEA) of R1 alone exerted the herbicidal action at the concentration present in R1. This was reproduced 3 times and outdoors, and in field also on a square meter of grass. After one week, the results were similar than after 120 h.
G alone did not show any herbicidal activity over 5 days, in contrast with the 3 compared formulations and the formulant family alone. Among the formulations, R1, containing POEA, appeared to be the family of compounds that was most toxic, in that it most quickly desiccated all leaves, despite its lower level in G (360 g/L), in comparison to R2 (450 g/L) and R3 (540 g/L). The formulant family F (POEA) was highly herbicidal and thus acted as the real toxic ingredient of the herbicide within 3 days under our conditions (72 h, Fig. 1). R1 and R2 formulations, which do not contain POEA but comparable petroleum distillates or derivatives, also fully killed the plants in 3–5 days. After 7 days, G alone began to desiccate and to whiten a few upper leaves, only starting to demonstrate minor herbicidal effects, while plants had already been killed by all the other treatments except water (control C). Hence G did not appear to be the main active substance of the herbicide, but rather the formulants. After one week, the results were similar than after 120 h. We confirmed that our observation was independent of the soil and of the hygrometry since it was observable in a garden and in a field of the University of Caen. The markers of plant health were the number of dead leaves, and can be at a molecular level in a future work. G alone has no visible action after a few days, like water; the formulants without G and Roundup had similar herbicidal actions. This must be further studied in the future; this experiment is very preliminary. It does not allow a general conclusion on the general herbicidal properties of glyphosate alone, it questions them.
3.2. Toxicity of glyphosate and formulations on human cells
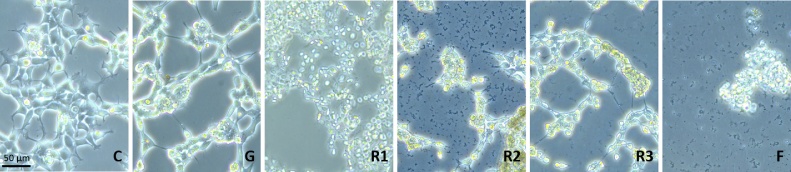
Human embryonic kidney cells (HEK 293) were also killed by the different GBH formulations within 90 min (Fig. 2), while G alone in these conditions did not demonstrate any cytotoxicity at similar levels as in R1 like in plants. The formulant family F had the same toxicity as formulations containing G. R1 appeared the most toxic over the short term, provoking cell death with shrinkage of cells, which became non-adhesive; before this step a lot of debris was visible for R2 and F, and to a lesser extent with R3. Dead cells no longer stuck to flasks, but floated in lumps. This was particularly visible for F (POEA) treatment, at a dose present in R1.
Fig. 2.
Human embryonic cells are killed by glyphosate-based formulations (GBH) and the formulant family, but not by G. The treatments for HEK293 cells over 90 min are like those of Fig. 1; chemicals are diluted in cell medium EMEM in quantities equivalent to the recommended agricultural use of 1.1% for R1.
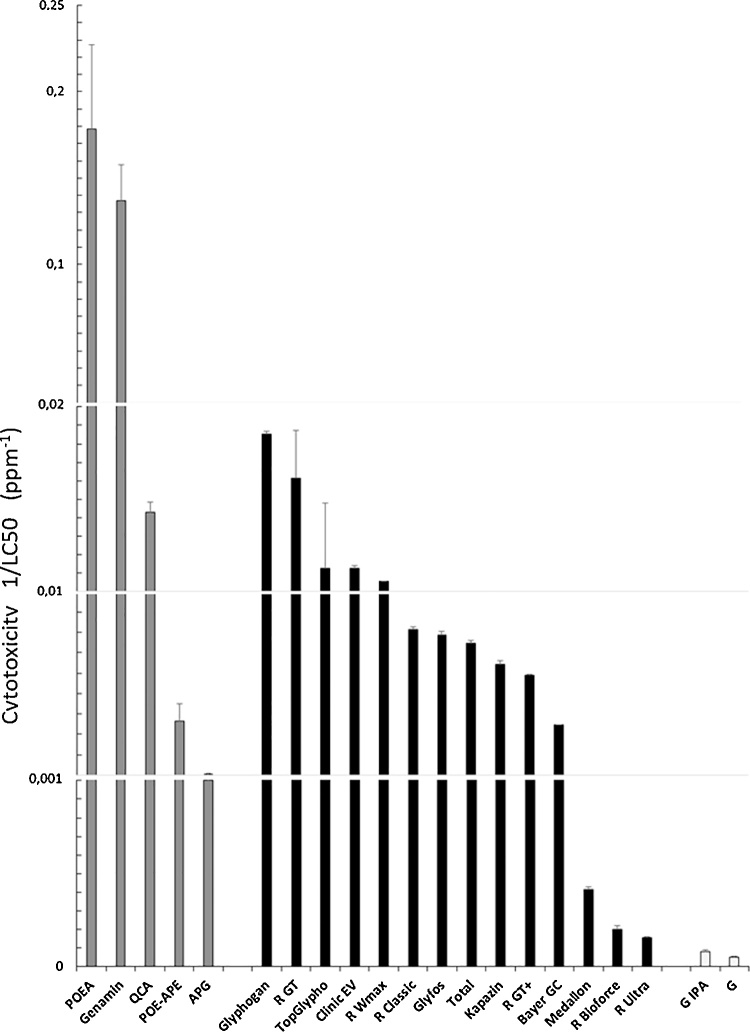
We performed a second experiment in order to understand in a more detailed way if the different formulations and formulants (Table 1) and the declared active principle of toxicity G acted in human cells correspondingly to what was observed in plants. We exposed human cells to 14 formulations at variable levels, 4 formulants and to G or its isopropylamine salt alone (Fig. 3) to quantify cytotoxicities by calculations of 1/LC50, with the highest number corresponding to the most toxic product.
Fig. 3.
Comparative cytotoxicities of 14 glyphosate-based herbicide (GBH) formulations, 5 formulants and G alone or its salt. Effects on mitochondrial succinate dehydrogenase (SD) activity, reflecting cell respiration inhibition, were measured in HEK293 human cells after 24 h exposure and expressed in ppm1 (1/LC50). LC50 values were calculated by a nonlinear regression using sigmoid (5-parameters) equation, SEM are indicated. Control is at 0. Formulants are in grey, formulations in black and isopropylamine salt of G (G IPA) and G alone in white. Genamin consists of 70% POEA.
Most formulations had toxicities in the same orders of magnitude as the formulants at comparable levels, and for all of them the toxicity did not depend on G or its salt, which appeared almost inert in comparison, in these conditions and at environmentally relevant levels. Formulations were 3–358 times more toxic than G, and 67–358 times more toxic if we exclude the three least toxic formulations (Fig. 3). The formulant family POEA alone was the most toxic, 3450 times more so than G, but was comparable to Glyphogan. It appears to be the real principle of toxicity, like in plants.
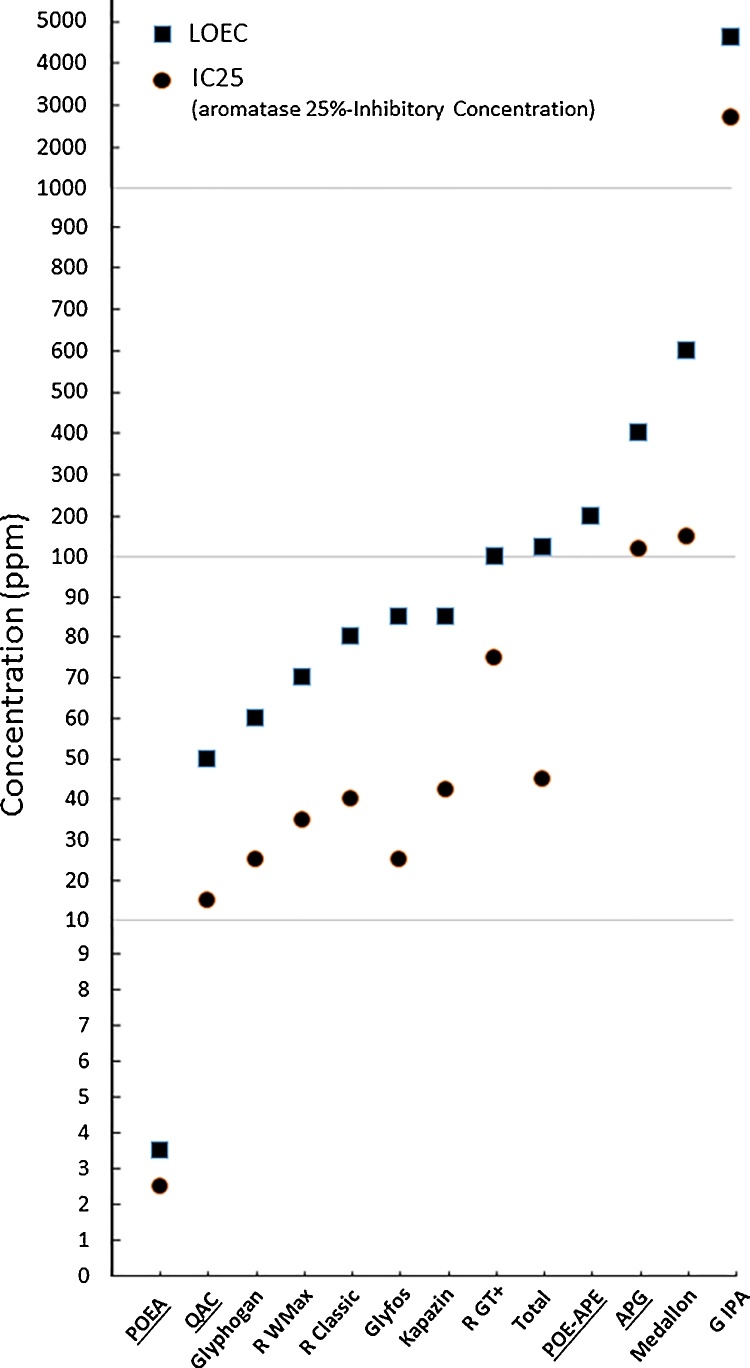
3.3. Formulations are endocrine disruptors below toxic levels
Around 10–100% below the LC50 of the pesticides, we assayed the lowest observed effect concentration (LOEC) and compared the latest to a threshold of around 25% (IC25) of aromatase inhibition (Fig. 4). This demonstrated that all pesticides in their formulations and their formulants, and even G, acted as cellular endocrine disruptors at lower levels than the levels that were cytotoxic. We made all these comparisons in this paper for the first time. The lowest LOEC corresponded to the maximal toxicity, and the lowest concentrations of aromatase inhibition in this experiment corresponded to the most cellular endocrine-disrupting products. Again, the strongest inhibitors were the 2 main formulants families, POEA and QAC, then the studied formulations, and the weakest disruptor was the G salt. The compounds of the formulants and not G appeared as the determinants for cellular endocrine disruption, as well as for toxicity to human cells and plants.
Fig. 4.
Endocrine disruptions occur below observable toxicity. Six representative formulations and 4 formulants (underlined in x axis), and G salt, were studied comparatively for their aromatase inhibition with the first threshold of around 25% (circles) and their lowest observable toxicity (squares), expressed in ppm of each product.
3.4. Heavy metals in formulations
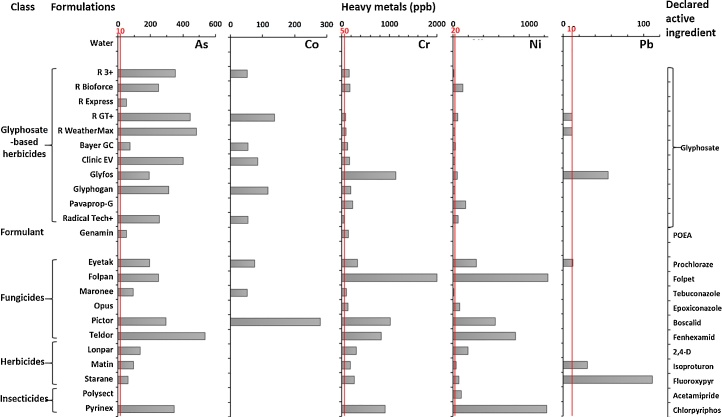
To determine whether the oxidized petroleum distillates forming most of the formulants were the only compounds responsible for toxicity and endocrine disruption, we measured in the formulations other contaminants present in petroleum, such as, among others, the heavy metals arsenic (As), cobalt (Co), chromium (Cr), nickel (Ni) and lead (Pb) (Fig. 5), which are known to be toxic and endocrine disruptors. Eleven GBH formulations were assessed, as well as 11 other pesticide formulations as comparators. Cadmium, mercury and aluminium were below detectable levels. Formulations from both groups were comparably and heavily contaminated (Fig. 5) with the heavy metal As, present in almost all samples, Cr to a lesser extent, and in a more sporadic manner Co and Pb; Ni levels were higher in non GBH herbicides.
Fig. 5.
Heavy metals in formulations of pesticides in comparison to admissible levels in water in the EU [17] (red lines) corresponding to the WHO guideline values [18] (As 10 ppb; Cr 50 ppb; Ni; Pb 10 ppb) except of Ni (20 ppb vs 70 ppb respectively); no existing value for Co (48).
In total, all except 3 formulations had 5–53 times the permitted level of As in water in European Union or USA; all except 1 had Cr above (up to 40 times) the permitted level; all except 1 contained Ni, with 19 samples being above the permitted level (up to 62 times); 6 contained up to 11 times the permitted level of Pb. Genamin (composed of 70% POEA) was contaminated with moderate levels of As and Cr only.
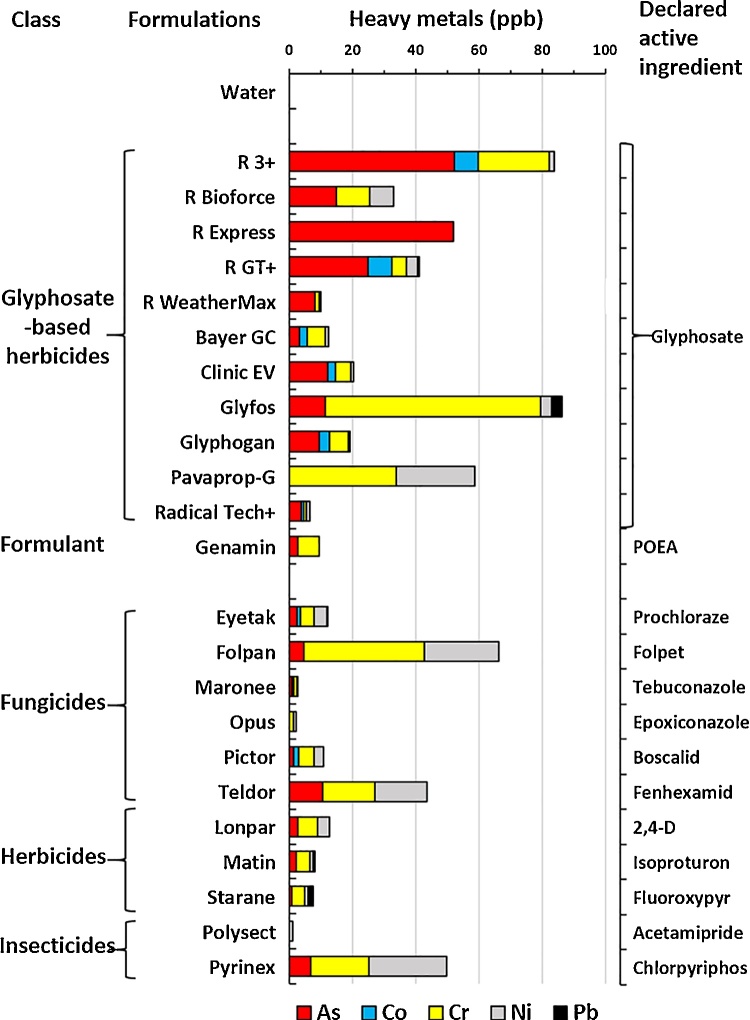
In Fig. 6, we observe that the sum of heavy metals in formulations after their different recommended dilutions can reach up to 80 ppb. It becomes obvious that the diluted GBH formulations are the most contaminated in general and pose a higher risk of contamination of soils and edible plants, especially in the case of As. Of 11 GBH, 6 exceeded the permitted levels in water even after recommended dilutions (1.5–15%) for agricultural or garden uses. Among the fungicides tested, Folpan was by far the most contaminated.
Fig. 6.
Heavy metals in formulations of pesticides at their recommended dilutions.
4. Discussion
G is known as a specific inhibitor of the shikimate pathway in plants, especially in vitro [19]. This is declared by the manufacturers to explain its widely claimed herbicidal activity. However, G is never used alone in agriculture but only mixed with formulants, which are mainly composed of various oxidized petroleum distillates or derivatives [16], [20],21]. These are supposed to be surfactants, diluents or adjuvants stabilizing G and allowing its penetration in plants [22]. However, the fact that their composition is considered confidential business information does not allow scientists to describe their mechanism of action either on non-target organisms or even on plants. They are declared as inert by manufacturers, because they are not considered to be directly responsible for the herbicidal activity. In this work, the main family of formulants was demonstrated to be herbicides when applied alone at agricultural dilutions, but G was not as we already suggested [23]. G alone may not penetrate or concentrate enough in plants, in contrast with the formulants, which act quickly and hence are not inert. We do not demonstrate in this work that G cannot inhibit the plant specific enzyme enolpyruvylshikimate phosphate synthase in vitro or in vivo, however at recommended 1% dilution of GBH formulants are more toxic on plants than G in a short term.
This is even more obvious in human cells, as we previously demonstrated in different models [7], [10], [12],16,21]. This work presents the most comprehensive comparison of the cytotoxicity of GBH formulations, formulants, and G and one of its salts. There is no instance in which G reaches the toxicity of any formulant, either in the formulations or alone. The mechanisms of actions of formulants have been described as membrane disruption [16], [21], [24],25], apoptosis [26], mitochondrial respiration inhibition [16,21,27], and DNA damaging [28,29]. G alone appears to enter the cells [30], but its toxic action is not well documented at this level and appears very secondary in time and effects in contrast with that of the formulants. The formulants also penetrate and bioaccumulate from the studies described above because of their chemical amphiphilic nature, as, for instance, with petroleum oxidative derivatives.
Evidence for penetration is the cellular endocrine disruption observed below toxic levels in this work. We demonstrate that non-target effects below toxicity thresholds are not due to the declared active ingredient G but undoubtedly to the formulants alone and in formulations, as previously documented for some instances [16]. Their endocrine-disrupting action can be explained by the membrane disruptions, where the steroidogenic enzyme aromatase is located in the endoplasmic reticulum, but may also be due to possible direct enzymatic interactions that could be synergistic with heavy metals (see below). The endocrine disruption by GBH has been observed in vivo by our group in several instances, on the androgen/estrogen balance that aromatase controls, after short-term Roundup treatment [31,32] and after long-term exposure [33].
The present results and others reviewed [1] show that the difference between “active ingredient” and “inert compound” is a regulatory assertion with no demonstrated toxicological basis. Indeed, the toxicity of formulants in pesticides has been well documented for years [[34], [35], [36], [37]]. They have been detected in large quantities in the environment [[38], [39], [40]] and food [[41], [42], [43]]. All the honey, pollen and wax samples monitored in a recent study were contaminated with high levels (up to 10 ppm) of nonylphenol polyethoxylates (NPEOs), a major family of formulants in pesticides [44]. Their absorption by organisms [45] and placental transfer into serum and brain have been demonstrated [46]. The assessment of a formulation should consider the toxicity of formulants over the long term, yet only their acute ocular and dermal properties are investigated at present in regulation for a few weeks. This has important regulatory consequences because the Acceptable Daily Intake (ADI) value is defined by the threshold of toxicity calculated with G alone. The ADI value thus does not consider the formulants present in the formulations.
Moreover, we searched for other known toxic and endocrine-disrupting elements in 22 pesticides, including 11 GBH. We report for the first time the presence of several heavy metals in most formulations, in particular As, Cr, and Ni. Pb and Co. All diluted formulations except one contained a cocktail of metals. This phenomenon thus appears to be widely distributed in the world, as our samples came from the European Union and North America. In Asia, large amounts of As were found in GBH in Sri Lanka [47].
Heavy metals may originate either from contamination of formulations due to their manufacturing process, for instance from petroleum, or from industrial waste. They may also be added intentionally as nanoparticles in pesticides [48] or as chemicals; this has been the case for As which has itself been considered to be a pesticide for decades [49,50]. Its use as a pesticide has been banned worldwide because As in groundwater and food is of major concern [51]. Admissible levels in drinking water have been lowered to 10 ppb (WHO guidelines value [18]) in nearly all countries within the last two decades [51]. The natural occurrence of As in soils is often discussed [51]; however, the intentional spraying of pesticides and GBH in particular may add significant As pollution in some environments. Folpan intensively sprayed in non-organic vineyards may also contaminate wines, water and soil with heavy metals. The toxicity and endocrine-disrupting effects are documented for As [49], [52]], Cr [[53], [54], [55], [56], [57], [58]], Ni [59,60], Pb [61], and Co [62,63].
Their toxicity must be considered in conjunction with that of the formulants already cited and of G, which can chelate such cations [64,65]. However, G appears the least toxic component of GBH, even in plants, in contrast with some formulants. All these results could shed a new light on the toxicity assessment of genetically modified plants tolerant to Roundup, because they could contain high levels of toxic formulants, and on the impact of these on the environment. Indeed, they are used for food and feed; and their assessment protocols should be upgraded [[66], [67]].
In conclusion, G being tested alone in chronic regulatory experiments to establish the ADI (RfD in USA) appears inappropriate, in light of these results. As a matter of fact, synergistic toxic effects undoubtedly occur, and therefore ADI calculations and other regulatory experiments should be performed with the full formulations and all components, especially should be declared and/or measured, because other active ingredients could be in formulants. To take that into account, ADIs could be divided by several orders of magnitude. In general, novel methodological approaches simulating real-life exposures must be applied for pesticides [[33], [68], [69], [70]]. In the meantime, toxicological studies have to be developed for a better environmental health [71].
Conflict of interest
The authors declare that there are no conflicts of interest.
Authors’ contributions
ND carried out the study, performed the statistical analyses, drew the figures and participated in the preparation of the manuscript. JSdV participated in the discussion. GES conceived the study, designed the work and directed the preparation of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank Dr. Robin Mesnage and Frédérique Hilary for valuable help. We also thank Gilbert Pigrée and Maxime Lemarchand from the platform IMOGERE, University of Caen Normandy, France. This work was supported by the University of Caen and the Committee of Research and Independent Information on Genetic Engineering (CRIIGEN). It received funding from Alibio Institute and Ekibio Foundation, the Regional Council Ile de France, the Regional Council Rhône-Alpes, JMG Foundation, Foundation Lea Nature, Nature Vivante, Malongo, and the Sustainable Food Alliance. The authors wish to thank the European Deputy Michèle Rivasi for the support to accomplish this work.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.toxrep.2017.12.025.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Mesnage R., Defarge N., Spiroux de Vendomois J., Seralini G.E. Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food Chem. Toxicol. 2015;84:133–153. doi: 10.1016/j.fct.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 2.James C. ISAAA Brief; 2015. Global Status of Commercialized Biotech/GM Crops: 2015; p. 51. [Google Scholar]
- 3.Portier C.J., Armstrong B.K., Baguley B.C., Baur X., Belyaev I., Belle R., Belpoggi F., Biggeri A. Differences in the carcinogenic evaluation of glyphosate between the International Agency for Research on Cancer (IARC) and the European Food Safety Authority (EFSA) J. Epidemiol. Commun. Health. 2016;70:741–745. doi: 10.1136/jech-2015-207005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guyton K.Z., Loomis D., Grosse Y., El Ghissassi F., Benbrahim-Tallaa L., Guha N., Scoccianti C., Mattock H., Straif K. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 2017;16(5):490–491. doi: 10.1016/S1470-2045(15)70134-8. [DOI] [PubMed] [Google Scholar]
- 5.EFSA Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J. 2015;13:4302–4409. [Google Scholar]
- 6.Gasnier C., Dumont C., Benachour N., Clair E., Chagnon M.C., Seralini G.E. Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology. 2009;262:184–191. doi: 10.1016/j.tox.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Richard S., Moslemi S., Sipahutar H., Benachour N., Seralini G.E. Differential effects of glyphosate and roundup on human placental cells and aromatase. Environ. Health Perspect. 2005;113:716–720. doi: 10.1289/ehp.7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letcher R.J., van Holsteijn I., Drenth H.J., Norstrom R.J., Bergman A., Safe S., Pieters R., van den Berg M. Cytotoxicity and aromatase (CYP19) activity modulation by organochlorines in human placental JEG-3 and JAR choriocarcinoma cells. Toxicol. Appl. Pharmacol. 1999;160:10–20. doi: 10.1006/taap.1999.8746. [DOI] [PubMed] [Google Scholar]
- 9.L’Azou B., Fernandez P., Bareille R., Beneteau M., Bourget C., Cambar J., Bordenave L. In vitro endothelial cell susceptibility to xenobiotics: comparison of three cell types. Cell Biol. Toxicol. 2005;21:127–137. doi: 10.1007/s10565-005-0172-8. [DOI] [PubMed] [Google Scholar]
- 10.Benachour N., Sipahutar H., Moslemi S., Gasnier C., Travert C., Seralini G. Time- and dose-dependent effects of roundup on human embryonic and placental cells. Arch. Environ. Contam. Toxicol. 2007;53:126–133. doi: 10.1007/s00244-006-0154-8. [DOI] [PubMed] [Google Scholar]
- 11.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 12.Benachour N., Seralini G.E. Glyphosate formulations induce apoptosis and necrosis in human umbilical, embryonic, and placental cells. Chem. Res. Toxicol. 2009;22:97–105. doi: 10.1021/tx800218n. [DOI] [PubMed] [Google Scholar]
- 13.Thompson E.A., Jr., Siiteri P.K. Utilization of oxygen and reduced nicotinamide adenine dinucleotide phosphate by human placental microsomes during aromatization of androstenedione. J. Biol. Chem. 1974;249:5364–5372. [PubMed] [Google Scholar]
- 14.Dintinger T., Gaillard J.L., Moslemi S., Zwain I., Silberzahn P. Androgen and 19-norandrogen aromatization by equine and human placental microsomes. J. Steroid Biochem. 1989;33:949–954. doi: 10.1016/0022-4731(89)90245-8. [DOI] [PubMed] [Google Scholar]
- 15.OECD . 2012. Draft Guidance Document on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption. (Available at http://www.oecd.org/chemicalsafety/testing/50459967.pdf) [Google Scholar]
- 16.Defarge N., Takacs E., Lozano V.L., Mesnage R., Spiroux de Vendomois J., Seralini G.E., Szekacs A. Co-formulants in glyphosate-based herbicides disrupt aromatase activity in human cells below toxic levels. Int. J. Environ. Res. Public Health. 2016;13:264. doi: 10.3390/ijerph13030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Commission . 1998. Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. (Available at http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:01998L0083-20151027&from=EN) [Google Scholar]
- 18.WHO . fourth edition. 2011. Guidelines for Drinking-Water Quality. (Available at http://apps.who.int/iris/bitstream/10665/44584/1/9789241548151_eng.pdf) [Google Scholar]
- 19.Boocock M.R., Coggins J.R. Kinetics of 5-enolpyruvylshikimate-3-phosphate synthase inhibition by glyphosate. FEBS Lett. 1983;154:127–133. doi: 10.1016/0014-5793(83)80888-6. [DOI] [PubMed] [Google Scholar]
- 20.Cox C., Surgan M. Unidentified inert ingredients in pesticides: implications for human and environmental health. Environ. Health Perspect. 2006;114:1803–1806. doi: 10.1289/ehp.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mesnage R., Bernay B., Seralini G.E. Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology. 2013;313:122–128. doi: 10.1016/j.tox.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Williams G.M., Kroes R., Munro I.C. Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul. Toxicol. Pharmacol. 2000;31:117–165. doi: 10.1006/rtph.1999.1371. [DOI] [PubMed] [Google Scholar]
- 23.Séralini G.E. Why glyphosate is not the issue with Roundup. A short overview of 30 years of our research. J. Biol. Phys. Chem. 2015;15:111–119. [Google Scholar]
- 24.Cserhati T. Alkyl ethoxylated and alkylphenol ethoxylated nonionic surfactants: interaction with bioactive compounds and biological effects. Environ. Health Perspect. 1995;103:358–364. doi: 10.1289/ehp.103-1519097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nobels I., Spanoghe P., Haesaert G., Robbens J., Blust R. Toxicity ranking and toxic mode of action evaluation of commonly used agricultural adjuvants on the basis of bacterial gene expression profiles. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zerin T., Song H., Gil H., Hong S. Surfactant 4-nonylphenyl-polyethylene glycol stimulates reactive oxygen species generation and apoptosis in human neuroblastoma cells. J. Environ. Sci. 2017;53:262–268. doi: 10.1016/j.jes.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Székács I., Fejes Á., Klátyik S., Takács E., Patkó D., Pomóthy J., Mörtl M., Horváth R., Madarász E., Darvas B., Székács A. Environmental and toxicological impacts of glyphosate with its formulating adjuvant. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2014;8:210–216. [Google Scholar]
- 28.Navarro C.D., Martinez C.B. Effects of the surfactant polyoxyethylene amine (POEA) on genotoxic, biochemical and physiological parameters of the freshwater teleost Prochilodus lineatus. Comp. Biochem. Physiol. C. 2014;165:83–90. doi: 10.1016/j.cbpc.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Zhao X., Yang G., Toyooka T., Ibuki Y. New mechanism of gamma-H2AX generation: surfactant-induced actin disruption causes deoxyribonuclease I translocation to the nucleus and forms DNA double-strand breaks. Mut. Res. 2015;794:1–7. doi: 10.1016/j.mrgentox.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Gasnier C., Laurant C., Decroix-Laporte C., Mesnage R., Clair E., Travert C., Seralini G. Defined plant extracts can protect human cells against combined xenobiotic effects. J. Occup. Med. Toxicol. 2011;6:3. doi: 10.1186/1745-6673-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassault-Meyer E., Gress S., Séralini G.-E., Galeraud-Denis I. An acute exposure to glyphosate-based herbicide alters aromatase levels in testis and sperm nuclear quality. Environ. Toxicol. Pharmacol. 2014;38:131–140. doi: 10.1016/j.etap.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Gress S., Laurant C., Defarge N., Travert C., Séralini G.É. Dig1 protects against locomotor and biochemical dysfunctions provoked by Roundup. BMC Complement. Altern. Med. 2016;16(July):234. doi: 10.1186/s12906-016-1226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Séralini G.E., Clair E., Mesnage R., Gress S., Defarge N., Malatesta M., Hennequin D., Spiroux de Vendômois J. Republished study: long-term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Environ. Sci. Eur. 2014;26:14. doi: 10.1186/s12302-014-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brausch J.M., Smith P.N. Toxicity of three polyethoxylated tallowamine surfactant formulations to laboratory and field collected fairy shrimp, Thamnocephalus platyurus. Arch. Environ. Contam. Toxicol. 2007;52:217–221. doi: 10.1007/s00244-006-0151-y. [DOI] [PubMed] [Google Scholar]
- 35.Eddleston M., Street J.M., Self I., Thompson A., King T., Williams N., Naredo G., Dissanayake K., Yu L.M., Worek F., John H., Smith S., Thiermann H., Harris J.B., Eddie Clutton R. A role for solvents in the toxicity of agricultural organophosphorus pesticides. Toxicology. 2012;294:94–103. doi: 10.1016/j.tox.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krogh K.A., Halling-Sorensen B., Mogensen B.B., Vejrup K.V. Environmental properties and effects of nonionic surfactant adjuvants in pesticides: a review. Chemosphere. 2003;50:871–901. doi: 10.1016/s0045-6535(02)00648-3. [DOI] [PubMed] [Google Scholar]
- 37.Tsui M.T., Chu L.M. Aquatic toxicity of glyphosate-based formulations: comparison between different organisms and the effects of environmental factors. Chemosphere. 2003;52:1189–1197. doi: 10.1016/S0045-6535(03)00306-0. [DOI] [PubMed] [Google Scholar]
- 38.Bergé A., Cladière M., Gasperi J., Coursimault A., Tassin B., Moilleron R. Meta-analysis of environmental contamination by alkylphenols. Environ. Sci. Pollut. Res. 2012;19:3798–3819. doi: 10.1007/s11356-012-1094-7. [DOI] [PubMed] [Google Scholar]
- 39.Krogh K.A., Vejrup K.V., Mogensen B.B., Halling-Sørensen B. Liquid chromatography-mass spectrometry method to determine alcohol ethoxylates and alkylamine ethoxylates in soil interstitial water, ground water and surface water samples. J. Chromatogr. A. 2002;957:45–57. doi: 10.1016/s0021-9673(02)00077-8. [DOI] [PubMed] [Google Scholar]
- 40.Vincent M.D., Sneddon J. Nonylphenol: an overview and its determination in oysters and wastewaters and preliminary degradation results from laboratory experiments. Microchem. J. 2009;92:112–118. [Google Scholar]
- 41.Ferrer E., Santoni E., Vittori S., Font G., Mañes J., Sagratini G. Simultaneous determination of bisphenol A, octylphenol, and nonylphenol by pressurised liquid extraction and liquid chromatography–tandem mass spectrometry in powdered milk and infant formulas. Food Chem. 2011;126:360–367. [Google Scholar]
- 42.Shao B., Han H., Li D., Ma Y., Tu X., Wu Y. Analysis of alkylphenol and bisphenol A in meat by accelerated solvent extraction and liquid chromatography with tandem mass spectrometry. Food Chem. 2007;105:1236–1241. [Google Scholar]
- 43.She Y., Wang J., Zheng Y., Cao W., Wang R., Dong F., Liu X., Qian M., Zhang H., Wu L. Determination of nonylphenol ethoxylate metabolites in vegetables and crops by high performance liquid chromatography–tandem mass spectrometry. Food Chem. 2012;132:502–507. doi: 10.1016/j.foodchem.2011.09.131. [DOI] [PubMed] [Google Scholar]
- 44.Chen J., Mullin C.A. Determination of nonylphenol ethoxylate and octylphenol ethoxylate surfactants in beehive samples by high performance liquid chromatography coupled to mass spectrometry. Food Chem. 2014;158:473–479. doi: 10.1016/j.foodchem.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Jobling S., Burn R.W., Thorpe K., Williams R., Tyler C. Statistical modeling suggests that antiandrogens in effluents from wastewater treatment works contribute to widespread sexual disruption in fish living in English rivers. Environ. Health Perspect. 2009;117:797–802. doi: 10.1289/ehp.0800197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doerge D.R., Twaddle N.C., Churchwell M.I., Chang H.C., Newbold R.R., Delclos K.B. Mass spectrometric determination of p-nonylphenol metabolism and disposition following oral administration to Sprague-Dawley rats. Reprod. Toxicol. 2002;16:45–56. doi: 10.1016/s0890-6238(01)00198-8. [DOI] [PubMed] [Google Scholar]
- 47.Jayasumana C., Fonseka S., Fernando A., Jayalath K., Amarasinghe M., Siribaddana S., Gunatilake S., Paranagama P. Phosphate fertilizer is a main source of arsenic in areas affected with chronic kidney disease of unknown etiology in Sri Lanka. SpringerPlus. 2015;4:90. doi: 10.1186/s40064-015-0868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kookana R.S., Boxall A.B., Reeves P.T., Ashauer R., Beulke S., Chaudhry Q., Cornelis G., Fernandes T.F., Gan J., Kah M., Lynch I., Ranville J., Sinclair C., Spurgeon D., Tiede K., Van den Brink P.J. Nanopesticides: guiding principles for regulatory evaluation of environmental risks. J. Agric. Food Chem. 2014;62:4227–4240. doi: 10.1021/jf500232f. [DOI] [PubMed] [Google Scholar]
- 49.Li Y., Ye F., Wang A., Wang D., Yang B., Zheng Q., Sun G., Gao X. Chronic arsenic poisoning probably caused by arsenic-based pesticides: findings from an investigation study of a household. Int. J. Environ. Res. Public Health. 2016;13:133. doi: 10.3390/ijerph13010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy E.A., Aucott M. An assessment of the amounts of arsenical pesticides used historically in a geographical area. Sci. Total Environ. 1998;218:89–101. [Google Scholar]
- 51.Kapaj S., Peterson H., Liber K., Bhattacharya P. Human health effects from chronic arsenic poisoning—a review. J. Environ. Sci. Health A. 2006;41:2399–2428. doi: 10.1080/10934520600873571. [DOI] [PubMed] [Google Scholar]
- 52.Ferrario D., Gribaldo L., Hartung T. Arsenic exposure and immunotoxicity: a review including the possible influence of age and sex. Curr. Environ. Health Rep. 2016;3:1–12. doi: 10.1007/s40572-016-0082-3. [DOI] [PubMed] [Google Scholar]
- 53.Banu S.K., Stanley J.A., Sivakumar K.K., Arosh J.A., Taylor R.J., Burghardt R.C. Chromium VI – induced developmental toxicity of placenta is mediated through spatiotemporal dysregulation of cell survival and apoptotic proteins. Reprod. Toxicol. 2016;68:171–190. doi: 10.1016/j.reprotox.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hemminki K., Kyyrönen P., Niemi M.L., Koskinen K., Sallmén M., Vainio H. Spontaneous abortions in an industrialized community in Finland. Am. J. Public Health. 1983;73:32–37. doi: 10.2105/ajph.73.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hemminki K., Niemi M.-L., Koskinen K., Vainio H. Spontaneous abortions among women employed in the metal industry in Finland. Int. Arch. Occup. Environ. Health. 1980;47:53–60. doi: 10.1007/BF00378328. [DOI] [PubMed] [Google Scholar]
- 56.Quansah R., Jaakkola J.J.K. Paternal and maternal exposure to welding fumes and metal dusts or fumes and adverse pregnancy outcomes. Int. Arch. Occup. Environ. Health. 2009;82:529–537. doi: 10.1007/s00420-008-0349-6. [DOI] [PubMed] [Google Scholar]
- 57.Sivakumar K.K., Stanley J.A., Arosh J.A., Pepling M.E., Burghardt R.C., Banu S.K. Prenatal exposure to chromium induces early reproductive senescence by increasing germ cell apoptosis and advancing germ cell cyst breakdown in the F1 offspring. Dev. Biol. 2014;388:22–34. doi: 10.1016/j.ydbio.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Y., Liu H., Xiang X.-h., Liu F.-y. Outline of occupational chromium poisoning in China. Bull. Environ. Contamin. Toxicol. 2013;90:742–749. doi: 10.1007/s00128-013-0998-3. [DOI] [PubMed] [Google Scholar]
- 59.Forgacs Z., Massanyi P., Lukac N., Somosy Z. Reproductive toxicology of nickel – review. J. Environ. Sci. Health A. 2012;47:1249–1260. doi: 10.1080/10934529.2012.672114. [DOI] [PubMed] [Google Scholar]
- 60.Kong L., Tang M., Zhang T., Wang D., Hu K., Lu W., Wei C., Liang G., Pu Y. Nickel nanoparticles exposure and reproductive toxicity in healthy adult rats. Int. J. Mol. Sci. 2014;15:21253–21269. doi: 10.3390/ijms151121253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wani A.L., Ara A., Usmani J.A. Lead toxicity: a review. Interdiscip. Toxicol. 2015;8:55–64. doi: 10.1515/intox-2015-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Behl M., Stout M.D., Herbert R.A., Dill J.A., Baker G.L., Hayden B.K., Roycroft J.H., Bucher J.R., Hooth M.J. Comparative toxicity and carcinogenicity of soluble and insoluble cobalt compounds. Toxicology. 2015;333:195–205. doi: 10.1016/j.tox.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 63.Roychoudhury S., Sirotkin A.V., Toman R., Kolesarova A. Cobalt-induced hormonal and intracellular alterations in rat ovarian fragments in vitro. J. Environ. Sci. Health B. 2014;49:971–977. doi: 10.1080/03601234.2014.951586. [DOI] [PubMed] [Google Scholar]
- 64.Madsen H.L., Christensen H., Gottlieb-Petersen C. Stability constants of copper (II), zinc, manganese (II), calcium, and magnesium complexes of N-(phosphonomethyl) glycine (glyphosate) Acta Chem. Scand. A. 1978;32:79–83. [Google Scholar]
- 65.Subramaniam V., Hoggard P.E. Metal complexes of glyphosate. J. Agric. Food Chem. 1988;36:1326–1329. [Google Scholar]
- 66.Tsatsakis A.M., Nawaz M.A., Tutelyan V.A., Golokhvast K.S., Kalantzi O.I., Chung D.H., Kang S.J., Coleman M.D., Tyshko N., Yang S.H., Chung G. Impact on environment, ecosystem, diversity and health from culturing and using GMOs as feed and food. Food Chem. Toxicol. 2017;107A:108–121. doi: 10.1016/j.fct.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 67.Tsatsakis A.M., Nawaz M.A., Kouretas D., Balias G., Savolainen K., Tutelyan V.A., Golokhvast K.S., Lee J.D., Yang S.H., Chung G. Environmental impacts of genetically modified plants: a review. Environ. Res. 2017;156:818–833. doi: 10.1016/j.envres.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 68.Hernandez A.F., Tsatsakis A.M. Human exposure to chemical mixtures: challenges for the integration of toxicology with epidemiology data in risk assessment. Food Chem. Toxicol. 2017;103:188–193. doi: 10.1016/j.fct.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 69.Tsatsakis A.M., Docea A.O., Tsitsimpikou C. New challenges in risk assessment of chemicals when simulating real exposure scenarios; simultaneous multi-chemicals’ low dose exposure. Food Chem. Toxicol. 2016;96:174–176. doi: 10.1016/j.fct.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 70.Tsatsakis A.M., Kouretas D., Tzatzarakis M.N., Stivaktakis P., Tsarouhas K., Golokhvast K.S., Rakitskii V.N. Simulating real-life exposures to uncover possible risks to human health: a proposed consensus for a novel methodological approach. Hum. Exp. Toxicol. 2017;36:554–564. doi: 10.1177/0960327116681652. [DOI] [PubMed] [Google Scholar]
- 71.Tsatsakis A.M., Lash L.H. Toxicology: the basic science for human well-being and environmental health. Toxicol. Rep. 2017;4:x–xi. doi: 10.1016/j.toxrep.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.