Abstract
Background
Clinical trials have shown promising results with the use of subcallosal cingulate gyrus deep brain stimulation (DBS) for treatment-resistant depression. However, strategies to manage patients who do not respond to this therapy have not been explored in detail. In rats, DBS in the ventromedial prefrontal cortex (vmPFC) induces a significant antidepressant-like response in the forced swim test (FST). We have used this test to investigate potential interactions between DBS and clinically used augmentative regimens.
Methods
Rats undergoing the FST were treated with vmPFC DBS along with different augmentative drugs, namely buspirone, risperidone and pindolol. Locomotor activity was tested in an open field.
Results
DBS induced a significant reduction in immobility scores as compared to saline treated controls. These antidepressant-like effects, however, were not potentiated by the co-administration of buspirone, risperidone or pindolol.
Limitations
Despite having good predictive validity, animal models are limited from a translational perspective.
Conclusions
Our results indicate that that the antidepressant-like effects of vmPFC DBS in the FST are not enhanced by augmentative therapies.
Keywords: Prefrontal cortex, Depression, Deep brain stimulation, Cingulate gyrus, Augmentative
1. Introduction
Deep brain stimulation (DBS) is currently being investigated as a potential therapy for treatment-refractory depression. One of the most promising DBS targets for this condition is the subcallosal cingulate gyrus (SCG) (Holtzheimer et al., 2012; Kennedy et al., 2011; Lozano et al., 2012, 2008; Mayberg et al., 2005). Open label series published to date have shown that approximately 50–60% of patients treated with stimulation respond to the procedure. Though encouraging, these results imply that approximately 40–50% of patients remain depressed after DBS. This is particularly worrisome if one considers that surgical candidates have tried and failed all approved therapeutic modalities. In this context, establishing strategies to potentiate the antidepressant effects of DBS may be of considerable value. A major difficulty to address this issue in the clinic is that patients included in surgical trials are often on multiple medications. Under these circumstances, isolating the effects of single therapeutic regimens on the patients’ clinical status is a complex task.
In rats, stimulation of the ventral medial prefrontal cortex (vmPFC), a region proposed to be the anatomical correlate of the SCG (Gabbott et al., 2003; Takagishi and Chiba, 1991), induces an antidepressant-like response in various behavioral paradigms, including the forced swim test (FST) (Hamani et al., 2010a, 2010b). Despite its caveats, the FST seems to have a good predictive validity and is one of the most commonly used screeners for testing the effects of antidepressant treatments in animals (Cryan et al., 2002; Hamani et al., 2010c; Hamani and Temel, 2012; Nestler et al., 2002; Nestler and Hyman, 2010).
Augmentative therapies involve the use of agents that are not considered to be standard antidepressants but enhance the therapeutic effects of these latter drugs (Carvalho et al., 2009). Here, we studied potential interactions between DBS and drugs commonly used during augmentative strategies (e.g. buspirone, risperidone and pindolol) in rats undergoing the FST.
2. Materials and methods
All protocols were approved by the Animal Care committee of the Centre for Addiction and Mental Health.
2.1. Forced swim test
On the first day of testing, male Sprague–Dawley rats (250–300 g) were individually placed for 15 min in a cylinder (25 cm diameter, 60 cm tall) filled with 25 ± 1 °C water to a depth of 40 cm. On the following day, rats underwent a 5 min swimming session. This was divided into 5 s segments and the predominant behavior (immobility, swimming, or climbing) during each segment was blindly scored (maximum of 60; 1 point per segment) (Detke and Lucki, 1996; Detke et al., 1995a). In the FST, low immobility is associated with an antidepressant-like effect.
2.2. Drug administration
The following medications and doses were selected based on their ability to potentiate the antidepressant-like effects of selective serotonin reuptake inhibitors in the FST (Cryan et al., 2005; Detke et al., 1995b; Wieland and Lucki, 1990): Buspirone (1 mg/kg; Tocris Bioscience), risperidone (0.1 mg/kg; Tocris Bioscience), and pindolol (6 mg/kg; Tocris Bioscience). Drugs or an equal volume vehicle were injected i.p. 1 and 5 h after the first swimming session on day 1 and 1 h prior to the FST on day 2, as previously described (Hamani et al., 2012a; Porsolt et al., 1978).
2.3. Surgical procedures and electrical stimulation
Rats were anesthetized with halothane and had electrodes implanted in the vmPFC at the following stereotaxic coordinates: anteroposterior (AP) + 3.0, lateral (L) ± 0.4, and depth (D) 5.6 mm (Paxinos and Watson, 1998). Monopolar stainless steel electrodes (250 μm in diameter and 0.75 mm of exposed surface) were used as cathodes. Epidural screws implanted over the somatosensory cortex were used as anodes (AP 0.5, L ± 1.5, D 1.0 mm) (Hamani et al., 2012a; Paxinos and Watson, 1998). Controls had holes drilled into the skull but were not implanted with electrodes. We have previously found that shams with electrodes implanted that did not receive stimulation had a non-significant reduction in immobility scores during the FST (Hamani et al., 2010a, 2010b). This effect, however, was never as striking as the one recorded after DBS. The main objective of the present study was to establish whether different augmentative regimens could potentiate the effects of vmPFC stimulation. As a result, we did not include sham groups in these experiments, as this would have increased the complexity of the study and the number of animals used without further contributing to answer our research questions.
Behavioral experiments were conducted seven days after surgical procedures. Stimulation was carried out with a portable device (ANS model 3510) at 100 μA, 130 Hz, and 90 μs. These settings were chosen because they were effective in our previous experiments using the FST (Hamani et al., 2010a, 2010b). Animals given DBS with or without the concomitant administration of drugs were stimulated for 4 h on day 1 and 2 h prior to the second swimming session on day 2.
2.4. Open field test
One week after swimming, animals in each group were given the same doses of medications received during the FST combined with DBS and tested in an open field (two doses separated by a 4 h interval on day 1 and one dose 1 h prior to testing on day 2) (Hamani et al., 2010b). Locomotor activity was assessed for 30 min in a squared 0.49 m2 apparatus (Med Associates) with infrared photo beams placed along the walls. Crossing of the beams provided counts of motor activity.
2.5. Histology
To assess electrode placement, brains were stained with cresyl violet. Electrode location in this study was similar to that published in our previous reports (Hamani et al., 2010a, 2010b, 2012b). Only animals with bilateral electrodes in the vmPFC were included in the final analyses. Based on this premise, four rats were excluded from the study, two from the DBS group (one with a lost cap and one with electrodes in the forceps minor), one from the DBS + buspirone group (electrodes in the forceps minor), and one from the DBS + risperidone group (one electrode in vmPFC and the other in the forceps minor).
2.6. Statistical analyses
One-way ANOVA (LSD post-hoc) was used to compare data across groups. Statistical significance was set at p ≤ 0.05.
3. Results
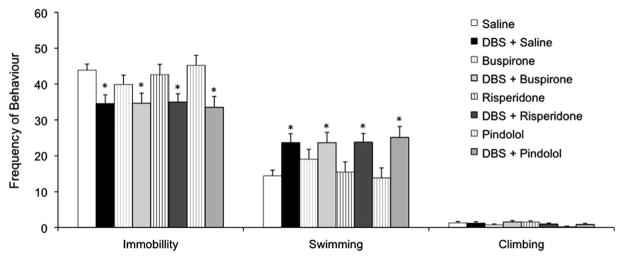
Overall, augmentative drugs by themselves did not induce an antidepressant-like effect at the selected doses (n = 16 per group; Fig. 1). As previously described, DBS induced a significant reduction in immobility scores in animals co-administered with saline (n = 16; p = 0.01) with swimming scores increasing in parallel (Hamani et al., 2010a, 2010b). Similar results have been recorded in groups of rats given DBS and buspirone (n = 16; p = 0.01), risperidone (n = 16; p = 0.02) or pindolol (n = 16; p = 0.02) (Fig. 1). In contrast, no significant differences were found when the percentage of improvement (i.e. reduction in immobility) recorded in animals from each DBS group was compared to that of rats given medications alone (Fig. 2). Locomotor activity in the open field was similar across groups (Supplementary Figure.).
Fig. 1.
Interaction between ventromedial prefrontal cortex deep brain stimulation (vmPFC DBS) and augmentative medications in the forced swim test (FST). DBS treated groups had a significant reduction in immobility scores when compared to saline treated controls. These effects, however, were not potentiated by buspirone, risperidone or pindolol. None of these drugs induced an antidepressant-like effect on their own. Data represent mean ± standard error. *p ≤ 0.05 when DBS + medication groups were compared saline treated controls. Sixteen animals were treated in each group.
Fig. 2.
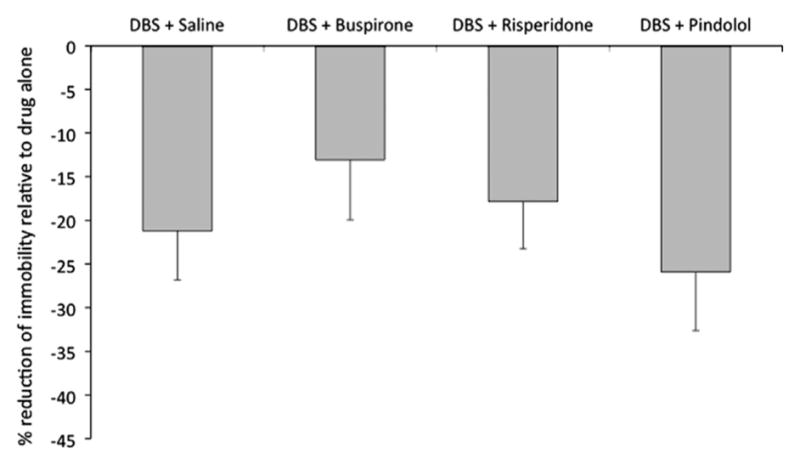
Antidepressant-like response in animals receiving ventromedial prefrontal cortex deep brain stimulation (vmPFC DBS) and augmentative medications in the forced swim test (FST). No significant differences were found when the percentage of improvement (i.e. reduction in immobility) recorded in animals from each DBS group was compared to that of rats given medications alone (i.e. DBS buspirone vs. buspirone; DBS risperidone vs. risperidone; DBS pindolol vs. pindolol). Data represent mean ± standard error. Sixteen animals were treated in each group.
4. Discussion
By definition, augmentative therapies involve the use of agents that are not considered standard antidepressants in order to enhance the therapeutic effects of these latter drugs (Carvalho et al., 2009). In rodents, treatment with medications commonly used in augmentative regimens enhances the response of antidepressants in the FST (Cryan et al., 2005; Detke et al., 1995b; Haddjeri et al., 2004; Moser and Sanger, 1999; Wieland and Lucki, 1990; Zaniewska et al., 2010). In our study, none of the drugs used for augmentation induced a significant antidepressant-like response in this model (although a 10% non-significant decrease in immobility was observed in animals given buspirone). With the low climbing scores recorded in our study however, it is possible that changes in behavior associated with the administration of drugs that act predominantly on the catecholaminergic system may not have been detected.
In view of these negative findings, a few aspects need to be addressed in further detail. The first refers to the choice of medication dosage. As mentioned earlier, doses were selected based on previous studies showing that they either induced an antidepressant-like effect or enhanced the response of selective serotonin reuptake inhibitors in the FST (Cryan et al., 2005; Detke et al., 1995b; Wieland and Lucki, 1990). Previous reports have studied the effects of different doses of medications in the FST. For buspirone and pindolol, we have selected doses that were considered to be in the high range to induce an effect (Cryan et al., 2005; Detke et al., 1995b). With risperidone, we have chosen a slightly lower dose as the use of this drug at higher concentrations induced a significant decrease in locomotor activity in the open field (data not shown).
Though the FST has several limitations, it has a good predictive validity for the screening of antidepressant therapies (Cryan et al., 2002, 2005; Hamani and Nobrega, 2012; Hamani and Temel, 2012). One caveat of this test is that it is not suitable to be used in test/retest paradigms. In this context, it does not allow one to isolate subpopulations of rats that do not respond to antidepressant medications for subsequent treatment with DBS. This differs from the clinical scenario in which only patients who fail multiple therapies and are considered to be “treatment-resistant” are offered DBS.
Our previous work suggests that both acute and chronic antidepressant-like effects of DBS in rats are dependent on the integrity of the serotonergic system (Hamani et al., 2010b, 2012b). Some of the drugs used in the present study are partial serotonergic agonists (e.g. buspirone and pindolol). As such, they may have competed with the serotonin released after DBS, not contributing to the enhancement of a stimulation response. One hypothesis to be tested in the future is whether doses of 5HT1A antagonists (e.g. WAY100635) known to block raphe autoreceptors may enhance serotonin levels after DBS and augment the antidepressant-like effects of this therapy.
To date, potential interactions between DBS and different classes of medications in the clinic are still unknown. Though this issue will only be resolved in clinical trials, preclinical studies may be helpful in pointing out more specific research directions. As an example, we have previously shown that monoamine oxidase inhibitors (MAOi) potentiate the effects of DBS in the FST (Hamani et al., 2012a). This has led us to add tranylcipromide to the medication regimen of a patient who was considered to be a non-responder in our DBS trial (Hamani et al., 2012a). After two weeks of treatment combining stimulation with this MAOi, the patient had a marked reduction in his depression, reaching levels compatible with a remission state (Hamani et al., 2012a).
Following the research paradigm described above, we have decided to investigate whether augmentative regimens could potentiate the effects of DBS in rats. Unfortunately, in contrast to our MAOi results, the co-administration of buspirone, risperidone or pindolol alongside DBS did not yield an enhancement of the antidepressant-like effects of stimulation. Though these results are not encouraging from a translational perspective, we stress that they are limited if one takes into account the caveats related to the use of animal models. It is possible that augmentative strategies may potentiate the effects of DBS in the clinic.
Supplementary Material
Acknowledgments
Role of funding source
Experimental work was supported by funds from the Canadian Institutes for Health Research (CIHR).
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.jad.2014.03.007.
Footnotes
Conflict of interest
C.H. is a consultant for St Jude Medical. The other authors do not have a conflict of interest.
References
- Carvalho AF, Machado JR, Cavalcante JL. Augmentation strategies for treatment-resistant depression. Curr Opin Psychiatry. 2009;22:7–12. doi: 10.1097/YCO.0b013e32831be9ef. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Lucki I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behav Brain Res. 1996;73:43–46. doi: 10.1016/0166-4328(96)00067-8. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berlin) 1995a;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Wieland S, Lucki I. Blockade of the antidepressant-like effects of 8-OH-DPAT, buspirone and desipramine in the rat forced swim test by 5HT1A receptor antagonists. Psychopharmacology (Berlin) 1995b;119:47–54. doi: 10.1007/BF02246053. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Bacon SJ. Areal and synaptic interconnectivity of prelimbic (area 32), infralimbic (area 25) and insular cortices in the rat. Brain Res. 2003;993:59–71. doi: 10.1016/j.brainres.2003.08.056. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Faure C, Lucas G, Mnie-Filali O, Chouvet G, Astier B, Renaud B, Blier P, Debonnel G. In-vivo modulation of central 5-hydroxytryptamine (5-HT1A) receptor-mediated responses by the cholinergic system. Int J Neuropsychopharmacol. 2004;7:391–399. doi: 10.1017/S1461145704004377. [DOI] [PubMed] [Google Scholar]
- Hamani C, Diwan M, Isabella S, Lozano AM, Nobrega JN. Effects of different stimulation parameters on the antidepressant-like response of medial prefrontal cortex deep brain stimulation in rats. J Psychiatr Res. 2010a;44:683–687. doi: 10.1016/j.jpsychires.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Hamani C, Diwan M, Macedo CE, Brandao ML, Shumake J, Gonzalez-Lima F, Raymond R, Lozano AM, Fletcher PJ, Nobrega JN. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol Psychiatry. 2010b;67:117–124. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Hamani C, Giacobbe P, Diwan M, Balbino ES, Tong J, Bridgman A, Lipsman N, Lozano AM, Kennedy SH, Nobrega JN. Monoamine oxidase inhibitors potentiate the effects of deep brain stimulation. Am J Psychiatry. 2012a;169:1320–1321. doi: 10.1176/appi.ajp.2012.12060754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Machado DC, Hipolide DC, Dubiela FP, Suchecki D, Macedo CE, Tescarollo F, Martins U, Covolan L, Nobrega JN. Deep brain stimulation reverses anhedonic-like behavior in a chronic model of depression: role of serotonin and brain derived neurotrophic factor. Biol Psychiatry. 2012b;71:30–35. doi: 10.1016/j.biopsych.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Nobrega JN. Preclinical studies modeling deep brain stimulation for depression. Biol Psychiatry. 2012;72:916–923. doi: 10.1016/j.biopsych.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Nobrega JN, Lozano AM. Deep brain stimulation in clinical practice and in animal models. Clin Pharmacol Ther. 2010c;88:559–562. doi: 10.1038/clpt.2010.133. [DOI] [PubMed] [Google Scholar]
- Hamani C, Temel Y. Deep brain stimulation for psychiatric disease: contributions and validity of animal models. Sci Transl Med. 2012;4:142rv148. doi: 10.1126/scitranslmed.3003722. [DOI] [PubMed] [Google Scholar]
- Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, Wint D, Craighead MC, Kozarsky J, Chismar R, Moreines JL, Mewes K, Posse PR, Gutman DA, Mayberg HS. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69:150–158. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Giacobbe P, Rizvi SJ, Placenza FM, Nishikawa Y, Mayberg HS, Lozano AM. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168:502–510. doi: 10.1176/appi.ajp.2010.10081187. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Giacobbe P, Hamani C, Rizvi SJ, Kennedy SH, Kolivakis TT, Debonnel G, Sadikot AF, Lam RW, Howard AK, Ilcewicz-Klimek M, Honey CR, Mayberg HS. A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression. J Neurosurg. 2012;116:315–322. doi: 10.3171/2011.10.JNS102122. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Moser PC, Sanger DJ. 5-HT1A receptor antagonists neither potentiate nor inhibit the effects of fluoxetine and befloxatone in the forced swim test in rats. Eur J Pharmacol. 1999;372:127–134. doi: 10.1016/s0014-2999(99)00202-2. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, Hen R, Koester S, Lederhendler I, Meaney M, Robbins T, Winsky L, Zalcman S. Preclinical models: status of basic research in depression. Biol Psychiatry. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego, London: 1998. [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Takagishi M, Chiba T. Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA-L study. Brain Res. 1991;566:26–39. doi: 10.1016/0006-8993(91)91677-s. [DOI] [PubMed] [Google Scholar]
- Wieland S, Lucki I. Antidepressant-like activity of 5-HT1A agonists measured with the forced swim test. Psychopharmacology (Berlin) 1990;101:497–504. doi: 10.1007/BF02244228. [DOI] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Wydra K, Filip M. Effects of serotonin (5-HT) 2 receptor ligands on depression-like behavior during nicotine withdrawal. Neuropharmacology. 2010;58:1140–1146. doi: 10.1016/j.neuropharm.2010.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.