Abstract
The field of complex microfluidic channels is rapidly expanding toward channels with variable cross-sections (i.e., beyond simple rounded channels with a constant diameter), as well as channels whose trajectory can be outside of a single plane. This paper introduces the use of three-dimensional (3D) printed soluble wax as cast molds for rapid fabrication of truly arbitrary microfluidic polydimethylsiloxane (PDMS) channels that are not achieved through typical soft lithography. The molds are printed directly from computer-aided design files, followed by simple dissolution using a solvent after molding PDMS, making rapid prototyping of microfluidic devices possible in hours. As part of the fabrication method, the solubility of several build materials in solvents and their effect on PDMS were investigated to remove the 3D-printed molds from inside the replicated PDMS microfluidic channels without damage. Technology limits, including surface roughness and resolution by comparing the designed channels with fabricated cylindrical channels with various diameters, are also characterized. We reproduced a 3D image of an actual human cerebral artery as cerebral artery-shaped PDMS channels with a diameter of 240 μm to prove the developed fabrication technique. It was confirmed that the fabricated vascular channels were free from any leakage by observing the fluorescence fluid fill.
I. INTRODUCTION
Microfluidics is a multidisciplinary field incorporating chemistry, physics, biology, medicine, and tissue engineering, allowing fine amounts of sample to be handled for short reaction times, leading to analysis and reproduction of flows in systems.1–5 An important application of microfluidics includes accurate studies of blood flow to mimic biology, which requires 3D geometries with biocompatible materials. For decades, polydimethylsiloxane (PDMS) has been a pivotal structural material in microfluidics owing to its positive attributes, which include low cost, nontoxicity, optical transparence, electrical and thermal insulation, gas permeability, and especially biocompatibility.6–8 In addition, PDMS is flexible and easy to manipulate.9 The typical fabrication method for PDMS microfluidic devices, which is called soft lithography, requires molds that are usually constructed by standard photolithography. Several studies have been conducted using soft lithography; however, the fabrication is generally labor intensive, time consuming, and costly because specialized facilities in a cleanroom are necessary.
The realization of a 3D microfluidic channel is important because it can accurately mimic rheology in small channels, which has been difficult when reproducing the biological microenvironment in a 2D structure.10 The 3D microfluidic channels allow accurate manipulation and analysis of fluids, as well as realistic vascular system for cell study, drug screening, and medical procedures.11–13 As one of the ways to fabricate the 3D microfluidic channel, planar 2D microfluidic structures fabricated by soft lithography were vertically stacked.14 Fabrication of the stacked 3D microfluidic channel, however, required a bonding process between the respective 2D structures. Thus, inevitable leaks between interfaces of the bonded structures might occur because the stacked 3D microfluidic channel was not unibody. In addition, it was still time consuming and costly because each 2D structure was based on soft lithography.
To overcome the issues inherent in soft lithography, alternatives for producing molds for 3D microfluidics have been studied. Instead of photoresists of soft lithography, slender nylon fibers15 and Ni-Cr alloy microwires16 were used as casting molds, which were removed physically to fabricate helical microfluidic channels. Agarose gel wires17 and sucrose fiber18 that could be dissolved by organic solvent were also used as molds to fabricate crossover microfluidic channels and vaso-mimetic microfluidic channels, respectively. Casting methods using wires did not require soft lithography and thus the bonding process anymore; nevertheless, shape change in a channel cross-section was restricted because the prefabricated wires with a fixed diameter were arranged to cast PDMS. Therefore, techniques using the wire molds were limited in manufacturing truly 3D microfluidic channels.
3D printing, which is also called additive manufacturing, is actively utilized to construct microfluidic channels because of several advantages: (1) reduction in time and cost for fabrication, (2) micrometer-scale minimum feature size that can provide suitable dimensions to microfluidic channels, and (3) unrestricted formation of the truly 3D geometry. Using the 3D printing technique, complex 3D microfluidic channels were simply and directly fabricated without additional processes such as casting and bonding;19 however, commercial printers are supposed to print only specified materials, most of which are proprietary. Therefore, the 3D printing technology can be applied to the PDMS microfluidic devices in various ways, but it is still limited as described below.
3D PDMS structures were indirectly realized using 3D cast molds constructed by commercial 3D printers. Szydzik et al. cast PDMS between two 3D-printed complementary molds to create complex multilayer microfluidic structures. The two complementary molds were peeled from cured PDMS, and then, both sides of PDMS were bonded with glass.20 The microchannel lacked uniform gas permeability and cell adhesion for biological applications because it did not entirely consist of PDMS. In another work, Hwang et al. achieved double-helical PDMS channels that were fabricated by casting PDMS around a 3D-printed structure and by removing the printed structure physically from the cured PDMS.21 Physical removal of the solid printed mold was simple and straightforward. However, it restricted creation of a complex channel because the mold had to be pulled towards the inlet or outlet of the channel once the PDMS was cured. Thus, in cases when the mold was larger than the entrance to the channel, the mold could not be extracted.
Alternatively, several studies have investigated ways to melt or chemically dissolve the 3D-printed molds for smooth removal. These technologies have great significance in that they overcome the previous limitations of creating restricted 3D structures. Here, the restricted 3D structure refers to channels with circular cross-sections that lie in a single plane22 and channels whose trajectory moves out of the plane but still have an inability to form arbitrary cross-sections.21 Parekh et al. fabricated 3D microfluidic channels using printed liquid metal on the PDMS sheet.23 The liquid metal in cured PDMS was removed by an electrochemical reaction, leaving the 3D microchannels. However, the printed liquid metal could be incompletely removed from the microchannels. Using a customized printing nozzle, Saggiomo and Velders cast PDMS around an acrylonitrile butadiene styrene (ABS) mold, which was dissolved with acetone.24 Owing to the low resolution (∼500 μm) of the custom-built system, not only were the printed and designed molds different but also the production of molds with arbitrary geometry was limited. Wang et al. produced PDMS virtual surgery models using a 3D-printed wax that was removed chemically in liquid n-hexane.25 Although they realized a unibody PDMS channel of 2 mm in diameter using wax mold, n-hexane for dissolving the printed wax mold could cause serious deformations on the microchannel surface because n-hexane swells the volume of PDMS to 35% according to a previous study.26 Hydrogel, carbohydrate, and polyvinyl alcohol have been reported as sacrificial molds that can be removed using water;17,27,28 however, they also did not go beyond the restricted 3D structure.
Recently, direct 3D printing of PDMS microchannels has been studied.29 A customized 3D printer realized PDMS channels in a hydrophilic Carbopol gel bath, which acted as a support material while extruding PDMS. The PDMS channel was released from Carbopol gel by liquefying it using a phosphate-buffered saline solution. The direct printing of PDMS was the most straightforward approach because no casting process was needed. However, there remained challenges to be settled such as poor resolution and lateral fusion between extruded PDMS.
This work reports truly 3D PDMS microchannels with a diameter of 240 μm and an arbitrarily shaped cross-section by using a 3D-printed soluble wax as a mold material for PDMS. Three steps were taken to develop the fabrication method described in this paper. First, combinations of 3D-printed build materials and organic solvents, which dissolved the printed material and hardly attacked PDMS, were investigated. Subsequently, 3D printer performance was evaluated by comparing relative differences between the designed and fabricated cylindrical channels with various diameters. As a proof of concept for this technique, real-size cerebral artery-shaped PDMS channels with a high resolution were realized using a 3D image of a cerebral artery reproduced from magnetic resonance angiography (MRA).
II. MATERIALS AND METHOD
A. Materials
It is important to identify solubility between commercial build materials for 3D printing and organic solvents to remove the 3D-printed mold surrounded by PDMS chemically without deforming PDMS. We prepared six commercial build materials supplied by manufacturers of 3D printers: ZR80 (ZRapid Technologies, China), Vero white plus (Stratasys, USA), Accura60, Visijet M3 crystal (3D Systems, USA), Visijet M3 Hi-cast (3D Systems, USA), and 3Z-model (Solidscape, USA). Nominal resolutions of 3D printers that use the six commercial build materials are listed in Table I. To identify combinations between the build materials and solvents that dissolve them, each of the commercial build materials was immersed into six solvents: acetone, methanol, ethanol, n-hexane, isopropyl alcohol (IPA), and dichloromethane (Sigma Aldrich, USA). PDMS (Sylgard 184, Dow Corning, USA) was used as a structure material of microfluidic channels. Scanning electron microscopy (TESCAN MIRA 3 LMH In-beam detector, Czech Republic) was used to investigate deformation of the PDMS surface due to organic solvents. The inside wall of the PDMS cerebral artery-shaped microchannel was observed using a 3D profiler (HRM-300, HUVITZ, Republic of Korea).
TABLE I.
Nominal specification of tested commercial 3D printers.
| Manufacturer | Model | Planar resolution (μm) | Layer thickness (μm) | Build material |
|---|---|---|---|---|
| Zrapid | SLA200 | 10 (2500 DPI) | 0.2 | ZR80 |
| Stratasys | Objet24 | 42 (600 DPI) | 28 | Vero white plus |
| 3DSystems | Viper SLA | 7.6 (3342 DPI) | 20 | Accura60 |
| 3DSystems | Projet3510SD | 67 (375 DPI) | 32 | Visijet crystal |
| 3DSystems | ProJet MJP 3600W | 33 (750 PDI) | 16 | Visijet M3 hi-cast |
| Solidscape | 3Z-STUDIO | 5 (5000 DPI) | 6.3 | 3Z-model |
B. Fabrication of a truly 3D microfluidic channel
A truly 3D microfluidic channel was fabricated using a 3D printing technique for soluble cast molds. Figure 1 illustrates how a truly 3D microfluidic channel was fabricated. The truly 3D structure was designed using computer-aided design (CAD) software, Autodesk Inventor (Autodesk Inc., USA) [Fig. 1(a)]. The 3D CAD data were digitally sliced into layers and saved to .STL format, which is recognized by most commercial 3D printers. After 3D printer software automatically generated support structures by considering the location and orientation of the designed model on its build platform, the printer built layers of the structure one by one with the build and support materials. After printing, the support materials were removed according to the recommended method for each 3D printer as follows: FullCure705 (Stratasys, USA) for the Objet24 was removed with a pressurized water jet and Visijet S300 (3D Systems, USA) for the Projet3510SD was melted in a heated oven. The support materials of the Viper SLA and SLA200 were mechanically removed with a sharp razor blade because they were automatically produced using the build materials. The support materials used by a wax 3D printer were immersed in solvents to remove them. Visijet S400 (3D Systems, USA) for the ProJet MJP 3600 W was immersed in solvent composed of isopropyl alcohol (IPA) and polypropylene glycol (PPG) in a 1:1 volume ratio at 45 °C. 3Z-support (Solidscape, USA), the support material of 3Z-STUDIO, was cleared by a heated BIOACT VSO (Vantage Specialty Chemicals, USA). Next, the PDMS mixture was prepared by mixing the base and agent at a 10:1 weight ratio and degassed for 30 min in a desiccator. The PDMS mixture was then cast around the printed molds, followed by degassing again for 30 min in the desiccator. The cast PDMS around the 3D-printed mold was cured at 50 °C for 6 h in an oven [Fig. 1(b)]. The cured PDMS block, inside which the 3D-printed mold was still contained, was immersed in organic solvent to dissolve the mold [Fig. 1(c)]. To dissolve the 3D-printed mold completely, the solvent was injected into the channel using a syringe. Finally, the replicated microfluidic channels were cleaned with DI (deionized) water dried using nitrogen gas, and stored in the desiccator for 1 h to ensure the solution evaporation [Fig. 1(d)].
FIG. 1.
The process of fabrication. (a) The 3D mold is printed by a commercial 3D printer. (b) Liquid PDMS is cast around the mold and cured in a heated oven. (c) The mold inside the PDMS block is dissolved by organic solvent. (d) Fabrication of a truly 3D PDMS microfluidic channel is finished after cleaning the channel with DI water.
III. RESULTS AND DISCUSSION
A. Soluble build materials
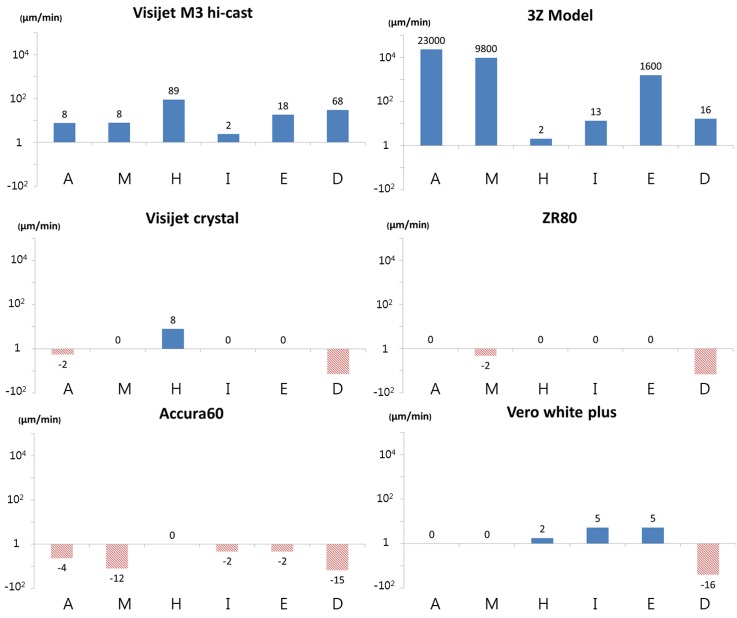
When designing a PDMS casting technique using a 3D printing material, the etch rate for each material must first be known. Chemical compositions of most of the commercial build materials for 3D printing are proprietary. Therefore, it is necessary to empirically develop methods that dissolve the 3D-printed molds without affecting PDMS. In this study, six materials for 3D printing—ZR80 (translucent acrylonitrile-butadiene-styrene copolymer), Visijet M3 crystal (translucent acrylic copolymer), Vero white plus (white acrylic copolymer), Accura60 (translucent polycarbonate copolymer), Visijet M3 Hi-cast (paraffine), and 3Z-model (o‐toluenesulfonamide and p‐ethylbenzenesulfonamide copolymer)—were used for testing the solubility of six organic solvents: acetone, methanol, ethanol, n-hexane, isopropyl alcohol, and dichloromethane. Each material printed with an identical size was immersed in each solvent for 1 min to measure the length change. Figure 2 shows the etch rates of the build materials for each of the solvents. It was observed that the two materials were substantially dissolved, while the other build materials were negligibly dissolved by the solvents; Visijet M3 Hi-cast dissolved at 89 μm/min and 68 μm/min when immersed in n-hexane and dichloromethane, respectively, and 3Z-model dissolved at 23 000 μm/min and 9800 μm/min when immersed in acetone and methanol, respectively. In contrast, some materials were found to increase in weight by absorbing solvents, which are presented in Fig. 2 as negative etch rates, implying that they cannot be dissolved by the solvents.
FIG. 2.
Etch rates of build materials of commercial 3D printers by each organic solvent. Visijet M3 Hi-cast dissolved at 89 μm/min in n-hexane. 3Z-model dissolved 23 000 μm/min and 9800 μm/min in acetone and methanol, respectively. The blue bar indicates the reduced volume change (i.e., etching by the solvent). The red bar with the comb pattern indicates the increased volume change (i.e., absorption of the solvent). Capital letters A, M, H, I, E, and D represent acetone, methanol, n-hexane, isopropyl alcohol, ethanol, and dichloromethane, respectively.
B. Effect of solvents on PDMS
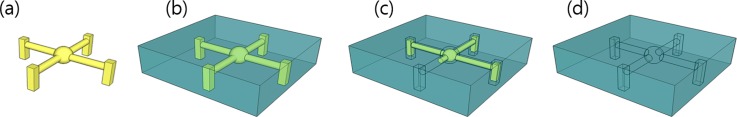
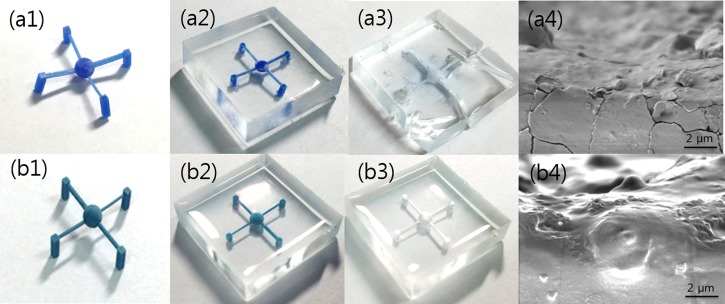
Considering the etch rates of the materials, 3D microfluidic channels were fabricated using two materials, Visijet M3 Hi-cast and 3Z-model to evaluate the effect of solvents on PDMS. Molds resembling a spider with four legs were designed; the central sphere was designed with a diameter of 600 μm, and the cylindrical channel of each leg was designed with various diameters ranging from 500 to 800 μm. Test structures were printed using ProJet MJP 3600 W and 3Z-STUDIO printers that used Visijet M3 Hi-cast and 3Z-model as build materials, respectively [Figs. 3(a1) and 3(b1)]. The PDMS mixture was cast around the printed molds and cured in a heated oven at 50 °C [Figs. 3(a2) and 3(b2)]. Next, according to the previous results, test structures formed with Visijet M3 Hi-cast and 3Z-model in a PDMS block were dissolved using n-hexane [Fig. 3(a3)] and acetone [Fig. 3(b3)], respectively.
FIG. 3.
PDMS channels fabricated using 3D-printed molds. (a1)–(a3) The mold is made of Visijet M3 hi-cast and dissolved by n-hexane. (b1)–(b3) The mold is made of 3Z-model and dissolved by acetone. The PDMS structure is severely torn by n-hexane (a3) but is completed by acetone without breakage (b3). SEM images show that the PDMS surface exposed to n-hexane had microcracks (a4), whereas the PDMS surface exposed to acetone barely had microcracks (b4).
Visijet M3 Hi-cast, however, encountered several issues for the PDMS casting. During the PDMS curing in the heated oven, Visijet M3 Hi-cast was melted and diffused into PDMS, turning the transparent PDMS block opaque [Fig. 3(a3)]. Not only did the dissolved Visijet M3 Hi-cast mold in n-hexane penetrate into PDMS25 but also n-hexane utilized to remove Visijet M3 Hi-cast molds swelled the volume of PDMS by ∼35%.26 In the case of a structure composed only of thin walls, part of the structure could be torn during chemical removal of the mold using n-hexane [Fig. 3(a3)]. Moreover, because the PDMS swelled and then returned to its original state as n-hexane evaporated, microcracks inevitably occurred on the channel wall [Fig. 3(a4)], causing leakage of fluid flowing in the channels. The microcracks could also distort microfluid flow because the microfluid flow was influenced by surface conditions of the channel.
On the other hand, when observed by the eye, the 3Z-model was not diffused into PDMS during the PDMS curing. Moreover, acetone and methanol utilized to remove the mold printed by the 3Z-model swelled PDMS by only 6% and 2%, respectively.26 Therefore, microcracks hardly occurred when the PDMS structure was immersed in acetone or methanol [Fig. 3(b4)]. Consequently, although both 3Z-model and Visijet M3 Hi-cast were dissolved remarkably by organic solvents, the 3Z-model was more suitable to fabricate the microfluidic channels in this study.
C. Characterization of the cast mold printed using 3Z-STUDIO
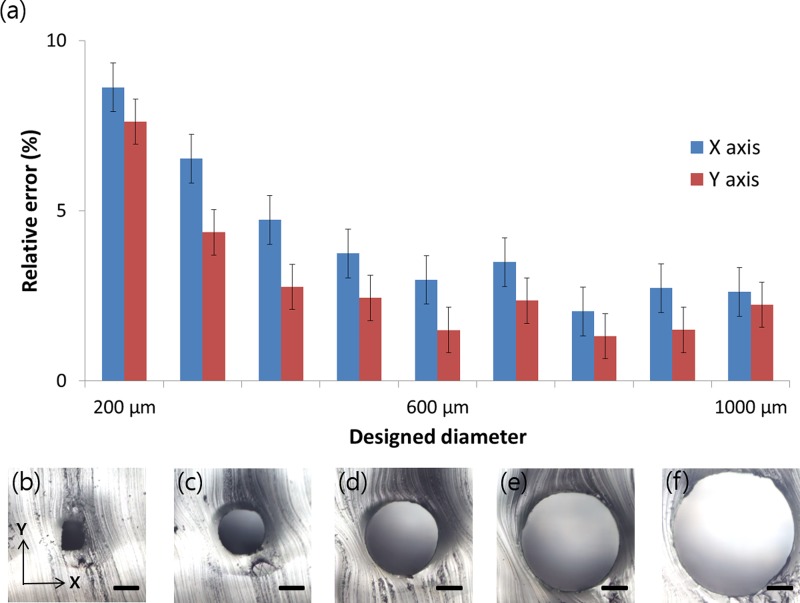
The empirical performance of the 3Z-STUDIO 3D printer, which builds structures composed of the 3Z-model, must be evaluated. This is because resolutions of 3D printers can differ according to the nominal nozzle size of the printer owing to the combined droplet size of the build material and reflow of the build material before curing.21 To compare cross-sections of the fabricated channels with the designed ones, cylindrical structures were designed with diameters from 200 μm to 1000 μm with 100-μm intervals and printed by the 3Z-Studio printer, whose printing layer thickness was set to 6 μm. The manufactured cylindrical PDMS channels were cut out, and then, their cross-sections were observed using an optical microscope. Figure 4 shows measured relative differences between the designed and fabricated channel widths (i.e., relative error). X- and y-axes indicate the direction perpendicular to the cylindrical channel mold and the direction in which each printed layer stacks, respectively. From 500 μm to 1000 μm in designed diameter, the relative errors of the fabricated channels for both the x-axis and the y-axis were 3% and 2%, fluctuating within ±1.8% and ±1.6%, respectively. The nominal specification of the 3Z-Studio allowed us to expect that the resolution in the x direction would be better than that in the y direction. However, the relative errors for the x direction were 1.3% larger than those for the y direction for the whole range of designed diameters. This is most likely an operating mechanism of the printer; vertical misalignment between printed layers causes relative error of the x-axis, whereas the vertical height determining the y-axis error is precisely controlled by the number of layers stacked. Smaller than 500 μm in designed diameter, the relative errors for both axes gradually increase. In the case of a cylindrical channel designed 200 μm in diameter, it had a relative error of 8.6% and a trapezoidal cross-section rather than a designed circular shape. Unfortunately, channels designed less than 100 μm in diameter failed to print although the nominal resolutions were lower.
FIG. 4.
(a) Relative error of diameters for the microchannels compared with the designed diameter, from 200 μm to 1000 μm, for the 3Z-Studio 3D printer. (b)–(f) Cross-sections of PDMS microchannels designed with diameters of 200 μm, 400 μm, 600 μm, 800 μm, and 1000 μm. As the diameter of the circular cross-section is designed smaller, the cross-section of the 3D-printed channel is gradually distorted to have a trapezoidal cross-section. The standard deviation in the data, which comprises measurements of six microchannels, is expressed as an error bar. Scale bars represent 200 μm.
The surface of the printed final structure was inherently not continuous but in the form of a staircase because the 3D printer stacked the build material layer by layer. The surface of the 3D-printed structure as cast molds was transferred directly to the PDMS; therefore, the wrinkled surface of the PDMS channel might affect the microfluid flow in the PDMS channel.30–32
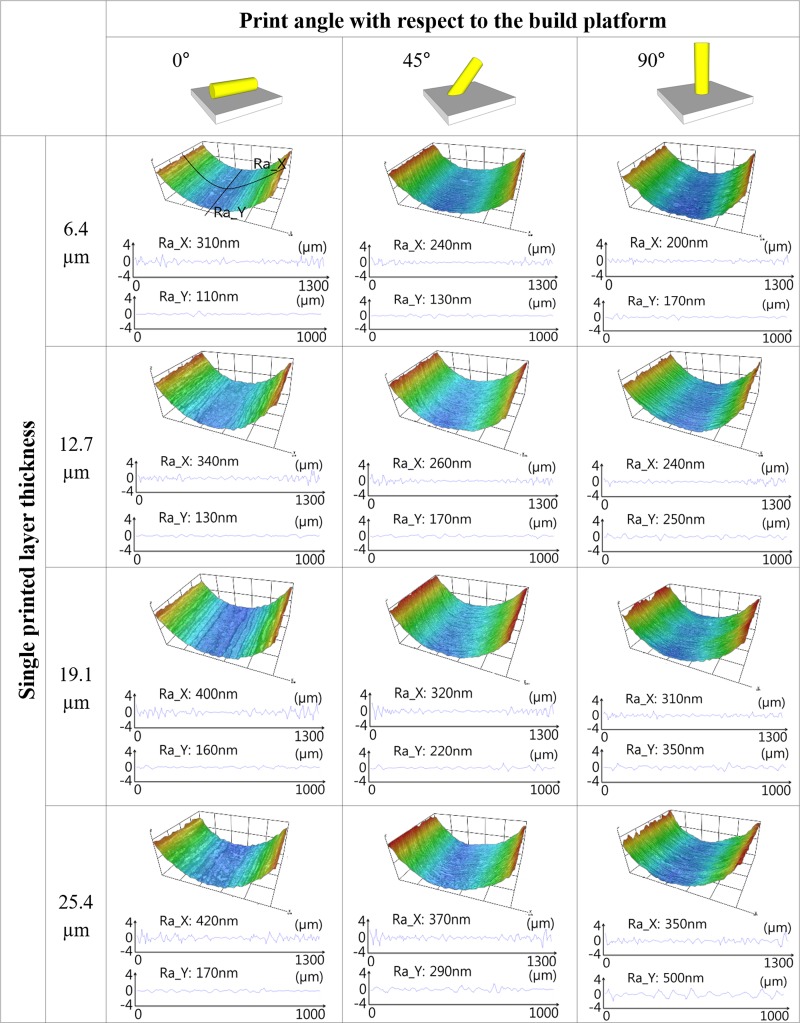
The surface roughness of the PDMS cylindrical microchannel was investigated for the single printed layer thickness and print angle with respect to the build platform because the surface of the printed mold could change according to 3D printer's resolution and printed direction. The 3Z-STUDIO stacked each layer vertically to the platform, and the single printed layer thickness of 3Z-STUDIO was user selectable as follows: 6.4 μm, 12.7 μm, 19.1 μm, and 25.4 μm. The cylindrical molds were created at 0°, 45°, and 90° with respect to the build platform for each condition about the printed layer thickness. Figure 5 shows the 3D profiles and surface roughness of each PDMS microchannel that were measured using the 3D profiler (HRM-300, HUVITZ, Republic of Korea). Red and blue in the 3D profiles indicated 400 μm and 0 μm in height, respectively. The surface roughness was measured about two directions. Ra_X indicates the surface roughness about the transverse direction to the microchannel, and Ra_Y was measured longitudinal to the microchannel. In the case of Ra_X, the greater printing angle resulted in a smoother surface. In contrast, the surface longitudinal to the microchannel got coarser as the printing angle was increased. In other words, it was confirmed that the stacking direction of the printer (corresponding to the transverse direction of the channel for the printing angle of 0° and to the longitudinal direction of the channel for a printing angle of 90°) appeared as wrinkles on the channel surface as it is. In both Ra_X and Ra_Y, the thicker the single layer printed, the rougher the printed surface, as expected.
FIG. 5.
Measured surface roughness and surface profile of the PDMS microchannel with different single printed layer thicknesses and different print angles with respect to the build platform of the 3D printer. The height of the microchannel is shown with a color map. (Red and blue indicate 400 μm and 0 μm, respectively.) As the first leftmost figure illustrates, Ra_X and Ra_Y represent the surface roughness of each microchannel about the direction transverse and longitudinal to the microchannel, respectively. The measured area was 1300 × 1000 μm2. In the insets at the top of the table, the gray plate and yellow cylinder represent the 3D printer's build platform and printed channel, respectively.
D. Cerebral artery-shaped microchannel
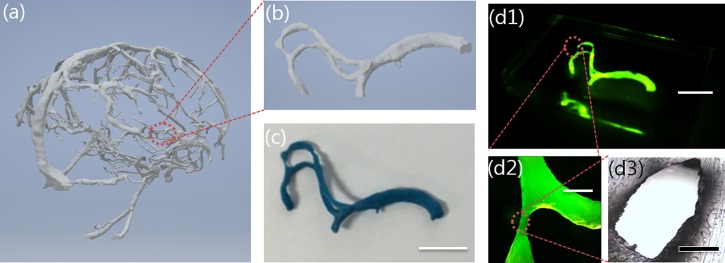
As a proof of concept for the proposed fabrication method, we reconstructed microfluidic channels of a human blood vessel shape with actual dimensions. A 3D image of a human cerebral artery [Fig. 6(a)] created by Ripley and Tatiana using magnetic resonance imaging (MRA) and provided by embodi3D33 was utilized. To print the blood-vessel-shaped wax mold, part of the 3D image of the cerebral artery was loaded into the 3Z-Studio 3D printer [Fig. 6(b)]. 3Z-Studio supported a build envelop of up to 152.4 × 152.4 × 101.6 mm3, and the 3D image (17.5 × 11.3 × 10.0 mm3) shown in Fig. 6(b) was printed by stacking a single layer of 6.4 μm thickness for 13.4 h with the automatically generated supporting material [Fig. 6(c)]. In the case of the free-standing block of the 3Z-model, the etch rate for acetone is 23 000 μm/mm as reported above. However, in the case of the cerebral artery-shaped PDMS channel with 23.5 mm long, acetone is supplied through only two inlets; therefore, it requires approximately 3 min to dissolve the printed mold inside the cast PDMS. Figures 6(d1)–6(d3) show the fabricated truly 3D PDMS channel filled with fluorescent liquid for visualization, demonstrating that the channel was defined in the PDMS without any leakage. Depending on the human blood vessel model, the reconstructed channel had various widths ranging from 240 μm to 1600 μm with relative error up to 7.9%.
FIG. 6.
(a) 3D image of human cerebral artery supplied from Embodi3D. (b) Magnified part of cerebral artery. (c) Cerebral artery-shaped mold made of 3Z-model after removing the supporting material. (d1) Cerebral artery-shaped PDMS microfluidic channel filled with fluorescent liquid for clear observation. (d2) Magnification of (d1). (d3) Cross-section of (d2) with a relative error of up to 7.9%. Scale bars are 5 mm in (c) and (d1), 1 mm in (d2), and 200 μm in (d3).
IV. CONCLUSIONS
We reported the soluble wax 3D printing and PDMS casting techniques for truly 3D microfluidic channels. Using this technique, microchannels were created with truly 3D geometry and smaller than 200 μm within 8.6% tolerance. To realize this technique, we investigated etch rates of the commercial build materials to the organic solvents and identified suitable combinations between build materials and solvents. The combination of the 3Z-model and acetone was found to have an etch rate of 23 000 μm/min while swelling the PDMS by only 6%. Relative error between the designed and fabricated microchannels was also measured to evaluate the empirical performance of the 3Z-Studio 3D printer. The 3Z-Studio 3D printer produced cylindrical structures with diameters ranging from 500 μm to 1000 μm with relative errors of 3% and 2%, fluctuating within ±1.8% and ±1.6% about the x-axis and the y-axis, respectively. When the cylindrical structure was designed smaller than 500 μm, the relative errors for both axes gradually increased, and for the structure designed with a diameter of 200 μm, it had a relative error of 8.6%.
As a proof of concept for this technique, we realized cerebral-artery-shaped PDMS channels using a 3D image of an actual human cerebral artery. The minimal width of the reconstructed PDMS vascular microchannel was 240 μm with an error of up to 7.9%.
Accurate study of blood flow using 3D microfluidic devices is one of the important applications of microfluidics. Nevertheless, conventional studies faced challenges in mimicking actual blood vessels via typical microfabrication methods. We believe that microchannels with actual blood vessel shapes might help to study the fluid characteristics of blood vessels, which have previously been limited.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant No. 2016R1D1A3B03931565). This research was also supported by the Korea University Future Research Grant.
References
- 1. Stone H. A. and Kim S., AlChE J. 47(6), 1250–1254 (2001). 10.1002/aic.690470602 [DOI] [Google Scholar]
- 2. Elvira K. S., Casadevall i Solvas X., and Wootton R. C. R., Nat. Chem. 5(11), 905–915 (2013). 10.1038/nchem.1753 [DOI] [PubMed] [Google Scholar]
- 3. Squires T. M. and Quake S. R., Rev. Mod. Phys. 77(3), 977 (2005). 10.1103/RevModPhys.77.977 [DOI] [Google Scholar]
- 4. Zhang Q. and Austin R. H., J. Bionanosci. 2(4), 277–286 (2012). 10.1007/s12668-012-0051-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi N. W., Cabodi M., Held B., Gleghorn J. P., Bonassar L. J., and Stroock A. D., Nat. Mater. 6(11), 908 (2007). 10.1038/nmat2022 [DOI] [PubMed] [Google Scholar]
- 6. Charati S. G. and Stern S. A., Macromolecules 31(16), 5529–5535 (1998). 10.1021/ma980387e [DOI] [Google Scholar]
- 7. Bélanger M. C. and Marois Y., J. Biomed. Mater. Res. Part A 58(5), 467–477 (2001). 10.1002/jbm.1043 [DOI] [PubMed] [Google Scholar]
- 8. Mata A., Fleischman A. J., and Roy S., Biomed. Microdevices 7(4), 281–293 (2005). 10.1007/s10544-005-6070-2 [DOI] [PubMed] [Google Scholar]
- 9. McDonald J. C. and Whitesides G. M., Acc. Chem. Res. 35(7), 491–499 (2002). 10.1021/ar010110q [DOI] [PubMed] [Google Scholar]
- 10. Knowlton S., Yu C. H., Ersoy F., Emadi S., Khademhosseini A., and Tasoglu S., Biofabrication 8(2), 025019 (2016). 10.1088/1758-5090/8/2/025019 [DOI] [PubMed] [Google Scholar]
- 11. Borenstein J. T., Tupper M. M., Mack P. J., Weinberg E. J., Khalil A. S., Hsiao J., and Garcia G., Cardena, Biomed. Microdevices 12(1), 71–79 (2010). 10.1007/s10544-009-9361-1 [DOI] [PubMed] [Google Scholar]
- 12. Kamei K., Mashimo Y., Koyama Y., Fockenberg C., Nakashima M., Nakajima M., Li J. J., and Chen Y., Biomed. Microdevices 17(2), 36 (2015). 10.1007/s10544-015-9928-y [DOI] [PubMed] [Google Scholar]
- 13. Calderon A. J., Heo Y. S., Huh D., Futai N., Takayama S., Fowlkes J. B., and Bull J. L., Appl. Phys. Lett. 89(24), 244103 (2006). 10.1063/1.2402898 [DOI] [Google Scholar]
- 14. Jo B. H., Van Lerberghe L. M., Motsegood K. M., and Beebe D. J., J. Microelectromech. Syst. 9(1), 76–81 (2000). 10.1109/84.825780 [DOI] [Google Scholar]
- 15. Verma M. K. S., Majumder A., and Ghatak A., Langmuir 22(24), 10291–10295 (2006). 10.1021/la062516n [DOI] [PubMed] [Google Scholar]
- 16. Jia Y., Jiang J., Ma X., Li Y., Huang H., Cai K., Cai S., and Wu Y., Chin. Sci. Bull. 53(24), 3928–3936 (2008). 10.1007/s11434-008-0528-6 [DOI] [Google Scholar]
- 17. Odera T., Hirama H., Kuroda J., Moriguchi H., and Torii T., Microfluid. Nanofluid. 17(3), 469–476 (2014). 10.1007/s10404-013-1327-1 [DOI] [Google Scholar]
- 18. Lee J., Paek J., and Kim J., Lab Chip 12(15), 2638–2642 (2012). 10.1039/c2lc40267j [DOI] [PubMed] [Google Scholar]
- 19. Lee W., Kwon D., Choi W., Jung G. Y., Au A. K., Folch A., and Jeon S., Sci. Rep. 5, 7717 (2015). 10.1038/srep07717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szydzik C., Niego B., Dalzell G., Knoerzer M., Ball F., Nesbitt W. S., Medcalf R. L., Khoshmanesh K., and Mitchell A., RSC Adv. 6(91), 87988–87994 (2016). 10.1039/C6RA20688C [DOI] [Google Scholar]
- 21. Hwang Y., Paydar O. H., and Candler R. N., Sens. Actuators A 226, 137–142 (2015). 10.1016/j.sna.2015.02.028 [DOI] [Google Scholar]
- 22. Nguyen T. Q. and Park W. T., Sens. Actuators B 235, 302–308 (2016). 10.1016/j.snb.2016.05.008 [DOI] [Google Scholar]
- 23. Parekh D. P., Ladd C., Panich L., Moussa K., and Dickey M. D., Lab Chip 16(10), 1812–1820 (2016). 10.1039/C6LC00198J [DOI] [PubMed] [Google Scholar]
- 24. Saggiomo V. and Velders A. H., Adv. Sci. 2(9), 1500125 (2015). 10.1002/advs.201500125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang H., Liu J., Zheng X., Rong X., Zheng X., Peng H., Silber-Li Z., Li M., and Liu L., Sci. Rep. 5, 10945 (2015). 10.1038/srep10945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee J. N., Park C., and Whitesides G. M., Anal. Chem. 75(23), 6544–6554 (2003). 10.1021/ac0346712 [DOI] [PubMed] [Google Scholar]
- 27. Miller J. S., Stevens K. R., Yang M. T., Baker B. M., Nguyen D. H. T., Cohen D. M., Toro E., Chen A. A., Galie P. A., Yu X., Chaturvedi R., Bhatia S. N., and Chen C. S., Nat. Mater. 11(9), 768–774 (2012). 10.1038/nmat3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakano T., Yoshida K., Ikeda S., Oura H., Fukuda T., Matsuda T., Negoro M., and Arai F., paper presented at the 2009 IEEE/RSJ International Conference on Intelligent Robots and Systems, 2009. [Google Scholar]
- 29. Hinton T. J., Hudson A., Pusch K., Lee A., and Feinberg A. W., ACS Biomater. Sci. Eng. 2(10), 1781–1786 (2016). 10.1021/acsbiomaterials.6b00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu Y., Werner C., and Li D., J. Fluids Eng. 125(5), 871–879 (2003). 10.1115/1.1598993 [DOI] [Google Scholar]
- 31. Rawool A. S., Mitra S. K., and Kandlikar S. G., Microfluid. Nanofluid. 2(3), 215–221 (2006). 10.1007/s10404-005-0064-5 [DOI] [Google Scholar]
- 32. Hwang Y., Seo D., Roy M., Han E., Candler R. N., and Seo S., J. Microelectromech. Syst. 25(2), 238–240 (2016). 10.1109/JMEMS.2016.2521858 [DOI] [Google Scholar]
- 33.embodi3D, https://www.embodi3d.com/ for human cerebral artery model, 2016.