Abstract
Purpose
Thumb carpometacarpal (CMC) osteoarthritis (OA) represents a major source of functional morbidity. The effects of early CMC OA on loading and use patterns potentially lead to changes in local bone density and micro-architecture. Hounsfield units (HU), a quantitative attenuation coefficient obtained from computed tomography (CT) scans, have been shown to be a reliable marker of bone density. We hypothesized that early CMC OA is associated with lower local bone density about the CMC joint as assessed by HU.
Methods
We examined HU units from CT scans in 23 asymptomatic subjects and 91 patients with early CMC OA. HU measurements were obtained within cancellous portions of the trapezium, capitate, first and third metacarpal bases, and distal radius. Linear regression models, with age and sex included as covariates, were used to assess the relationship between CMC OA and HU values at each anatomic site.
Results
Early OA patients had significantly lower HU than asymptomatic subjects within the trapezium (mean 377 vs 436) and first metacarpal bases (265 vs 324). No significant group differences were noted at the capitate, third metacarpal, or distal radius. Male sex and younger age were associated with significantly higher HU at all the anatomic sites, except the first metacarpal base, where age had no significant effect.
Conclusions
Subjects presenting with early CMC OA had significantly lower bone density as assessed with HU at the thumb CMC joint (trapezium and first metacarpal base). Early thumb CMC OA and discomfort may lead to diminished loading across the basal joint, producing focal disuse osteopenia. These findings in symptomatic early arthritis suggest a relationship between symptoms, functional use of the CMC joint, and local bone density.
Level of Evidence
Diagnostic Level II
Keywords: Hounsfield Units, Osteoarthritis, Thumb
INTRODUCTION
Thumb carpometacarpal (CMC) osteoarthritis (OA) represents a major source of functional morbidity1–3. Pain is commonly associated with pinching and other tasks that load the CMC joint; the literature supports the association of lower pinch strength with CMC OA4. Other reports associate hand OA and decreased grip strength5,6. Julius Wolff, a 19th century German anatomist and surgeon, is often credited with first observing that normal bone architecture adapts and remodels in direct response to loads placed upon it7. The inverse is also true, as disuse can lead to osteopenia resulting from a lack of mechanical stimulus8.
Local changes in bone density and micro-architecture in early CMC OA potentially adheres to Wolff’s Law and bone loading properties. Hounsfield units (HU), a quantitative attenuation marker obtained from computed tomography (CT) scans, have been shown to be a reliable marker of bone density9–12. HU represent a standardized linear attenuation coefficient of tissue based on a defined scale of 0 for water and −1000 for air. Local bone density measurements can be conveniently obtained by HU at any region of interest from diagnostic CT scans, with no additional cost or radiation exposure to the patient.
HU measurements have been shown to have excellent intra- and inter-observer reliability9–11, and present a method for assessing regional bone density. It has previously been demonstrated that early CMC OA is associated with reduced CMC joint loading, due to activity modification and decreased strength4. Since loading has been demonstrated to affect local bone density and micro-architecture within bone, we hypothesize that early CMC OA is associated with lower local bone density in the region of the CMC joint.
MATERIALS AND METHODS
Cohort
Patients with early CMC OA and asymptomatic subjects were recruited as part of a larger investigation on the biomechanics of thumb CMC OA1,4,13. The sub-cohorts analyzed in the current study consisted of 114 individuals (91 patients with early CMC OA and 23 asymptomatic subjects) (Table 1). Institutional review board approval was obtained at both participating sites. Inclusion criteria for subjects in the early CMC OA cohort included pain or discomfort at the base of the thumb, clinical signs of OA such as a positive grind test, and radiographs demonstrating modified Eaton Stage 0/1 CMC OA (either normal radiographs or mild arthritic changes including slight subchondral sclerosis and/or joint space narrowing)14,15. A board-certified orthopaedic hand surgeon made all clinical and radiographic assessments. Subjects with a history of previous ipsilateral hand surgery, wrist and thumb fractures, ligamentous injury, metabolic bone disease, radiographically evident OA in other joints of the wrist and trapezium, inflammatory arthritis, or connective tissue disease were excluded from the study. The control cohort was comprised of healthy community volunteers and patients seen for conditions unrelated to hand osteoarthritis. Inclusion criteria for asymptomatic controls included no complaints of thumb pain, normal radiographs (modified Eaton Stage 0)15, and an examination by a board-certified orthopaedic hand surgeon to rule out clinical evidence of CMC pathology.
Table 1.
Summary statistics (mean ± SD) and results of bone mineral density, as measured in Hounsfield Units, at each of the five anatomic sites assessed.
| Variable | Control Subjects mean (SD) n=23 | Osteoarthritis Subjects mean (SD) n=91 | p value |
|---|---|---|---|
| Age (years) | 56.6 (8.0) | 58.2 (7.3) | 0.354 |
| Male sex (%) | 43 | 47 | 0.749 |
| Female sex (%) | 57 | 43 | 0.749 |
| BMI (kg/m2) | 28.6 (6.0) | 26.9 (5.2) | 0.172 |
| Trapezium HU | 420.4 (77.3) | 369.1 (90.6) | <0.05 |
| 1st Metacarpal Base HU | 321.9 (55.8) | 268.9 (74.8) | <0.05 |
| 3rd Metacarpal Base HU | 403.2 (101.3) | 394.8 (106.6) | 0.706 |
| Capitate HU | 601.2 (103.5) | 609.8 (119.4) | 0.746 |
| Distal Radius HU | 269.8 (68.2) | 270.1 (82.8) | 0.989 |
Hounsfield Unit Methodology
Unenhanced CT scans of the wrist and thumb were performed in both control and early OA subjects as part of a larger longitudinal study of CMC OA progression1,4,13 with a 16-slice clinical CT scanner (GE LightSpeed 16, General Electric, Milwaukee, WI). CT parameters included a slice thickness of 0.625-mm, an in-plane resolution of at least 0.4-mm × 0.4-mm, a tube voltage of 80 kVp, a tube current of 80 mA, and a bone reconstruction algorithm (window width/window level, −3000/300). Two-dimensional reconstructions were obtained in the coronal and sagittal planes.
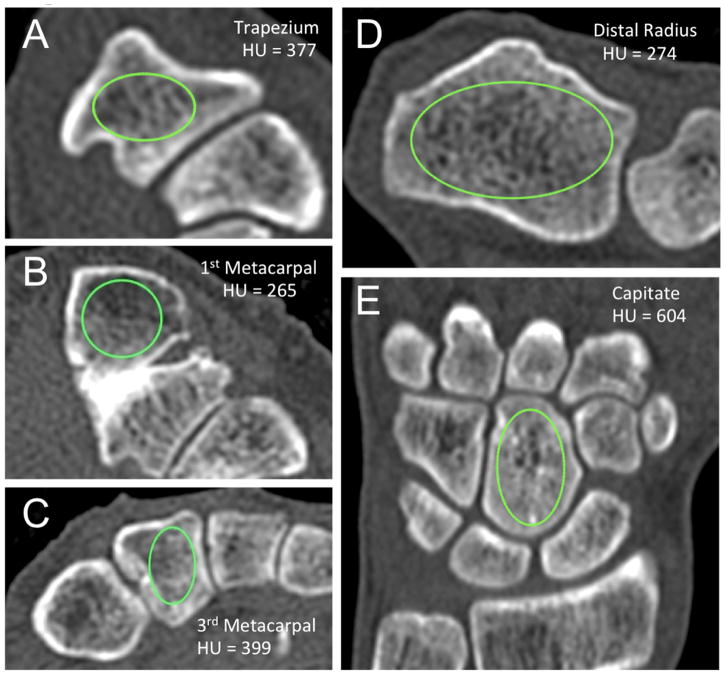
The methodology for HU measurements adhered to previously reported techniques optimized for assessment of bone density9,10. All measurements were made with OsiriX DICOM viewer (Pixmeo, Bernex, Switzerland), with an elliptical region-of-interest tool used to calculate an average HU value at each anatomic site. Measurements were isolated to cancellous regions of bone at all sites, with the largest ellipse possible used, while avoiding cortical regions to prevent volume biasing (Figure 1). An average of measurements from three consecutive slices was utilized at each osseous site. For the distal radius, axial images proximal to the physeal scar and within the widest portion of the metaphysis were utilized. Trapezium HU measurements were made from axial images at the mid-portion of the bone from proximal to distal, which corresponded to the trapezial ridge landmark. Metacarpal base values were obtained from the proximal metaphyseal regions of the thumb and third metacarpal, while avoiding sclerosis seen in the immediate subchondral layer, and remaining proximal to the diaphyseal narrowing. Coronal images were used to make HU measurements of the capitate at its widest mid-coronal portion in the volar to dorsal axis. Two observers (one orthopaedic hand surgeon, one medical student) ascertained HU measurements independently at each site for all patients in both cohorts, and their values were averaged. During data collection of HU measurements, CT scans were blinded as to which cohort the patient belonged.
Figure 1.
Technique for obtaining Hounsfield Unit measurements. An elliptical region of interest was localized to cancellous portions of bone on axial computed tomography cuts of the A) trapezium, B) first metacarpal base, C) third metacarpal base and D) distal radius metaphysis. E) Coronal images were utilized for Hounsfield Unit measurements within the capitate. An average measurement from three slices was used at each osseous site in each subject to generate an assessment of local bone density.
Statistical Analysis
Summary statistics including means and standard deviations were generated for all variables in both the early CMC OA and asymptomatic control cohorts. A linear regression analysis was used to assess the strength and direction of the relationship between CMC OA and HU at each anatomic site. Bone density (HU) was the dependent variable, whereas OA status, sex, and age were independent variables. Given the possibility for variation in manual measurements of bone density, we first assessed the inter-observer reliability of our methodology. Interobserver reliability of HU measurements was assessed using an intraclass correlation coefficient (ICC), which was reported along with 95% confidence intervals (CI). A value of greater than 0.8 is considered an “excellent” interrater correlation16.
RESULTS
Interobserver reliability was excellent at the trapezium (ICC = .82 [95% CI, 0.75–0.87], p < 0.05), first metacarpal base (ICC = .90 [95% CI, 0.86–0.93], p < 0.05), third metacarpal base (ICC = .92 [95% CI, 0.88–0.94], p < 0.05), capitate (ICC = .92 [95% CI, 0.87–0.94], p < 0.05), and distal radius (ICC = .96 [95% CI, 0.93–0.97], p < 0.05).
There were no differences between the control and early OA cohorts in terms of age, sex, or body mass index (kg/m2) (Table 1). Controlling for age and sex in the linear regression models, there were significant differences in HU between the early OA and asymptomatic control cohort at both the first metacarpal base and trapezium (Table 1). There were no observed differences between the two cohorts at the third metacarpal base, capitate, and distal radius. Male sex and younger age were significantly correlated with higher HU (p < 0.05) at all anatomic sites except within the first metacarpal base, where sex and age had no effects (p = 0.056 and p = 0.171 respectively).
DISCUSSION
We found that patients with symptomatic, early CMC OA, exhibit lower local bone density than asymptomatic subjects, suggesting a relationship between symptoms, functional use of the CMC joint4–6, and bone adaption in the form of locally decreased bone density. Patients with symptomatic CMC OA modify activities and demonstrate functional changes as measured by loss of pinch and grip strength4–6. Activity modification, relative disuse, and decreased axial loading potentially contribute to decreased local bone mineral density (BMD) about the CMC joint in patients with early OA. Regional bone loading correlates to regional bone density at anatomic sites throughout the skeleton7,17. Our findings support this concept. In this same cohort, McQuillan and colleagues noted a 14% decrease in key pinch strength in early symptomatic CMC OA patients as compared to asymptomatic subjects4. This magnitude is similar to the HU decrease at the trapezium (14%) and first metacarpal base (18%) observed in the same CMC OA cohort presented here.
Bone is a dynamic tissue that continually remodels to meet its mechanical demands. This happens through a complex interplay between osteocytes, the primary mechanosensors in normal bone, and resultant effects on progenitor cells and the relative activity of osteoblasts and osteoclasts17. We suggest that decreased forces on the local micro-architecture over time could explain the lower HU measured in the trapezium and first metacarpal base that was not seen elsewhere throughout the hand and wrist.
Whereas disuse and mechanical aberrations contribute to osteopenia and diminished bone density, our symptomatic subjects also had a competing variable: the presence of OA. Advanced OA often presents regional variability in bone density and quality. Osteophytes and subchondral sclerosis represent regional areas of increased bone density, whereas cystic arthritic areas represent decreased bone density, with both demonstrating abnormal bone quality. The association between osteoarthritis and osteoporosis is controversial, and it is generally agreed that it differs by disease state and anatomic site18. The CMC OA cohort studied here was relatively early in the degenerative process as demonstrated by modified Eaton Stage 0/1 radiographic findings14,15. Our cohort with early radiographic OA potentially represents a more subtle and homogenous degenerative process compared to advanced arthritis. Marked subchondral sclerosis, which would increase HU values, was rarely seen on the CT scans in this cohort. While we demonstrated decreased HU about the CMC joint in subjects with early CMC OA, it is unknown if this association would be diminished, or even reversed, in a cohort with more advanced CMC OA. Decreased HU may be the cause or the result of bony remodeling from the OA process, or could be cause by something different, such as disuse osteopenia or other biomechanical phenomena. Longitudinal evaluation of an inception cohort before the onset of CMC OA may provide further insight regarding the interplay between CMC OA and local bone density.
In addition to the potential effects of use, loading, and osteoarthritis on local bone density – several other factors are known to affect bone density and quality, including age, sex, and medical co-morbidities. Similar to dual x-ray absorptiometry (DXA) results, previous studies have shown that HU values at various anatomic sites are lower in females and decrease with increasing age9,11,12. We found female subjects in both cohorts to have significantly lower HU values than males at all anatomic sites, and found that increased age was associated with decreased HU values at all sites except the first metacarpal base.
There is no firmly established methodology for assessing local bone quality and density about the CMC joint. In the spine literature, correlations between HU and T scores, BMD, compressive strength and fracture risk have been firmly established9,12,19,20. Thresholds have also been identified for “opportunistic” diagnosis of osteopenia and osteoporosis from clinical CT scans9,12. HU provides a volumetric assessment of bone that can be localized to any region, as opposed to a planar evaluation of bone density provided by DXA20. As such, when used in the setting of degenerative changes and OA, it may provide a more accurate assessment of local BMD, as sclerosis and osteophyte formation can lead to artificially elevated DXA scores21. However, while degenerative changes (osteophytes and sclerosis) may artificially elevate DXA scores, the results of the present study suggest that HU values may also be affected by local degenerative changes and alterations in joint loading. The variable effect of arthritis on local bone density is worth considering in future studies evaluating HU as a marker of bone quality.
In the upper extremity, HU measurements from CT scans have been used to diagnose osteoporosis at the proximal humerus11, and lower HU values at the distal radius and capitate have been associated with increased risk of distal radius fracture10. Additionally, HU measurements at the capitate have been correlated with BMD and t scores at the hip, femoral neck, and spine, suggesting that capitate HU may be used to screen for global osteopenia and osteoporosis22. The measurement technique has been shown to be reliable at several anatomic sites, with excellent inter- and intra-observer reliability9–11,22.
Several limitations exist in our study. DXA results were unavailable in both asymptomatic and symptomatic cohorts; whether global BMD differences exist is unknown. However, results at the capitate were similar between the cohorts, and capitate HU has previously been correlated global BMD22. We do not have comprehensive data on splint use in the early OA cohort, and do not know how this may affect regional HU values. Additionally, our regression analysis did not account for other factors that may affect bone quality, including various co-morbidities, medication profile, social history, including alcohol and caffeine consumption, and ethnicity. Multiple anatomic sites remote from the CMC joint (capitate, third metacarpal, distal radius), however, were used as internal controls and demonstrated no differences between the cohorts. The subjects were relatively homogenous in their stage of OA, so while decreased BMD was seen in this early OA cohort, it is unknown how the interplay of these variables will change with advancing disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Halilaj E, Moore DC, Laidlaw DH, et al. The morphology of the thumb carpometacarpal joint does not differ between men and women, but changes with aging and early osteoarthritis. J Biomech. 2014;47(11):2709–2714. doi: 10.1016/j.jbiomech.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ladd AL, Crisco JJ, Hagert E, Rose J, Weiss AP. The 2014 ABJS Nicolas Andry Award: The puzzle of the thumb: mobility, stability, and demands in opposition. Clin Orthop Relat Res. 2014;472(12):3605–3622. doi: 10.1007/s11999-014-3901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Heest AE, Kallemeier P. Thumb carpal metacarpal arthritis. J Am Acad Orthop Surg. 2008;16(3):140–151. doi: 10.5435/00124635-200803000-00005. [DOI] [PubMed] [Google Scholar]
- 4.McQuillan TJ, Kenney D, Crisco JJ, Weiss AP, Ladd AL. Weaker Functional Pinch Strength Is Associated With Early Thumb Carpometacarpal Osteoarthritis. Clin Orthop Relat Res. 2016;474(2):557–561. doi: 10.1007/s11999-015-4599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dominick KL, Jordan JM, Renner JB, Kraus VB. Relationship of radiographic and clinical variables to pinch and grip strength among individuals with osteoarthritis. Arthritis Rheum. 2005;52(5):1424–1430. doi: 10.1002/art.21035. [DOI] [PubMed] [Google Scholar]
- 6.Bagis S, Sahin G, Yapici Y, Cimen OB, Erdogan C. The effect of hand osteoarthritis on grip and pinch strength and hand function in postmenopausal women. Clin Rheumatol. 2003;22(6):420–424. doi: 10.1007/s10067-003-0792-4. [DOI] [PubMed] [Google Scholar]
- 7.Wolff J. The Law of Bone Remodeling (translation of the German 1892 edition) Berlin Heidelberg New York: Springer; 1986. [Google Scholar]
- 8.Bloomfield SA. Disuse osteopenia. Curr Osteoporos Rep. 2010;8(2):91–97. doi: 10.1007/s11914-010-0013-4. [DOI] [PubMed] [Google Scholar]
- 9.Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG. Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am. 2011;93(11):1057–1063. doi: 10.2106/JBJS.J.00160. [DOI] [PubMed] [Google Scholar]
- 10.Schreiber JJ, Gausden EB, Anderson PA, Carlson MG, Weiland AJ. Opportunistic Osteoporosis Screening - Gleaning Additional Information from Diagnostic Wrist CT Scans. J Bone Joint Surg Am. 2015;97(13):1095–1100. doi: 10.2106/JBJS.N.01230. [DOI] [PubMed] [Google Scholar]
- 11.Pervaiz K, Cabezas A, Downes K, Santoni BG, Frankle MA. Osteoporosis and shoulder osteoarthritis: incidence, risk factors, and surgical implications. J Shoulder Elbow Surg. 2013;22(3):e1–8. doi: 10.1016/j.jse.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 12.Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med. 2013;158(8):588–595. doi: 10.7326/0003-4819-158-8-201304160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halilaj E, Rainbow MJ, Got C, et al. In vivo kinematics of the thumb carpometacarpal joint during three isometric functional tasks. Clin Orthop Relat Res. 2014;472(4):1114–1122. doi: 10.1007/s11999-013-3063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eaton RG, Glickel SZ. Trapeziometacarpal osteoarthritis. Staging as a rationale for treatment. Hand Clin. 1987;3(4):455–471. [PubMed] [Google Scholar]
- 15.Ladd AL, Messana JM, Berger AJ, Weiss AP. Correlation of clinical disease severity to radiographic thumb osteoarthritis index. J Hand Surg Am. 2015;40(3):474–482. doi: 10.1016/j.jhsa.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 17.Chen JH, Liu C, You L, Simmons CA. Boning up on Wolff’s Law: mechanical regulation of the cells that make and maintain bone. J Biomech. 2010;43(1):108–118. doi: 10.1016/j.jbiomech.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Im GI, Kim MK. The relationship between osteoarthritis and osteoporosis. J Bone Miner Metab. 2014;32(2):101–109. doi: 10.1007/s00774-013-0531-0. [DOI] [PubMed] [Google Scholar]
- 19.Meredith DS, Schreiber JJ, Taher F, Cammisa FP, Jr, Girardi FP. Lower preoperative Hounsfield unit measurements are associated with adjacent segment fracture after spinal fusion. Spine (Phila Pa 1976) 2013;38(5):415–418. doi: 10.1097/BRS.0b013e31826ff084. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber JJ, Anderson PA, Hsu WK. Use of computed tomography for assessing bone mineral density. Neurosurg Focus. 2014;37(1):E4. doi: 10.3171/2014.5.FOCUS1483. [DOI] [PubMed] [Google Scholar]
- 21.Rand T, Seidl G, Kainberger F, et al. Impact of spinal degenerative changes on the evaluation of bone mineral density with dual energy X-ray absorptiometry (DXA) Calcif Tissue Int. 1997;60(5):430–433. doi: 10.1007/s002239900258. [DOI] [PubMed] [Google Scholar]
- 22.Johnson CC, Gausden EB, Weiland AJ, Lane JM, Schreiber JJ. Using Hounsfield Units to Assess Osteoporotic Status on Wrist Computed Tomography Scans: Comparison With Dual Energy X-Ray Absorptiometry. J Hand Surg Am. 2016;41(7):767–774. doi: 10.1016/j.jhsa.2016.04.016. [DOI] [PubMed] [Google Scholar]