Abstract
BACKGROUND: Most tumor cell lines exhibited low-dose hyperradiosensitivity (LDHRS) to radiation doses lower than 0.3 Gy. Pulsed low–dose rate radiotherapy (PLDR) took advantage of LDHRS and maximized the tumor control process. In this study, we retrospectively analyzed patients receiving PLDR for refractory malignancies. PATIENTS AND METHODS: In total, 22 patients were included in our study: 9 females and 13 males. The median age was 61 years old. All the patients previously received multiline treatments and failed with an estimated survival less than 6 months. Thus, palliative PLDR was given. The PLDR was delivered using 10 fractions of 2 Gy/day, with an interval of 3 minutes, for 5 days per week. The dose rate was 6.67 cGy/min. The median follow-up was 1 year (range 8-30 months). Nine patients underwent PLDR for reirradiation due to locally recurrent diseases. The time interval from last irradiation was 11 to 168 months. Ten patients received PLDR due to poor performance status. Three patients were given PLDR for bulky tumor. The irradiated sites included primary disease (seven patients), locally recurrent disease (nine patients), and retroperitoneal adenopathy (six patients). RESULTS: Five patients developed grade 3 or 4 toxicities. No grade 5 toxicities occurred. All the toxicities recovered after treatments. In general, the 1-year local-regional control rate was approximately 40%, and almost all the patients developed progression at the second year after PLDR. The 6-month survival rate was 76%, and the 1-year survival rate was 69%. For the three patients given PLDR for bulky tumor, all of them achieved partial remission 1 month after the PLDR, and one patient achieved complete response at the fourth month. CONCLUSION: PLDR is an effective and safe option not only for reirradiation but also for patients with poor performance status or bulky tumors. A prospective clinical trial (NCT03061162) is ongoing to validate our results.
Introduction
Both the prognosis and quality of life are poor for patients with recurrent diseases after multiple-line treatments for malignancies [1]. There is a lack of standard treatments for these patients. Generally, surgery or further aggressive treatments are intolerable to patients with refractory malignancies. Radiotherapy is an option for such patients. However, these patients, who always have received radiotherapy previously and developed in-field recurrences or are with bulky tumors, are not suitable for conventional radiotherapy.
Pulsed low-dose radiation therapy (PLDR) has been demonstrated as a novel treatment for radioresistant tumors by taking advantage of low-dose hyperradiosensitivity (LDHRS). The LDHRS defines the enhanced killing response of tumor cells when the dose is ≤0.25 Gy. The potential mechanism is that X-ray doses ≤ 0.3 Gy weakly induced ataxia‐telangiectasia mutated activity and doses > 0.5 Gy promoted the obvious activity of ataxia‐telangiectasia mutated, which conferred radioresistance by enhancing the deoxyribonucleic acid repair [2], [3]. In a murine orthotopic model of glioblastoma, Marples' group found that PLDR was more efficacious than the standard fractionated treatment [4].
PLDR not only exerts increased tumor control capacity but also favors normal tissue sparing and the preservation of vascular network. In the lung cancer murine model, Zhang et al. found that PLDR could control tumors as effectively as the conventional radiotherapy but results in much less normal tissue toxicities than the conventional radiotherapy [5]. The preclinical study demonstrated that the combination of PLDR and temozolomide was associated with increased vascularization and fewer degenerating neurons compared with standard fractionated treatment plus temozolomide [2]. For the head and neck squamous cell carcinoma murine models, Marples' group also found that the PLDR group exerted fewer tumor hypoxia compared to the conventional radiotherapy group [6]. Similar to the findings in glioblastoma, PLDR showed more preservation of vascular network in the head and neck squamous cell carcinoma murine model [7]. PLDR caused less vascular damage, and the preserved vascular network could improve tumor oxygenation and could explain the improved therapeutic effect. Some clinical studies demonstrated the efficacy and safety of PLDR applied for reirradiation in patients with recurrent tumors to achieve palliative benefits. PLDR also showed an increased capacity of normal tissue sparing. These clinical studies mainly focused on breast cancer, head and neck cancer, and glioma [8], [9], [10], [11].
However, the clinical experiences using PLDR in patients with other kinds of tumors or bulky tumor are limited. In this study, patients with late stage were included. The estimated survivals for these patients were less than 6 months. We developed a technique where a series of 0.2-Gy pulses was separated by 3-minute intervals, creating a dose rate of 6.67 cGy/min according to the previous studies [9], [12]. This study described the treatment responses and related toxicities in patients given PLDR for recurrent tumors or bulky tumors in our institute. The primary tumors were various, including gastric cancer, lung cancer, esophageal cancer, colorectal cancer, bladder cancer, glioma, chondrosarcoma, and cholangiocarcinoma.
Methods
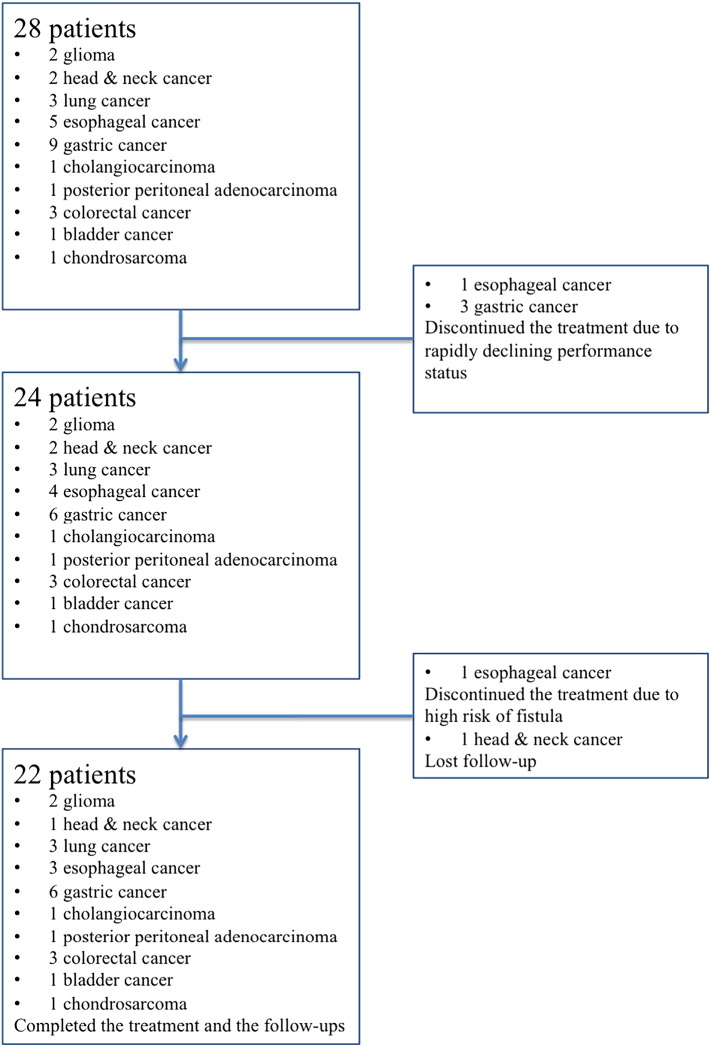
As shown in Figure 1, this study initially included 28 patients with recurrent tumors treated with PLDR between May 2014 and November 2015 at Drum Tower Hospital. The ethics committee of Drum Tower Hospital has approved our study. Informed consents for all the patients were obtained before treatment. Eligibility criteria were 1) pathologically confirmed malignant tumors; 2) previously received radiotherapy and developed in-field recurrence or with bulky tumors; or 3) with poor performance status unsuitable for conventional radiotherapy after evaluation by the multidisciplinary team. All the patients received multiline treatments previously but failed. No standard treatments were available. Three patients with metastatic gastric cancer and one patient with discontinued the treatment. These patients were at the end stage, with progressive cachexia and rapidly enlarging tumor leading to the rapidly declining performance status. The estimated survival was extremely short, and it was not appropriate to perform any antitumor treatment after balancing the poor tolerance and the potential gains. Then, the PLRT was discontinued. One patient with esophageal tumor did complete the computed tomographic simulation for the PLRT. After the reevaluation by the images from the simulation, the risk of fistula was very high, and this patient therefore did not complete the planned PLRT. In total, 23 patients completed the treatment. There were two patients with glioma, two patients with head and neck cancer, three patients with lung cancer, three patients with esophageal cancer, six patients with gastric cancer, one patient with cholangiocarcinoma, one patient with posterior peritoneal adenocarcinoma, three patients with colorectal cancer, one patient with bladder cancer, and one patient with chondrosarcoma. The follow-up of one patient with head and neck cancer was lost after the treatment. Finally, 22 patients completed the PLDR and the follow-ups.
Figure 1.
Flowchart of the recruitment process.
PLDR was administered to the region of recurrent disease. The irradiation treatment was delivered using 10 fractions of 2 Gy/day, with interval of 3 minutes, for 5 days per week. Approximately 30 minutes was taken to complete the treatment every day. The dose rate was 6.67 cGy/min. This pattern is most commonly used [13]. The treatment response was assessed 1 month later after the treatment end by computed tomography with contrast or positron emission tomography scans if applicable. Then, the follow-up was updated every 2 months. The local regional relapse-free survival (LRRFS) and overall survival (OS) were assessed after the end of the treatment. The acute and late toxicities were scored via the Common Terminology Criteria for Adverse Events v3.0. RECIST was referred to for evaluating the treatment responses. Kaplan-Meier estimates and plots were generated via SPSS 21.
Results
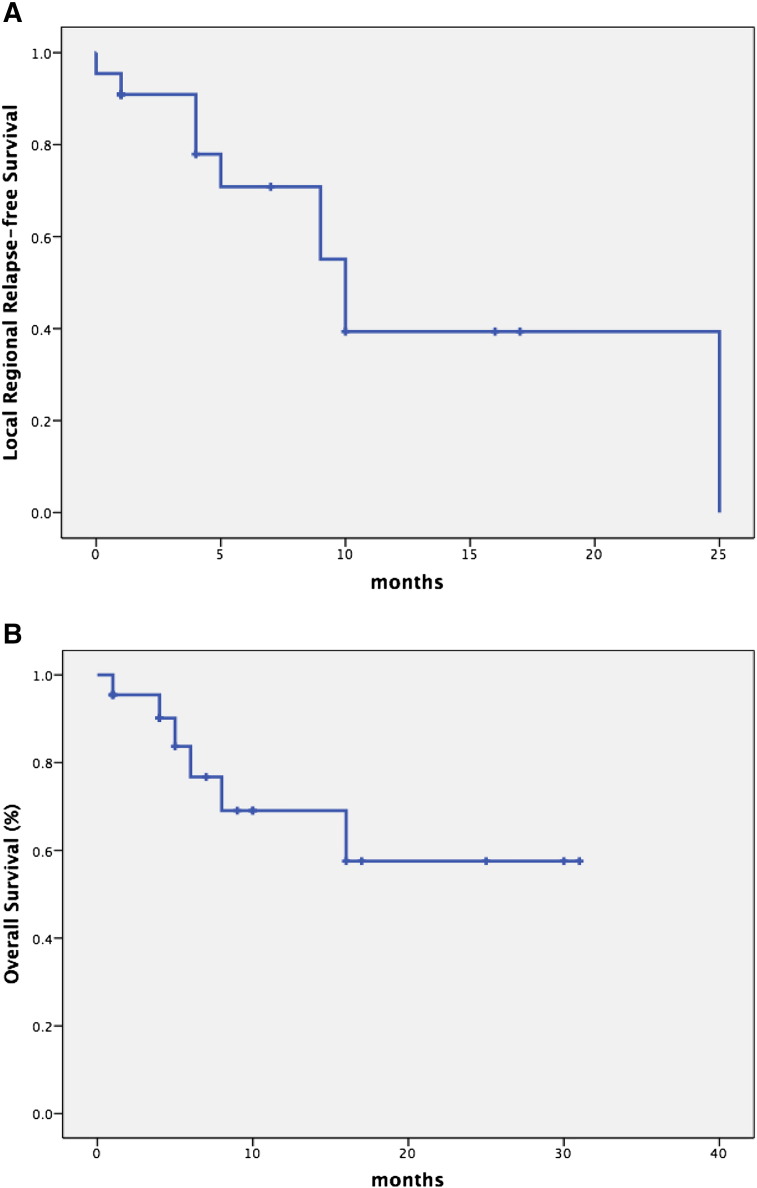
The flowchart of the enrollment was shown in Figure 1 and described in detailed in “Methods.” The multidisciplinary team evaluated every patient before the enrollment of the PLDR group. All the included patients have received multiline treatments and still suffered from the progressive disease. These patients were at the end stage with an estimated survival less than 6 months. In the studied population, there were 9 females and 13 males (Table 1). The median age was 61 years old (range 26-79 years old). The median follow-up was 1 year (range 8-30 months). The ECOG performance status varied from 0 to 3. Seven patients achieved partial remission at 1 month after PLDR. As for the grade 3 or 4 toxicity, three patients developed grade 3 bone marrow suppression, one patient developed grade 4 bone marrow suppression, and one patient developed grade 3 enteritis (Table 1). After supportive care, all the patients recovered from the toxicities without any complication of life-threatening infections or chronic injuries. The Kaplan-Meier estimated 1-year local-regional control rate was approximately 40% (Figure 2A), and almost all the patients developed progression at the second year after PLDR (Figure 2A). As shown in Figure 2B, the 6-month survival rate was 76% and the 1-year survival rate was 69%.
Table 1.
Clinical Characteristics of the Studied Population
| Patient No. | Gender | Age | Primary Tumor | PLRT Total Dose | Enrollment Reason Code | Irradiation Site | Radiation Related Toxicities | Concurrent Therapy | Time Interval from the Last Radiotherapy | Total Dose of the Last Radiotherapy |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 46 | Head and neck cancer | 60 Gy/30 f | Reirradiation | Locally recurrent disease | Grade 1 bone marrow suppression Grade 2 stomatitis |
Chemotherapy | 50 months | 70 Gy |
| 2 | F | 26 | Lung cancer | 50 Gy/25 f | Reirradiation | Locally recurrent disease | Grade 1 pneumonitis | Chemotherapy | 22 months | 60 Gy |
| 3 | M | 67 | Lung cancer | 56 Gy/28 f | Poor performance status | Primary tumor | Grade 1 bone marrow suppression | Chemotherapy | NA | |
| 4 | M | 74 | Lung cancer | 50 Gy/25 f | Reirradiation | Locally recurrent disease | None | None | 24 months | 59.4 Gy |
| 5 | M | 78 | Cholangiocarcinoma | 54 Gy/27 f | Poor performance status | Primary tumor | None | Adoptive immune cell therapy | NA | |
| 6 | F | 50 | Posterior peritoneal adenocarcinoma | 50 Gy/25 f | Bulky tumor | Primary tumor | Grade 1 diarrhea | Chemotherapy | NA | |
| 7 | F | 61 | Colorectal cancer | 40 Gy/20 f | Poor performance status | Primary tumor | Grade 2 nausea and vomiting | None | NA | |
| 8 | F | 75 | Colorectal cancer | 50 Gy/25 f | Re-irradiation | Locally recurrent disease | None | None | 30 months | 39 Gy |
| 9 | M | 48 | Colorectal cancer | 50 Gy/25 f | Poor performance status | Primary tumor | Grade 3 bone marrow suppression | None | NA | |
| 10 | M | 61 | Glioma | 50 Gy/25 f | Reirradiation | Locally recurrent disease | Mild brain swelling | Targeted therapy | 60 months; 12 months | 56 Gy; 60 Gy |
| 11 | M | 28 | Glioma | 48 Gy/24 f | Reirradiation | Locally recurrent disease | None | None | 36 months | 64 Gy |
| 12 | F | 52 | Bladder cancer | 2 Gy × 20 f | Bulky tumor | Primary tumor | Grade 2 bone marrow suppression Grade 2 diarrhea |
Chemotherapy | NA | |
| 13 | M | 51 | Esophageal cancer | 50 Gy/25 f | Reirradiation | Locally recurrent disease | None | None | 30 months | 60 Gy |
| 14 | M | 63 | Esophageal cancer | 60 Gy/30 f | Bulky tumor | Primary tumor | Grade 3 bone marrow suppression | Chemotherapy | NA | |
| 15 | M | 74 | Esophageal cancer | 50 Gy/25 f | Reirradiation | Locally recurrent disease | None | None | 52 months | 54gy |
| 16 | F | 52 | Gastric cancer | 50 Gy/25 f | Poor performance status | Retroperitoneal adenopathy | None | None | NA | |
| 17 | F | 36 | Gastric cancer | 2 Gy × 25 f | Poor performance status | Retroperitoneal adenopathy | Grade 1 diarrhea Grade 2 bone marrow suppression |
Chemotherapy | NA | |
| 18 | F | 70 | Gastric cancer | 44 Gy/22 f | Poor performance | Retroperitoneal adenopathy | Grade 4 bone marrow suppression Grade 3 enteritis |
Chemotherapy | NA | |
| 19 | F | 67 | Gastric cancer | 50 Gy/25 f | Poor performance status | Retroperitoneal adenopathy | Grade 3 bone marrow suppression | Chemotherapy | NA | |
| 20 | M | 79 | Gastric cancer | 60 Gy/30 f | Poor performance status | Retroperitoneal adenopathy | None | Targeted therapy | NA | |
| 21 | M | 66 | Gastric cancer | 50 Gy/25 f | Poor performance status | Retroperitoneal adenopathy | None | Chemotherapy | NA | |
| 22 | M | 52 | Chondrosarcoma | 54 Gy/27 f | Re-irradiation | Locally recurrent disease | None | None | 168 months | 50 Gy |
NA, not applicable.
Figure 2.
(A) Kaplan-Meier analysis of local regional-free survival. (B) Kaplan-Meier analysis of overall survival.
Patients Who Received PLDR for Reirradiation
Nine patients underwent PLDR for reirradiation due to locally recurrent diseases (Table 1). Of the nine patients, one was with head and neck cancer, two with lung cancer, one with colorectal cancer, two with glioma, two with esophageal cancer, and one with chondrosarcoma. These nine patients completed PLDR successfully without interruption. The time interval from last irradiation was 11 to 168 months. The previously delivered dose range was 39 to 116 Gy (one patient [no. 10 in Table 1] with glioma received twice cerebral irradiation with doses of 56 Gy in 2009 and 60 Gy in 2013). The dose range delivered by PLDR in this study was 48 to 60 Gy. The cumulative dose range was 104 to 166 Gy. Among patients receiving reirradiation, four patients received concurrent therapy: two with chemotherapy, one with targeted therapy, and one with the adoptive immune cell transfer (Table 1). One patient (no. 1 in Table 1) with head and neck cancer receiving concurrent chemotherapy developed grade 1 bone marrow suppression and grade 2 stomatitis. One lung cancer patient (no. 2 in Table 1) with concurrent chemotherapy developed grade 1 pneumonitis. Mild brain swelling occurred in one patient (no. 10 in Table 1) receiving concurrent targeted therapy for recurrent glioma. Five patients underwent merely PLDR for the locally recurrent disease, and treatment-related toxicities were not observed. At 1 month after the end of PLDR, three patients (nos. 1, 2, and 10 in Table 1) achieved partial remission (PR), one patient (No. 13 in Table 1) developed progressive disease, and five patients remained with stable disease. The treatment response rate was 33% (3/9). For the patients achieving PR, the patient with head and neck cancer (no. 1 in Table 1) still remained disease-free on the last follow-up on 5/5/2017; the patient with lung cancer (no. 2 in Table 1) even achieved complete response at the seventh month after the end of PLDR, and the disease-free status lasted for 10 months. In summary, PLDR was effective and safe for reirradiation in patients with locally recurrent diseases. Concurrent chemotherapy or targeted therapy could increase the risk of treatment-related toxicities. The LRRFS could last relatively long once PR was achieved.
Patients Who Received PLDR Due to Poor Performance Status
Ten patients received PLDR due to poor performance status (Table 1). One patient was with lung cancer (no. 3 in Table 1), one with cholangiocarcinoma (no. 5 in Table 1), two with colorectal cancer (nos. 7 and 9 in Table 1), and six with gastric cancer (no. 16-21 in Table 1). The irradiation sites of patients (nos. 3, 5, 7, and 9 in Table 1) were the primary tumors, and the other patients (nos. 16-21 in Table 1) received PLDR at retroperitoneal adenopathy. Most patients (7/10) underwent concurrent treatments. About 60% (6/10) of patients developed treatment-related toxicities. Most toxicities were ≤ grade 3, and no grade 5 toxicities occurred. Only patient no. 18 developed grade 4 bone marrow suppression, which recovered after treatments. Two patients (2/10) achieved PR 1 month after the PLDR.
Patients Who Received PLDR for Bulky Tumors
There were three patients (nos. 6, 12, and 14 in Table 1) given PLDR for bulky tumor. All the patients (3/3) achieved PR 1 month after the PLDR. One patient (no. 6 in Table 1) with posterior peritoneal adenocarcinoma received concurrent chemotherapy and developed grade 1 diarrhea. This patient achieved PR 1 month after the PLDR and remained progression-free till the last follow-up on 5/5/2017. The LRRFS lasted for 16 months. One patient with bladder cancer (no. 12 in Table 1) achieved complete response (CR) at 4 months after the PLDR, and the LRRFS lasted for 10 months. In the patients with bulky tumor, one developed grade 3 bone marrow suppression, one developed grade 1 diarrhea, and one had grade 2 bone marrow suppression.
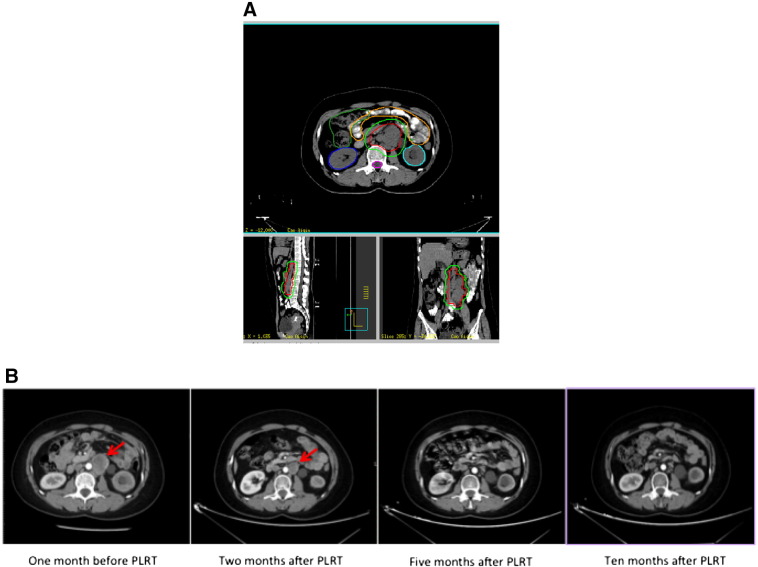
Taking patient no. 6 as an example, this patient was a 61-year-old female with posterior peritoneal adenocarcinoma diagnosed in November 2013. Initially, she received chemotherapy, but she remained with stable disease after chemotherapy. The tumor was 5 cm × 4 cm × 12 cm. Then, she received PLRT due to the bulky tumor. The PLRT began on 11/12/2015 and ended on 12/29/2015 without interruption. As shown in Figure 3A, the gross tumor volume was delineated, and the gross tumor volume to planning tumor volume (PTV) margin was 0.5 cm. A total dose of 50 Gy was delivered by PLRT. Only grade 1 diarrhea occurred during PLRT. At the second month after PLRT, the tumor regressed obviously, and this patient achieved PR (Figure 3B). Following this, this patient was given four cycles of chemotherapy (irinotecan plus tegafur). Five months after PLRT, this patient achieved nearly complete response (Figure 3B), and this patient remained disease-free until the last follow-up on 5/5/2017.
Figure 3.
(A) Delineation of the bulky disease. (B) Evaluation of treatment response by computed tomography.
Discussion
Refractory malignancies present clinical challenges and are indicative of poor outcomes. This study found that PLDR was effective and with acceptable toxicities for patients with refractory malignancies. PLDR is an effective and safe option not only for reirradiation but also for patients with poor performance status or bulky tumors to achieve palliative benefits. As far as we know, this is the first study trying to illustrate the role of PLDR in patients with poor performance status or bulky tumors. Based on the encouraging results of this retrospective study, a prospective clinical trial (NCT03061162) has been initiated by our institute in February 2017.
Our findings are clinically supported by the previous studies. Adkison et al. found that PLDR was safe for the retreatment of larger volumes to high doses in patients with recurrent glioma. The median survival for patients undergoing PLDR was 22 weeks. Age at the initial diagnosis, initial low-grade disease, and performance status were significant predictors of survival after initiation of PLDR [10]. In this study, patient no. 11 with recurrent glioma had a relatively longer LRRFS of 8 months maybe due to the young age at diagnosis, use of targeted therapy bevacizumab, and good performance status. In a case report, Li et al. reported that a 56-year-old man with nasopharyngeal carcinoma was treated with PLDR plus cetuximab for a recurrent lesion in the neck. The recurrent lesion had a CR with no apparent treatment-related toxicities [14]. Similar to this case report, patient no. 1 in our study was also with recurrent neck lesion after radical chemoradiotherapy for nasopharyngeal carcinoma. The concurrent regimen was chemotherapy with gemcitabine. This patient also achieved CR until the last follow-up on 5/5/2017. The LRRFS lasted for 17 months. Richards et al. found that PLDR was an effective retreatment strategy for breast cancer recurrence in the previously irradiated chest wall, axilla, or supraclavicular region [9]. Our study did not include patients with recurrent breast cancer. Based on the results of our study and the study from Richards [9], it will be reasonable to include recurrent breast cancer in the future clinical trials.
Our findings were also biologically reasonable. PLDR potentially exploits LDHRS. LDHRS has been characterized in over 40 human cell lines [15], [16], [17]. A series of 0.2-Gy pulses was applied in our study. In HT-29 adenocarcinoma cells, 2 Gy in 10 subfractions of 0.2 Gy favored a statistically significant decrease in cell survival compared to a single 2-Gy fraction [18]. Patient no. 6 in this study who had bulky tumor before treatment achieved PR 1 month after the PLDR and remained progression-free till the last follow-up on 5/5/2017. The therapeutic benefit may be due to LDHRS and improvement of hypoxia induced by PLDR.
Strengths of our study included the following: various kinds of malignancies were observed; the outcomes of each patient were intensively followed up; patients with a bulky tumor or poor performance status were also included. The limitation of our study was the single-institution nature. Thus, these findings require validation in studies of other populations at other institutions.
Conclusions
PLDR is an effective and safe option not only for reirradiation but also for patients with poor performance status or bulky tumors. We are currently validating these results in an ongoing prospective trial.
Ethics Approval and Consent to Participate
The ethics committee of Drum Tower Hospital has approved our study. Informed consents for all the patients were obtained before treatment.
Consent for Publication
Consents were obtained for publication from every patient involved.
Availability of Data and Supporting Materials Section
The data sets generated during the current study are not publicly available due the personal information but are available from the corresponding author on reasonable request.
Competing Interests
The authors do not see any conflicts of interest.
Funding
The National Key Research and Developmental Program of China supported this study (No. 2017YFC1308900).
Authors' Contributions
B. L. designed the study. J. Yan and J. Yang collected and analyzed the data. J. Yan and J. Yang wrote the manuscript. Y. Y., W. K., and W. R. organized the data. J. L., S. S. L., and B. G. designed the treatment plans. M. Y., X. Q., and L. Z. helped enroll patients. All authors read and approved the final manuscript.
Acknowledgements
The authors are grateful to all the patients receiving treatments in our department. We have learned great experiences when helping them battle the tumors, which will help more patients in the future.
Footnotes
Conflict of interest: The authors do not see any conflict of interest.
Funding: This study was supported by the National Key Research and Developmental Program of China (No. 2017YFC1308900).
References
- 1.Morris DE. Clinical experience with retreatment for palliation. Semin Radiat Oncol. 2000;10:210–221. doi: 10.1053/s1053-4296(00)80039-9. [DOI] [PubMed] [Google Scholar]
- 2.Lee DY, Chunta JL, Park SS. Pulsed versus conventional radiation therapy in combination with temozolomide in a murine orthotopic model of glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2013;86:978–985. doi: 10.1016/j.ijrobp.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 3.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 4.Park SS, Chunta JL, Robertson JM. MicroPET/CT imaging of an orthotopic model of human glioblastoma multiforme and evaluation of pulsed low-dose irradiation. Int J Radiat Oncol Biol Phys. 2011;80:885–892. doi: 10.1016/j.ijrobp.2011.01.045. [DOI] [PubMed] [Google Scholar]
- 5.Zhang P, Wang B, Chen X. Local tumor control and normal tissue toxicity of pulsed low-dose rate radiotherapy for recurrent lung cancer: an in vivo animal study. Dose Response. 2015;13 doi: 10.1177/1559325815588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wobb J, Krueger SA, Kane JL. The effects of pulsed radiation therapy on tumor oxygenation in 2 murine models of head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2015;92:820–828. doi: 10.1016/j.ijrobp.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Meyer K, Krueger SA, Kane JL. Pulsed radiation therapy with concurrent cisplatin results in superior tumor growth delay in a head and neck squamous cell carcinoma murine model. Int J Radiat Oncol Biol Phys. 2016;96:161–169. doi: 10.1016/j.ijrobp.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 8.Cannon GM, Tome WA, Robins HI, Howard SP. Pulsed reduced dose-rate radiotherapy: case report: a novel re-treatment strategy in the management of recurrent glioblastoma multiforme. J Neurooncol. 2007;83:307–311. doi: 10.1007/s11060-007-9329-z. [DOI] [PubMed] [Google Scholar]
- 9.Richards GM, Tome WA, Robins HI. Pulsed reduced dose-rate radiotherapy: a novel locoregional retreatment strategy for breast cancer recurrence in the previously irradiated chest wall, axilla, or supraclavicular region. Breast Cancer Res Treat. 2009;114:307–313. doi: 10.1007/s10549-008-9995-3. [DOI] [PubMed] [Google Scholar]
- 10.Adkison JB, Tome W, Seo S. Reirradiation of large-volume recurrent glioma with pulsed reduced-dose-rate radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:835–841. doi: 10.1016/j.ijrobp.2009.11.058. [DOI] [PubMed] [Google Scholar]
- 11.Pierquin B, Calitchi E, Mazeron JJ. A comparison between low dose rate radiotherapy and conventionally fractionated irradiation in moderately extensive cancers of the oropharynx. Int J Radiat Oncol Biol Phys. 1985;11:431–439. doi: 10.1016/0360-3016(85)90172-5. [DOI] [PubMed] [Google Scholar]
- 12.Tome WA, Howard SP. On the possible increase in local tumour control probability for gliomas exhibiting low dose hyper-radiosensitivity using a pulsed schedule. Br J Radiol. 2007;80:32–37. doi: 10.1259/bjr/15764945. [DOI] [PubMed] [Google Scholar]
- 13.Dilworth JT, Krueger SA, Dabjan M. Pulsed low-dose irradiation of orthotopic glioblastoma multiforme (GBM) in a pre-clinical model: effects on vascularization and tumor control. Radiother Oncol. 2013;108:149–154. doi: 10.1016/j.radonc.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 14.Li GH, Zhu B, Yang F. Use of cetuximab in combination with pulsed reduced dose-rate radiotherapy in a patient with recurrence of nasopharyngeal carcinoma in the neck. Exp Ther Med. 2012;3:869–872. doi: 10.3892/etm.2012.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marples B. Is low-dose hyper-radiosensitivity a measure of G2-phase cell radiosensitivity? Cancer Metastasis Rev. 2004;23:197–207. doi: 10.1023/B:CANC.0000031761.61361.2a. [DOI] [PubMed] [Google Scholar]
- 16.Joiner MC, Marples B, Lambin P. Low-dose hypersensitivity: current status and possible mechanisms. Int J Radiat Oncol Biol Phys. 2001;49:379–389. doi: 10.1016/s0360-3016(00)01471-1. [DOI] [PubMed] [Google Scholar]
- 17.Marples B, Lambin P, Skov KA, Joiner MC. Low dose hyper-radiosensitivity and increased radioresistance in mammalian cells. Int J Radiat Biol. 1997;71:721–735. doi: 10.1080/095530097143725. [DOI] [PubMed] [Google Scholar]
- 18.Lin PS, Wu A. Not all 2 Gray radiation prescriptions are equivalent: cytotoxic effect depends on delivery sequences of partial fractionated doses. Int J Radiat Oncol Biol Phys. 2005;63:536–544. doi: 10.1016/j.ijrobp.2005.06.010. [DOI] [PubMed] [Google Scholar]