Abstract
We demonstrate an implantable MEMS drug delivery device to conduct controlled and on-demand, ex vivo drug transport to human eye tissue. Remotely operated drug delivery to human post-mortem eyes was performed via a MEMS device. The developed curved packaging cover conforms to the eyeball thereby preventing the eye tissue from contacting the actuating membrane. By pulsed operation of the device, using an externally applied magnetic field, the drug released from the device accumulates in a cavity adjacent to the tissue. As such, docetaxel (DTX), an antiangiogenic drug, diffuses through the eye tissue, from sclera and choroid to retina. DTX uptake by sclera and choroid were measured to be 1.93±0.66 and 7.24±0.37 μg/g tissue, respectively, after two hours in pulsed operation mode (10 s on/off cycles) at 23°C. During this period, a total amount of 192 ng DTX diffused into the exposed tissue. This MEMS device shows great potential for the treatment of ocular posterior segment diseases such as diabetic retinopathy by introducing a novel way of drug administration to the eye.
INTRODUCTION
Diabetes is now a global epidemic. One of the associated complications, diabetic retinopathy, is the major cause of visual loss in middle-aged adults. In proliferative diabetic retinopathy, unwanted proliferation of capillary cells in the retina (ocular angiogenesis) compromises retina function, causing vision loss [1]. In the United States alone, approximately 4.2 million individuals with diabetes have diabetic retinopathy [2]. This number is expected to double by 2020.
Conventional laser ablation therapy (panretinal laser photocoagulation) slows the progression of diabetic retinopathy, however its effectiveness is temporary and brings with it negative side effects. Currently, delivery of therapeutic agents to the posterior segment of the eye is via intravitreal administration. This involves injecting a drug solution or placing a slow-release polymeric implant directly into the vitreous. However, drugs are most effective if they can be delivered to the posterior portion of the eye, the area which requires treatment (i.e. choroid and retina). Consequently, effective delivery of therapeutic agents to disease sites in the posterior segment of the eye is critical to attaining treatment efficacy. Other ocular drug delivery methods such as polymeric systems and microneedles have been proposed for targeted delivery [3]. In most of these implants, drug release is passive and dosing cannot be stopped after implantation, except by surgical removal of the implant. It is essential to have active control of drug release rate and switchable on-off mode capabilities, especially when unexpected changes in the physiological conditions of the patient require corresponding adjustments in dosing. Adjusting drug release profiles (i.e. timing, duration, and dose) externally provides tremendous benefits and ensures drug concentrations are maintained within the therapeutic window. Furthermore, delivering antiproliferative drugs such as DTX locally, adjacent to the surface of sclera, minimizes the toxicities associated with systemic delivery of the drug.
MEMS-based drug delivery devices have distinct advantages over polymeric and thin film systems in providing precise drug doses [4]. However, on-chip batteries often limit operation and further miniaturization, especially in area of ophthalmology. Previously, the concept of electricity-free, magnetically controlled drug delivery devices have been demonstrated in vitro [5, 6] and compared to manually-actuated devices [7]. In this work we demonstrate DTX delivery to the ex vivo target eye tissue via the MEMS device (Fig. 1). This work further presents advancements in the curved package cover and pulse operation mode designed for the ex vivo tests. Formerly, an antiangiogenic effect of DTX at low nanomolar concentrations was demonstrated in vitro. This is the first ex vivo demonstration of DTX uptake in sclera delivered by a MEMS device.
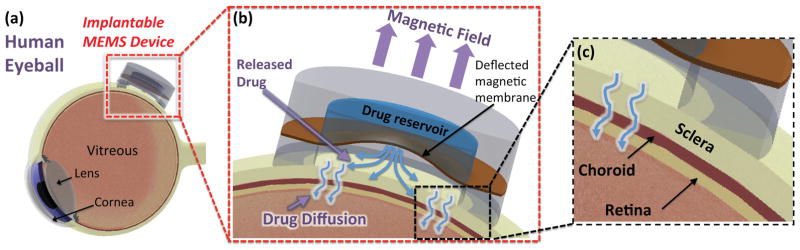
Figure 1.
(a) Conceptual illustration of the MEMS drug delivery device sutured to the posterior eyeball, (b, c) Working principle of the device; the deformed magnetic membrane in a magnetic field discharges the drug solution into the cavity followed by drug penetration into the eye tissue layer.
CONCEPT
Figure 1a conceptually depicts the MEMS device implanted against the sclera at the posterior eye to provide the antiangiogenic drug to the eye tissue. The device consists of a drug-loaded reservoir (Ø6 mm×580 μm), sealed by a 40 μm-thick elastic magnetic PDMS membrane with a laser-drilled aperture (100×100 μm2). The membrane is protected by a curved ring of PDMS (Ø8 mm×1 mm) that conforms to the eyeball and allows a minimum distance of 0.5 mm between the membrane and the eye tissue to prevent the sclera from contacting the membrane. When the device is actuated by an externally applied magnetic field (Fig. 1b) the force causes the membrane to deform and discharge the drug solution into the cavity enclosed by the eyeball and membrane. The accumulated drug in that cavity then diffuses through the eye tissue layers, penetrating through the sclera and choroid to the retina (Fig. 1c).
METHODS AND MATERIALS
Docetaxel powder was obtained from Phyton Biotech, Inc. (Delta, BC, Canada) and radiolabeled docetaxel (3H-DTX) (specific activity 23 Ci/mM) was purchased from Moravek Biochemicals and Radiochemicals (Brea, CA). Human post-mortem eyes were obtained from the Eye Bank at UBC. The Protocol for handling human tissue was approved by the Clinical Research Ethics Board (CREB) at UBC.
Fabrication
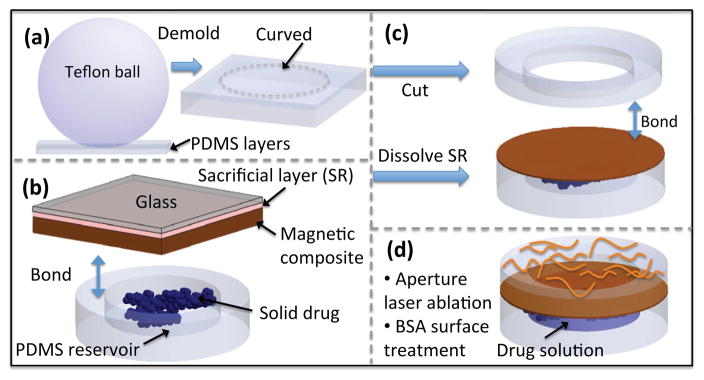
The fabrication process for the MEMS drug delivery device is summarized in Fig. 2. The drug reservoir was fabricated by micro molding PDMS (Sylgard 184, Dow Corning Co.) using a SU-8 2150 (MicroChem Corp., MA, USA) master. The thickness of the PDMS reservoir layer was under 2 mm by adjusting the volume of PDMS used in the mold. The magnetic membrane composite was prepared by incorporating 40%w/w of coated iron oxide nanoparticles (10 nm), EMG 1200 (Ferrotec, MA, USA), in a PDMS matrix [5]. The composite was spin-coated on a glass substrate with a poly(acrylic acid) sacrificial layer (20%w/v in water). A mixture of tritium-labeled DTX (3H-DTX) and unlabeled DTX at the desired concentration of 2 mg/ml was prepared in a 50/50 solution of ethanol and dichloromethane to be deposited in the drug reservoirs. The radiolabeled drug content was 1.7% of unlabeled drug. After the magnetic membrane and reservoir surfaces were treated with oxygen plasma, the drug was deposited in the drug reservoir and the membrane was irreversibly bonded to the reservoir layer. The device was detached from the substrate by dissolving the sacrificial layer in water.
Figure 2.
Fabrication process: (a) curved packaging, (b) drug delivery device, and (c, d) MEMS device assembly, aperture laser ablation and surface treatment.
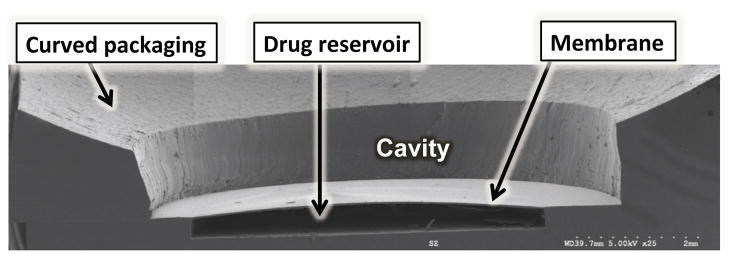
The curved PDMS ring was fabricated using a molding technique. A teflon ball with a diameter of 25 mm was used to form a curved PDMS layer with a curvature similar to human eyeball curvature. The curved layer consists of two layers of PDMS fused together. The first layer of PDMS was cured with a desired thickness of 0.5 mm. The thickness of this layer determines the size of the gap between the magnetic membrane and the eyeball and ideally should be greater than the maximum deflection of the membrane to avoid contact with the eyeball. Next, a second layer of PDMS was poured around a Teflon ball placed on the first layer, degassed, and cured for 3 hours at 65°C. The fused PDMS layers were then demolded and a 8 mm in diameter hole was cut at the center (Harris UNI-CORE™). The resulting curved ring was bonded to the membrane using oxygen plasma. Next, a 100×100 μm2 aperture was ablated with a UV laser (355 nm wavelength) using Quicklaze laser system (New Wave Research, Sunnyvale, CA). The device was immersed in a 40 mg/ml bovine serum albumin (BSA, Sigma-Aldrich, USA) in phosphate buffered saline (PBS) solution and incubated at 37°C. The drug reservoir gradually filled with solution as a result of the surface-tension driven flow through the aperture [5]. Figure 3 shows a SEM of the cross-section of the device.
Figure 3.
SEM image of the device cross-section.
Drug Release Studies
For the in vitro constant release study, the MEMS drug delivery device was placed in a petri dish and actuated in 9 ml of an aseptically filtered solution of 1%w/v BSA in PBS (pH 7.4) (referred to as BSA solution subsequently). In the in vitro “pulsed operation” mode, a teflon ball was placed on the device to mimic the condition with a human eyeball, and the device was actuated in 9 ml of BSA solution. This actuation scheme includes multiple on and off cycles for a desired period. The accumulated drug in the cavity between the membrane and the teflon ball was measured after the actuation period. The radioactive DTX content was measured by transferring 400 μl of media directly into scintillation fluid in vials. Disintegrations per minute (DPM) were measured using a scintillation counter (LS-6500 series, Beckman Coulter, Inc., Brea, CA).
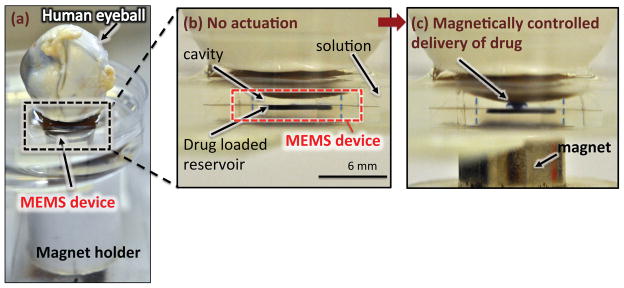
The setup for the ex vivo study is shown in Fig. 4a. In these studies, the posterior area of post-mortem human eyes was positioned on top of the device in a petri dish filled with BSA solution. The exposed area corresponded to the desired implantation location of the device. The device was actuated by mechanically moving a cylindrical NdFeB permanent magnet [6]. The eyeball was removed from the top of the device at each measurement data point in the ex vivo studies. The surface of sclera was rinsed with fresh BSA solution to remove residual drug adsorbed to the surface of the sclera that had not diffused into the tissue. The exposed area was dissected out and the retina, choroid, and sclera were separated from the vitreous humor using tweezers. Next, the tissues were rinsed with ice-cold saline, blotted dry and weighed followed by dissolution overnight at 60°C in 1 ml of Solvable (Perkin Elmer, Woodbridge, ON, Canada). To de-colour the samples, 50 ml of 200 mM EDTA and 200 ml of 30% hydrogen peroxide (Fisher Scientific, Ottawa, ON, Canada) were added followed by heating to 60°C for 1 hour. Samples were allowed to cool to room temperature before adding 5 ml of scintillation cocktail (Ultima Gold, Perkin Elmer, Woodbridge, ON, Canada). Drug uptake in the tissues was determined by scintillation counting and the values were normalized to the tissue weight.
Figure 4.
(a) Optical image of the actuation setup with human eyeball on the device, (b) No actuation, (c) Mixture of drug with a dye was released into the cavity following magnetic actuation at ~270 mT.
RESULTS AND DISCUSSION
On-demand release profiles
The device was actuated in two distinct modes: constant release rate operation and pulsed cumulative release operation.
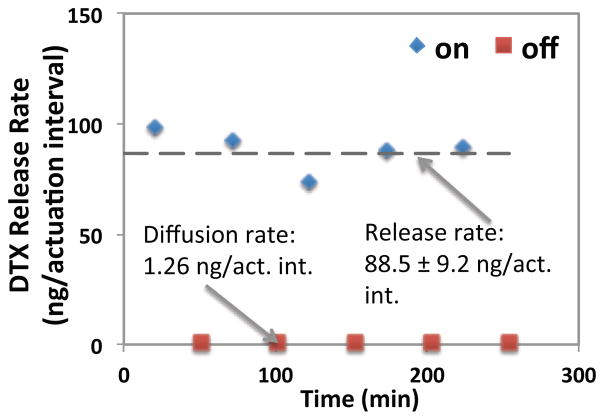
The consistent operation of the device was characterized in vitro using the constant release rate operation mode. This actuation scheme involves using two time constants to account for drug discharge time (in the presence of a magnetic field) and mixing time (when the membrane relaxes back and the pumped-in solution mixes with reservoir content). Therefore, in this actuation scheme, each actuation cycle was programmed to allow 50 s for drug solution discharge, and 200 s for the mixing of pumped-in solution (in the absence of the magnetic field) in each cycle. Figure 5 shows that a constant release rate of 88.5±9.2 ng/actuation interval was achieved by magnetic actuation in a 270 mT field. Here, each actuation interval includes five consecutive actuation cycles. In the no-actuation periods, the device was left in BSA solution. In this “off” state, drug release is expected to occur via background diffusion through the laser-drilled aperture. Drug release in the “off” state was measured to be 1.26 ng/actuation interval showing the background leakage of the drug solution through the aperture was 70-fold less than the amount released during the actuation mode (i.e. “on” state).
Figure 5.
Controlled release of DTX in ~270 mT magnetic field (each actuation interval includes five consecutive actuation cycles of 50 s on-200 s off).
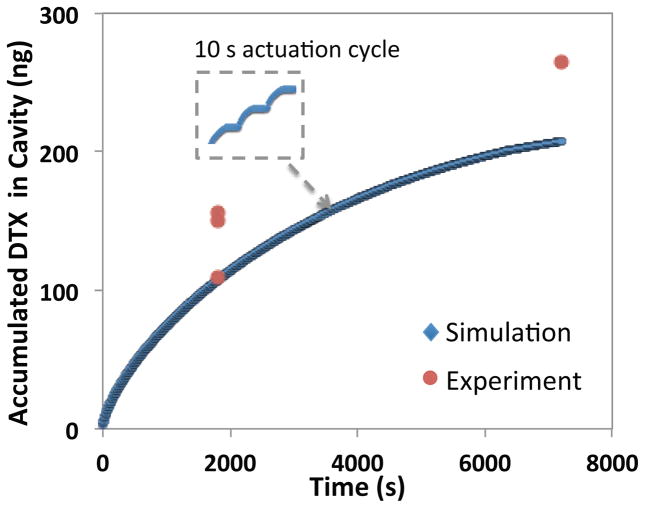
In the pulsed cumulative release operation mode, drug is continually released and accumulated in the cavity enclosed by the membrane and the teflon ball. This actuation scheme involves the periodic actuation of the device using on and off cycles of 10 s each (i.e. 10 s actuation, 10 s no actuation). This pulsed operation mode was characterized in vitro (Fig. 6) and used in the ex vivo study (Fig. 7). Figures 4b and 4c illustrate the release of a mixture of drug with Trypan Blue (TB) from the reservoir before and after device actuation, respectively. The use of TB in the reservoir was for visualization purposes only. Figure 6 demonstrates the cumulative drug release data over time in a ~270 mT field, experimentally and using simulations. In this actuation scheme, the drug concentration inside the cavity increased with actuation time. Therefore, by adjusting the duration of pulses, the amount of drug available to diffuse into the eye tissue, is precisely controlled. This drug dose regulation allows for adjustments, which may be required due to change in the patient’s treatment plan. Two-dimensional axisymmetric COMSOL simulation was used to estimate the cavity concentration (Fig. 6). The simulation considers mass transport based on isotropic diffusion of DTX from the volume displaced by the deflected membrane to the cavity between the membrane and the ball.
Figure 6.
Cumulative drug release profile with 10 s on/off cycles in a ~270 mT magnetic field.
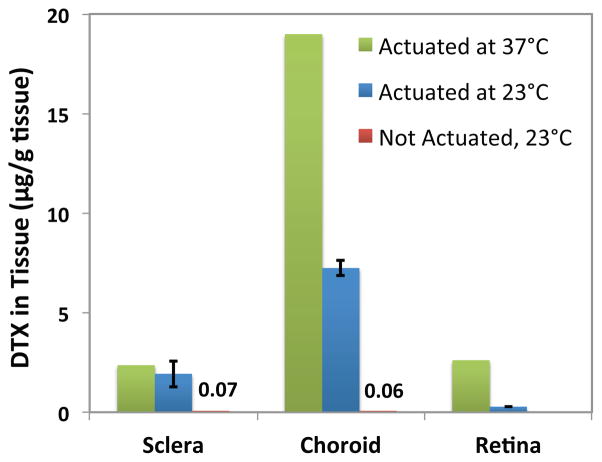
Figure 7.
Diffusion of DTX into ex vivo human eyeballs following a two hour periodic actuation at ~270 mT, using on and off cycles of 10 s each at 37°C and 23°C.
Ex vivo study
The ex vivo study was performed to investigate the distribution of DTX in the three tissue layers of the posterior segment of the eye (i.e. sclera, choroid, and retina). Drug accumulation in each layer was measured after the device was periodically actuated at ~270 mT for two hours with “on” and “off” cycles of 10 s each (i.e. 10 s actuation, 10 s no actuation), similar to the actuation scheme used in the in vitro pulsed cumulative release study. Figure 7 describes the amount of DTX accumulated and retained in sclera, choroid, and retina per gram of tissue, performed at 37°C and 23°C, for two eyeballs. The amount of drug uptake by the sclera and choroid at 23°C was 1.93±0.66 and 7.24±0.37 μg/g tissue, respectively. Since the antiangiogenic effect of DTX occurs at concentrations as low as 10nM (approximately equivalent to 10 ng/g tissue) these drug levels are therapeutically relevant. Based on these measurements the total amount of drug that diffused into the exposed posterior eyeball tissue in the two-hour experiment window was 192 ng. This shows that ~73% of the accumulated drug in the cavity diffused into the tissue. Therefore, the ex vivo results suggest that DTX delivered by the MEMS device could successfully pass through the sclera to reach target tissues of choroid and retina. In the absence of the magnetic field (i.e. off state) the drug uptake in tissue after two hours was lower than the minimum therapeutic efficacy of DTX at 0.07 and 0.06 ng/g tissue. Therefore, the background leakage through the aperture does not interfere with the planned drug release via on-demand activation of the device.
CONCLUSION
We demonstrated on-demand and controlled delivery of DTX to ex vivo human eyeballs using a MEMS device. DTX was released adjacent to the target tissue by actuating the device with an externally applied magnetic field and diffused into eye tissue layers: sclera, choroid, and retina. Two operating schemes for both constant and cumulative releases were used. It was demonstrated in vitro that the dosing could be regulated on-demand and by adjusting the actuation period. Ex vivo results demonstrated significant DTX penetration into the ocular tissues following actuation of the MEMS device. This study demonstrates the successful application of a MEMS device for drug delivery to the eye.
Acknowledgments
This work was funded in part by the Collaborative Health Research Projects, Natural Science and Engineering Research Council of Canada, the Canadian Institutes of Health Research, and an Academic Excellence Alliance grant awarded by the KAUST Office of Competitive Research Funds. Mu Chiao is supported by the Canada Research Chairs Program. Authors would like to thank Mr. Tom Brubaker and Ms. Kye Lee, undergraduate students in the Lin lab, for their assistance.
References
- 1.Rajappa M, Saxena P, Kaur J, Gregory SM. Elsevier, editor. Ocular angiogenesis: mechanisms and recent advances in therapy. In: Makowski GS, editor. Advances in Clinical Chemistry. Vol. 50. 2010. pp. 103–121. [PubMed] [Google Scholar]
- 2.National Diabetes Statistics. 2011 Available: http://diabetes.niddk.nih.gov/dm/pubs/statistics/?control=Pubs.
- 3.Thrimawithana TR, Young S, Bunt CR, Green C, Alany RG. Drug delivery to the posterior segment of the eye. Drug Discovery Today. 2011;16:270–277. doi: 10.1016/j.drudis.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Santini JT, Cima MJ, Langer R. A controlled-release microchip. Nature. 1999;397:335–338. doi: 10.1038/16898. [DOI] [PubMed] [Google Scholar]
- 5.Pirmoradi FN, Jackson JK, Burt HM, Chiao M. A magnetically controlled MEMS device for drug delivery: design, fabrication, and testing. Lab on a Chip. 2011;11:3072–3080. doi: 10.1039/c1lc20438f. [DOI] [PubMed] [Google Scholar]
- 6.Pirmoradi FN, Jackson JK, Burt HM, Chiao M. On-demand controlled release of docetaxel from a battery-less MEMS drug delivery device. Lab on a Chip. 2011;11:2744–2752. doi: 10.1039/c1lc20134d. [DOI] [PubMed] [Google Scholar]
- 7.Lo R, Li PY, Saati S, Agrawal R, Humayun M, Meng E. A passive MEMS drug delivery pump for treatment of ocular diseases. Biomedical Microdevices. 2009;11:959–970. doi: 10.1007/s10544-009-9313-9. [DOI] [PubMed] [Google Scholar]