Abstract
Background & Aims
During late embryonic development and through weaning, enterocytes of the ileum are highly endocytic. Defects in endocytosis and trafficking are implicated in neonatal disease, however, the mechanisms regulating trafficking during the developmental period are incompletely understood. The apical endosomal protein endotubin (EDTB) is highly expressed in the late embryonic and neonatal ileum. In epithelial cells in vitro, EDTB regulates both trafficking of tight junction proteins and proliferation through modulation of YAP activity. However, EDTB function during the endocytic stage of development of the intestine is unknown.
Methods
By using Villin-CreERT2, we induced knockout of EDTB during late gestation and analyzed the impact on endocytic compartments and enterocyte structure in neonates using immunofluorescence, immunocytochemistry, and transmission electron microscopy.
Results
Deletion of the apical endosomal protein EDTB in the small intestine during development impairs enterocyte morphogenesis, including loss of the apical endocytic complex, defective formation of the lysosomal compartment, and some cells had large microvillus-rich inclusions similar to those observed in microvillus inclusion disease. There also was a decrease in apical endocytosis and mislocalization of proteins involved in apical trafficking.
Conclusions
Our results show that EDTB-mediated trafficking within the epithelial cells of the developing ileum is important for maintenance of endocytic compartments and enterocyte integrity during early stages of gut development.
Keywords: Trafficking, Endotubin, Rab, Tight Junction, Endosomes
Abbreviations used in this paper: AEC, apical endocytic complex; AP, alkaline phosphatase; CRISPR/Cas9, clustered regularly interspaced short palindromic repeats/cas9 endonuclease; EDTB, endotubin; EEA1, early endosomal antigen 1; G, guide; GFP, green fluorescent protein; GTPase, guanosine triphosphatase; KO, knockout; LAMP1, lysosome-associated membrane protein 1; MAMDC4, MAM domain containing 4; MVID, microvillus inclusion disease; P, postnatal day; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; SDS, sodium dodecyl sulfate; TBST, tris-buffered saline with 0.1% tween-20; TEM, transmission electron microscopic; TJ, tight junction
Graphical abstract
See editorial on page 167.
Summary.
The apical endosomal protein endotubin is highly expressed during enterocyte development. Here, we show that endotubin is required for membrane trafficking and enterocyte morphogenesis. We propose that endotubin serves as a scaffold that modulates apical membrane traffic.
The intestinal epithelium serves as a selective barrier to macromolecules and pathogens, and dysfunction of the enterocytes can lead to disease in both neonates and adults.1, 2, 3, 4, 5, 6, 7, 8 During early development of the ileum, the enterocytes of the villus undergo a stage in which they are highly endocytic, with a well-developed tubular apical endosomal complex and extensive lysosomal system.9, 10 In rodents, the apical endocytic complex (AEC) forms late in gestation and is present in the apical cytoplasm through weaning.9, 10, 11, 12, 13 These membranes consist of both tubular and vesicular endosomes adjacent to the apical plasma membrane, with the giant lysosome filling the majority of the apical cytoplasm. This endocytic complex may provide nutrition through the uptake of macromolecules from the lumen and also facilitates the transfer of growth factors such as nerve and epidermal growth factors from the lumen to the circulation.13, 14, 15 Both the AEC and giant lysosome are lost at the time of weaning.
The importance of vesicular trafficking in enterocyte development has been established in studies with knockout of the small guanosine triphosphatases (GTPases) Rab8a and Rab11a, which result in defective localization of apical enzymes and a poorly developed microvillus border.16, 17, 18, 19 Studies with knockout of the transcription factor Cdx2 showed that Cdx2 regulates a network of genes that are involved in endosome/lysosome trafficking.20 Finally, microvillus inclusion disease (MVID) has been shown to be caused by a mutation in myosin Vb or syntaxin 3, proteins known to mediate apical trafficking, and loss of the actin nucleator Arp2/3 results in defects in endolysosomal assembly and vesicle trafficking.2, 21, 22, 23, 24, 25 Endotubin (EDTB) also known as MAM domain containing 4 (MAMDC4) is an integral membrane protein that is concentrated in the tubular apical endosomes of developing intestine.11, 12 In the rodent, EDTB begins to be expressed late in embryonic development and retains high expression levels until weaning.11 It still is expressed in enterocytes after weaning, but at approximately one half the levels found during development. Despite its endosomal location, experiments in epithelial cells in vitro have shown that EDTB regulates the assembly and maintenance of tight junctions (TJs) and interacts with TJ proteins and transcriptional co-factors.26, 27 However, the role of EDTB in the regulation of membrane trafficking and function in the developing intestine is unknown.
To better understand the importance of apical membrane trafficking during intestinal development, we used inducible intestinal epithelial knockout of EDTB. EDTB was knocked out late in gestation before assembly of the AEC and was evaluated shortly after birth. We found that loss of EDTB resulted in disruption of the AEC and the giant lysosome. Furthermore, we observed loss of some TJ proteins from the lateral membranes and the occasional formation of large microvillus-containing inclusions. These results implicate EDTB as an essential regulator of membrane trafficking during intestinal development.
Materials and Methods
Mice
The EDTB (MAMDC4) targeting vector was generated containing a neomycin selectable marker and loxP recombination sites flanking exons 2 and 10. Deletion of exons 2 through 10 results in a frame shift mutation and premature stop codon. The mouse colony containing MAMDC4fl/+ and MAMDC4fl/fl offspring was generated and maintained by the Experimental Mouse Shared Resource (EMSR) at the University of Arizona Cancer Center and supported by the National Cancer Institute of the National Institutes of Health P30 CA023074. Villin-CreErt2 and MAMDC4fl/fl animals were crossed to generate MAMDC4fl/+/Villin-CreErt2 males. MAMDC4fl/+/Villin-CreErt2 males then were mated with MAMDC4fl/fl females. Pregnant females were subjected to intraperitoneal injections with 100 uL of 10 mg/mL 4-hydroxytamoxifen (cat. T176-50; Sigma, St. Louis, MO) on day 15 of gestation to induce expression of Cre recombinase. Offspring were collected at postnatal day 3 (P3).
Genotyping
Tail clips were obtained and digested in 90 μL tail digestion buffer (100 mmol/L Tris, pH 8.0, 0.5 mmol/L EDTA, 0.2% sodium dodecyl sulfate [SDS], 200 mmol/L NaCl, 100 μg proteinase K) for 1 hour at 55°C. After digestion, 300 μL H2O was added, samples were vortexed, and then incubated at 95°C for 15 minutes. Debris was removed by centrifugation and 2 μL of supernatant was used for polymerase chain reaction (PCR) reactions using TopTaq Master Mix Kit (cat. 200403; Qiagen).
The primers used were as follows: mouse EDTB neo forward, GGGGTTTGCTCGACATTG; mouse EDTB wild type reverse, ATACAGCTTTGATGGGGCTTC; mouse EDTB wild type forward, GTGGCGGTTCTTGGTATATGTC; villin-cre forward, CAAGCCTGGCTCGACGGCC; and villin-cre reverse, CGCGAACATCTTCAGGTTCT.
Tissue Preparation
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Arizona. Animals were euthanized and the abdominal wall was incised. The intestine was exposed and the ileum was flushed with ice-cold phosphate-buffered saline (PBS) and divided into proximal and distal segments. Tissue was fixed in 4% paraformaldehyde in PBS for 4 hours at room temperature for paraffin embedding. After fixation, tissue was washed within PBS and dehydrated through an ethanol series (2 × 30 min in 50%, 70%, and 100%) followed by 50:50 ETOH:xylene and 100% xylene. Tissue was transferred to paraffin at 60°C for 1 hour and to fresh paraffin overnight at 60°C. For electron microscopy, ileum was fixed in 2% glutaraldehyde in 0.1 mol/L cacodylate buffer (pH 7.4). Postfixation was performed in aqueous 1% OsO4 for 2 hours at 4°C, and en bloc stained with 0.5% uranyl acetate, followed by dehydration in ethanol and embedding in Spurrs resin (Electron Microscopy Sciences, Hatfield, PA). Sections were stained with uranyl acetate and lead citrate and examined using a Philips 410STEM at 80 kV. Images were acquired using an AMT-XR40 digital camera (Advanced Microscopy Techniques).
Protein Analysis
Ileum segments were placed in RIPA buffer (20 mmol/L Tris, pH 7.4, 1% Triton X-100, 1% deoxycholate, 0.1% SDS, 100 mmol/L NaCl) containing protease inhibitors (Complete Mini, cat. 11374600; Roche, Basel, Switzerland) and the phosphatase inhibitors calyculin A and sodium orthovanadate (Sigma, St. Louis, MO) and homogenized. Debris was removed by centrifugation and protein concentration was determined using the Pierce 660-nm Protein Assay Reagent (cat. 1861426; Thermo Scientific). For immunoblotting, 15–20 μg of protein was separated on a SDS–polyacrylamide gel electrophoresis, transferred to nitrocellulose, blocked in tris-buffered saline with 0.1% tween-20 (TBST) + 5% milk, and incubated in primary antibody overnight at 4°C (Table 1). Blots were washed in TBST at room temperature and incubated with secondary antibody followed by imaging using the LICOR-Odyssey system.
Table 1.
Primary Antibodies Used in This Study
| Antibody | Source | Dilution | Antigen retrieval |
|---|---|---|---|
| Mouse anti-endotubin (5F11) | Wilson Lab (University of Arizona, Tucson, AZ) | IF 1:3 Immunoblot 1:5 |
None |
| Rabbit anti-Rab 11a | Invitrogen (cat. 71-5300) | ICC 1:100 | Citrate, pH 6.0 |
| Rabbit anti-Rab 14 | Sigma (cat. R0656) | ICC 1:500 | Tris/EDTA, pH 9.0 |
| Rabbit anti–claudin 1 | Invitrogen (cat. 51-9000) | ICC 1:50 Immunoblot 1:200 |
Tris/EDTA, pH 9.0 |
| Rabbit anti-claudin 2 | Invitrogen (cat. 51-6100) | IF 1:200 Immunoblot 1:500 |
Tris/EDTA, pH 9.0 |
| Rabbit anti-Lamp1 | Abcam (Cambridge, MA) (cat. ab24170) | IF 1:200 | Citrate, pH 6.0 |
| Rabbit anti-NHE3 | Kiela Lab (University of Arizona, Tucson, AZ) | IF 1:1000 | Tris/EDTA, pH 9.0 |
| Rabbit anti-EEA1 | Abcam (cat. ab2900) | ICC 1:500 | Citrate pH, 6.0 |
ICC, immunocytochemistry; IF, immunofluoresence.
Tissue Labeling
Sections (5 μm) were cut and dried on a slide warmer followed by rehydration. Antigen retrieval, if required, was performed using a vegetable steamer. Antigen retrieval buffers included 10 mmol/L citrate buffer, 0.05% Tween-20, pH 6.0, or 10 mmol/L Tris, 1 mmol/L EDTA, and 0.05% Tween-20, pH 9.0. Slides were incubated for 20–40 minutes in the vegetable steamer in prewarmed antigen retrieval buffer. Tissue was blocked in TBST/5% fetal bovine serum for 30 minutes at room temperature followed by primary antibody overnight at 4°C. For fluorescent labeling, sections were incubated in secondary antibody in blocking solution for 30 minutes at room temperature and mounted using Prolong Gold with 4′,6-diamidino-2-phenylindole (cat. P36971; Invitrogen, Carlsbad, CA). For immunohistochemistry, slides were incubated in 3% H2O2 solution before secondary antibody addition. Signal Boost (cat. 8114S; Cell Signaling Technology, Danvers, MA) and DAB (3,3′-diaminobenzidine tetra hydrochloride) detection kits (cat. 8059S; Cell Signaling Technology, Danvers, MA) were used for visualization, following the manufacturer's instructions. Slides were washed in H2O, dehydrated through an ethanol series, and mounted using SignalStain Mounting media (cat. 14177S; Cell Signaling Technology).
Alkaline Phosphatase Histochemistry
Tissue was deparaffinized as described earlier and incubated in alkaline phosphatase buffer (100 mmol/L NaCl, 100 mmol/L Tris, 5 mmol/L MgCl2, pH 9.5) for 15 minutes followed by staining solution containing 40 μL nitroblue tetazloium (NBT) at 50 mg/mL (cat. N6876; Sigma) and 40 μL 5-bromo-4-chloro-3-indolylphosphate (BCIP) at 50 mg/mL (cat. B-6149; Sigma) in 5 mL.
Microscopy
For conventional light microscopy, images were obtained using a Leica DMI6000 microscope and Leica LAS-X software to capture bright field images. For immunofluorescence, sections were imaged using an Olympus FluoView 1200 confocal microscope with a 60 Å, NA 1.43 oil immersion objective. Identical imaging parameters were used within a single experiment. Images were processed using Adobe Photoshop.
Caco2–EDTB (MAMDC4) Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR associated protein 9 (CRISPR/Cas9)
An EDTB CRISPR gene knockout kit was purchased from Origene (cat. KN216843). Constructs containing the targeting sequence along with green fluorescent protein (GFP) and a puromycin selection cassette were transfected into Caco2BBE using Lipofectamine 2000 (Invitrogen). Constructs included guide (G)1, G2, and a scramble control to generate 2 EDTB knockout (KO) and a single control cell line. Flow cytometry using a FACScanto II (BD Biosciences, San Jose, CA) was used to isolate GFP-positive CRISPR cells from both the EDTB KO and control cell lines. After expansion of the mixed population of GFP-expressing cells, loss of EDTB expression was verified by PCR and immunoblotting. Control and Caco2 CRISPR EDTB KO cell lines were maintained in Dulbecco's modified Eagle medium (Invitrogen) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA), 1000 U of penicillin, 1 mg/mL streptomycin, 20 mmol/L L-glutamine, 10 mmol/L HEPES, and 2 μg/mL puromycin.
Dextran Uptake
For evaluation of endocytosis, Caco2BBE CRISPR cell lines were plated in a 6-well plate at 65% confluency. Twenty-four hours after plating the media was replaced with Dulbecco's modified Eagle medium containing 1 mg/mL of 4 kilodaltons fluorescein isothiocyanate dextran and incubated at 37°C for 30 minutes. Cells were washed twice with ice-cold PBS and lysed with 1 mL PBS containing 0.17% Triton X-100. After centrifugation, supernatant was collected and fluorescence intensity was measured using a Veriscan fluorometer (Horiba Scientific, Edison, NJ).
Statistical Analysis
Statistical comparisons were performed using Excel (Microsoft, Redmond, WA) and the Student t test. Data are expressed as means ± SEM.
Results
Intestinal Epithelial KO of EDTB Disrupts the Morphology of the Intestinal Epithelium
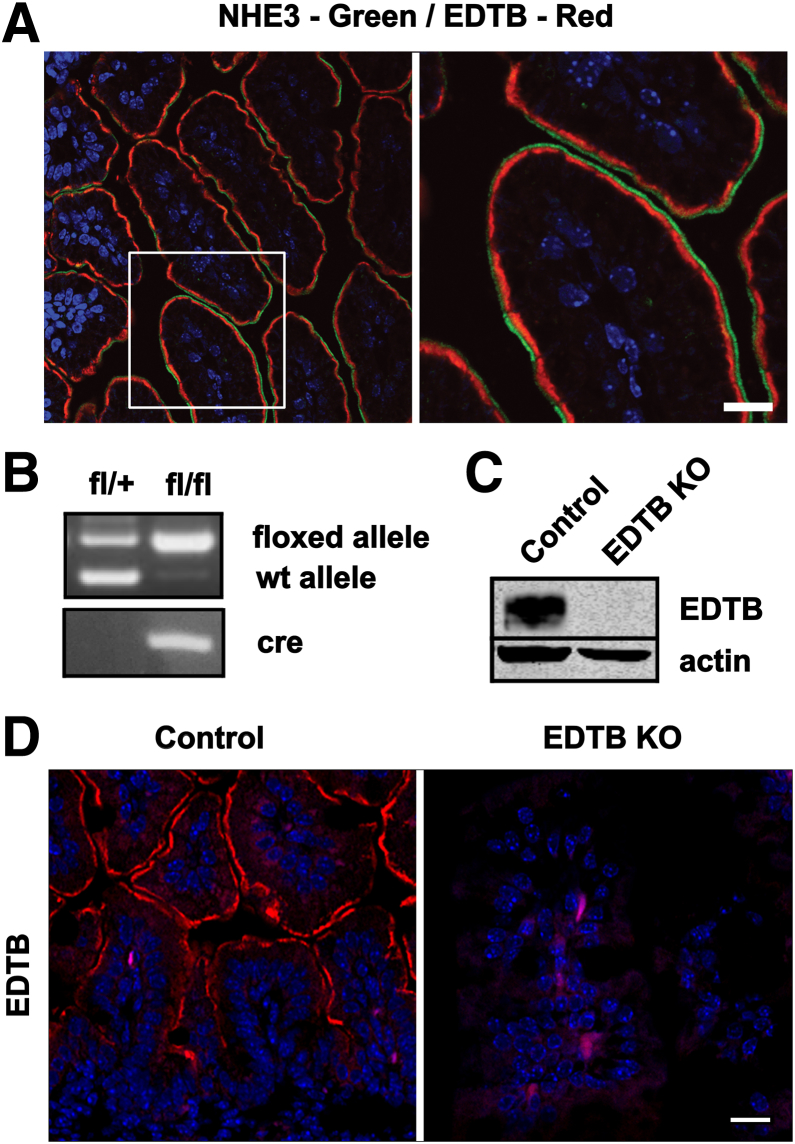
EDTB marks the endocytic stage of intestinal development of many species.28, 29 Immunoelectron microscopy has localized EDTB to the tubular endosomes of the AEC in the small intestine during development and the suckling period.11, 12 EDTB labeling is subapical and distinct from the microvilli because double labeling with antibodies against EDTB and the apical transporter NHE3 is closely associated but nonoverlapping (Figure 1A). To determine the role that EDTB plays in development of the ileum we used a conditional intestine-specific KO of EDTB in mice (Figure 1B). Expression of Cre recombinase under control of the villin promoter was induced at embryonic day 15 through injection of 4-hydroxytamoxifen into the pregnant females and the ileum was collected at P3. At this time in development, the ileum is a simple columnar epithelium with villi, but crypt invagination has not yet begun. Genomic DNA was used for PCR analysis to verify the incorporation of the EDTBfl/fl alleles and the presence of the Cre recombinase, and loss of EDTB expression was verified by Western blot analysis of ileum lysates (Figure 1B and C). Furthermore, immunofluorescence microscopy showed that EDTB is absent from the apical domain of epithelial cells along the length of the villi (Figure 1D).
Figure 1.
EDTB is present in apical endosomes and is deleted upon tamoxifen induction of the Cre-recombinase. (A) P3 ileum labeled with antibodies against NHE3 (green) and EDTB (red). EDTB labeling is adjacent to NHE3 and localized in the apical cytoplasm (boxed area is magnified). Nuclei (4′,6-diamidino-2-phenylindole, blue). (B) PCR genotyping of P3 pups shows the presence of the floxed alleles and CreERT2 in KO animals. (C) Lysates from the proximal ileum were analyzed by Western blot and confirm loss of EDTB protein in KO intestine. (D) Immunofluorescence of distal ileum shows apical localization of EDTB (red) in control animals and loss of expression in KO animals. Nuclei (4′,6-diamidino-2-phenylindole, blue). Scale bars: 25 μm.
EDTB KO Disrupts Intestinal Morphology
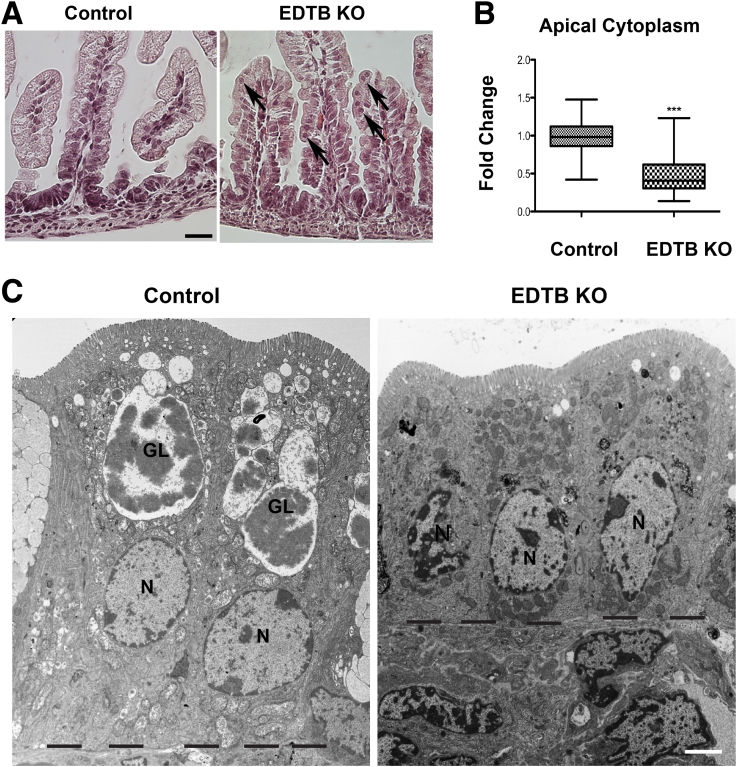
Upon dissection of the small intestine of control and knockout mice, there were no obvious differences in the length or gross morphology of the intestines. To assess the impact of EDTB KO on the cellular characteristics of the developing ileum, we performed histologic and transmission electron microscopic (TEM) analysis. H&E staining of sections showed that villi from control mice were uniform with columnar enterocytes, whereas the villi of the KO animals were irregular in appearance, suggesting a variability in cell height along the villus axis (Figure 2A). Furthermore, in KO animals, the nuclei appeared irregularly located within the enterocytes, with many displaced adjacent to the apical border (Figure 2A, arrows). This miss-localization of the nuclei was quantified by measuring the distance from the nuclei to the apical border and showed that there is decreased apical cytoplasm in the epithelial cells (Figure 2C). Ultrastructural analysis showed that KO of EDTB does not cause a loss of epithelial character of the enterocytes (Figure 2C). Unlike mice that have been knocked out for Cdx2, Rab8a, or Rab11a,18, 19, 20 there does not appear to be a defect in the assembly of the brush border or formation of microvilli along the lateral membranes (Figure 3). This may reflect age differences because early after birth, KO of Rab8a did not affect microvillus height.18 In addition to the reduction in apical cytoplasm (Figure 2B), there was a decrease in the overall cell height and absence of the giant lysosome. Together, these results suggest that EDTB is critical for the normal differentiation of developing enterocytes.
Figure 2.
Disruption of enterocyte morphology after EDTB KO. (A) H&E staining of control and EDTB KO ileum. In control animals, the enterocytes have basal nuclei. In contrast, in knockout animals the nuclei are displaced, with many nuclei close to the brush border (arrows). Scale bar: 25 μm. (B) Whisker plot representation showing fold change in cell height from the nuclei to the apical border of enterocytes. ***P < .005. (C) TEM of enterocytes of control and EDTB KO animals. In both cases the enterocytes are a simple epithelium with a well-developed brush border. However, in KO animals the cells are shorter and lack the giant lysosome. Dashed line marks the basal border of the enterocytes. Scale bar: 5 μm. GL, giant lysosome; N, nucleus.
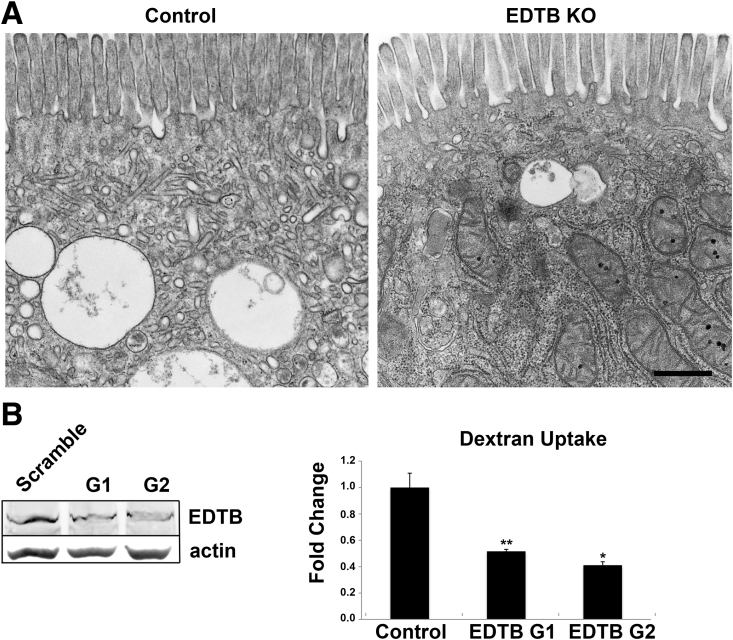
Figure 3.
Disruption of apical endocytic compartment after EDTB KO. (A) TEM of the apical domain of control and EDTB KO enterocytes at P3. Control animals have a well-developed AEC. EDTB KO animals largely lack apical endosomal tubules and vesicles. Scale bar: 0.5 μm. (B) CRISPR/Cas9 genome editing tool was used to disrupt EDTB in Caco2BBE cells followed by pooling of cells. Loss of EDTB expression was verified by Western blot. The presence of some EDTB likely is owing to the mixed population. (C) Uptake of 4 kilodaltons of fluorescent dextran for 30 minutes followed by lysis and fluorometric analysis. Dextran uptake was decreased after EDTB KO in Caco2BBE cells. *P < .05, **P < .01.
Endocytosis Is Decreased After EDTB KO
EDTB is highly expressed in membranes of the AEC, and its up-regulation is coincident with the development of these membranes.11 To determine the effect of EDTB KO on these membranes, we examined the enterocytes by transmission electron microscopy. In control animals, the apical endocytic complex was present in the apical cytoplasm just below the brush border (Figure 3A). This compartment comprised an extensive tubular/vesicular network of endosomes. KO of EDTB results in a dramatic loss of apical endocytic vesicles and tubules, suggesting that EDTB is required for the formation and/or maintenance of this compartment (Figure 3A).
Because EDTB is highly expressed during the endocytic stage of development, and TEM suggests that loss of EDTB results in loss of the AEC and giant lysosome, we next tested whether EDTB could play a role in endocytosis. To test this, we used an in vitro uptake assay using Caco2BBE cells that had been subjected to Crispr/Cas9-mediated deletion of EDTB. Caco2BBE cells expressing GFP were isolated using flow cytometry. These mixed populations of Crispr/Cas9 EDTB KO cells were expanded and designated G1 and G2, representing independent guide RNAs used to generate the EDTB KO. Control and EDTB KO cells were incubated with fluorescent dextran followed by cell lysis and fluorometric quantification. As shown in Figure 3C, knockout of EDTB results in decreased uptake of this tracer.
Giant Lysosome Formation Is Impaired in EDTB KO Ileum
A morphologic feature unique to developing enterocytes is the giant lysosome.9, 10, 11 The giant lysosome is a transient compartment that mediates degradation of milk proteins during the suckling period. The giant lysosome forms through fusion of multiple apical lysosomes and occupies the region of the cell just basal to the EDTB-expressing AEC.11
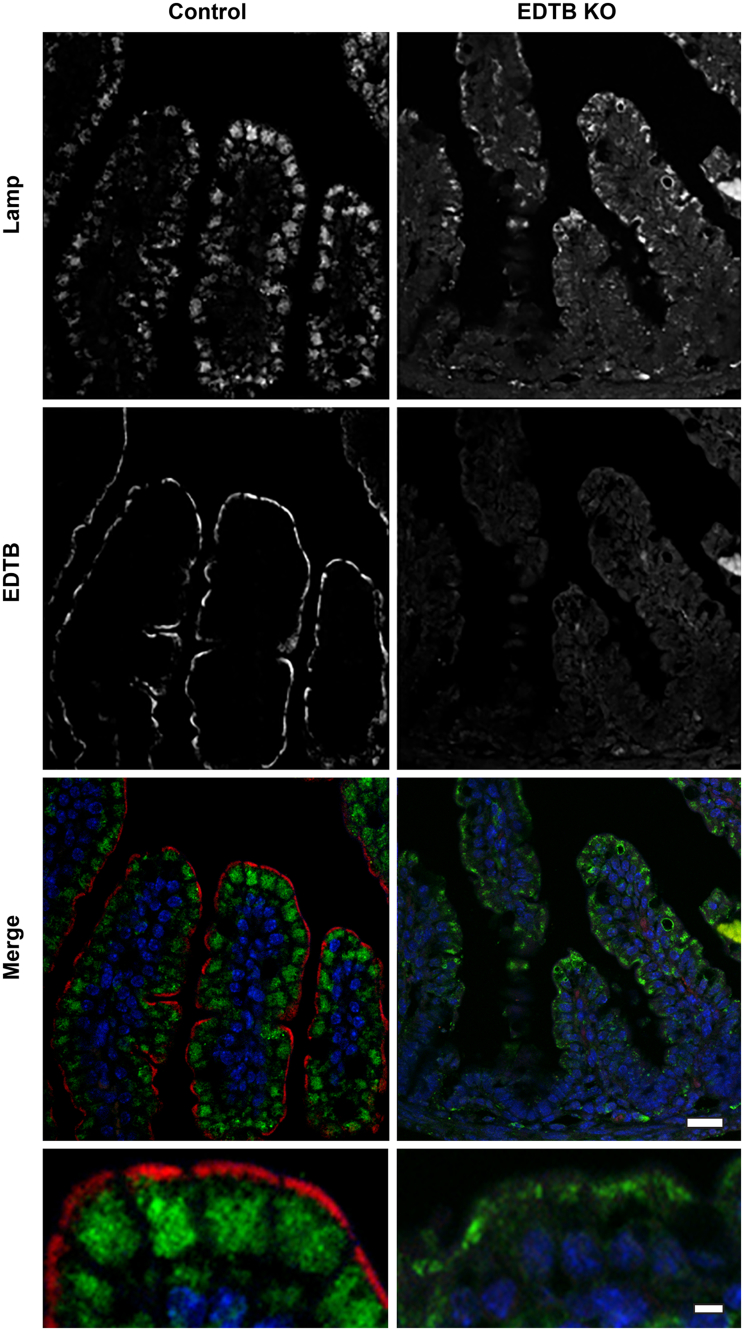
As shown earlier (Figure 2) by TEM, the giant lysosome is absent from the enterocytes of EDTB KO animals. To determine if the lysosomal membranes were dispersed to other domains in the cell, we used an antibody against the lysosome-associated membrane protein 1 (LAMP1). In control ileum, LAMP1 labeling is present in the space between the nucleus and brush border, consistent with the position of the giant lysosome (Figure 4). However, after EDTB KO, LAMP1 labeling is irregular and compact and often is displaced apically (Figure 4). Interestingly, this contrasts with Cdx2 KO mice, which appear to have aberrant development of an apical giant lysosome before birth.20 Whether this structure represents a true giant lysosome or is owing to the failure of lysosomal enzymes and transporters to localize to the lysosomal vacuole is not clear. In Rab8a KO mice after weaning, large apical inclusions are positive for LAMP1, perhaps reflecting decreased recycling.18
Figure 4.
EDTB KO disrupts lysosomes. Immunofluorescent labeling of ileum using the lysosome marker LAMP1 (green). In control animals, LAMP1 labeling is present in large structures above the nucleus but distinct from the EDTB-positive AEC (red). After EDTB KO, LAMP1 labeling is more compact and displaced apically. Nuclei (blue). Scale bars: 20 μm (6 upper panels) and 5 μm (2 lower panels).
Apical Membrane Markers in EDTB KO Ileum
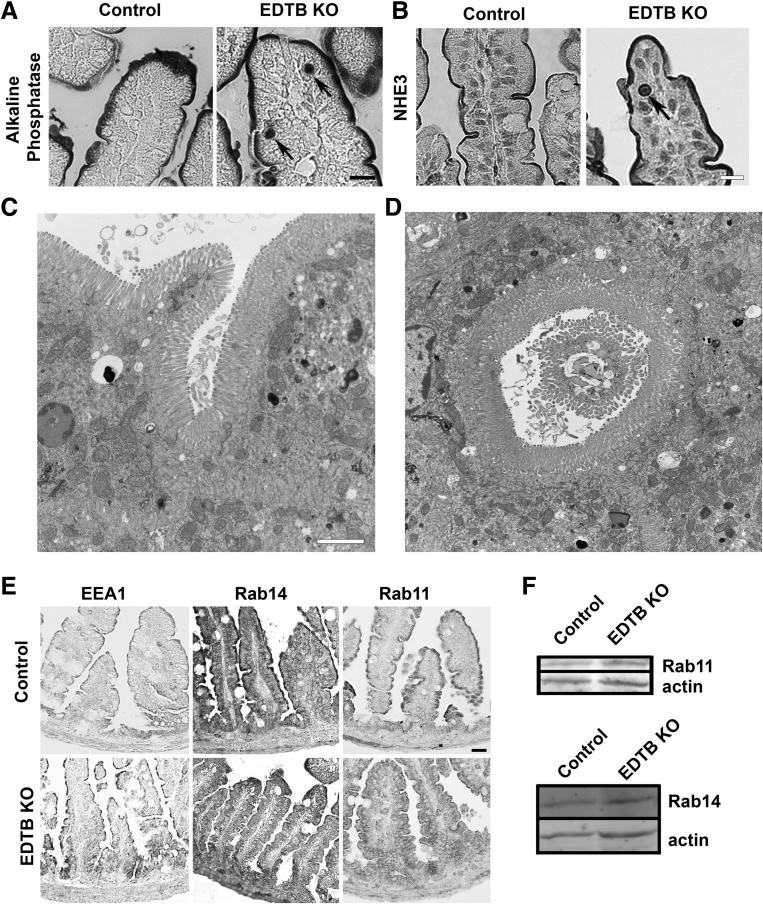
Because EDTB KO results in loss of the AEC, we next tested the localization of the apical plasma membrane markers alkaline phosphatase (AP) and the Na+/H+ exchanger, NHE3. In Cdx2, Rab8a, or Rab11a KO mice, AP localizes to the apical membrane but also is displaced into the apical cytoplasm.2, 21, 22, 23, 24, 25 In control and EDTB KO ileum, AP also is present at the apical plasma membrane (Figure 5). However, in animals with EDTB KO, in some enterocytes AP accumulates in large vacuoles. These apical vacuoles are rare, but largely (25 of 33 observed) present in the supranuclear cytoplasm. Interestingly, alkaline phosphatase histochemistry in adult EDTB KO animals does not result in these large inclusions (data not shown). With respect to NHE3, there was some nonspecific labeling of the nuclei in both control and KO animals. However, there also were large vacuoles that contained NHE3 in the KO animals (Figure 5B, arrows). Large, membrane-bound inclusions are found in individuals with MVID, which is caused by mutations to myosin Vb or syntaxin3,2, 16, 21, 24, 30, 31 and is thought to reflect a defect in recycling to the apical plasma membrane. Interestingly, we occasionally observed large microvillus-containing vacuoles in the enterocytes of EDTB KO intestine (Figure 5D).
Figure 5.
EDTB KO results in intracellular accumulation of apical proteins in some enterocytes. (A) Alkaline phosphatase histochemistry of P3 ileum of control and EDTB KO enterocytes. Alkaline phosphatase is present on the apical membrane and AP-positive inclusions are present in EDTB KO enterocytes (arrows). Scale bar: 25 μm. (B) NHE3 labeling of P3 ileum of control and EDTB KO enterocytes. NHE3 inclusions are present in EDTB KO enterocytes (arrow). Scale bar: 25 μm. (C and D) TEM of enterocytes shows large invaginations and inclusions containing microvilli. Scale bar: 5 μm. (E) Labeling with antibodies against EEA1, Rab11a, and Rab14. EEA1 is localized to the apical cytoplasm in control and EDTB KO ileum. Both Rab11 and Rab14 are enriched in the apical cytoplasm of control enterocytes. After EDTB KO, the labeling is diffuse within the enterocytes. Scale bar: 50 μm. (F) Lysates of proximal ileum were analyzed by Western blot to examine levels of Rab protein. The levels of both Rab11 and Rab14 appear unchanged.
In fibroblasts and epithelial cells in culture, EDTB localized to an early endosomal compartment that was distinct from the early endosomes marked by the endosomal marker early endosomal antigen 1 (EEA1).32 To determine the effect of EDTB knockdown on EEA1-positive early endosomes in developing intestine, we labeled control and KO ileum with antibody against EEA1. EEA1 labeling was enriched at the apical border in both control and KO animals, suggesting that the distinct early endosomal compartments identified in vitro also were present in vivo (Figure 5E). To further assess early endosomal compartments, we examined the distribution of Rab small GTPases. EDTB interacted with the small GTPase Rab14, an apical endosomal Rab, and Rab14 impacted the targeting of apical membrane, TJ, and polarity proteins.33, 34, 35 Furthermore, Rab11 is essential for normal membrane trafficking in enterocytes.16, 19 Because of these relationships, we labeled the ileum of control and KO mice with antibodies against Rab11a and Rab14. As shown in Figure 5E, Rab11a and Rab14 are present in the apical cytoplasm of villus enterocytes in control animals. However, after EDTB KO, both Rab11a and Rab14 were dispersed in the cells (Figure 5E). However, the protein levels of these Rabs were unaffected by EDTB KO (Figure 5F).
Mislocalization of TJ Proteins After EDTB KO
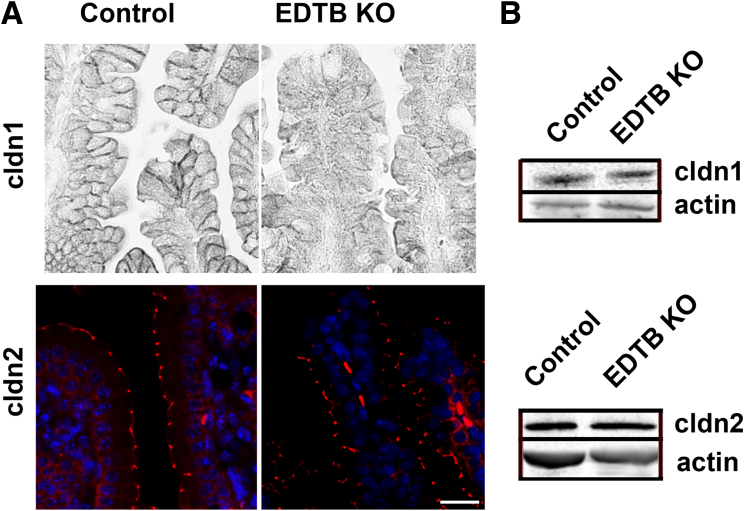
EDTB interacts with occludin and impacts the trafficking of TJ proteins.26, 27 We next used immunohistochemistry to determine the localization of the TJ proteins in the ileum after EDTB KO. There is no change in the relative amounts of claudin 1 or 2 proteins in KO ileum (Figure 6B), however, claudin 1 localization at the tight junction is lost with deletion of EDTB (Figure 6A). Claudin 2, on the other hand, remains localized at the junction, suggesting the EDTB plays a role in trafficking of a subset of TJ proteins.
Figure 6.
EDTB KO disrupts TJ protein localization. (A) Claudin (cldn) 1 and claudin 2 labeling of control and EDTB KO ileum. Claudin 1 is present at the lateral membrane of control cells. However, in EDTB KO ileum the labeling is diffuse, with limited labeling at the lateral membrane. Claudin 2 is localized at the apical membrane in both control and EDTB KO ileum. Scale bar: 25 μm. (B) Lysates of the proximal ileum were analyzed by Western blot to examine levels of TJ proteins. The levels of claudin 1 and claudin 2 are unchanged.
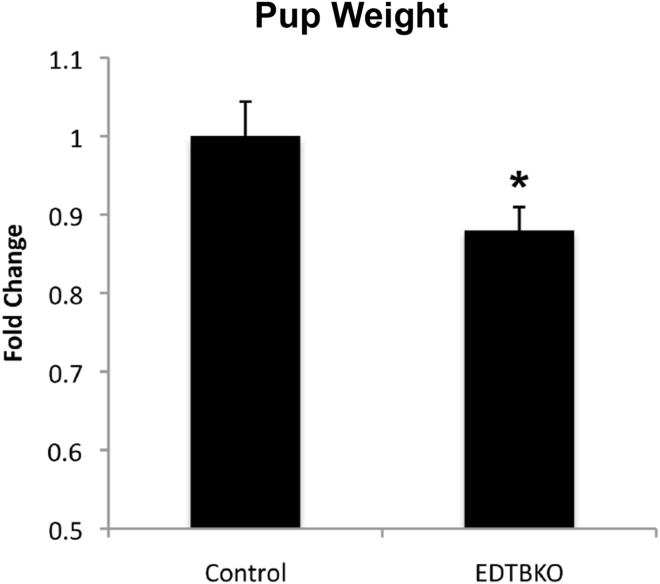
Apical endocytosis is a major route of nutrient absorption in the suckling rodent. Because EDTB KO resulted in loss of the AEC and decreased endocytosis, we tested the effect of EDTB KO on the size of the neonatal pups. A total of 20 pups were collected at P3 from 3 separate litters. Eleven of the pups were EDTBfl/fl and cre positive. There was a 12% reduction in weight compared with littermate controls (Figure 7). These results suggest that EDTB function is important for nutrient uptake and growth. Interestingly, after P15, the animals appear to recover to the same body weight as littermate controls (data not shown).
Figure 7.
Reduced growth after endotubin KO. Control and KO mice were weighed on P3. Mice with EDTB KO are lighter than controls. *P < .05.
Discussion
EDTB was discovered as an integral membrane protein that is highly expressed during the development of the intestine and is enriched in the membranes of the AEC; its expression is coincident with the development of the apical endocytic complex.11, 12 We show here that EDTB plays an integral role in the differentiation and structure of enterocytes in the developing intestine. The AEC has been shown to be important during the suckling period for both uptake of luminal contents and for sorting of growth factors for transcytosis.13, 14, 15, 36 Here, we found that EDTB is essential for the establishment of the AEC and its loss results in decreased endocytosis, aberrant trafficking of apical membrane components and the giant lysosome, and mislocalization of TJ proteins.
Membrane trafficking has been established as an essential process during morphogenesis of the small intestine.2, 16, 17, 18, 19, 20, 30, 37 KO of the transcription factor Cdx2 that controls the expression of several components of the trafficking machinery results in defects in cell polarity, tissue morphogenesis, and differentiation.20, 38 However, it does not appear that expression of EDTB is regulated directly by Cdx2 because it is not present in the published data set. However, it is possible that EDTB was not present in the array used to assess Cdx2 targets. Small GTPases such as Cdc42, Rab8a, and Rab11a have been identified as important mediators of polarity and apical membrane trafficking machinery in enterocytes.16, 17, 18, 19, 37, 39, 40 Knockout of Rab8a or Rab11 in the intestine results in mislocalization of apical transporters and enzymes such as dipeptidylpeptidase IV, NHE3, and alkaline phosphatase.16, 17, 18, 19 Interestingly, knockout of Rab8a or Cdc42 resulted in the formation of apical inclusions, but knockout of Rab11a resulted in microvillar defects but no microvillus inclusions.16, 17, 18, 19, 37, 39, 40 A recent comprehensive study examining the effects of double knockout of Rab8a and Rab11a in enteroctyes showed that these proteins modulated similar but slightly distinct pathways that nonetheless could compensate for partial loss of the function of one of the pair.39 However, the morphology of the enteroctyes after knockdown of these proteins was different from that observed after EDTB knockout, suggesting that different points of the endocytic process were being impacted by EDTB knockout.
After weaning, the apical endosomal complex is less elaborate, and the giant lysosome is lost. This transition is regulated at least in part by the transcription factor Blimp1,41 and knockout of Blimp1 results in decreased expression of EDTB. Interestingly, although loss of EDTB results in a more mature enterocyte phenotype, we did not observe expression of adult brush-border enzymes such as sucrase isomaltase (data not shown), suggesting that EDTB loss does not simply result in a conversion to the adult enterocyte.
How loss of endotubin mediates the pleiotropic effects observed here is not completely understood. EDTB regulates the maintenance of TJ and interacts with both the small GTPase Rab14 and occludin.26, 27, 35 In nonpolarized cells, Rab14 has been shown to mediate trafficking between the Golgi apparatus and endosomes/plasma membrane, as well as in lysosomal maturation.42, 43, 44, 45, 46 We found that Rab14 is essential for apical targeting of a subset of proteins in polarized cells and for defining the apical membrane domain during the early establishment of polarity.34, 35 Therefore, loss of EDTB may compromise the ability of Rab14 to mediate apical trafficking. Alternatively, EDTB may serve as a scaffolding protein that helps to organize multiple aspects of the apical endosomal complex, and its loss may result in the defects observed here.
In some cells, EDTB KO resulted in the formation of large microvilli- and alkaline phosphatase–positive inclusions. These structures are reminiscent of inclusions seen in MVID, which is, in most cases, caused by mutations in myosin Vb or syntaxin 3.2, 21, 22, 24, 30 However, MVID also manifests as a wholesale redistribution of apical enzymes, which is likely to be the primary cause of the disease. The formation of inclusions is thought to be owing to the failure of myosin Vb to interact with Rabs11a and 8 to mediate recycling of internalized proteins.30 We did not observe these types of redistributions and in fact our data on EDTB and Rab14 support a model of decreased endocytosis rather than recycling.47 Because knockout of EDTB impacts formation of early endosomal membranes, the localization of Rab11, as well as TJ proteins and lysosomal biogenesis, we propose that EDTB serves as a scaffold to organize the endosomal compartment and direct apical membrane traffic.
These results underscore the importance of vesicular trafficking in the differentiation of enterocytes during the development of the small intestine. These results have implications for understanding the etiology of developmental diseases that lead to poor barrier function and failure to thrive.
Footnotes
Author contributions Christopher M. Cox drafted the manuscript, analyzed and interpreted the data, performed the statistical analysis, and acquired data; Ruifeng Lu acquired, analyzed, and interpreted the data; Kaan Salcin acquired data; and Jean M. Wilson was responsible for the study concept and design, revision of the manuscript for content, and obtained funding.
Conflicts of interest The authors disclose no conflicts.
Funding This project was funded in part by a grant to the University of Arizona Cancer Center, and a grant from the National Cancer Institute of the National Institutes of Health (P30 CA023074). The authors received funding from the National Institutes of Health grants RO1 DK084047 and DK109701 (J.M.W.), and by a Science Education Award from the Howard Hughes Medical Institute to Macalester College (K.S.). Support also was provided by the University of Arizona BIO5 Institute’s Genetic Engineering of Mouse models core, which made the AEG conditional knockout mouse, and the matching funds program of the University of Arizona Cancer Center (National Cancer Institute Cancer Center Support Grant P30 CA023074). Also supported by a grant for flow cytometry and maintenance of the mouse colony (P30 CA023074).
References
- 1.Bjarnason I., Peters T.J. In vitro determination of small intestinal permeability: demonstration of a persistent defect in patients with coeliac disease. Gut. 1984;25:145–150. doi: 10.1136/gut.25.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erickson R.P., Larson-Thome K., Valenzuela R.K., Whitaker S.E., Shub M.D. Navajo microvillous inclusion disease is due to a mutation in MYO5B. Am J Med Genet A. 2008;146A:3117–3119. doi: 10.1002/ajmg.a.32605. [DOI] [PubMed] [Google Scholar]
- 3.Hackam D.J., Upperman J.S., Grishin A., Ford H.R. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:49–57. doi: 10.1053/j.sempedsurg.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Halpern M.D., Holubec H., Saunders T.A., Dvorak K., Clark J.A., Doelle S.M., Ballatori N., Dvorak B. Bile acids induce ileal damage during experimental necrotizing enterocolitis. Gastroenterology. 2006;130:359–372. doi: 10.1053/j.gastro.2005.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Overeem A.W., Posovszky C., Rings E.H., Giepmans B.N., van I.S.C. The role of enterocyte defects in the pathogenesis of congenital diarrheal disorders. Dis Model Mech. 2016;9:1–12. doi: 10.1242/dmm.022269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landy J., Ronde E., English N., Clark S.K., Hart A.L., Knight S.C., Ciclitira P.J., Al-Hassi H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J Gastroenterol. 2016;22:3117–3126. doi: 10.3748/wjg.v22.i11.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore S.A., Nighot P., Reyes C., Rawat M., McKee J., Lemon D., Hanson J., Ma T.Y. Intestinal barrier dysfunction in human necrotizing enterocolitis. J Pediatr Surg. 2016;51:1907–1913. doi: 10.1016/j.jpedsurg.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pope J.L., Ahmad R., Bhat A.A., Washington M.K., Singh A.B., Dhawan P. Claudin-1 overexpression in intestinal epithelial cells enhances susceptibility to adenomatous polyposis coli-mediated colon tumorigenesis. Mol Cancer. 2014;13:167. doi: 10.1186/1476-4598-13-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knutton S., Limbrick A.R., Robertson J.D. Regular structures in membranes. I. Membranes in the endocytic complex of ileal epithelial cells. J Cell Biol. 1974;62:679–694. doi: 10.1083/jcb.62.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wissig S.L., Graney D.O. Membrane modifications in the apical endocytic complex of ileal epithelial cells. J Cell Biol. 1968;39:564–579. doi: 10.1083/jcb.39.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson J.M., Whitney J.A., Neutra M.R. Biogenesis of the apical endosome-lysosome complex during differentiation of absorptive epithelial cells in rat ileum. J Cell Sci. 1991;100:133–143. doi: 10.1242/jcs.100.1.133. [DOI] [PubMed] [Google Scholar]
- 12.Wilson J.M., Whitney J.A., Neutra M.R. Identification of an endosomal antigen specific to absorptive cells of suckling rat ileum. J Cell Biol. 1987;105:691–703. doi: 10.1083/jcb.105.2.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonnella P.A., Neutra M.R. Membrane-bound and fluid-phase macromolecules enter separate prelysosomal compartments in absorptive cells of suckling rat ileum. J Cell Biol. 1984;99:909–917. doi: 10.1083/jcb.99.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siminoski K., Gonnella P., Bernanke J., Owen L., Neutra M., Murphy R.A. Uptake and transepithelial transport of nerve growth factor in suckling rat ileum. J Cell Biol. 1986;103:1979–1990. doi: 10.1083/jcb.103.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonnella P.A., Siminoski K., Murphy R.A., Neutra M.R. Transepithelial transport of epidermal growth factor by absorptive cells of suckling rat ileum. J Clin Invest. 1987;80:22–32. doi: 10.1172/JCI113051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knowles B.C., Weis V.G., Yu S., Roland J.T., Williams J.A., Alvarado G.S., Lapierre L.A., Shub M.D., Gao N., Goldenring J.R. Rab11a regulates syntaxin 3 localization and microvillus assembly in enterocytes. J Cell Sci. 2015;128:1617–1626. doi: 10.1242/jcs.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato T., Iwano T., Kunii M., Matsuda S., Mizuguchi R., Jung Y., Hagiwara H., Yoshihara Y., Yuzaki M., Harada R., Harada A. Rab8a and Rab8b are essential for several apical transport pathways but insufficient for ciliogenesis. J Cell Sci. 2014;127:422–431. doi: 10.1242/jcs.136903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato T., Mushiake S., Kato Y., Sato K., Sato M., Takeda N., Ozono K., Miki K., Kubo Y., Tsuji A., Harada R., Harada A. The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature. 2007;448:366–369. doi: 10.1038/nature05929. [DOI] [PubMed] [Google Scholar]
- 19.Sobajima T., Yoshimura S., Iwano T., Kunii M., Watanabe M., Atik N., Mushiake S., Morii E., Koyama Y., Miyoshi E., Harada A. Rab11a is required for apical protein localisation in the intestine. Biol Open. 2014;4:86–94. doi: 10.1242/bio.20148532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao N., Kaestner K.H. Cdx2 regulates endo-lysosomal function and epithelial cell polarity. Genes Dev. 2010;24:1295–1305. doi: 10.1101/gad.1921510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller T., Hess M.W., Schiefermeier N., Pfaller K., Ebner H.L., Heinz-Erian P., Ponstingl H., Partsch J., Rollinghoff B., Kohler H., Berger T., Lenhartz H., Schlenck B., Houwen R.J., Taylor C.J., Zoller H., Lechner S., Goulet O., Utermann G., Ruemmele F.M., Huber L.A., Janecke A.R. MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat Genet. 2008;40:1163–1165. doi: 10.1038/ng.225. [DOI] [PubMed] [Google Scholar]
- 22.Schneeberger K., Vogel G.F., Teunissen H., van Ommen D.D., Begthel H., El Bouazzaoui L., van Vugt A.H., Beekman J.M., Klumperman J., Muller T., Janecke A., Gerner P., Huber L.A., Hess M.W., Clevers H., van Es J.H., Nieuwenhuis E.E., Middendorp S. An inducible mouse model for microvillus inclusion disease reveals a role for myosin Vb in apical and basolateral trafficking. Proc Natl Acad Sci U S A. 2015;112:12408–12413. doi: 10.1073/pnas.1516672112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weis V.G., Knowles B.C., Choi E., Goldstein A.E., Williams J.A., Manning E.H., Roland J.T., Lapierre L.A., Goldenring J.R. Loss of MYO5B in mice recapitulates microvillus inclusion disease and reveals an apical trafficking pathway distinct to neonatal duodenum. Cell Mol Gastroenterol Hepatol. 2016;2:131–157. doi: 10.1016/j.jcmgh.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiegerinck C.L., Janecke A.R., Schneeberger K., Vogel G.F., van Haaften-Visser D.Y., Escher J.C., Adam R., Thoni C.E., Pfaller K., Jordan A.J., Weis C.A., Nijman I.J., Monroe G.R., van Hasselt P.M., Cutz E., Klumperman J., Clevers H., Nieuwenhuis E.E., Houwen R.H., van Haaften G., Hess M.W., Huber L.A., Stapelbroek J.M., Muller T., Middendorp S. Loss of syntaxin 3 causes variant microvillus inclusion disease. Gastroenterology. 2014;147:65–68 e10. doi: 10.1053/j.gastro.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Zhou K., Sumigray K.D., Lechler T. The Arp2/3 complex has essential roles in vesicle trafficking and transcytosis in the mammalian small intestine. Mol Biol Cell. 2015;26:1995–2004. doi: 10.1091/mbc.E14-10-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox C.M., Mandell E.K., Stewart L., Lu R., Johnson D.L., McCarter S.D., Tavares A., Runyan R., Ghosh S., Wilson J.M. Endosomal regulation of contact inhibition through the AMOT: YAP pathway. Mol Biol Cell. 2015;26:2673–2684. doi: 10.1091/mbc.E15-04-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarter S.D., Johnson D.L., Kitt K.N., Donohue C., Adams A., Wilson J.M. Regulation of tight junction assembly and epithelial polarity by a resident protein of apical endosomes. Traffic. 2010;11:856–866. doi: 10.1111/j.1600-0854.2010.01052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trahair J.F., Wilson J.M., Neutra M.R. Identification of a marker antigen for the endocytic stage of intestinal development in rat, sheep, and human. J Pediatr Gastroenterol Nutr. 1995;21:277–287. doi: 10.1097/00005176-199510000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Pasternak A.J., Hamonic G.M., Van Kessel A., Wilson H.L. Postnatal regulation of MAMDC4 in the porcine intestinal epithelium is influenced by bacterial colonization. Physiol Rep. 2016;4:21. doi: 10.14814/phy2.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knowles B.C., Roland J.T., Krishnan M., Tyska M.J., Lapierre L.A., Dickman P.S., Goldenring J.R., Shub M.D. Myosin Vb uncoupling from RAB8A and RAB11A elicits microvillus inclusion disease. J Clin Invest. 2014;124:2947–2962. doi: 10.1172/JCI71651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kravtsov D., Mashukova A., Forteza R., Rodriguez M.M., Ameen N.A., Salas P.J. Myosin 5b loss of function leads to defects in polarized signaling: implication for microvillus inclusion disease pathogenesis and treatment. Am J Physiol Gastrointest Liver Physiol. 2014;307:G992–G1001. doi: 10.1152/ajpgi.00180.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson J.M., de Hoop M., Zorzi N., Toh B.H., Dotti C.G., Parton R.G. EEA1, a tethering protein of the early sorting endosome, shows a polarized distribution in hippocampal neurons, epithelial cells, and fibroblasts. Mol Biol Cell. 2000;11:2657–2671. doi: 10.1091/mbc.11.8.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu R., Dalgalan D., Mandell E.K., Parker S.S., Ghosh S., Wilson J.M. PKCiota interacts with Rab14 and modulates epithelial barrier function through regulation of claudin-2 levels. Mol Biol Cell. 2015;26:1523–1531. doi: 10.1091/mbc.E14-12-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu R., Wilson J.M. Rab14 specifies the apical membrane through Arf6-mediated regulation of lipid domains and Cdc42. Sci Rep. 2016;6:38249. doi: 10.1038/srep38249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitt K.N., Hernandez-Deviez D., Ballantyne S.D., Spiliotis E.T., Casanova J.E., Wilson J.M. Rab14 regulates apical targeting in polarized epithelial cells. Traffic. 2008;9:1218–1231. doi: 10.1111/j.1600-0854.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonnella P.A., Neutra M.R. Glycoconjugate distribution and mobility on apical membranes of absorptive cells of suckling rat ileum in vivo. Anat Rec. 1985;213:520–528. doi: 10.1002/ar.1092130408. [DOI] [PubMed] [Google Scholar]
- 37.Sakamori R., Das S., Yu S., Feng S., Stypulkowski E., Guan Y., Douard V., Tang W., Ferraris R.P., Harada A., Brakebusch C., Guo W., Gao N. Cdc42 and Rab8a are critical for intestinal stem cell division, survival, and differentiation in mice. J Clin Invest. 2012;122:1052–1065. doi: 10.1172/JCI60282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao N., White P., Kaestner K.H. Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev Cell. 2009;16:588–599. doi: 10.1016/j.devcel.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng Q., Bonder E.M., Engevik A.C., Zhang L., Tyska M.J., Goldenring J.R., Gao N. Disruption of Rab8a and Rab11a causes formation of basolateral microvilli in neonatal enteropathy. J Cell Sci. 2017;130:2491–2505. doi: 10.1242/jcs.201897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melendez J., Liu M., Sampson L., Akunuru S., Han X., Vallance J., Witte D., Shroyer N., Zheng Y. Cdc42 coordinates proliferation, polarity, migration, and differentiation of small intestinal epithelial cells in mice. Gastroenterology. 2013;145:808–819. doi: 10.1053/j.gastro.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muncan V., Heijmans J., Krasinski S.D., Buller N.V., Wildenberg M.E., Meisner S., Radonjic M., Stapleton K.A., Lamers W.H., Biemond I., van den Bergh Weerman M.A., O'Carroll D., Hardwick J.C., Hommes D.W., van den Brink G.R. Blimp1 regulates the transition of neonatal to adult intestinal epithelium. Nat Commun. 2011;2:452. doi: 10.1038/ncomms1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuijl C., Savage N.D., Marsman M., Tuin A.W., Janssen L., Egan D.A., Ketema M., van den Nieuwendijk R., van den Eeden S.J., Geluk A., Poot A., van der Marel G., Beijersbergen R.L., Overkleeft H., Ottenhoff T.H., Neefjes J. Intracellular bacterial growth is controlled by a kinase network around PKB/AKT1. Nature. 2007;450:725–730. doi: 10.1038/nature06345. [DOI] [PubMed] [Google Scholar]
- 43.Ueno H., Huang X., Tanaka Y., Hirokawa N. KIF16B/Rab14 molecular motor complex is critical for early embryonic development by transporting FGF receptor. Dev Cell. 2011;20:60–71. doi: 10.1016/j.devcel.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Reed S.E., Hodgson L.R., Song S., May M.T., Kelly E.E., McCaffrey M.W., Mastick C.C., Verkade P., Tavare J.M. A role for Rab14 in the endocytic trafficking of GLUT4 in 3T3-L1 adipocytes. J Cell Sci. 2013;126:1931–1941. doi: 10.1242/jcs.104307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sadacca L.A., Bruno J., Wen J., Xiong W., McGraw T.E. Specialized sorting of GLUT4 and its recruitment to the cell surface are independently regulated by distinct Rabs. Mol Biol Cell. 2013;24:2544–2557. doi: 10.1091/mbc.E13-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Junutula J.R., De Maziere A.M., Peden A.A., Ervin K.E., Advani R.J., van Dijk S.M., Klumperman J., Scheller R.H. Rab14 is involved in membrane trafficking between the Golgi complex and endosomes. Mol Biol Cell. 2004;15:2218–2229. doi: 10.1091/mbc.E03-10-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu R., Johnson D.L., Stewart L., Waite K., Elliott D., Wilson J.M. Rab14 regulation of claudin-2 trafficking modulates epithelial permeability and lumen morphogenesis. Mol Biol Cell. 2014;25:1744–1754. doi: 10.1091/mbc.E13-12-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]