Abstract
Background
Deep brain stimulation (DBS) is being investigated as a treatment for major depression, but its mechanisms of action are still unknown. We have studied the effects of ventromedial prefrontal cortex (vmPFC) stimulation in a chronic model of depression and assessed the involvement of the serotonergic system and brain derived neurotrophic factor (BDNF) in a DBS response.
Methods
Rats were subjected to chronic unpredictable mild stress during 4 weeks. Decline in preference for sucrose solutions over water, an index suggested to reflect anhedonic-like behavior, was monitored on a weekly basis. The outcome of chronic vmPFC stimulation alone (8 hours/day for 2 weeks) or combined with serotonin-depleting lesions was characterized. BDNF levels were measured in the hippocampus.
Results
Stress induced a significant decrease in sucrose preference as well as hippocampal BDNF levels as compared with those recorded in control subjects. vmPFC stimulation completely reversed this behavioral deficit and partially increased BDNF levels. In contrast, DBS did not improve stress-induced anhedonic-like behavior in animals bearing serotonin-depleting raphe lesions with associated normal hip-pocampal BDNF levels.
Conclusions
vmPFC stimulation was effective in a chronic model of depression. Our results suggest that the integrity of the serotonergic system is important for the anti-anhedonic-like effects of DBS but question a direct role of hippocampal BDNF.
Keywords: BDNF, cingulate gyrus, deep brain stimulation, depression, prefrontal cortex, psychiatry, serotonin
Subcallosal cingulate gyrus (SCG) deep brain stimulation (DBS) is currently being investigated as a potential therapy for treatment-resistant depression. Despite the promising results of initial trials (1–4), mechanisms underlying an antidepressant DBS response remain largely unknown (4). We have recently shown that DBS applied to the ventromedial prefrontal cortex (vmPFC), the rodent homologue of the human SCG, induces an antidepressant-like effect in rats undergoing the forced swim test (FST) (5,6). Although the FST is a well-established screener to assess the antidepressant activity of clinical therapies, a common criticism to its face validity is the short timeframe for an antidepressant-like response (e.g., 1–2 days). In this context, models such as chronic unpredictable mild stress (CUS) would be more suitable for the investigation of chronic effects of DBS. As commonly applied, CUS involves subjecting rodents to a series of unpredictable stressors over a period of weeks. A decline in the preference for sucrose solutions over water has been suggested to reflect an anhedonic-like behavior (7–12).
In the present study, we report on a significant anti-anhedonic-like effect of vmPFC DBS in rodents undergoing CUS. The kinetics of a DBS response and mechanisms for the effects of vmPFC stimulation are investigated and discussed from a translational perspective.
Methods and Materials
Protocols were approved by the Animal Care Committees of the Centre for Addiction and Mental Health and the Universidade Federal de São Paulo (UNIFESP), Brazil. Forty male 250–275-g Wistar rats (UNIFESP, Sao Paulo, Brazil) were housed in individual cages and initially trained to drink a 1% sucrose solution with a two-bottle choice procedure. Animals were paired according to a sucrose preference index (SPI), to balance the groups to undergo stress or no-stress. This is defined as (sucrose)/(sucrose + water) consumed during 1 hour. In a second set of experiments to study mechanisms of DBS, 45 animals were used. Of every three animals, two were randomly assigned to receive stress and one was randomly assigned to become a control.
CUS
Rats were subjected to 12 different stressors on a weekly schedule. These included four periods of water deprivation (16 hours, 19 hours, 20 hours, and 30 hours), two periods of food deprivation (16 hours and 30 hours), two periods in a soiled cage (wet bedding, 17 hours and 18 hours), two periods of cage tilt (45°–60°, 21 hours and 41 hours), two periods of paired housing (18 hours and 19 hours), two 5-hour and one 4-hour periods of continuous noise (85 dB), three periods of exposure to a cloth with cat smell (1 hour), one period of continuous illumination in the dark phase (12 hours), two periods of darkness in the light phase (12 hours), two 16-hour periods of partial food deprivation (30 g of food), two 1-hour periods with an empty water bottle, and two 3-hour periods of stroboscopic illumination. The SPI measurements were obtained once/week.
Four weeks after the beginning of stress, all rats (including non-stressed control subjects) were once again matched for SPI scores. Members of each pair were randomly assigned to receive DBS or sham surgery.
Surgical Procedures
Rats were anesthetized with ketamine/xylazine (100/7.5 mg/kg IP) and had monopolar vmPFC stainless steel electrode implanted (anteroposterior + 3.0; medial-lateral ± .4; dorsal-ventral 5.2 mm) (13). These had 250 μm in diameter and .75 mm of exposed surface and were used as cathodes. Electrodes connected to an epidural screw were used as anodes. Control subjects (sham surgery) were anesthetized and had holes drilled into the skull but were not implanted with electrodes.
Serotonin (5-HT) depletion was achieved by injections of 5,7-dihydroxytryptamine (5,7-DHT) into the dorsal and median raphe nuclei (5). A 30-gauge needle was first lowered into the median raphe (AP −7.8, L ± 0, D8.8 mm) and .2 μL of a solution containing 4 μg of 5,7-DHT creatinine sulphate (Sigma-Aldrich, Oakville, ON, Canada) in .1% ascorbic acid was injected over 4 min. After an additional 4 min, the needle was raised by 2.2 mm, and the process was repeated in the dorsal raphe. Control subjects received .1% ascorbic acid (5). During the same surgical procedure, animals were either implanted with vmPFC electrodes or had holes drilled into their skull, as described in the preceding text.
DBS and Postoperative Assessment
Animals were allowed 1 week to recover from surgery before stress was restarted. The DBS was only commenced 1 week later (on the third postoperative week), when SPI measurements were similar to preoperative levels. Stimulation was conducted with a hand-held device (St. Jude Medical 3510, St. Jude Medical, St. Paul, Minnesota) at 200 μA, 130 Hz, and 90 μsec of pulse width. These settings were chosen because they were effective in our previous FST studies (5,6) and have been suggested to approximate those used in clinical practice (5,6,14). Stimulation was applied daily from 9:00 AM to 5:00 PM for 2 weeks. Rats were not stimulated during SPI measurements or when they were paired-housed. Animals continued to receive stress for an additional 3 weeks, to study the recurrence of anhedonic-like behavior after DBS offset.
Histology
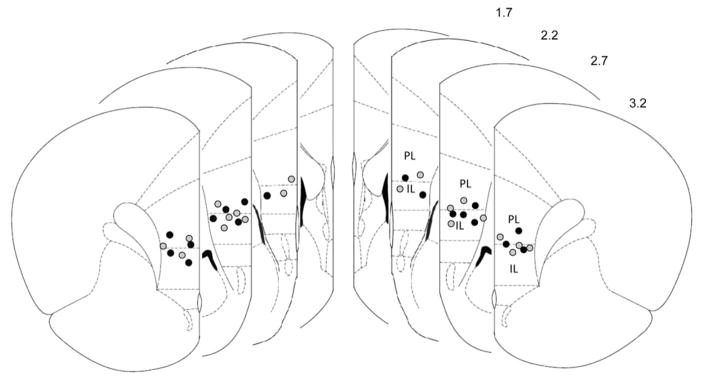
In the first set of experiments, rats were sacrificed 3 weeks after stimulation was discontinued and had their brains stained with cresyl violet (5,6). One animal lost the stimulation cap, and one died during CUS. Both were excluded from the analysis. The location of the electrodes in these animals is presented in Figure 1. Because the exposed tip of our electrodes covered .75 mm, most of the current was delivered to the ventral prelimbic cortex (vPL). This region was chosen as a target on the basis of our previous studies showing that stimulation of the vPL induces significant antidepressant-like effects in the FST (6,15,16).
Figure 1.
Location of electrodes in the ventromedial prefrontal cortex in animals undergoing deep brain stimulation. Schematic representation of coronal brain sections showing the location of the tip of the electrodes implanted in stressed (black circles) and nonstressed (gray circles) animals. Because the exposed tip of our electrodes covered .75 mm, most of the current was delivered to ventral regions of the prelimbic cortex (PL). Numbers to the right denote distance from bregma. IL, infralimbic cortex. Reprinted from Paxinos and Watson (13), with permission from Elsevier, Copyright 1998.
In experiments designed to investigate mechanisms of DBS, animals were decapitated immediately after stimulation was discontinued (2 weeks after DBS onset). Two animals lost the stimulation cap and was excluded from the study. Two animals died during CUS. After removal from the skull, brains were quickly frozen in dry ice. Hippocampi were bilaterally dissected for the analysis of brain derived neurotrophic factor (BDNF) and 5-HT levels (one hemisphere each). For BDNF, tissue was homogenized in lysis buffer and centrifuged. Protein levels in the supernatant were detected with enzyme-linked immunosorbent assay (Promega Corporation, Madison, Wisconsin) (17). The extent of 5-HT depletion in animals undergoing 5,7-DHT lesions was assessed by capillary electrophoresis with laser-induced fluorescence detection (CE-LIF) (18,19). The 5-HT levels in these animals may be found in Figure S1 in Supplement 1. Three animals that did not have a significant decrease in 5-HT levels after 5,7-DHT were excluded from behavioral and neurochemical analyses (two in the stress alone group, and one in the DBS stress group).
Statistical Analysis
One-way analysis of variance was used to compare data when three or more independent groups were considered (Fisher post hoc). For experiments with only 2 samples, independent or paired Student t tests were used. Statistical significance was set at p = .05. Results in the text and figures are expressed as mean ± SEM.
Results
Effects of vmPFC DBS in the CUS Model: Kinetics and Timeframe of Response
As shown in Figure 2, SPI at baseline was approximately 66%. One week after CUS, differences between stressed and nonstressed rats were already noticeable. These became particularly pronounced after 2 weeks of stress. During weeks 3 and 4, SPI stabilized at approximately 30%–40% in the stressed group. Animals were then implanted with electrodes or had sham surgery. One week later, the CUS protocol was restarted.
Figure 2.
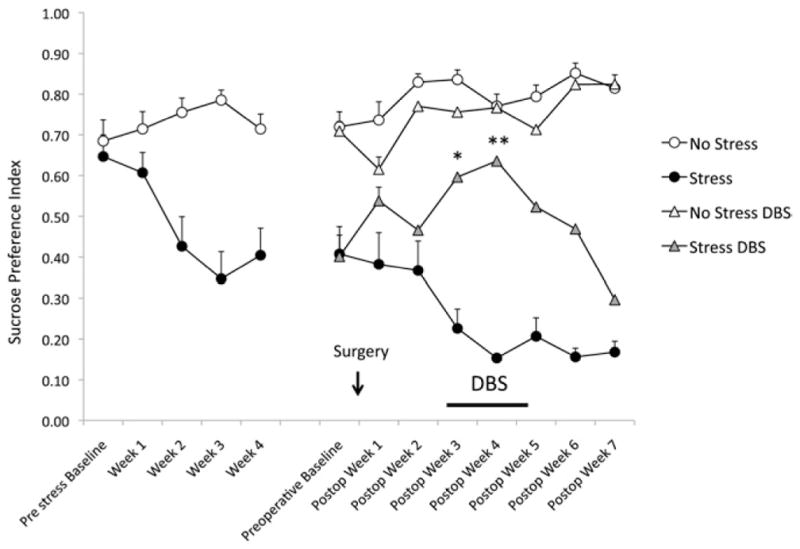
Changes in sucrose preference index (SPI) as a function of stress and deep brain stimulation (DBS). The figure is divided in two parts, before and after surgery (arrow). Before surgery, rats were exposed to chronic unpredictable mild stress or remained in their home cages for 4 weeks. Except for baseline, all points were statistically different between stressed and nonstressed animals (p < .03). Indicators of statistical significance were omitted for clarity. In the preoperative baseline assessments immediately before surgery, one may notice that SPI in stressed and nonstressed animals to undergo DBS or sham surgery were similar. Stress was reinitiated 1 week after the procedure. DBS (8 hours/day) was commenced on the third postoperative (postop) week and continued for 2 weeks (horizontal bar). On the first week after stimulation onset, SPI in stress DBS animals (n = 9) was significantly different from those recorded in the stress alone group (n = 9, p < .002*). Differences were even more accentuated on the second week of stimulation (p < .0001 vs. stress alone**) with SPI in DBS-treated stressed animals, reaching levels comparable to those of nonstressed control subjects. Thereafter, stimulation was discontinued for the assessment of changes in hedonic-like behavior. Decrease in sucrose preference was gradual, with values approximating preoperative baseline levels only 3 weeks later. No significant differences were observed between nonstressed animals that did (n = 10) or did not receive DBS (n = 10).
On the first postoperative assessment, stressed animals with implanted electrodes had a slight increase in SPI scores (54% vs. 40% before surgery; p > .05). Return to near baseline levels was only observed on the second postoperative week. A 2-week regimen of DBS (8 hours/day) was then commenced. On the first post-DBS measurement, a significant increase in SPI was observed when stressed animals that had or did not have DBS were compared (60% vs. 23%, respectively, p < .002). Results became even more pronounced on the second week of stimulation (64% vs. 15%, p = .0001), with SPI in DBS-treated stressed animals reaching levels similar to those in nonstressed control subjects. After discontinuing DBS, the recurrence of an anhedonic-like behavior in stressed animals was gradual. Three weeks after stimulation was ceased, SPI scores declined by 30% but were still significantly higher than those recorded in sham-treated stressed rats (17%). Of note, DBS had no effect on SPI in nonstressed control subjects.
Changes in sucrose consumption were similar to the ones described in the preceding text for the SPI (Figure S2 in Supplement 1). Stressed rats treated with DBS had a significant increase in sucrose consumption (p < .001) and a significant decrease in water intake (p < .001) as compared with nonstimulated stressed control subjects. After 4 weeks of CUS, body weight in stressed animals was 12% lower than in nonstressed control subjects (p < .01). Two weeks after DBS onset, body weight in stressed animals that did and did not receive DBS were 18% and 13% lower than in nonstressed control subjects, respectively (p < .01 for both comparisons). Differences in body weight between groups receiving stress alone or DBS + stress were not significant.
Mechanisms for the Effects of vmPFC DBS: 5-HT Depletion and BDNF Levels
We found that the anti-anhedonic-like effects of vmPFC stimulation were abolished in rats bearing 5-HT depleting raphe lesions (Figure 3). In contrast, 5-HT depletion did not affect sucrose preference in either sham stressed animals or nonstressed control subjects.
Figure 3.
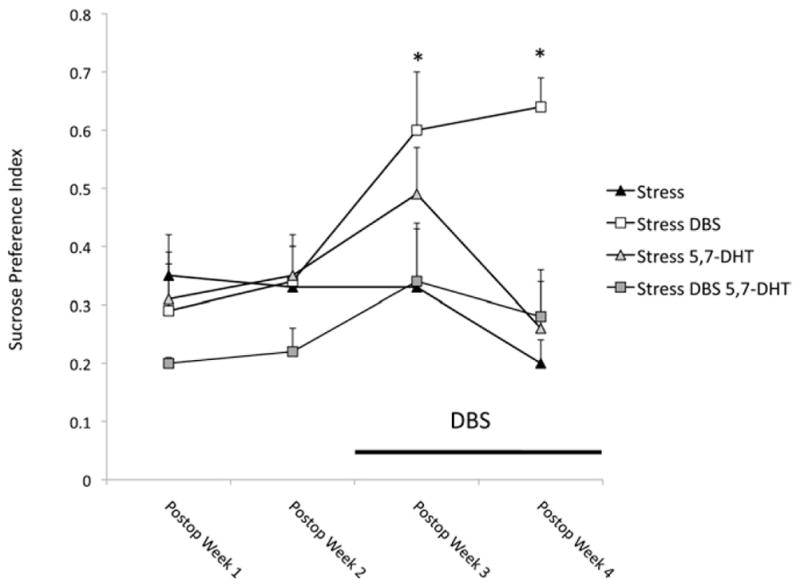
Changes in SPI as a function of stress and DBS in animals undergoing 5,7-dihydroxytryptamine (5,7-DHT) raphe lesions. As in our previous experiment, DBS (n = 5) induced a significant increase in SPI as compared with the nonstimulated stressed group (n = 6; p = .05 and p < .001 for the first and second weeks of stimulation, respectively). The effects of stimulation were blocked in stressed animals given raphe microinjections of 5,7-DHT, a toxin for serotonergic neurons (n = 6). Differences in SPI were not significant when stressed (n = 6) and nonstressed (n = 5) serotonin-depleted animals were compared with their respective control subjects (results of nonstressed animals were not depicted in the figure for the sake of clarity). *Statistically significant differences. Abbreviations as in Figure 2.
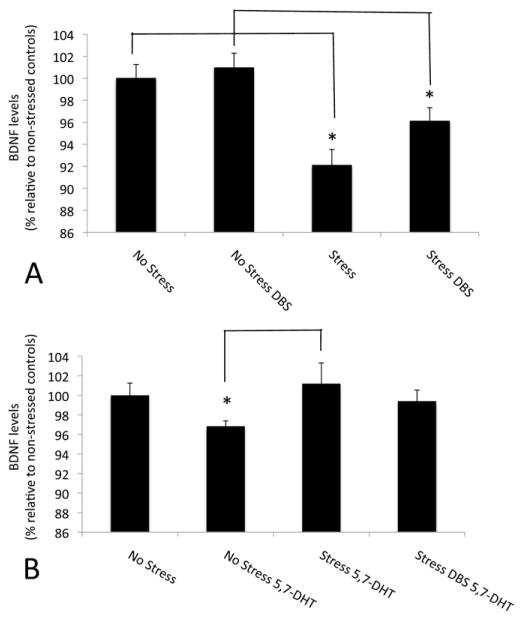
As expected, hippocampal BDNF levels in animals undergoing CUS were significantly lower than in nonstressed control subjects (p = .0007) (Figure 4A). DBS partially reversed this deficit with a trend toward an increase in BDNF levels (p = .055) (Figure 4A). In contrast, stimulation did not exert a significant effect on BDNF levels in nonstressed animals.
Figure 4.
Brain derived neurotrophic factor (BDNF) levels in the hippocampus of animals receiving ventromedial prefrontal cortex stimulation during chronic unpredictable mild stress. (A) Hippocampal BDNF levels were significantly lower in stressed animals as compared with nonstressed control subjects (p < .0007). DBS partially reversed this deficit with a trend toward an increase in BDNF levels (p = .055). (B) In stressed animals given 5,7-DHT raphe injections, BDNF levels were higher than in corresponding serotonin-depleted control subjects (p = .02). In contrast, BDNF levels in 5,7-DHT stressed animals with or without DBS were similar to those recorded in nonstressed, nonlesioned control subjects. Values in the graph represent percentages relative to animals that did not undergo chronic unpredictable mild stress or 5,7-DHT injections. *Statistically significant. Abbreviations as in Figure 3.
Stressed animals injected with 5,7-DHT showed a small but significant increase in BDNF levels relative to 5-HT depleted control subjects (p = .02) (Figure 4B). In contrast, BDNF levels in 5,7-DHT stressed animals with or without DBS were similar to those recorded in nonstressed, nonlesioned control subjects. This is of importance, because DBS did not exert an anti-anhedonic-like response in 5-HT-depleted animals, despite normal BDNF levels.
Discussion
In the present study, chronic vmPFC DBS improved anhedonic-like behavior in animals undergoing CUS. This shows that DBS is effective in ameliorating this cardinal symptom of depression in rodents, in addition to resulting in a general antidepressant-like response. As in our previous report (5), rats tolerated DBS very well and behaved normally during stimulation. In addition, chronic DBS was found to have no effect on SPI in nonstressed control subjects, thus ruling out the possibility of a stimulation-induced nonspecific increase in sucrose preference.
Translation of data from animal models to humans must always be considered with caution. That being said, we believe our findings might yield interesting parallels and possibly raise issues to be considered in clinical trials. One observation was that rats implanted with electrodes showed a slight increase in SPI right after the surgery in the absence of stimulation. In a parallel scenario, a so-called “insertional effect” has been characterized as an improvement in clinical symptoms after the implantation of electrodes in the absence of stimulation (20,21). This effect should be taken into account when data from clinical trials is interpreted and when experiments with DBS in animal models are conducted.
In our study, a significant improvement in sucrose preference after DBS was already apparent after 1 week of treatment. On the second week, anhedonic-like behavior was remarkably improved, with SPI levels approximating those recorded in nonstressed control subjects. This short timeframe for a DBS response suggests that the mechanisms involved in the effects of stimulation might be different from the ones reported for medications, which might require weeks for a positive outcome (8–11).
Animals continued to receive CUS for 3 additional weeks, so that recurrence of anhedonia after termination of DBS could be studied. We found that SPI began to decline a few weeks after simulation was ceased, suggesting that vmPFC stimulation might be associated with a carry-over effect (i.e., persistence of a response after treatment is discontinued). In the clinical scenario, Holtzheimer et al. (22) have recently conducted a study in which a few depression patients receiving DBS had stimulation discontinued. Symptom recurrence in these subjects did not occur immediately but in a few weeks. Crossover studies are commonly used in the DBS literature (23). Trials with such design involve the administration of either active or sham stimulation for a period of time, after which patients receive the inverse treatment. Our findings suggest that a washout period without stimulation in between arms might be of importance and crossover only be carried out when symptom recurrence is recorded.
Neural substrates commonly thought to be involved in mechanisms of antidepressant therapies include monoaminergic systems, neurotrophins, and neurogenesis (24–27). The relatively short timeframe required for a behavioral DBS response in the CUS model suggests that neurogenesis might not be involved (e.g., this interval would be too short for newly born cells to differentiate and become incorporated into the hippocampal circuitry). We therefore decided to focus our initial investigation on the serotonergic system and BDNF.
We have previously shown that vmPFC stimulation induces a fourfold increase in hippocampal 5-HT release and that the antide-pressant-like effects of DBS in the FST are dependent on the integrity of the serotonergic system (5). Our current results are in line with those findings, because the anti-anhedonic-like effects of stimulation in stressed animals were abolished after 5-HT-depleting raphe lesions. However, although in our initial report naïve animals received DBS for only a few hours, in the present study stressed rats were stimulated for 2 weeks. These results suggest that the serotonergic system might be involved in both acute and chronic effects of vmPFC DBS in rats. Mechanisms underlying the antidepressant-like effects of vmPFC DBS remain unclear but might involve the modulation of prefrontal projections to the raphe, a key structure in 5-HT synthesis and release (6,15,16,28). Future studies are still required to characterize the effects of chronic stimulation on the serotonergic system and how DBS modulates activity of raphe nuclei.
BDNF levels are significantly reduced in rodents undergoing stress and in patients with depression (29–31). Commonly used antidepressant treatments such as medications and electroconvulsive therapy upregulate BDNF in various brain regions (31,32). In our study, vmPFC DBS partially reversed the stress-induced reduction in hippocampal BDNF levels observed in animals undergoing CUS. This is in line with previous reports showing an increase in BDNF after electrical stimulation of the ventral tegmental area, nucleus accumbens, and vPL in rodents (33,34). In one of these studies, increased BDNF levels after vPL stimulation were positively correlated with sucrose preference scores in animals undergoing CUS (34).
Reports on interactions between BDNF and 5-HT have mainly focused on changes in 5-HT levels in transgenic animals and preparations that increase or decrease BDNF (35–39). Studies addressing the consequences of 5-HT depletion on BDNF levels are far less common, particularly in stressed animals. In our study, 5-HT-depleting raphe lesions prevented the reduction in BDNF levels observed in animals undergoing CUS. Furthermore, BDNF levels in these animals were significantly higher than in nonstressed rats undergoing 5,7-DHT injections. These findings are in line with previous reports showing that 5-HT depletion might interfere with mechanisms of stress adaptation, leading to an increase in the expression of hippocampal glucocorticoid receptors and BDNF (40). Perhaps the most intriguing aspect of our study was that vmPFC DBS did not improve SPI scores in 5-HT-depleted stressed animals, despite the concurrent presence of normal hippocampal BDNF levels. These results question a direct role for hippocampal BDNF in the anti-anhedonic-like effects of vmPFC DBS. That being said, it is possible that an increase in BDNF might contribute to the effects of DBS in conjunction with other substrates and that this neurotrophin might play a role in animals with an intact serotonergic system.
A few methodological considerations need to be addressed in our study. The first includes the timeframe of surgery and stimulation. In an initial study, Friedman et al. stimulated the ventral tegmental area in naïve and Flinders rats at settings designed to mimic the neuronal firing pattern of the nucleus (33). Deep brain stimulation administered 20 min before testing induced an antidepressant-like response in different behavioral paradigms (33). In a more recent study, rats undergoing CUS were treated with vPL and nucleus accumbens stimulation at settings suggested to mimic those used for transcranial magnetic stimulation in humans (10 min/day, 5-sec pulses at 20 Hz with 20-sec pauses for 10 days) (34). Deep brain stimulation in either target but not the dorsal prelimbic cortex (PL) induced an antidepressant/anti-anhedonic-like effect (34). In the present study, our goal was to mimic the clinical scenario, in which depressed patients receive DBS continuously for a long time (3). In this context, electrodes were implanted in rats that were already stressed, and stimulation was conducted for 8 hours/day during 2 weeks. Because our DBS system is external and we could not leave the animals connected to the equipment at night, we were unable to conduct experiments with stimulation being delivered 24 hours/day. As mentioned in Methods, frequency and pulse width in our study were similar to those used in clinical practice. Current was selected on the basis of our previous experiments in the FST (6).
Another important aspect to be discussed is the stimulation target. Although the anatomy of the PFC varies considerably across species (41,42), when anatomical connections and cytoarchitectural features are considered, ventral regions of the medial prefrontal cortex (e.g., infralimbic cortex [IL] and vPL) are suggested as the anatomical correlates of the SCG (43,44). In our previous studies, DBS in PL was associated with a more prominent antidepressant-like response in the FST as compared with the IL (6). In this context, we decided to implant the tip of our electrodes in the IL/PL border so that most of the exposed surface of the electrodes would be placed within the vPL.
Finally, the CUS protocol in our study was similar to that described by others (11). Although stressors in our protocol were largely mild, some might be considered somewhat stronger (e.g., paired housing and food deprivation). We do not know whether DBS would still be effective in animals only subjected to severe forms of stress.
In summary, we report an anti-anhedonic-like effect of vmPFC DBS in a chronic model of depression. The kinetics involved in such response were somewhat similar to those described in clinical practice, including the presence of insertional and carryover effects. Our study also shows that the effects of DBS in the CUS model were abolished in animals bearing 5-HT-depleting lesions. This suggests that, as for the antidepressant-like effects of DBS in the FST, the integrity of the serotonergic system is important for the chronic effects of vmPFC stimulation in CUS. We found that, in contrast to stressed animals, rats receiving 5,7-DHT raphe injections with or without DBS had normal hippocampal BDNF levels. This is of importance, because in 5-HT-depleted animals DBS did not exert an anti-anhedonic-like response, despite normal BDNF levels. These results question a direct role for hippocampal BDNF in the anti-anhedonic-like effects of vmPFC DBS. Future studies are still required to further address this issue.
Supplementary Material
Acknowledgments
CH is a consultant for St. Jude Medical. JNN and CH are supported in part with funds from the National Alliance for Research on Schizophrenia and Depression, the Ontario Mental Health Foundation, and the Canadian Institutes of Health Research. LC received funds from FAPESP (São Paulo research Foundation). DS is the recipient of a research fellowship from the Brazilian National Research Council. DCH received support from AFIP (Associação Fundo de Incentivo à Pesquisa). All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online.
References
- 1.Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 2.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy SH, Giacobbe P, Rizvi SJ, Placenza FM, Nishikawa Y, Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression: Follow-up after 3 to 6 years. Am J Psychiatry. 2011;168:502–510. doi: 10.1176/appi.ajp.2010.10081187. [DOI] [PubMed] [Google Scholar]
- 4.Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011;69:301–308. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 5.Hamani C, Diwan M, Macedo CE, Brandao ML, Shumake J, Gonzalez-Lima F, et al. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol Psychiatry. 2010;67:117–124. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 6.Hamani C, Diwan M, Isabella S, Lozano AM, Nobrega JN. Effects of different stimulation parameters on the antidepressant-like response of medial prefrontal cortex deep brain stimulation in rats. J Psychiatr Res. 2010;44:683–687. doi: 10.1016/j.jpsychires.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry. 2007;62:496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Willner P. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 9.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 10.Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- 11.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 12.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1988. [Google Scholar]
- 14.Hamani C, Nobrega JN. Deep brain stimulation in clinical trials and animal models of depression. Eur J Neurosci. 2010;32:1109–1117. doi: 10.1111/j.1460-9568.2010.07414.x. [DOI] [PubMed] [Google Scholar]
- 15.Hamani C, Diwan M, Macedo CE, Brandao ML, Shumake J, Gonzalez-Lima F, et al. Reply to: Electrical brain stimulation in depression: Which target(s)? Biol Psychiatry. 2011;69:e7–8. doi: 10.1016/j.biopsych.2010.09.056. [DOI] [PubMed] [Google Scholar]
- 16.Lim LW, Tan SK, Groenewegen HJ, Temel Y. Electrical brain stimulation in depression: Which target(s)? Biol Psychiatry. 2011;69:e5–6. doi: 10.1016/j.biopsych.2010.09.056. [DOI] [PubMed] [Google Scholar]
- 17.Vines A, Delattre AM, Lima MM, Rodrigues LS, Suchecki D, Machado RB, et al. The role of 5-HT(1A) receptors in fish oil-mediated increased BDNF expression in the rat hippocampus and cortex: A possible antidepressant mechanism [published online ahead of print June 29] Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Huang YF, Chiang CK, Lin YW, Liu K, Hu CC, Bair MJ, et al. Capillary electrophoretic separation of biologically active amines and acids using nanoparticle-coated capillaries. Electrophoresis. 2008;29:1942–1951. doi: 10.1002/elps.200700534. [DOI] [PubMed] [Google Scholar]
- 19.Macedo CE, Castilho VM, de Souza e Silva MA, Brandao ML. Dual 5-HT mechanisms in basolateral and central nuclei of amygdala in the regulation of the defensive behavior induced by electrical stimulation of the inferior colliculus. Brain Res Bull. 2002;59:189–195. doi: 10.1016/s0361-9230(02)00862-6. [DOI] [PubMed] [Google Scholar]
- 20.Mann JM, Foote KD, Garvan CW, Fernandez HH, Jacobson CE, 4th, Rodriguez RL, et al. Brain penetration effects of microelectrodes and DBS leads in STN or GPi. J Neurol Neurosurg Psychiatry. 2009;80:794–797. doi: 10.1136/jnnp.2008.159558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pourfar M, Tang C, Lin T, Dhawan V, Kaplitt MG, Eidelberg D. Assessing the microlesion effect of subthalamic deep brain stimulation surgery with FDG PET. J Neurosurg. 2009;110:1278–1282. doi: 10.3171/2008.12.JNS08991. [DOI] [PubMed] [Google Scholar]
- 22.Holtzheimer PE, Filkowski M, Kelley ME, Gross RE, Mayberg HS. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Biol Psychiatry. 2011;69:110S. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359:2121–2134. doi: 10.1056/NEJMoa0708514. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 26.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Mongeau R, Blier P, de Montigny C. The serotonergic and noradrenergic systems of the hippocampus: Their interactions and the effects of antidepressant treatments. Brain Res Brain Res Rev. 1997;23:145–195. doi: 10.1016/s0165-0173(96)00017-3. [DOI] [PubMed] [Google Scholar]
- 28.Hamani C, Nobrega JN. Deep brain stimulation in an animal model of depression. Eur J Neurosci. 2010;32:1109–1117. doi: 10.1111/j.1460-9568.2010.07414.x. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54:70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 30.Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altar CA, Whitehead RE, Chen R, Wortwein G, Madsen TM. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol Psychiatry. 2003;54:703–709. doi: 10.1016/s0006-3223(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 33.Friedman A, Frankel M, Flaumenhaft Y, Merenlender A, Pinhasov A, Feder Y, et al. Programmed acute electrical stimulation of ventral tegmental area alleviates depressive-like behavior. Neuropsychopharmacology. 2009;34:1057–1066. doi: 10.1038/npp.2008.177. [DOI] [PubMed] [Google Scholar]
- 34.Gersner R, Toth E, Isserles M, Zangen A. Site-specific antidepressant effects of repeated subconvulsive electrical stimulation: Potential role of brain-derived neurotrophic factor. Biol Psychiatry. 2010;67:125–132. doi: 10.1016/j.biopsych.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Benmansour S, Deltheil T, Piotrowski J, Nicolas L, Reperant C, Gardier AM, et al. Influence of brain-derived neurotrophic factor (BDNF) on serotonin neurotransmission in the hippocampus of adult rodents. Eur J Pharmacol. 2008;587:90–98. doi: 10.1016/j.ejphar.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 36.Deltheil T, Guiard BP, Cerdan J, David DJ, Tanaka KF, Reperant C, et al. Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice. Neuropharmacology. 2008;55:1006–1014. doi: 10.1016/j.neuropharm.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Deltheil T, Guiard BP, Guilloux JP, Nicolas L, Delomenie C, Reperant C, et al. Consequences of changes in BDNF levels on serotonin neurotransmission, 5-HT transporter expression and function: Studies in adult mice hippocampus. Pharmacol Biochem Behav. 2008;90:174–183. doi: 10.1016/j.pbb.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 38.Gardier AM. Mutant mouse models and antidepressant drug research: focus on serotonin and brain-derived neurotrophic factor. Behav Pharmacol. 2009;20:18–32. doi: 10.1097/FBP.0b013e3283243fcd. [DOI] [PubMed] [Google Scholar]
- 39.Guiard BP, David DJ, Deltheil T, Chenu F, Le Maitre E, Renoir T, et al. Brain-derived neurotrophic factor-deficient mice exhibit a hippocampal hyperserotonergic phenotype. Int J Neuropsychopharmacol. 2008;11:79–92. doi: 10.1017/S1461145707007857. [DOI] [PubMed] [Google Scholar]
- 40.Zhou J, Li L, Tang S, Cao X, Li Z, Li W, et al. Effects of serotonin depletion on the hippocampal GR/MR and BDNF expression during the stress adaptation. Behav Brain Res. 2008;195:129–138. doi: 10.1016/j.bbr.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 41.Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: Evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 43.Gabbott PL, Warner TA, Jays PR, Bacon SJ. Areal and synaptic interconnectivity of prelimbic (area 32), infralimbic (area 25) and insular cortices in the rat. Brain Res. 2003;993:59–71. doi: 10.1016/j.brainres.2003.08.056. [DOI] [PubMed] [Google Scholar]
- 44.Takagishi M, Chiba T. Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: An anterograde tracer PHA-L study. Brain Res. 1991;566:26–39. doi: 10.1016/0006-8993(91)91677-s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.