Abstract
Recent studies in patients with treatment-resistant depression have shown similar results with the use of deep brain stimulation (DBS) in the subcallosal cingulate gyrus (SCG), ventral capsule/ventral striatum (VC/VS) and nucleus accumbens (Acb). As these brain regions are interconnected, one hypothesis is that by stimulating these targets one would just be influencing different relays in the same circuitry. We investigate behavioral, immediate early gene expression, and functional connectivity changes in rats given DBS in homologous regions, namely the ventromedial prefrontal cortex (vmPFC), white matter fibers of the frontal region (WMF) and nucleus accumbens. We found that DBS delivered to the vmPFC, Acb but not WMF induced significant antidepressant-like effects in the FST (31%, 44%, and 17% reduction in immobility compared to controls). Despite these findings, stimulation applied to these three targets induced distinct patterns of regional activity and functional connectivity. While animals given vmPFC DBS had increased cortical zif268 expression, changes after Acb stimulation were primarily observed in subcortical structures. In animals receiving WMF DBS, both cortical and subcortical structures at a distance from the target were influenced by stimulation. In regard to functional connectivity, DBS in all targets decreased intercorrelations among cortical areas. This is in contrast to the clear differences observed in subcortical connectivity, which was reduced after vmPFC DBS but increased in rats receiving Acb or WMF stimulation. In conclusion, results from our study suggest that, despite similar antidepressant-like effects, stimulation of the vmPFC, WMF and Acb induces distinct changes in regional brain activity and functional connectivity.
Keywords: Prefrontal cortex, Nucleus accumbens, Anterior capsule, Depression, Deep brain stimulation, Connectivity
Introduction
Deep brain stimulation (DBS) is currently being investigated as a potential therapy for treatment-refractory depression. Targets commonly studied in different trials include the subcallosal cingulate gyrus (SCG), ventral capsule/ventral striatum (VC/VS) and nucleus accumbens (Acb) (Bewernick et al., 2010; Hamani and Nobrega, 2010; Holtzheimer et al., 2011b; Lozano et al., 2008; Malone et al., 2009; Mayberg et al., 2005). Though no clinical study has been conducted comparing the efficacy of DBS in different targets, approximately 50% of the patients seem to respond to the procedure, independent of the stimulated brain site (Bewernick et al., 2010; Hamani and Nobrega, 2010; Holtzheimer et al., 2011b; Lozano et al., 2008; Malone et al., 2009; Mayberg et al., 2005).
Cortical regions involved in the pathophysiology of psychiatric disorders include the prefrontal and orbitofrontal cortices (Drevets et al., 2008; Ongur and Price, 2000; Price and Drevets, 2010; Price et al., 1996). These project the ventral striatum and the head of the caudate, which in turn send GABAergic efferents to the limbic and associative regions of the globus pallidus and substantia nigra reticulata. Outflow structures of the basal ganglia innervate the mediodorsal and ventral anterior thalamic nuclei, which in turn project back to the prefrontal and orbitofrontal regions (Drevets et al., 2008; Ongur and Price, 2000; Price and Drevets, 2010; Price et al., 1996). In primates, the main fiber pathways interconnecting the above-mentioned structures are the white matter fibers of the frontal lobe, the anterior limb of the internal capsule and the inferior thalamic peduncle (Lehman et al., 2011; Velasco et al., 2005). Considering the similar antidepressant effects of SCG, Acb and VC/VS DBS in open label trials and the fact that prefrontal projections to subcortical structures run, at least in part, through the anterior limb of the internal capsule, a plausible hypothesis would be that by stimulating such targets one would just be influencing different relays of the same circuitry.
Animal models have been suggested to have both predictive and construct validity for translational DBS studies in depression (Hamani and Nobrega, 2012; Hamani and Temel, 2012). In rodents, the antidepressant-like effects of DBS in the forced swim test (FST) and chronic unpredictable mild stress (CUS) occur upon stimulation settings that approximate those used in humans and targets analogous to those used in clinical practice (e.g. the Acb or ventromedial prefrontal cortex; vmPFC) (Gersner et al., 2010; Hamani et al., 2010a, 2010b, 2012; Li et al., 2011).
In the present study we tested the hypothesis that DBS in the vmPFC, Acb, or white matter fibers of the frontal region (WMF) induces comparable antidepressant-like effects in rats by modulating a similar set of brain regions. The first two structures are homologous to the subgenual cingulum and nucleus accumbens in humans (Groenewegen et al., 1990; Hamani and Temel, 2012; Hamani et al., 2011; Heimer et al., 1997; Uylings et al., 2003). As the rodent internal capsule does not have an anterior limb, we have applied stimulation to the largest white matter structure in the anterior portion of the rodent brain, namely the forceps minor. The FST was used as the main behavioral paradigm as it has proven to be very sensitive to DBS alone or in combination with other manipulations (Hamani and Nobrega, 2010, 2012; Hamani and Temel, 2012; Hamani et al., 2010c; Temel et al., 2007). To obtain an index of neuronal activity following stimulation at each anatomical site we examined local expression of zif268. This immediate early gene (IEG) was chosen as, in addition to being very well characterized, it is known to capture both increases and decreases of regional brain activity (Covington et al., 2005; Creed et al., 2013; Guzowski et al., 2001; Horner and Keefe, 2006; Lonergan et al., 2010; Schiltz et al., 2007; Yano and Steiner, 2005). In a preliminary effort to assess possible changes in functional connectivity, we compared intercorrelation patterns of zif268 signal following DBS at different targets.
Methods
All protocols were approved by the Animal Care committee of the Centre for Addiction and Mental Health and complied with Canadian Council on Animal Care (CCAC) and NIH standards and guidelines.
Surgical procedures
Male Sprague–Dawley rats (250–300 g) were anesthetized with halothane and had electrodes implanted in one of the following targets (Paxinos and Watson, 1998): 1) vmPFC; anteroposterior (AP) +3.0 mm, lateral (L) ±0.4 mm, and depth (D) 5.6 mm; 2) white matter fibers of the frontal region (forceps minor) AP +2.7 mm, L 1.4 mm, and D 5.6 mm; and 3) Acb AP +1.6 mm, L 1.2 mm, and D 8.0 mm. Monopolar stainless steel electrodes (250 μm in diameter and 0.5 mm of exposed surface) were used as cathodes. An epidural screw placed over the somatosensory cortex was used as anode (AP 0.5, L ± 1.5, D 1.0 mm) (Paxinos and Watson, 1998). Controls had holes drilled into the skull but were not implanted with electrodes. In behavioral and zif268 experiments we also tested a sham treated group with electrodes implanted that did not receive stimulation. Results were similar to those recorded in non-stimulated controls (Supplementary Fig. 1 and Supplementary Table 1).
Electrical stimulation
DBS was conducted with a handheld device (ANS model 3510) at 100 μA, 130 Hz, and 90 μs. Though we recognize that different targets may require different stimulation amplitudes for a clinical effect, we decided to use a standard current for several reasons. First, with our electrode system these settings generate a charge density similar to that used in patients undergoing DBS (Hamani et al., 2010b, 2012). Second, by using fixed stimulation parameters we could more easily compare data across groups (as the only different variable was the surgical target). Third, at currents above 200 μA animals receiving Acb DBS presented stereotypic behavior. Finally, by using 100 μA the volume of tissue activated by DBS was relatively restricted to the target region (see details below).
Behavioral testing
The FST was conducted one week after electrode implantation. On testing day 1, rats were individually placed for 15 min in a cylinder filled with 25 ± 1 °C water (Detke and Lucki, 1996; Detke et al., 1995). Thereafter, DBS-treated animals received continuous stimulation for 4 h. On the following day, rats in the DBS groups received 2 h of stimulation followed by a 5 min swimming session during which the predominant behavior of the animals was scored every 5 s (immobility, swimming, or climbing) (Detke and Lucki, 1996; Detke et al., 1995). The timeframe of stimulation was selected based on our previous studies (Hamani et al., 2010a, 2010b) and on literature showing that 1–2 doses of antidepressants after swimming on day 1 and one dose before swimming on day 2 reduce immobility (Porsolt et al., 1978). As our systems deliver electrical stimulation, swimming sessions were conducted with the animals disconnected from the swivels (i.e. immediately after receiving DBS). Under these circumstances, it is possible that behavioral responses might have been due to a rebound phenomenon related to the discontinuation of current delivery.
One week after the FST, animals received stimulation for 4 h on day 1 and 2 h on day 2. Thereafter, locomotor activity was assessed in a square 0.49 m2 open field (Med Associates) with infrared photo beams placed along the walls. In this study, we subdivided the open field test into two. The first 5 min were used to evaluate an anxiety-like behavioral response to a novel environment. Overall results of the 30 min of testing were considered to measure general locomotion. After the experiments, electrode location was checked in brain slices processed with cresyl violet (Hamani et al., 2010b).
Computational prediction of neural activation
The computational model used to establish the effects of stimulation has been previously described in detail (Butson et al., 2007). The electric field was calculated using a finite element model constructed in COMSOL v4.3 (COMSOL, Lenexa, KS), taking into account the geometry of the stimulation electrode and the configuration of anodes and cathodes. For simplicity, the voltage in the tissue medium was interpolated as if the medium was homogeneous (Holsheimer et al., 2000). The estimated volume of tissue activated was based on the assumption that electrodes were implanted in an ideal location. We note that this approach is somewhat limited, as it does not reflect individual values. It does, however, provide a general sense of the spillover of current as applied in average electrode locations. Simulations were conducted using NEURON (Hines and Carnevale, 1997). Threshold criteria were used to determine the model-predicted volume of tissue activated.
In situ hybridization and histology
One week after surgery, a batch of animals that did not undergo behavioral testing received stimulation for 4 h on day 1 and 2 h on day 2. Sacrifice was carried out immediately after by decapitation following ketamine/xylazine anesthesia. Hybridization was performed using 35S-UTP labeled riboprobes complementary to zif268, as previously described (Creed et al., 2012). After hybridization, slides were exposed to Kodak BioMax film for 6 days at 4 °C along with calibrated radioactivity standards. Film analyses were conducted with an MCID system (Interfocus, UK).
To assess electrode placement brains were stained with cresyl violet (Supplementary Fig. 2).
3D modeling of structures expressing zif268
Atlas plates (Paxinos and Watson, 1998) were exported as individual pages in TIFF file format. A custom Matlab script (MathWorks Inc., Natick, MA) was used to position each slice in 3D space according to bregma coordinates (as indicated in the atlas). The script allowed us to trace the boundaries of each nucleus and create a continuous curve. Surface reconstruction was done using Pro/Engineer (PTC, Needham, MA), a parametric solid and surface-modeling package. Once contours were created, the SweptBlend function was used to generate closed surfaces. Files were then exported in IGES format and converted to STL using FreeCad. After surfaces were converted into meshes, duplicate vertices, edges and overlapping surfaces were removed with Meshlab. Finally we used ParaView (Kitware Inc.) an open-source, multi-platform data analysis and visualization application. We imported each structure and gave it a distinct color. Surface normals were applied to give it a smooth appearance. Once an image of the desired nuclei was established, it was saved as a ParaView state file as well as exported as VRML (Virtual Reality Modeling Language). The VRML format allows the entire image to be imported into Adobe Acrobat. Each surface was annotated and the final version exported in PDF format.
Correlation matrices and network construction
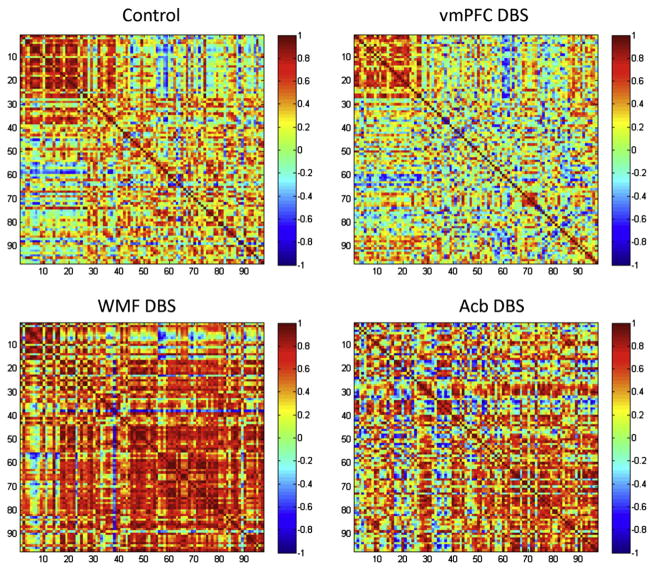
Details on the technique used for correlation analyses and network construction may be found elsewhere (Wheeler et al., 2014). Using the zif268 signal as a dependent variable, all possible pairwise correlations between 98 brain regions expressing this immediate early gene were computed using the Pearson correlation coefficients in controls and the DBS groups separately. High correlation between pairs was noted when zif268 expression in one structure was strongly related to the other in individuals from the same group. Each complete set of correlations was displayed as color-coded correlation matrices using the Matlab software (Mathworks Inc., Natick, MA).
Networks were constructed by considering the strongest inter-regional correlations in each group of animals (Wheeler et al., 2013, 2014). In selecting a threshold criterion for inclusion in the network, we examined a progressive series of increasing r values and the corresponding series of decreasing numbers of brain areas to be treated as nodes in the network. In an effort to balance a stringent r threshold with a reasonable number of network nodes to be included, the decision was made to retain correlations that correspond to a p value of less than 0.01 in each group. This corresponded to a network r > ±0.83 for controls, r > ±0.79 for the vmPFC DBS group, r > ±0.93 for the WMF DBS group, and r > ±0.93 for the Acb DBS group. The nodes in the resulting networks represent brain regions and the correlations that survived the threshold criterion were considered to be functional connections. Netdraw software (Analytic Technologies: Lexington, KY) was used to visualize the resulting networks, where node size was set proportional to degree (number of connections) and connection line weights reflect the strength of the correlation.
Statistical analyses
ANOVA (LSD post-hoc) was used to compare behavioral and zif268 data across groups. Statistical significance was set at p ≤0.05. Correlation analysis was used to measure functional connectivity, as described above.
Results
Forced swim test and open field
Overall, we found a significant treatment effect of DBS in the FST (p = 0.01, F3,39 = 6.36 for immobility; p = 0.02, F3,39 = 6.27 for swimming) (Fig. 1A). This was due to a significant decrease in immobility scores in animals receiving vmPFC (n = 9; p = 0.02) and Acb stimulation (n = 10; p = 0.001) as compared to controls (n = 14; 31% and 44%, respectively). In contrast, only a non-significant 17% antidepressant-like effect was recorded after WMF DBS (n = 10; p = 0.1). When the three targets were compared, differences in immobility, swimming and climbing scores were not significant.
Fig. 1.
Outcome of deep brain stimulation (DBS) delivered to the ventromedial prefrontal cortex (vmPFC), nucleus accumbens (Acb) and white matter fibers of the frontal region (WMF) of rats undergoing the forced swim test (FST) and open field. A) During the FST, rats were treated with DBS at 130 Hz, 90 μs and 100 μA. For scoring, the predominant behavior (immobility, swimming, or climbing) during the 5 min of testing was recorded every 5 s (maximal score of 60). Animals receiving vmPFC and Acb DBS had a significant reduction in immobility, the hallmark of an antidepressant-like response, as compared to controls (p = 0.02 and p = 0.001, respectively). In contrast, the antidepressant-like effects of WMF DBS were not statistically significant. B) In the open field, scoring was subdivided into two. The first 5 min of testing were used to evaluate the anxiety of the animals while exploring a novel environment (B). The overall 30 min were considered to be a measure of locomotor activity (C). In neither circumstance, DBS induces significant effect. In all figures of the panel, numbers in parenthesis represent the number of animals per group.
In contrast to the FST, no significant treatment effect was recorded in the open field at either the 5 or 30 min interval (Fig. 1B). That being said, DBS in all three targets induced a 20–30% non-significant increase in locomotion in the first 5 min, suggesting a mild anti-anxiety effect. At the 30 min interval, scores of DBS-treated animals were similar to those of non-stimulated controls, suggesting that DBS did not induce a significant increase in locomotion (Fig. 1C).
Volume of tissue activated by DBS
To investigate whether a spillover of current into adjacent structures could have been responsible for our behavioral findings, we calculated the volume of tissue activated by DBS using computer modeling. As can be seen in Fig. 2, current spread with stimulation parameters and electrodes used in our study was limited to the target region. Aside from a small spillover of current to lateral regions of the vmPFC after WMF DBS, no overlap among target structures primarily influenced by stimulation was observed. In other words, current spread after vmPFC or Acb DBS was limited to the vmPFC and Acb, respectively. In animals receiving WMF DBS, in addition to the forceps minor, current has also directly influenced regions of the claustrum and infralimbic cortex. As the exposed surface and length of the tip of our electrodes were relatively large, current delivered to the Acb in our animals has often spread through both core and shell subdivisions.
Fig. 2.
Volume of tissue activated in animals receiving DBS in the ventromedial prefrontal cortex (vmPFC), nucleus accumbens (Acb) and white matter fibers of the frontal region (WMF). As can be appreciated, with the stimulation settings and electrodes used in our study current spread (gray spheres) was limited to the target region. PL — prelimbic cortex; IL — infralimbic cortex; Cl — claustrum; AcbC — nucleus accumbens core; AcbSh — nucleus accumbens shell. Numbers in the upper right corner of the plates represent distance from bregma (Paxinos and Watson, 1998) (reprinted with permission from Elsevier). Gray bars represent the electrodes while the dark end represents the exposed surface.
Brain regions modulated by DBS
To assess the brain circuits modulated by vmPFC, WMF, or Acb DBS, we have studied the expression of zif268 in regions involved in mechanisms of psychiatric disorders (Supplementary Table 2). Overall, stimulation in each brain site influenced a different set of structures at a distance from the target (Fig. 3, Supplementary Figs. 3–7). As compared to controls, vmPFC DBS increased zif268 expression exclusively in cortical regions, including the prelimbic, cingulate, lateral entorhinal, piriform, and orbitofrontal cortices, as well as the temporal association area (Fig. 3, Supplementary Fig. 3). In contrast, Acb DBS increased zif268 expression mainly in subcortical structures (and also the piriform cortex). These included the globus pallidus, reticular nucleus of the thalamus, zona incerta, lateral hypothalamus, red nucleus, and ventral tegmental area (Fig. 3, Supplementary Figs. 3–7). After Acb DBS, decreased zif268 expression was observed in the pre and prosubiculum. DBS applied to the WMF induced more widespread effect as compared to the other targets, increasing zif268 expression in the cortical structures (cingulate, piriform and orbitofrontal cortices), basal ganglia (globus pallidus and substantia nigra reticulata), thalamus (mediodorsal and paracentral nuclei), zona incerta, and brain stem (red nucleus, dorsal raphe, median raphe, pedunculopontine nucleus and ventral tegmental area) (Fig. 3, Supplementary Figs. 3–7). Levels of zif268 in the ventral CA1 were decreased after WMF stimulation.
Fig. 3.
Differences in zif268 expression between non-stimulated controls and animals receiving DBS in the ventromedial prefrontal cortex (vmPFC), nucleus accumbens (Acb) and white matter fibers of the frontal region (WMF). In the left, 3D reconstructions give an overview of structures with an increase (red for cortical and orange for subcortical) or a decrease (blue) in zif268 expression after DBS. In the middle and right columns, these same structures were individually labeled. As can be clearly appreciated, the administration of DBS to different targets influenced different brain structures and circuits. vmPFC stimulation significantly increased zif268 expression in cortical regions. Acb DBS induced significant changes in zif268 expression mainly in subcortical structures. Stimulation of the WMF was associated with a more complex pattern of changes in zif268 expression, altering levels of this immediate early gene in cortical and subcortical regions. CA1 ventral; Cg1 — cingulate gyrus, area 1; Cg2 — cingulate gyrus, area 2; DRV — dorsal raphe, ventral; LGP — lateral globus pallidus; LH — lateral hypothalamus; LO — lateral orbital cortex; MD — mediodorsal nucleus of the thalamus; MGP — medial globus pallidus; MO — medial orbital cortex; MS — medial septum; PC — paracentral nucleus of the thalamus; Pir — piriform cortex; Post — postsubiculum; PPTg — pedunculopontine tegmental nucleus; PrL — prelimbic cortex; Prs — presubiculum; RPC — red nucleus, parvicellular; Rt — reticular nucleus of the thalamus; SNr — substantia nigra reticulata; TeA — temporal association cortex; VO — ventral orbital cortex; VTA — ventral tegmental area; ZI — zona incerta.
In summary, DBS delivered to different targets influenced different brain structures and circuits. vmPFC stimulation increased the expression of zif268 in cortical areas, whereas Acb DBS induced changes in zif268 expression in a subset of subcortical structures. Stimulation of the WMF induced a mixed and more complex pattern of zif268 expression, altering levels of this immediate early gene in cortical and subcortical regions. Of note, the piriform cortex was the only region in which zif268 expression was increased with the delivery of DBS to all targets.
DBS and functional connectivity
After determining local differences in zif268 expression, we examined possible changes in functional connectivity in animals receiving vmPFC, WMF or Acb DBS. For this, intercorrelations of zif268 levels measured in 98 brain regions were computed (for a complete list of structures see Supplementary Table 3). Significant correlations are presented in Figs. 4 and 5. Stimulation of each specific target induced distinct changes in functional connectivity. After vmPFC DBS, correlations across brain regions were globally reduced. Strikingly, this was more pronounced in cortical regions presenting an increase in zif268 levels after stimulation. Correlations across brain sites were also reduced after Acb DBS, except for the thalamus and brain stem. In contrast, animals given WMF DBS had a widespread increase in functional connectivity in subcortical structures but reduced intercorrelations among frontal cortical regions.
Fig. 4.
Correlation matrices in rats given DBS in the ventromedial prefrontal cortex (vmPFC), nucleus accumbens (Acb) or white matter fibers of the frontal region (WMF). Column and row numbers correspond to brain regions indicated in Supplementary Table 3. Permutation testing was used to compare DBS and control matrices. Significant differences are shown graphically. Overall, we found that correlation between zif268 expressions across brain regions was globally reduced after vmPFC DBS as compared to controls. In contrast, correlation across brain sites after Acb DBS was increased in the thalamus and brain stem (numbers 55–98 in the matrix). In animals given WMF DBS, functional connectivity was increased in most subcortical structures (areas 24–98 of the matrix) but reduced in frontal cortical regions (areas 1–23).
Fig. 5.
Functional networks in rats given DBS in the ventromedial prefrontal cortex (vmPFC), nucleus accumbens (Acb) or white matter fibers of the frontal region (WMF). Networks were constructed by retaining the top 1% strongest correlations for DBS and control groups. This corresponded to a network r > ±0.83 for controls, r > ±0.79 for the vmPFC DBS group, r > ±0.93 for the WMF DBS group, and r > ±0.93 for the Acb DBS group. Nodes in the resulting networks represent brain regions. Correlations that survived the threshold criterion were considered to be functional connections. Nodes in the networks represent brain regions and the connections reflect super threshold correlations. The number of connections is reflected by the size of the node and the connection strength by line thickness. As in Fig. 4, one can clearly appreciate that after vmPFC DBS, correlation between zif268 expressions across brain regions was globally reduced. Correlations across brain sites were also reduced after Acb DBS, except for the thalamus and brain stem. In animals given WMF DBS functional connectivity was increased in subcortical structures but reduced in frontal cortical regions.
In summary, our findings suggest that DBS in different targets leads to distinct changes in functional connectivity. Interestingly, the highest increases in zif268 expression and brain connectivity were seen after WMF DBS, a treatment that was associated with the lowest degree of antidepressant-like effect in the forced swim test. Common to all three targets was a decrease in functional connectivity among frontal cortical regions.
Discussion
The main findings from this study were twofold. First, DBS delivered to the vmPFC and Acb induced comparable antidepressant-like effects in the FST. Second, despite of a lack of overlap in regions directly modulated by DBS, stimulation to each different target influenced activity and connectivity in a distinct set of brain structures and circuits. This suggests that, despite inducing similar antidepressant-like effects, stimulation of the vmPFC, WMF and Acb does not necessarily rely on similar circuits for their mechanisms of action.
Anatomical considerations and stimulation parameters
To mimic the clinical scenario, our choice of target in the region of the Acb was relatively straightforward. Electrodes were placed in the transition between shell and core so that most of the exposed surface would be implanted in the latter (Bewernick et al., 2010; Schlaepfer et al., 2008). In contrast, determining regions homologous to the human SCG or anterior capsule in rodents was far more complex. Based on cytoarchitectural features and anatomical projections, the rodent ventromedial prefrontal cortex (IL and ventral PL) has been suggested to correspond to the human subgenual cingulum (Hamani et al., 2011; Uylings et al., 2003; Vertes, 2004). In rodents, the anterior limb of the internal capsule is not well developed. White matter projections in frontal brain regions comprise the forceps minor of the corpus callosum and the anterior commissure. While acknowledging that none of these structures would resemble the anterior limb of the internal capsule in humans, we chose to implant electrodes in the forceps minor, as this structure contains both commissural and projection fibers. In humans, the anterior limb of the internal capsule is composed in part by cortical projections that innervate the thalamus, basal ganglia and brain stem pontine nuclei (Paxinos and Watson, 1998). Though pathways running through the rodent forceps minor have not been characterized in detail, our stimulation results suggest that they may include projections that innervate the cerebral cortex, basal ganglia, thalamus and brain stem structures. At this point we cannot rule out that some of these structures may have been recruited through polysynaptic circuits. However, we find this unlikely as changes in zif268 expression and functional connectivity were recorded in numerous brain regions after WMF DBS. Since not many brain structures have such wide patterns of innervation, it seems more reasonable to think that such changes derived from the activation of a common source (in this case fibers from the forceps minor) rather than multiple brain sites.
As for stimulation parameters, our choice was based on charge density, side effects and the volume of tissue activated by DBS. With similar settings being delivered to each target, we could standardize the delivery of current while minimizing spread to adjacent structures. That being said, we recognize that different parameters may be required for an optimal effect of WMF DBS in the FST. For example, in patients with obsessive–compulsive disorder initial studies used high current intensities during VC/VS stimulation (Nuttin et al., 2003). However, with accumulation of experience and a better characterization of the optimal region for electrode placement, the current required for a good postoperative outcome was dramatically reduced. In depression, stimulation parameters across DBS targets are comparable (high frequency stimulation with current intensities in the order of 3–5 V) and in the range of those used in the current study (Bewernick et al., 2010; Lozano et al., 2008; Malone et al., 2009).
Regional brain differences induced by DBS
One of the main findings of our study was that changes in zif268 expression after stimulation varied according to target. This suggests that by delivering DBS to the vmPFC, Acb and WMF we were influencing different sets of structures and not simply simulating relays of the same circuitry. Despite similar behavioral results, levels of zif268 after vmPFC DBS were only increased in cortical regions, whereas after Acb DBS zif268 expression was primarily increased in subcortical structures. WMF DBS influenced both cortical and subcortical structures but did not induce a significant antidepressant-like response.
In our study, the only common region presenting increased zif268 expression after DBS in all three targets was the piriform cortex, a structure primarily involved in processing olfactory information (Isaacson, 2010; Wilson and Sullivan, 2011). Most of the research implicating this cortical region in depression has been conducted after olfactory bulb lesions in rodents (Holmes et al., 1998; Jarosik et al., 2007; Wang et al., 2007). In the FST, studies using microPET and c-fos in situ hybridization have shown reduced activity in the piriform cortex of rats treated with fluoxetine or imipramine (Jang et al., 2009; Sairanen et al., 2007). This is in contrast with the increased zif268 expression recorded after DBS in our study. Though further investigation is needed, these findings suggest that the piriform cortex may be a region in which DBS and medications exert opposite effects.
Distinctions in brain regions expressing zif268 in animals receiving WMF DBS versus Acb or vmPFC that could explain our behavioral findings include those in the dorsal raphe (DR) and some thalamic nuclei. The DR provides serotonergic innervation to a number of cortical and subcortical structures (Hensler, 2006; Lowry et al., 2008; Michelsen et al., 2007; Molliver, 1987). It has been extensively studied in the context of depression, since a number of commonly used antidepressant medications increase serotonin release in different brain sites (Lowry et al., 2008; Mann, 2005; Michelsen et al., 2007). In previous work, we have shown that the antidepressant-like effects of DBS in the vmPFC are largely dependent on the integrity of the serotonergic system (Hamani et al., 2010b). Bearing this in mind, a question that emerges is why WMF DBS would induce an increase in raphe activity but exert no significant behavioral effects. Though zif268 is commonly used as a marker of local brain activity, mRNA measures cannot by themselves be used to characterize the cell type involved. Frontal cortex-raphe fibers innervate both serotonergic projection cells and GABArgic interneurons (Hajos et al., 1998; Varga et al., 2001; Warden et al., 2012). One possibility is that WMF stimulation might have predominantly influenced the latter, with a subsequent inhibition of serotonin release. This would be in contrast with Acb or vmPFC DBS, which has been previously shown to induce serotonin release in various brain regions (Hamani et al., 2010b; Juckel et al., 1999; van Dijk et al., 2012). Another unique feature of WMF DBS was that it increased thalamic activity, particularly in the mediodorsal nucleus (MD). The MD is a relay structure that projects to the orbitofrontal and prefrontal cortical regions. It has been largely implicated in mechanisms of stress, impulsivity and addictive behavior. In the clinic, stimulation of the inferior thalamic peduncle (a fiber pathway that interconnects the MD with the orbitofrontal cortex) has been used for the treatment of depression with promising results (Jimenez et al., 2005; Velasco et al., 2005). In rodents, glutamatergic projections from the prefrontal and orbitofrontal regions to the MD innervate primarily glutamatergic neurons (Ray and Price, 1992; Ray et al., 1992). Whether increased MD activity might be responsible for the less pronounced behavioral results of WMF DBS remains to be demonstrated. However, should that prove to be the case, it is possible that focal inactivation of the MD (i.e. through local injections of GABAegic agonists or even DBS) could have antidepressant-like effects.
Interconnectivity patterns
In addition to inducing local changes in zif268 expression, DBS in the vmPFC, WMF and Acb induced distinct changes in functional connectivity. Common to all three targets, however, was a DBS-induced decrease in the correlation of cortical zif268 expression. This is in contrast to the differences observed in subcortical connectivity in animals given DBS to the vmPFC, Acb and WMF. Though comparisons of connectivity data between naïve rodents and humans need to be approached with caution, we find it intriguing that, similar to our DBS findings, electroconvulsive therapy and antidepressant medications also reduce cortical connectivity in patients with depression (McCabe et al., 2011; Perrin et al., 2012; Scheidegger et al., 2012). Results from our study certainly need to be corroborated in other models of depression, in animals undergoing chronic stimulation, and in patients treated with DBS. They do suggest, however, that changes in cortical brain function and connectivity should be further explored as a mechanism of antidepressant therapies.
Limitations
A few limitations of studies such as ours include the use of naïve rats and the translational nature of animal models of psychiatric disorders (Hamani and Nobrega, 2012; Hamani and Temel, 2012). One of the limitations of the forced swim test is the short timeframe required for an antidepressant-like response. In addition, animals do not have genotype and/or phenotype features that may resemble a depressive-like state prior to testing. That being said, the FST is the most commonly used screener for the assessment of the efficacy of antidepressant interventions, having excellent predictive validity (Cryan and Slattery, 2007; Cryan et al., 2002; Krahl et al., 2004; Li et al., 2007; Porsolt et al., 1977, 1978).
Despite the caveats described above, preclinical models seem to have a very good predictive validity for the study the antidepressant-like effects of DBS and may offer a unique contribution to the field (Hamani and Temel, 2012). In humans, the appraisal of the clinical effects of DBS is often carried out through the use of subjective scales. As such, placebo effects are difficult to measure in open label or smaller scale studies. Preclinical research can help us characterize the biological effects of DBS so that clinical trials may be perfected. As an example, we have recently shown that the behavioral and neurochemical effects of DBS may persist after stimulation is discontinued (Hamani et al., 2010b, 2012). This suggests that in clinical trials contemplating a crossover design (i.e. patients receiving active or sham stimulation followed by the inverse treatment) a washout phase is mandatory. Though studies in animal models by all means substitute clinical research, they may certainly provide a guide so that time and resources may be better allocated.
In our study the pattern of immediate early gene expression after DBS has been characterized in naïve rats. Though this does not reflect the scenario observed in “depressive-like states”, our study design may have distinct advantages. In the clinic, it is difficult to appreciate whether particular metabolic changes in patients given stimulation are primarily due to DBS, the clinical effects of stimulation or an interaction of both. By studying naïve rats we were able to dissect the subject and demonstrate specific patterns of activation of DBS related exclusively to treatment.
Finally, structures being investigated as DBS targets in more recent studies are the lateral habenula and medial forebrain bundle (MFB) (Sartorius and Henn, 2007; Sartorius et al., 2010; Schlaepfer et al., 2013). In the former, antidepressant-like effects of stimulation have been described in preclinical models (Li et al., 2011). Whether MFB stimulation induces an antidepressant-like effect in rodents remains to be demonstrated.
Conclusions
In conclusion, we show that DBS delivered to the vmPFC and Acb induced comparable antidepressant-like effects in the FST. Immobility scores in animals that had DBS applied to the WMF were slightly higher than those recorded after vmPFC or Acb DBS but not significantly different from that of non-stimulated controls. Bearing in mind that PFC regions heavily project to the Acb and some of the projections from PFC to subcortical areas run through white matter fibers of the frontal cortex, we expected some degree of overlap in the expression of immediate early genes after DBS in these three different targets. Contrary to our expectations, however, we found that stimulation of the vmPFC, WMF and Acb influenced activity and connectivity in a distinct set of brain structures and circuits. This suggests that, despite inducing similar antidepressant-like effects, stimulation of these three targets does not necessarily rely on similar circuits for their mechanisms of action. The fact that DBS in different regions induces specific circuitry changes may have important implications to the field. For example, this therapy may be individualized according to the patients’ predominant symptoms and the specific circuits that require modulation. In other words, different depressive phenotypes may require DBS in different targets.
At present, DBS for depression remains investigational. Despite the promising results of open label studies, double blind evaluations comparing the effects of active versus sham stimulation in patients treated with VC/VS DBS have been somewhat disappointing (Holtzheimer et al., 2011a). Rather than discouragement, the field should look more into basic research and predictors of a response. Several preclinical studies have shown that DBS does induce antidepressant-like responses and modulates neurochemical substrates involved in depression. At present, we cannot rule out that the antidepressant effects of DBS are due to a placebo response. That said, the fact that electrical stimulation induces concrete biological effects suggests that, rather than a simple placebo effect, we still do not understand who are the ideal candidates, optimal targets and appropriate kinetics of for a DBS response.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nbd.2014.08.007.
Supplementary Material
Acknowledgments
This work was supported in part with funds from the Brain & Behavior Research Foundation, the Ontario Mental Health Foundation and the Canadian Institutes of Health Research.
Abbreviations
- Acb
nucleus accumbens
- DBS
deep brain stimulation
- FST
forced swim test
- vmPFC
ventromedial prefrontal cortex
- WMF
white mater fibers
Footnotes
Available online on ScienceDirect (www.sciencedirect.com).
Disclosures
C.H. and C.R.B. are consultants for St Jude Medical. C.R.B. is a consultant for Boston Scientific, Advanced Bionics, Intellect Medical and NeuroPace. The other authors do not have a conflict of interest.
References
- Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, Axmacher N, Lemke M, Cooper-Mahkorn D, Cohen MX, Brockmann H, Lenartz D, Sturm V, Schlaepfer TE. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67:110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Butson CR, Cooper SE, Henderson JM, McIntyre CC. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage. 2007;34:661–670. doi: 10.1016/j.neuroimage.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, III, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr, Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Creed MC, Hamani C, Nobrega JN. Early gene mapping after deep brain stimulation in a rat model of tardive dyskinesia: comparison with transient local inactivation. Eur Neuropsychopharmacol. 2012;22:506–517. doi: 10.1016/j.euroneuro.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Creed MC, Hamani C, Nobrega JN. Effects of repeated deep brain stimulation on depressive- and anxiety-like behavior in rats: comparing entopeduncular and sub-thalamic nuclei. Brain Stimul. 2013;6:506–514. doi: 10.1016/j.brs.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Slattery DA. Animal models of mood disorders: recent developments. Curr Opin Psychiatry. 2007;20:1–7. doi: 10.1097/YCO.0b013e3280117733. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Lucki I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behav Brain Res. 1996;73:43–46. doi: 10.1016/0166-4328(96)00067-8. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersner R, Toth E, Isserles M, Zangen A. Site-specific antidepressant effects of repeated subconvulsive electrical stimulation: potential role of brain-derived neurotrophic factor. Biol Psychiatry. 2010;67:125–132. doi: 10.1016/j.biopsych.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Wolters JG, Lohman AH. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. Prog Brain Res. 1990;85:95–116. doi: 10.1016/s0079-6123(08)62677-1. (discussion 116–118) [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos M, Richards CD, Szekely AD, Sharp T. An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience. 1998;87:95–108. doi: 10.1016/s0306-4522(98)00157-2. [DOI] [PubMed] [Google Scholar]
- Hamani C, Nobrega JN. Deep brain stimulation in clinical trials and animal models of depression. Eur J Neurosci. 2010;32:1109–1117. doi: 10.1111/j.1460-9568.2010.07414.x. [DOI] [PubMed] [Google Scholar]
- Hamani C, Nobrega JN. Preclinical studies modeling deep brain stimulation for depression. Biol Psychiatry. 2012;72:916–923. doi: 10.1016/j.biopsych.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Temel Y. Deep brain stimulation for psychiatric disease: contributions and validity of animal models. Sci Transl Med. 2012;4:142rv8. doi: 10.1126/scitranslmed.3003722. [DOI] [PubMed] [Google Scholar]
- Hamani C, Diwan M, Isabella S, Lozano AM, Nobrega JN. Effects of different stimulation parameters on the antidepressant-like response of medial prefrontal cortex deep brain stimulation in rats. J Psychiatr Res. 2010a;44:683–687. doi: 10.1016/j.jpsychires.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Hamani C, Diwan M, Macedo CE, Brandao ML, Shumake J, Gonzalez-Lima F, Raymond R, Lozano AM, Fletcher PJ, Nobrega JN. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol Psychiatry. 2010b;67:117–124. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Hamani C, Nobrega JN, Lozano A. Deep brain stimulation in clinical practice and animal models. Clin Pharmacol Ther. 2010c;88:559–562. doi: 10.1038/clpt.2010.133. [DOI] [PubMed] [Google Scholar]
- Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011;69:301–308. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Hamani C, Machado DC, Hipolide DC, Dubiela FP, Suchecki D, Macedo CE, Tescarollo F, Martins U, Covolan L, Nobrega JN. Deep brain stimulation reverses anhedonic-like behavior in a chronic model of depression: role of serotonin and brain derived neurotrophic factor. Biol Psychiatry. 2012;71:30–35. doi: 10.1016/j.biopsych.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS. The accumbens: beyond the core–shell dichotomy. J Neuropsychiatry Clin Neurosci. 1997;9:354–381. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- Hensler JG. Serotonergic modulation of the limbic system. Neurosci Biobehav Rev. 2006;30:203–214. doi: 10.1016/j.neubiorev.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Hines ML, Carnevale NT. The NEURON simulation environment. Neural Comput. 1997;9:1179–1209. doi: 10.1162/neco.1997.9.6.1179. [DOI] [PubMed] [Google Scholar]
- Holmes PV, Davis RC, Masini CV, Primeaux SD. Effects of olfactory bulbectomy on neuropeptide gene expression in the rat olfactory/limbic system. Neuroscience. 1998;86:587–596. doi: 10.1016/s0306-4522(98)00029-3. [DOI] [PubMed] [Google Scholar]
- Holsheimer J, Demeulemeester H, Nuttin B, de Sutter P. Identification of the target neuronal elements in electrical deep brain stimulation. Eur J Neurosci. 2000;12:4573–4577. [PubMed] [Google Scholar]
- Holtzheimer PE, Dougherty DD, Schlaepfer T, Mayberg H. Acute effects of deep brain stimulation for treatment-resistant depression: using variability across targets to inform on the neurobiology of mood regulation. Biol Psychiatry. 2011a;69:202S. [Google Scholar]
- Holtzheimer PE, Filkowski M, Kelley ME, Gross RE, Mayberg HS. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Biol Psychiatry. 2011b;69:110S. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner KA, Keefe KA. Regulation of psychostimulant-induced preprodynorphin, c-fos and zif/268 messenger RNA expression in the rat dorsal striatum by mu opioid receptor blockade. Eur J Pharmacol. 2006;532:61–73. doi: 10.1016/j.ejphar.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Isaacson JS. Odor representations in mammalian cortical circuits. Curr Opin Neurobiol. 2010;20:328–331. doi: 10.1016/j.conb.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang DP, Lee SH, Park CW, Lee SY, Kim YB, Cho ZH. Effects of fluoxetine on the rat brain in the forced swimming test: a [F-18]FDG micro-PET imaging study. Neurosci Lett. 2009;451:60–64. doi: 10.1016/j.neulet.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Jarosik J, Legutko B, Unsicker K, von Bohlen Und Halbach O. Antidepressant-mediated reversal of abnormal behavior and neurodegeneration in mice following olfactory bulbectomy. Exp Neurol. 2007;204:20–28. doi: 10.1016/j.expneurol.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Jimenez F, Velasco F, Salin-Pascual R, Hernandez JA, Velasco M, Criales JL, Nicolini H. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005;57:585–593. doi: 10.1227/01.neu.0000170434.44335.19. (discussion 585–593) [DOI] [PubMed] [Google Scholar]
- Juckel G, Mendlin A, Jacobs BL. Electrical stimulation of rat medial prefrontal cortex enhances forebrain serotonin output: implications for electroconvulsive therapy and transcranial magnetic stimulation in depression. Neuropsychopharmacology. 1999;21:391–398. doi: 10.1016/S0893-133X(98)00097-9. [DOI] [PubMed] [Google Scholar]
- Krahl SE, Senanayake SS, Pekary AE, Sattin A. Vagus nerve stimulation (VNS) is effective in a rat model of antidepressant action. J Psychiatr Res. 2004;38:237–240. doi: 10.1016/j.jpsychires.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Lehman JF, Greenberg BD, McIntyre CC, Rasmussen SA, Haber SN. Rules ventral prefrontal cortical axons use to reach their targets: implications for diffusion tensor imaging tractography and deep brain stimulation for psychiatric illness. J Neurosci. 2011;31:10392–10402. doi: 10.1523/JNEUROSCI.0595-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Suemaru K, Cui R, Araki H. Repeated electroconvulsive stimuli have long-lasting effects on hippocampal BDNF and decrease immobility time in the rat forced swim test. Life Sci. 2007;80:1539–1543. doi: 10.1016/j.lfs.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan ME, Gafford GM, Jarome TJ, Helmstetter FJ. Time-dependent expression of arc and Zif268 after acquisition of fear conditioning. Neural Plast. 2010 doi: 10.1155/2010/139891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann N Y Acad Sci. 2008;1148:86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Malone DA, Jr, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, Rauch SL, Rasmussen SA, Machado AG, Kubu CS, Tyrka AR, Price LH, Stypulkowski PH, Giftakis JE, Rise MT, Malloy PF, Salloway SP, Greenberg BD. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65:267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ. The medical management of depression. N Engl J Med. 2005;353:1819–1834. doi: 10.1056/NEJMra050730. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Filippini N, Cowen PJ, Taylor MJ, Harmer CJ. SSRI administration reduces resting state functional connectivity in dorso-medial prefrontal cortex. Mol Psychiatry. 2011;16:592–594. doi: 10.1038/mp.2010.138. [DOI] [PubMed] [Google Scholar]
- Michelsen KA, Schmitz C, Steinbusch HW. The dorsal raphe nucleus — from silver stainings to a role in depression. Brain Res Rev. 2007;55:329–342. doi: 10.1016/j.brainresrev.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Molliver ME. Serotonergic neuronal systems: what their anatomic organization tells us about function. J Clin Psychopharmacol. 1987;7:3S–23S. [PubMed] [Google Scholar]
- Nuttin BJ, Gabriels LA, Cosyns PR, Meyerson BA, Andreewitch S, Sunaert SG, Maes AF, Dupont PJ, Gybels JM, Gielen F, Demeulemeester HG. Long-term electrical capsular stimulation in patients with obsessive–compulsive disorder. Neurosurgery. 2003;52:1263–1272. doi: 10.1227/01.neu.0000064565.49299.9a. (discussion 1272–1264) [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Merz S, Bennett DM, Currie J, Steele DJ, Reid IC, Schwarzbauer C. Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proc Natl Acad Sci U S A. 2012;109:5464–5468. doi: 10.1073/pnas.1117206109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Carmichael ST, Drevets WC. Networks related to the orbital and medial prefrontal cortex; a substrate for emotional behavior? Prog Brain Res. 1996;107:523–536. doi: 10.1016/s0079-6123(08)61885-3. [DOI] [PubMed] [Google Scholar]
- Ray JP, Price JL. The organization of the thalamocortical connections of the mediodorsal thalamic nucleus in the rat, related to the ventral forebrain–prefrontal cortex topography. J Comp Neurol. 1992;323:167–197. doi: 10.1002/cne.903230204. [DOI] [PubMed] [Google Scholar]
- Ray JP, Russchen FT, Fuller TA, Price JL. Sources of presumptive glutamatergic/aspartatergic afferents to the mediodorsal nucleus of the thalamus in the rat. J Comp Neurol. 1992;320:435–456. doi: 10.1002/cne.903200403. [DOI] [PubMed] [Google Scholar]
- Sairanen M, O’Leary OF, Knuuttila JE, Castren E. Chronic antidepressant treatment selectively increases expression of plasticity-related proteins in the hippocampus and medial prefrontal cortex of the rat. Neuroscience. 2007;144:368–374. doi: 10.1016/j.neuroscience.2006.08.069. [DOI] [PubMed] [Google Scholar]
- Sartorius A, Henn FA. Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med Hypotheses. 2007;69:1305–1308. doi: 10.1016/j.mehy.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, Henn FA, Meyer-Lindenberg A. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, Boesiger P, Henning A, Seifritz E. Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One. 2012;7:e44799. doi: 10.1371/journal.pone.0044799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltz CA, Bremer QZ, Landry CF, Kelley AE. Food-associated cues alter forebrain functional connectivity as assessed with immediate early gene and proenkephalin expression. BMC Biol. 2007;5:16. doi: 10.1186/1741-7007-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Bewernick BH, Kayser S, Madler B, Coenen VA. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73:1204–1212. doi: 10.1016/j.biopsych.2013.01.034. [DOI] [PubMed] [Google Scholar]
- Temel Y, Boothman LJ, Blokland A, Magill PJ, Steinbusch HW, Visser-Vandewalle V, Sharp T. Inhibition of 5-HT neuron activity and induction of depressive-like behavior by high-frequency stimulation of the subthalamic nucleus. Proc Natl Acad Sci U S A. 2007;104:17087–17092. doi: 10.1073/pnas.0704144104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- van Dijk A, Klompmakers AA, Feenstra MG, Denys D. Deep brain stimulation of the accumbens increases dopamine, serotonin, and noradrenaline in the prefrontal cortex. J Neurochem. 2012;123:897–903. doi: 10.1111/jnc.12054. [DOI] [PubMed] [Google Scholar]
- Varga V, Szekely AD, Csillag A, Sharp T, Hajos M. Evidence for a role of GABA interneurones in the cortical modulation of midbrain 5-hydroxytryptamine neurones. Neuroscience. 2001;106:783–792. doi: 10.1016/s0306-4522(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Velasco F, Velasco M, Jimenez F, Velasco AL, Salin-Pascual R. Neurobiological background for performing surgical intervention in the inferior thalamic peduncle for treatment of major depression disorders. Neurosurgery. 2005;57:439–448. doi: 10.1227/01.neu.0000172172.51818.51. (discussion 439–448) [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Wang D, Noda Y, Tsunekawa H, Zhou Y, Miyazaki M, Senzaki K, Nabeshima T. Behavioural and neurochemical features of olfactory bulbectomized rats resembling depression with comorbid anxiety. Behav Brain Res. 2007;178:262–273. doi: 10.1016/j.bbr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, Adhikari A, Tye KM, Frank LM, Deisseroth K. A prefrontal cortex–brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–432. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler AL, Teixeira CM, Wang AH, Xiong X, Kovacevic N, Lerch JP, McIntosh AR, Parkinson J, Frankland PW. Identification of a functional connectome for long-term fear memory in mice. PLoS Comput Biol. 2013;9:e1002853. doi: 10.1371/journal.pcbi.1002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler AL, Creed MC, Voineskos AN, Nobrega JN. Changes in brain functional connectivity after chronic haloperidol in rats: a network analysis. Int J Neuropsychopharmacol. 2014:1–10. doi: 10.1017/S1461145714000042. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Cortical processing of odor objects. Neuron. 2011;72:506–519. doi: 10.1016/j.neuron.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Steiner H. Methylphenidate (Ritalin) induces Homer 1a and zif 268 expression in specific corticostriatal circuits. Neuroscience. 2005;132:855–865. doi: 10.1016/j.neuroscience.2004.12.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.