Abstract
This review considers the “promise” of exploiting the proton-coupled folate transporter (PCFT) for selective therapeutic targeting of cancer. PCFT was discovered in 2006 and was identified as the principal folate transporter involved in the intestinal absorption of dietary folates. The recognition that PCFT was highly expressed in many tumors stimulated substantial interest in using PCFT for cytotoxic drug targeting, taking advantage of its high level transport activity under the acidic pH conditions that characterize many tumors. For pemetrexed, among the best PCFT substrates, transport by PCFT establishes its importance as a clinically important transporter in malignant pleural mesothelioma and non-small cell lung cancer. In recent years, the notion of PCFT-targeting has been extended to a new generation of tumor-targeted 6-substituted pyrrolo[2,3-d]pyrimidine compounds that are structurally and functionally distinct from pemetrexed, and that exhibit near exclusive transport by PCFT and potent inhibition of de novo purine nucleotide biosynthesis. Based on compelling preclinical evidence in a wide range of human tumor models, it is now time to advance the most optimized PCFT-targeted agents with the best balance of PCFT transport specificity and potent antitumor efficacy to the clinic to validate this novel paradigm of highly selective tumor targeting.
Keywords: Antifolate, folate, glycinamide ribonucleotide formyltransferase, hypoxia, proton-coupled folate transporter, purine, reduced folate carrier, targeted therapy
INTRODUCTION
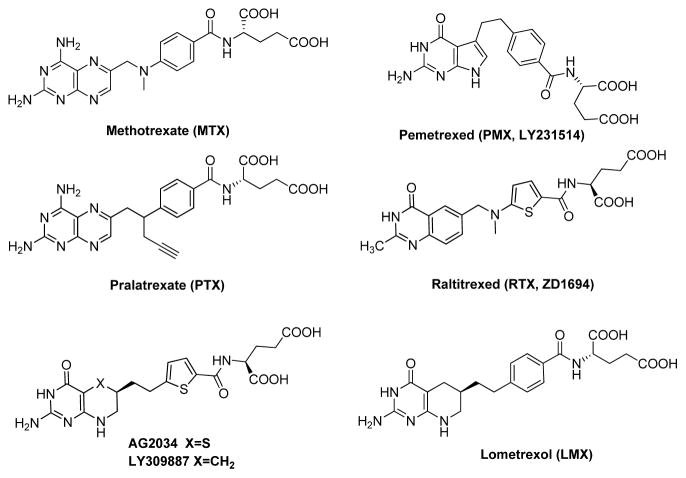
Folates are hydrophilic molecules that are anions at physiologic pH and that cross membranes poorly by diffusion. Reflecting this, mammalian cells have evolved sophisticated transport systems for facilitating cellular uptake of folate cofactors. The ubiquitously expressed reduced folate carrier (RFC or SLC19A1) is widely considered to be the major transport system for folates in mammalian cells [1]. RFC mediates concentrative uptake of folate substrates such as (6S)-5-methyl tetrahydrofolate (THF), its physiologic substrate. Notably, RFC is also an important transporter for antifolate drugs approved for cancer therapy, including methotrexate (MTX), pemetrexed (PMX), pralatrexate (PTX), and raltitrexed (RTX) [1–3] (Figure 1).
Figure 1.
Structures of classic antifolates, including methotrexate (MTX), pemetrexed (PMX), pralatrexate (PTX), raltitrexed (RTX), lometrexol (LMX), AG2034, and LY30987.
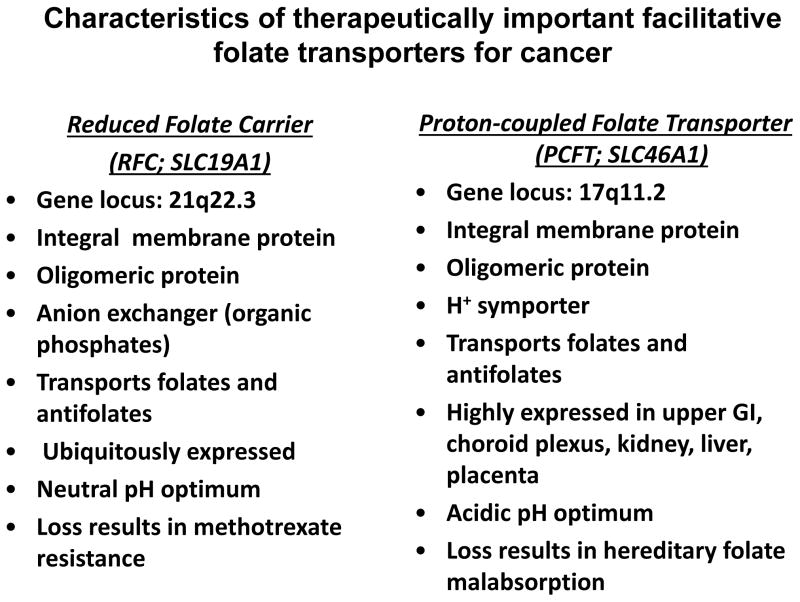
In 2006, a previously uncharacterized facilitative transporter (SLC46A1), the proton-coupled folate transporter (PCFT), was reported to mediate uptake of dietary folates in the duodenum, with mutations in PCFT resulting in a rare autosomal recessive disorder, hereditary folate malabsorption [4]. PCFT is distantly related to RFC and is distinct in terms of its substrate specificities, mechanism, and pH optimum, and its patterns of expression in tissues and tumors (Figure 2) [1,2,5]. These differences have provided the impetus for studies of the biology of PCFT including its physiology, pharmacology, and structure and function [6–29], and the development of novel PCFT-targeted cytotoxic antifolates for selective tumor targeting [30–41].
Figure 2.
Characteristics of therapeutically important facilitative folate transporters for cancer including the reduced folate carrier and proton-coupled folate transporter.
In this review, we explore the promise and potential challenges of exploiting the PCFT for selective therapeutic targeting of cancer.
Folate-based therapies for cancer and the evolution of antifolates that target de novo purine nucleotide biosynthesis
In the modern era of targeted therapies, antifolates (Figure 1) remain versatile and widely used agents for treating cancer and other diseases. MTX is used for treating acute lymphoblastic leukemia (ALL), osteogenic sarcoma, lymphoma, and breast cancer [3]. RTX is used outside the US for treating advanced colorectal cancer, particularly in patients with fluoropyrimidine-induced cardiotoxicity or a significant history of cardiac disease [42]. PMX was approved in the US for treating malignant pleural mesothelioma (2004) [43] and non-small cell lung cancer (2009) [44]. Most recently, PTX was FDA-approved (2009) for treating relapsed or refractory peripheral T-cell lymphoma [45].
MTX and PTX are inhibitors of dihydrofolate reductase (DHFR) [46,3], whereas PMX and RTX are thymidylate synthase (TS) inhibitors [42,47,48]. For PMX, 5-aminoimidazole-4-carboxamide ribonucleotide (ZMP) formyltransferase (AICARFTase) and glycinamide ribonucleotide formyltransferase (GARFTase) in de novo purine nucleotide biosynthesis, and DHFR are secondary targets [49,50,48,47]. In PMX-treated tumor cells, accumulation of the AICARFTase substrate ZMP (reflecting AICARFTase inhibition) results in activation of 5′ adenosine monophosphate-activated protein kinase (AMPK) and mTOR inhibition [49,50]. However, unlike the direct effects of ZMP in activating AMPK, the effects of PMX on mTORC1 reflected AMPK-mediated phosphorylation of Raptor and were independent of TSC2 and p53 [51].
Other antifolates have been described which exclusively target de novo purine nucleotide biosynthesis at GARFTase. Lometrexol (LMX) (Figure 1) was introduced in 1985 by Eli Lilly Corporation as a bona fide GARFTase inhibitor and showed promising in vitro and in vivo antitumor activities in assorted preclinical tumor models associated with depletion of purine nucleotide pools [52,47,53,54]. When LMX progressed to a Phase I clinical trial [53,55], patients experienced dose-limiting myelosuppression and mucositis, thus hampering further clinical development. Toxicity was reduced by administering folic acid [53]. For 2nd generation GARFTase inhibitors (i.e., LY309887, AG2034) (Figure 1), replacement of the 1,4-phenyl by a 2,5-thienyl ring increased drug potency over LMX [56,53]. Unfortunately, in phase I clinical trials, LY309887 and AG2034 showed similar toxicities to those encountered with LMX [57,58].
The notion of targeting de novo purine nucleotide biosynthesis with folate analogs assumes that depletion of purines can limit nucleotides for DNA synthesis and repair, while also impacting ATP and GTP stores required for cellular energetics. GARFTase inhibitors kill tumors independent of wild-type/mutant p53 status [59,60], and selectively target tumors secondary to 5′-deoxy-5′-methylthioadenosine (MTA) phosphorylase (MTAP) deletions in many cancers (e.g., non-small cell lung cancer), as MTAP-expressing normal tissues are protected by MTA [61,62].
As described below, recent studies have described the discovery of a new generation of novel tumor-targeted anti-purine antifolates that target GARFTase, with tumor selectivity based on their preferential transport into tumors over normal tissues.
Facilitative folate transporters and the malignant phenotype: the role of RFC and PCFT in transport and antitumor efficacy of antifolate therapeutics
The ubiquitously expressed RFC is the major transport route for folate cofactors and classical antifolate drugs (such as MTX, PTX, and RTX) into both tumors and normal tissues [1–3]. For DHFR inhibitors such as MTX, RFC transport is essential for generating sufficient intracellular drug to maximize DHFR inhibition and to support the synthesis of antifolate polyglutamates required for cellular retention [63,3]. Differences in the extent of MTX polyglutamylation between normal tissues and tumors likely contribute to drug selectivity and antitumor efficacy, and to the selectivity of leucovorin rescue from MTX toxicity [63,3]. With antifolates such as PMX or LMX that inhibit enzymes other than DHFR, drug polyglutamates typically bind to these cellular targets with much higher affinities than their non-polyglutamyl drug forms [47,53,48].
Loss of RFC transport is an important contributing factor in MTX resistance in preclinical tumor models, and has been implicated as causal in clinical resistance to MTX in ALL and osteogenic sarcoma [63,1,64]. In cancer cell lines, antifolate resistance due to loss of RFC function results from decreased RFC expression, or from synthesis of mutant RFC with impaired transport function [63,1,64]. For MTX, loss of RFC transport often accompanies other cellular defects including decreased drug polyglutamate synthesis and/or elevated levels of DHFR [64,63]. Loss of RFC transport has also been reported for other antifolates such as GW1843 [64]. Interestingly, for antifolates that are sufficiently good folylpolyglutamate synthetase (FPGS) substrates such as LMX, drug accumulation and chemosensitivity can be significantly preserved toward MTX resistant cells in spite of a major loss of RFC transport activity [65].
PCFT is highly expressed in apical brush border membranes in the proximal jejunum and duodenum; however, levels are substantially reduced in other segments of the intestine and colon [4,66,67]. PCFT expression is elevated in the choroid plexus, liver and kidney, but PCFT appears to be expressed modestly in most other human tissues and is undetectable in the bone marrow [68,30,67].
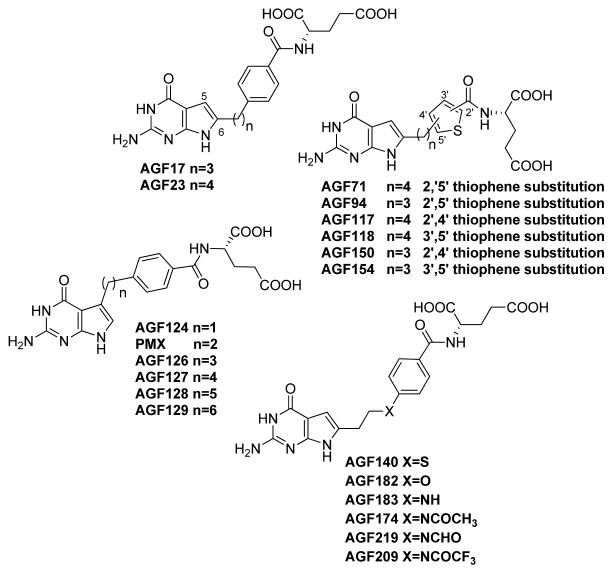
Growing evidence suggests an association between PCFT levels and function, and the malignant phenotype. A comprehensive analysis of folate transporter expression by real-time RT-PCR in 80 cancer cell lines derived from human solid tumors (n=53) and leukemias (n=27) detected substantial PCFT expression in 52 of 53 tumor cells [33]. PCFT transcript levels were elevated in hepatoma cells, and in epithelial ovarian cancer, malignant pleural mesothelioma, non-small cell lung cancer and pancreatic cancer cells [33]. PCFT levels were low-to-undetectable in leukemias (including ALL and acute myeloid leukemia) [33]. Low level PCFT expression in leukemia cells appears at least in part to involve promoter methylation [16]. Relative differences in PCFT transcripts between tumor cell lines generally paralleled levels of PCFT protein on western blots and transport activity at pH 5.5, although there were outliers [30]. While this suggests possible posttranslational regulation of PCFT, to date, the only posttranslational modification reported for PCFT involves its glycosylation [69]. However, glycosylation of PCFT does not appreciably impact its transport function [69]. In lung cancer cells, expression of PCFT proteins was accompanied by robust transport activity at pH 5.5 with a PCFT-selective radiolabeled substrate (AGF154) (Figure 3) [40]. Analogous results were reported in epithelial ovarian cancer cell lines (IGROV1, SKOV3, A2780) expressing an ~11-fold range of FRα levels, along with PCFT [41].
Figure 3.
Structures of novel 5- and 6-pyrrolo[2,3-d]pyrimidine antifolates.
For primary human tumors, including 26 non-small cell lung cancer [40], 47 epithelial ovarian cancer [41], and 124 malignant pleural mesothelioma [70] specimens, PCFT transcripts were detected by real-time RT-PCR. By immunohistochemistry, PCFT protein was detected with polyclonal PCFT antibody [41,40,70].
In both test (n=73) and validation (n=51) cohorts of mesothelioma patients treated with PMX, patients with low PCFT transcripts had significantly lower rates of disease control and shorter overall survival [70]. In 35 malignant pleural mesothelioma specimens from patients treated with PMX, low PCFT protein levels were accompanied by significantly shorter overall survival [70]. Collectively, these results strongly imply that PCFT is an important determinant of PMX clinical efficacy in malignant mesothelioma.
Net tissue and tumor uptake of classical antifolate drugs confers antitumor efficacy and toxicity, and reflects substrate specificities for RFC and/or PCFT. Other factors include expression levels of RFC and PCFT, their tissue/tumor localization which determines their access to circulating drug, and the pH of the tissue/tumor microenvironment relative to the pH optima for transport by RFC or PCFT.
With certain tumors such as epithelial ovarian cancer, folate receptor (FR) α may also contribute to antifolate drug uptake [71,72]. This has been extended to include cytotoxic folate conjugates (e.g., vintafolide) [73] and small molecule inhibitors (e.g., ONX0801) [74] that selectively target FR-expressing tumors. While FRs are also expressed in normal epithelial tissues such as renal tubules (i.e., FRα) and thymus (FRβ), these are either inaccessible to the circulation (FRα) or are non-functional (FRβ) [71]. Agents that are transported by FRs and not by other processes such as RFC would be expected to exhibit tumor selectivity and limited toxicity to normal tissues.
For RFC substrates such as MTX, there is a finite selectivity toward tumors over normal proliferative tissues (e.g., bone marrow) since both tumors and normal tissues express RFC [1]. Indeed, it now seems likely that the substantial toxicities encountered in clinical trials with the early generation of GARFTase inhibitors such as LMX [55,53] were at least partly due to their RFC-mediated transport, along with drug polyglutamylation that results in drug retention in susceptible normal tissues.
On this basis, PCFT-selective tumor-targeted therapeutics were developed with a singular goal of exploiting the substantial levels of PCFT expression in many tumors compared to most normal tissues, and the significant transport activity of PCFT at the acidic pH of the tumor microenvironment [31–34,30,36,38–41]. This is considered below.
Functional and molecular characterization of PCFT
PCFT is a folate-proton symporter that transports folates and related molecules under acidic conditions characterizing the small intestine and the microenvironment of many solid tumors [30]. PCFT was identified in 2006 as a high affinity folate transporter [4], even though it had been originally described as a low affinity heme transporter [75].
Like RFC, PCFT belongs to the major facilitator superfamily (MFS) of secondary transporters, although it is only distally related (PCFT shares ~14% amino acid identity with RFC) [76,4]. The characteristics of PCFT and RFC are summarized in Figure 2. As with other MFS proteins, PCFT is a homo-oligomer and oligomerization is important to its optimal transport function [77,7,78].
PCFT is unique from RFC in that it has an acidic rather than a neutral pH optimum [30,67,2,5,79]. Whereas RFC is optimally active at physiologic pH (pH 7.2–7.4), PCFT transport is maximal at pH 5–5.5 and significant activity is detectable up to pH 6.8 [80,79,5]. Above pH 7, PCFT transport activity is very low, yet the extent of this residual transport varies for different substrates (see below). In Xenopus oocytes, PCFT transport is electrogenic [4] and results in intracellular acidification [20]. At acidic pH, protons are transported via PCFT in the absence of folate substrates, a phenomenon termed “slippage” [20,22].
Interestingly, physiologic levels of bicarbonate are potently inhibitory of PCFT transport at neutral pH [27]. Other univalent anions such as bisulfite and nitrite were also inhibitory, due to their effects on the collapse of the transmembrane pH gradient [27]. The inhibitory effects of bicarbonate raise yet another factor which contributes to the modest transport by PCFT in systemic tissues where it is expressed. However, with decreasing pH, bicarbonate decreases as well, thus favoring PCFT-mediated transport.
Transport changes with pH reflect both Kt (Michaelis constant) and Vmax (maximum velocity) values for individual substrates [79,15,33,31,32,34]. RFC substrates such as (6S)5-methyl and (6S)5-formyl THFs, and the antifolates MTX, PMX, RTX and PTX are all transported by PCFT [30,2,79,5]. Conversely, excellent RFC substrates such as the antifolates PT523 and GW1843U89 are poor PCFT substrates [31,32,79,80]. Folic acid is poorly transported by RFC but is a reasonably good PCFT substrate [79]. PMX is among the very best PCFT substrates [30,2,79,5]. A series of novel 6-substituted pyrrolo[2,3-d]pyrimidine antifolates was described as excellent PCFT substrates with affinities approximating that for PMX and with PCFT-selectivity over RFC [30,33,32,34,38].
Certain drugs such as diclofenac, indomethacin, and sulfasalazine were reported to inhibit folate uptake by PCFT [67]. Since patients with rheumatoid arthritis are administered sulfasalazine with MTX, this combination could impact the oral bioavailability of MTX, as well as of dietary folates.
Discovery of tumor-targeted cytotoxic folate analogs with PCFT transport selectivity
The extracellular pH (pHe) of the tumor microenvironment can be as low as ~6.7–7.1, whereas intracellular pH (pHi) is ≥ 7.4 [81,82]. For normal differentiated cells, pHe is ~7.3 and pHi is ~7.2. PCFT is expressed in human tumors (above) and is significantly active at pH 6.5–6.8, although the pH optimum is 5–5.5 [80,67,79,5]. At neutral pH, PCFT activity is modest and is inhibited by physiologic concentrations of bicarbonate [27].
These features, combined with the demonstrated clinical efficacy of PMX with non-small cell lung cancer and malignant pleural mesothelioma, suggested a unique opportunity for selective therapeutic targeting tumors via PCFT should compounds be developed with high level substrate activity and selectivity for PCFT over RFC [30]. Indeed, if it was possible to develop cytotoxic PCFT-selective agents without transport by the ubiquitously expressed RFC, these should exhibit far greater tumor selectivity and much less toxicity toward normal tissues than either PMX or MTX, since PCFT is highly expressed in a limited subset of normal tissues (in contrast to RFC) and most normal tissues do not experience the acidic microenvironment most conducive to PCFT transport [30,2].
PMX is a 5-substituted 2-amino-4-oxo-pyrrolo[2,3-d]pyrimidine analog with a side chain comprised of a 2-carbon bridge attached to a p-aminobenzoyl L-glutamate [47] (Figures 1 and 3). PMX is efficiently transported by both RFC and PCFT and shows potent antitumor activity associated with TS inhibition, along with secondary enzyme targets (e.g., GARFTase, AICARFTase) [47,2]. Both shortening (1 carbon; AGF124) and lengthening (3–6 carbons; AGF126, AGF127, AGF128, AGF129) (Figure 3) the bridge region profoundly decreased anti-proliferative activity and PCFT transport, whereas RFC transport was modestly affected up to 5 bridge carbons [83].
The 6-substituted pyrrolo[2,3-d]pyrimidine regioisomer of PMX is pharmacologically inert [84,85]. However, when the bridge region is lengthened to 3 (AGF17) or 4 (AGF23) carbons (Figure 3), thus providing greater conformational flexibility, growth inhibition of PCFT-expressing cells occurred at low nmolar concentrations and PCFT selectivity over RFC was observed [31]. PCFT transport decreased upon further increasing bridge lengths up to 6 carbons. Notably, both AGF17 and AGF23 also preserved substantial inhibitory activity toward FR-expressing cells [86].
Further studies identified additional structure-activity determinants for PCFT transport. Hence, isosteric heteroatom substitutions at C10 in AGF17, including N, O, and S (AGF183, AGF182, and AGF140, respectively), (Figure 3) preserved PCFT transport, albeit with slightly decreased (~4-fold) antiproliferative activities toward PCFT-expressing cells [39]. N-substitution with formyl (AGF219), acetyl (AGF174) or trifluoroacetyl (AGF209) groups had no significant impact on PCFT targeting compared to the N-unsubstituted AGF183 (Figure 3). None of these analogs with heteroatom bridge substitutions were RFC substrates [39].
AGF17 and AGF23 analogs with side-chain thienoyl-for-benzoyl replacements (AGF94 and AGF71, respectively) (Figure 3) showed greater PCFT-targeted activities than the parent compounds, and afforded some of the most potent PCFT-active agents yet discovered, even though substantial FR-targeted activity was preserved, as well [36,41,33,87,32,34,40]. AGF94 (3-carbon bridge) was approximately 10-fold more potent toward PCFT-expressing cells than AGF71 (4-carbon bridge), although this was unrelated to differences in PCFT transport [87,34]. Kis for PCFT transport of AGF71 and AGF94 were nearly identical at pHs 5.5 and 6.8, and were only slightly higher than the Kis for PMX. For AGF94, but not AGF71, modest uptake activity only partly attributed to RFC was also reported [34].
Replacement of L-glutamate in AGF94 with L-aspartate or with unnatural amino acids (α-amino adipate, 4-amino butanoate, α-amino pentanoate) abolished PCFT transport [37]. Based on the antitumor activity profiles for the 2′,5′ thienoyl analogs AGF71 and AGF94, 2′,4′ (AGF117 and AGF150, respectively) and 3′,5′ (AGF118 and AGF154, respectively) thienoyl regioisomers (Figure 3) were synthesized; all were potent inhibitors of cell proliferation, directly attributable to their selective transport by PCFT over RFC [35,38].
Antitumor activity of PCFT-targeted 6-substituted pyrrolo[2,3-d]pyrimidine antifolates is associated with inhibition of de novo purine nucleotide biosynthesis
Based on their potent and PCFT-selective cell inhibition profiles, AGF71 [33,32], AGF94 [87,34] and AGF154 [38] were used as prototype analogs to confirm selective PCFT transport and metabolism to polyglutamates. When HeLa cells were incubated with AGF71 and AGF94 (16 h, pH 6.8), AGF94 polyglutamates accumulated to an ~8-fold greater extent than for AGF71, paralleling differences (~10-fold) in antiproliferative activities [87].
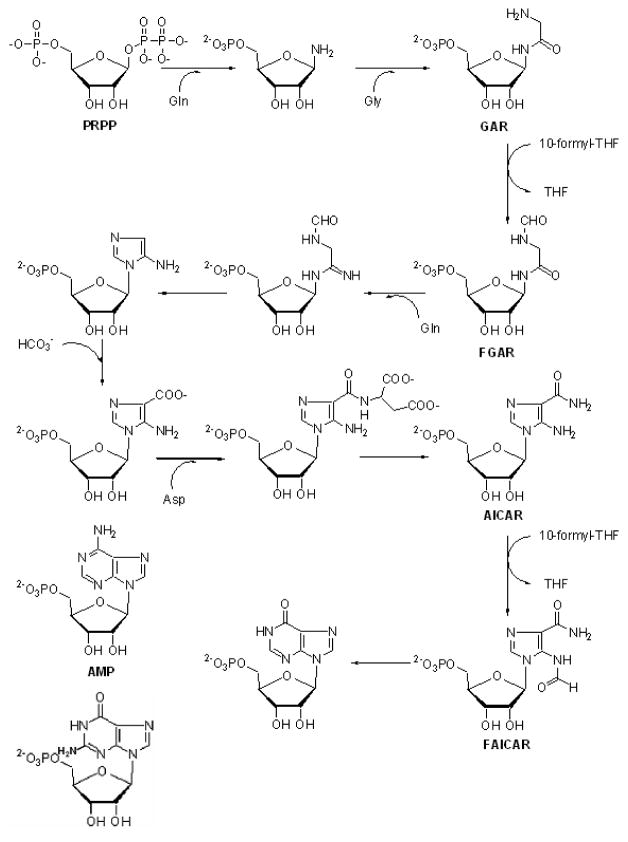
Following cellular uptake by PCFT, the 6-substituted pyrrolo[2,3-d]pyrimidine compounds [AGF17, AGF23, AGF71, AGF94, AGF140, AGF150, AGF154, AGF174, AGF182, AGF183, AGF209, and AGF219] all targeted GARFTase, the first folate-dependent step in the 10 step sequence from phosphoribosyl pyrophosphate (PRPP) to inosine monophosphate (IMP) (Figure 4) [39,32,35,34,38,40,31,33], resulting in inhibition of de novo purine nucleotide biosynthesis and ATP depletion [33,31,34].
Figure 4. De novo purine nucleotide biosynthesis pathway.
The de novo purine nucleotide biosynthetic pathway including 10 steps from phosphoribosyl pyrophosphate (PRPP) to inosine monophosphate (IMP) is shown. Folate-dependent reactions (steps 3 and 9) in which 10-formyl THF (10-CHO-THF) serves as the one-carbon donor are catalyzed by GARFTase and AICARFTase. Undefined abbreviations: FAICAR, 5-formamidoimidazole-4-carboxamide ribotide; FGAR, formyl glycinamide ribonucleotide; GAR, glycinamide ribonucleotide; PRPP, phosphoribosyl pyrophosphate.
By in vitro assays with purified human GARFTase (formyltransferase domain), inhibition by 6-substituted pyrrolo[2,3-d]pyrimidine analogs including AGF23, AGF71, AGF94, AGF117, AGF118, AGF150, AGF154 and AGF183 was confirmed with IC50 values in the low-to-mid nanomolar concentration range [88,39,38]. From crystal structures of ternary complexes of human GARFTase with β-GAR and monoglutamyl pyrrolo[2,3-d]pyrimidine compounds, in vitro inhibitory potencies correlated with drug binding and positioning of the terminal carboxylates within the enzyme active site [88,39,38].
AGF94 and AGF154 were also potent inhibitors of the human epithelial ovarian cancer cell lines (IGROV1, SKOV3, A2780), expressing an ~11-fold range of FRα with similar and significant levels of PCFT [41]. In IGROV1 cells, FRα knockdown preserved substantial inhibition of cell proliferation by both AGF94 and AGF154 in vitro that was directly attributable to PCFT [41]. Thus, dual FR- and PCFT-targeted analogs such as AGF94 and AGF154 would offer significant advantages over current iterations of exclusively FRα-targeted agents in clinical development for epithelial ovarian cancer [73], which would be expected to be less efficacious toward tumors expressing modest levels of FRα, along with high FRα-expressing tumors [41].
Toward H2452 malignant pleural mesothelioma [36] and lung cancer cells (H460, H1437, A549, H1650, H2030) [40], AGF94 and AGF154 were also potent inhibitors. By colony-forming assays with H460 and IGROV1 cells treated with AGF94 under conditions that favor PCFT transport (24 h, pH 6.8), AGF94 was cytotoxic to a far greater extent than PMX [41,40] (Figure 5 shows results with IGROV1 cells). In vivo antitumor efficacies were confirmed for AGF94 and/or AGF154 with early and late-stage H2452 malignant pleural mesothelioma [36] (Figure 6), early-stage H460 non-small cell lung cancer [40], and IGROV1 epithelial ovary cancer [41,38] (subcutaneous) xenografts in severe-combined immunodeficient (SCID) mice. Toxicity was minimal and consisted of modest weight loss that was completely reversible upon completion of therapy.
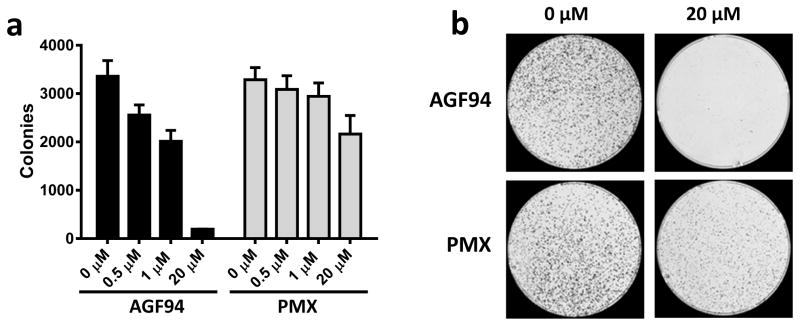
Figure 5. Cytotoxicity of AGF94 and PMX toward IGROV1 epithelial ovarian cancer cells.
The cytotoxic effects of AGF94 and PMX toward the IGROV1 epithelial ovarian cancer subline were assessed with colony-forming assays. IGROV1 cells (10,000 cells) were treated with AGF94 or PMX (0, 0.1, 0.5, 1, 5, 20 μM) for 24 h, washed, then incubated in drug-free media for 12 days. The colonies were stained, rinsed and electronically counted. Results (n=3) are shown for the numbers of colonies counted relative to controls without drug (Panel A). Representative images of colony formation at 0 and 20 μM of AGF94 or PMX are shown (Panel B). From Hou et al. [41] with permission.
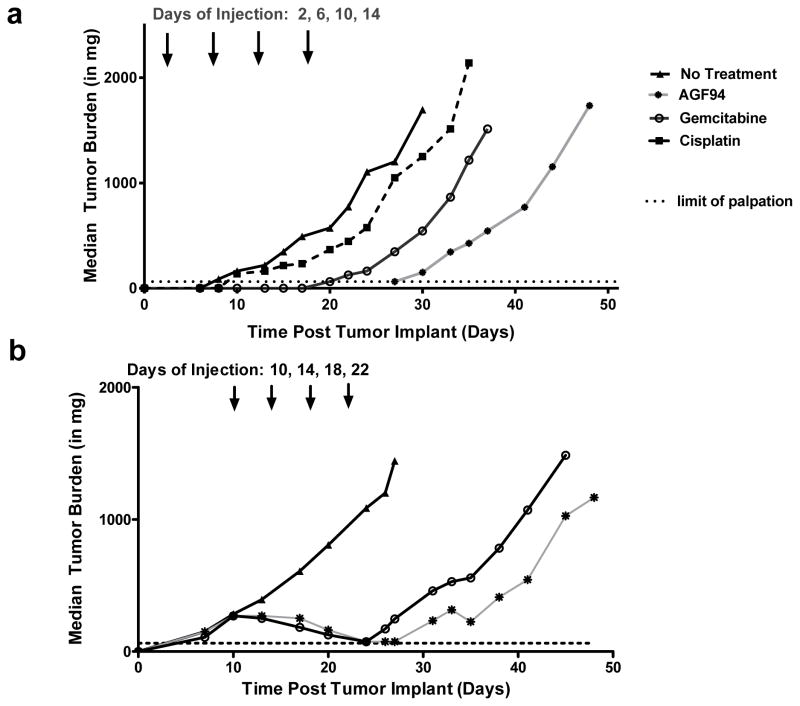
Figure 6. In vivo efficacy of AGF94 toward H2452 malignant pleural mesothelioma xenografts.
Panel A shows an early-stage efficacy trial for female ICR SCID mice implanted with H2452 xenografts (5 mice/group) and treated intravenously after 2 days with AGF94 (32 mg/kg/dose), gemcitabine (150 mg/kg/dose), or cisplatin (2.8 mg/kg/dose) on a schedule of 4 doses over 12 days. Panel B shows an analogous advanced stage disease trial in which chemotherapy was administered beginning 10 days after tumor implantation when tumors were measurable (270–310 mg median tumor burden for treatment groups) at 32 mg/kg/dose for AGF94 and 150 mg/kg/dose for gemcitabine as 4 doses over 12 days. For early and advanced stage tumors, AGF94 produced potent antitumor efficacies exceeding those for standard agents. All mice tolerated the treatment regimens well, as there were no drug-related lethalities or adverse symptoms other than a reversible weight loss. From Cherian et al. [36] with permission.
Collectively, these results provide a compelling argument for the potential value of PCFT-targeted therapies for cancer. The normal tissue milieu is generally associated with a neutral pH which favors membrane transport by RFC even if PCFT is present. In such case, even if PCFT is expressed, the reduced electrochemical proton gradient combined with the inhibitory effects of physiologic bicarbonate [27] would result in limited accumulation of PCFT-targeted cytotoxic antifolates such as AGF94, and limited cytotoxicity toward normal tissues. This would be further enhanced by RFC transport of extracellular reduced folates into normal tissues that would protect cells from antifolate cytotoxicity by competing for polyglutamylation at FPGS, and/or for binding to drug targets such as GARFTase. Additional factors include decreased activity of FPGS [89] and/or increased levels or altered cellular distribution of ABC transporters [90] in response to increased cellular folate pools.
By contrast, in tumors characterized by an acidic pH, the elevated electrochemical proton gradient would favor high levels of membrane transport of cytotoxic PCFT substrates such as AGF94 which can be metabolized to polyglutamates and inhibit critical intracellular targets such as GARFTase and de novo purine biosynthesis independent of p53 mutation status [59,60]. Selectivity of GARFTase inhibition toward tumor versus normal tissues is further augmented by differences in rates of de novo purine nucleotide biosynthesis versus purine salvage between normal tissues and tumors [91,92]. MTAP deficient tumors would be especially sensitive to the cytotoxic effects of 6-substituted pyrrolo[2,3-d]pyrimidine GARFTase inhibitors, whereas intact purine salvage through MTAP should selectively protect normal tissues [61,62]. Cancers with mutant BCRA would also likely show increased sensitivities to inhibitors of de novo purine biosynthesis [93].
For tumors that express FRα along with PCFT such as epithelial ovarian cancer and drugs such as AGF94 that are transported by both PCFT and FRs, PCFT would sustain potent GARFTase inhibition and antitumor activity over a wide range of FRα levels [41].
Is hypoxia and acidosis accompanying the malignant phenotype limiting to the therapeutic potential of PCFT-targeted antifolates?
An acidic microenvironment is a hallmark of cancer [82,94–96]. Rapid tumor growth and/or ischemia can result in hypoxic conditions, associated with acidification of the cytosol and increased pumping of protons into the extracellular environment [97,96]. However, tumors have elevated glucose uptake even under “normoxic” conditions, reflecting increased glycolysis and generation of lactic acid (“Warburg effect”) [94,98]. Furthermore, expression of carbonic acid anhydrases 9/12 on the tumor surface results in extracellular trapping of acid by hydrating CO2 to HCO3− and H+ [99].
Ischemia and acidosis promote tumor progression, invasion and metastases, decrease drug activity through ion-trapping, and result in evasion of immune recognition and apoptosis, and cell cycle arrest [82,100–105]. Drug resistance associated with hypoxia results from: (i) cells that are distant from blood vessels [106,107] and decreased proliferation of tumor cells with increasing distance from blood vessels [108]; (ii) loss of sensitivity to p53-mediated apoptosis [109]; (iii) increased mutation rates [110]; (iv) reduced generation of free radicals [111,112]; and (v) increased activity of DNA repair enzymes and expression of genes important for tumor cell growth and survival, including vascular endothelial growth factor (VEGF), glycolytic enzymes, and signaling molecules [113,114,110,104,115–118].
With this backdrop, the wisdom of developing PCFT-targeted drugs with selective activity toward acidic and hypoxic tumors might seem questionable, as further implied by the study of Raz et al. [119] which reported that under severe hypoxia (<0.1% O2) or in the presence of dimethyloxallylglycine (a hypoxia mimetic agent that stabilizes HIF-1α), expression of genes encoding major transporters (RFC and PCFT), and cytosolic targets (TS, DHFR, and GARFTase) are all down-regulated, accompanying cell cycle arrest and complete (reversible) loss of cytotoxic activities for PMX and related antifolates [119].
However, the relevance of these findings to PCFT-selective targeting of tumors in vivo is uncertain given that Raz et al. [119] used tumors that were severely hypoxic and below the range of hypoxia often reported in human tumors [120,121]. Other considerations include the extreme heterogeneity of oxygenation within tumors, whereby variations in oxygenation occur chronically, as well acutely [121,120,122,123]. For more oxygenated regions, likely closer to functional capillaries, oxygen levels are sufficient to permit cell division which is further augmented by increased glycolysis via Warburg metabolism. With increasing distance from the capillaries, cells are more chronically hypoxic and eventually die, resulting in tissue necrosis [120].
Of course, the strongest evidence for the potential of PCFT-targeted therapeutics lies in their demonstrated in vivo antitumor efficacies toward a wide range of preclinical tumor xenografts, including epithelial ovarian [41,38,39] and lung [40] cancers, hepatoma [31], and malignant pleural mesothelioma [36] (Figure 6). Very recent findings have extended these results to MIA PaCa-2 pancreatic ductal adenocarcinoma xenografts (J. Fruehauf, L. Polin, A. Gangjee, L.H. Matherly, unpublished), a hypoxic tumor model which shows early responses to the hypoxia-activated bioreductive prodrug TH-302 [124,125]. Subcutaneous tumor models are reported to display extensive hypoxia and thus may under predict potential antitumor efficacies of PCFT-targeted therapies toward autochthonous tumors in patients [126]. Collectively, these results provide compelling proof-of-principle evidence of the clinical feasibility of selective therapeutic targeting PCFT-expressing tumors with novel cytotoxic 6-substituted pyrrolo[2,3-d]pyrimidine analogs, in combination with other agents and/or as adjuvant therapies.
CONCLUSIONS
This review summarizes the “promise” of exploiting the PCFT for selective therapeutic targeting of cancer. PCFT was discovered in 2006 and was identified as the principal folate transporter involved in the intestinal absorption of dietary folates and as causal in the rare autosomal condition, hereditary folate malabsorption [4]. The recognition that PCFT was highly expressed in malignant cells [33], including primary specimens from some of the most deadly cancers [41,40,70], stimulated interest in using PCFT for cytotoxic drug targeting to tumors, taking advantage of its substantial transport activity under the acidic pH conditions that characterize many tumors [30,67,79].
Targeting tumor acidity for therapy is an attractive therapeutic strategy, as PCFT-targeted agents would show unique tumor selectivity and circumvent dose-limiting toxicities encountered with standard chemotherapy drugs. The finding that PMX is an excellent substrate for PCFT [47,79], coupled with high level PCFT expression in primary malignant pleural mesothelioma [70] and non-small cell lung cancer [40] specimens, validates its importance as a clinically relevant transporter that contributes to antitumor efficacy of PMX. The notion of PCFT-targeting was further established with in vitro and in vivo models by the discovery of novel 6-substituted pyrrolo[2,3-d]pyrimidine analogs, which are structurally and functionally unique from PMX [30–41]. This manifests as high level and near exclusive PCFT transport and very limited transport by RFC, combined with potent inhibition of de novo purine nucleotide biosynthesis at the reaction catalyzed by GARFTase.
There still remain important unresolved areas to consider in order to maximize the potential of PCFT-targeted therapies for cancer. For instance, (i) how are PCFT levels and function regulated in normal tissues and tumors, including transcriptional, posttranscriptional and posttranslational controls, and what is the role of PCFT oligomerization [77,7,78]? Better understanding of these processes may lead to improved therapeutic strategies for PCFT-targeted agents. Although CpG methylation of the PCFT promoter been described [16,19,70], can antitumor efficacies of PCFT-targeted therapeutics be augmented by co-administering demethylating agents (i.e., azacitidine and decitabine), as recently shown for H2452 mesothelioma cells in vitro [70]? (ii) While cytotoxic tumor-targeted 6-substituted pyrrolo[2,3-d]pyrimidine analogs with selective membrane transport by PCFT over RFC have been reported, to date, all of these compounds show some level of substrate activity toward FRs, as well. As these dual FR- and PCFT-targeted analogs potently inhibit epithelial ovarian cancer cells expressing a wide range of FRα levels [41], is there any benefit of developing PCFT-selective substrates without FR activity? (iii) How does resistance to PCFT-targeted agents manifest and to what extent do cytotoxic PCFT-targeted agents circumvent resistance to standard chemotherapy agents for cancer including PMX? Further, are tumors with high level expression of PCFT less likely to develop PMX resistance? (iv) Can PCFT-targeted agents be used in the therapy of pancreatic cancer and/or brain tumors that express ample levels of PCFT? (v) Can PCFT-targeted GARFTase inhibitors be combined with other therapeutic agents in treating cancers such as non-small cell lung cancer, for instance kinase (e.g., EGFR) inhibitors, to provide enhanced or even synergistic activity? The vast number of clinical trials of kinase inhibitors with cytotoxic agents attests to the potential significance of this approach. (vi) Can PCFT-selective analogs be developed that target intracellular enzyme targets other than GARFTase? Our recent studies (A. Dekhne, J. Fruehauf, Z. Hou, A. Gangjee, L.H. Matherly, unpublished results) establish the therapeutic value of targeting mitochondrial one-carbon metabolism at serine hydroxymethyltransferase 2 (SHMT2) with compounds that are transported by PCFT, a particularly intriguing finding given the frequent upregulation of SHMT2 in many cancers [127], and reports of SHMT2 as a potential cancer driver [128,129]. (vii) Finally, given their unique selectivity for acidic tumors, can PCFT-targeted agents be adapted to other applications such tumor imaging with non-invasive tools such as positron emission tomography (PET)? Such molecules may offer a unique non-invasive strategy for stratifying patients for therapy, including PCFT-targeted cytotoxic agents.
Although there clearly remain many areas for continued study, the compelling preclinical evidence in multiple tumor models suggests it is now time to advance the most optimized PCFT-targeted agents with best balance of selective PCFT transport and potent antitumor efficacies to the clinic to validate the paradigm of tumor-targeting via selective uptake by PCFT over RFC.
Acknowledgments
Funding: This study was supported by grants from the National Cancer Institute, National Institutes of Health [R01 CA53535 (LHM, ZH), R01 CA152316 (LHM, AG), R01 CA166711 (AG, LHM)], the Eunice and Milton Ring Endowed Chair for Cancer Research (LHM), and the Duquesne University Adrian Van Kaam Chair in Scholarly Excellence (AG).
Footnotes
Conflict of Interest: Larry H. Matherly declares that he has no conflict of interest. Zhanjun Hou declares that he has no conflict of interest. Aleem Gangjee declares that he has no conflict of interest.
Ethical Approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
References
- 1.Matherly LH, Hou Z, Deng Y. Human reduced folate carrier: translation of basic biology to cancer etiology and therapy. Cancer Metastasis Rev. 2007;26(1):111–128. doi: 10.1007/s10555-007-9046-2. [DOI] [PubMed] [Google Scholar]
- 2.Matherly LH, Wilson MR, Hou Z. The major facilitative folate transporters SLC19A1 and SLC46A1: biology and role in antifolate chemotherapy of cancer. Drug Metab Dispos. 2014;42(4):632–649. doi: 10.1124/dmd.113.055723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visentin M, Zhao R, Goldman ID. The antifolates. Hematol Oncol Clin North Am. 2012;26(3):629–648. doi: 10.1016/j.hoc.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127(5):917–928. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 5.Zhao R, Aluri S, Goldman ID. The proton-coupled folate transporter (PCFT-SLC46A1) and the syndrome of systemic and cerebral folate deficiency of infancy: Hereditary folate malabsorption. Mol Aspects Med. 2017;53:57–72. doi: 10.1016/j.mam.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin DS, Zhao R, Yap EH, Fiser A, Goldman ID. A P425R mutation of the proton-coupled folate transporter causing hereditary folate malabsorption produces a highly selective alteration in folate binding. Am J Physiol Cell Physiol. 2012;302(9):C1405–C1412. doi: 10.1152/ajpcell.00435.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao R, Shin DS, Fiser A, Goldman ID. Identification of a functionally critical GXXG motif and its relationship to the folate binding site of the proton-coupled folate transporter (PCFT-SLC46A1) American journal of physiology Cell physiology. 2012;303(6):C673–681. doi: 10.1152/ajpcell.00123.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin DS, Zhao R, Fiser A, Goldman ID. The role of the fourth transmembrane domain in proton-coupled folate transporter (PCFT) function as assessed by the substituted cysteine accessibility method. Am J Physiol Cell Physiol. 2013;304:C1159–1167. doi: 10.1152/ajpcell.00353.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson MR, Hou Z, Matherly LH. Substituted cysteine accessibility reveals a novel transmembrane 2–3 reentrant loop and functional role for transmembrane domain 2 in the human proton-coupled folate transporter. J Biol Chem. 2014;289(36):25287–25295. doi: 10.1074/jbc.M114.578252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visentin M, Unal ES, Najmi M, Fiser A, Zhao R, Goldman ID. Identification of Tyr residues that enhance folate substrate binding and constrain oscillation of the proton-coupled folate transporter (PCFT-SLC46A1) American journal of physiology Cell physiology. 2015;308(8):C631–641. doi: 10.1152/ajpcell.00238.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson MR, Hou Z, Wilson LJ, Ye J, Matherly LH. Functional and mechanistic roles of the human proton-coupled folate transporter transmembrane domain 6–7 linker. Biochem J. 2016;473(20):3545–3562. doi: 10.1042/BCJ20160399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao R, Najmi M, Fiser A, Goldman ID. Identification of an extracellular gate for the proton-coupled folate transporter (PCFT-SLC46A1) by cysteine cross-linking. J Biol Chem. 2016;291(15):8162–8172. doi: 10.1074/jbc.M115.693929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao R, Najmi M, Aluri S, Goldman ID. Impact of posttranslational modifications of engineered cysteines on the substituted cysteine accessibility method: evidence for glutathionylation. American journal of physiology Cell physiology. 2017;312(4):C517–C526. doi: 10.1152/ajpcell.00350.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao R, Min SH, Qiu A, Sakaris A, Goldberg GL, Sandoval C, Malatack JJ, Rosenblatt DS, Goldman ID. The spectrum of mutations in the PCFT gene, coding for an intestinal folate transporter, that are the basis for hereditary folate malabsorption. Blood. 2007;110(4):1147–1152. doi: 10.1182/blood-2007-02-077099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao R, Qiu A, Tsai E, Jansen M, Akabas MH, Goldman ID. The proton-coupled folate transporter: impact on pemetrexed transport and on antifolates activities compared with the reduced folate carrier. Mol Pharmacol. 2008;74(3):854–862. doi: 10.1124/mol.108.045443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonen N, Bram EE, Assaraf YG. PCFT/SLC46A1 promoter methylation and restoration of gene expression in human leukemia cells. Biochem Biophys Res Commun. 2008;376(4):787–792. doi: 10.1016/j.bbrc.2008.09.074. S0006-291X(08)01838-X [pii] [DOI] [PubMed] [Google Scholar]
- 17.Lasry I, Berman B, Straussberg R, Sofer Y, Bessler H, Sharkia M, Glaser F, Jansen G, Drori S, Assaraf YG. A novel loss-of-function mutation in the proton-coupled folate transporter from a patient with hereditary folate malabsorption reveals that Arg 113 is crucial for function. Blood. 2008;112(5):2055–2061. doi: 10.1182/blood-2008-04-150276. blood-2008-04-150276 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Stark M, Gonen N, Assaraf YG. Functional elements in the minimal promoter of the human proton-coupled folate transporter. Biochem Biophys Res Commun. 2009;388(1):79–85. doi: 10.1016/j.bbrc.2009.07.116. S0006-291X(09)01480-6 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Diop-Bove NK, Wu J, Zhao R, Locker J, Goldman ID. Hypermethylation of the human proton-coupled folate transporter (SLC46A1) minimal transcriptional regulatory region in an antifolate-resistant HeLa cell line. Molecular cancer therapeutics. 2009;8(8):2424–2431. doi: 10.1158/1535-7163.MCT-08-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unal ES, Zhao R, Chang MH, Fiser A, Romero MF, Goldman ID. The functional roles of the His247 and His281 residues in folate and proton translocation mediated by the human proton-coupled folate transporter SLC46A1. J Biol Chem. 2009;284(26):17846–17857. doi: 10.1074/jbc.M109.008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unal ES, Zhao R, Goldman ID. Role of the glutamate 185 residue in proton translocation mediated by the proton-coupled folate transporter SLC46A1. American journal of physiology Cell physiology. 2009;297(1):C66–74. doi: 10.1152/ajpcell.00096.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahadeo K, Diop-Bove N, Shin D, Unal ES, Teo J, Zhao R, Chang MH, Fulterer A, Romero MF, Goldman ID. Properties of the Arg376 residue of the proton-coupled folate transporter (PCFT-SLC46A1) and a glutamine mutant causing hereditary folate malabsorption. American journal of physiology Cell physiology. 2010;299(5):C1153–1161. doi: 10.1152/ajpcell.00113.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin DS, Min SH, Russell L, Zhao R, Fiser A, Goldman ID. Functional roles of aspartate residues of the proton-coupled folate transporter (PCFT-SLC46A1); a D156Y mutation causing hereditary folate malabsorption. Blood. 2010;116(24):5162–5169. doi: 10.1182/blood-2010-06-291237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao R, Unal ES, Shin DS, Goldman ID. Membrane topological analysis of the proton-coupled folate transporter (PCFT-SLC46A1) by the substituted cysteine accessibility method. Biochemistry (Mosc) 2010;49(13):2925–2931. doi: 10.1021/bi9021439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin DS, Mahadeo K, Min SH, Diop-Bove N, Clayton P, Zhao R, Goldman ID. Identification of novel mutations in the proton-coupled folate transporter (PCFT-SLC46A1) associated with hereditary folate malabsorption. Mol Genet Metab. 2011;103(1):33–37. doi: 10.1016/j.ymgme.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao R, Shin DS, Diop-Bove N, Ovits CG, Goldman ID. Random mutagenesis of the proton-coupled folate transporter (SLC46A1), clustering of mutations, and the bases for associated losses of function. J Biol Chem. 2011;286(27):24150–24158. doi: 10.1074/jbc.M111.236539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao R, Visentin M, Suadicani SO, Goldman ID. Inhibition of the proton-coupled folate transporter (PCFT-SLC46A1) by bicarbonate and other anions. Mol Pharmacol. 2013;84:95–103. doi: 10.1124/mol.113.085605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Najmi M, Zhao R, Fiser A, Goldman ID. Role of the tryptophan residues in proton-coupled folate transporter (PCFT-SLC46A1) function. Am J Physiol Cell Physiol. 2016;311(1):C150–157. doi: 10.1152/ajpcell.00084.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aluri S, Zhao R, Fiser A, Goldman ID. Residues in the eighth transmembrane domain of the proton-coupled folate transporter (SLC46A1) play an important role in defining the aqueous translocation pathway and in folate substrate binding. Biochim Biophys Acta. 2017;1859(11):2193–2202. doi: 10.1016/j.bbamem.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desmoulin SK, Hou Z, Gangjee A, Matherly LH. The human proton-coupled folate transporter: Biology and therapeutic applications to cancer. Cancer Biol Ther. 2012;13(14):1355–1373. doi: 10.4161/cbt.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kugel Desmoulin S, Wang Y, Wu J, Stout M, Hou Z, Fulterer A, Chang MH, Romero MF, Cherian C, Gangjee A, Matherly LH. Targeting the proton-coupled folate transporter for selective delivery of 6-substituted pyrrolo[2,3-d]pyrimidine antifolate inhibitors of de novo purine biosynthesis in the chemotherapy of solid tumors. Mol Pharmacol. 2010;78(4):577–587. doi: 10.1124/mol.110.065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Cherian C, Desmoulin SK, Polin L, Deng Y, Wu J, Hou Z, White K, Kushner J, Matherly LH, Gangjee A. Synthesis and antitumor activity of a novel series of 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate inhibitors of purine biosynthesis with selectivity for high affinity folate receptors and the proton-coupled folate transporter over the reduced folate carrier for cellular entry. J Med Chem. 2010;53(3):1306–1318. doi: 10.1021/jm9015729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kugel Desmoulin S, Wang L, Hales E, Polin L, White K, Kushner J, Stout M, Hou Z, Cherian C, Gangjee A, Matherly LH. Therapeutic targeting of a novel 6-substituted pyrrolo [2,3-d]pyrimidine thienoyl antifolate to human solid tumors based on selective uptake by the proton-coupled folate transporter. Mol Pharmacol. 2011;80(6):1096–1107. doi: 10.1124/mol.111.073833. mol.111.073833 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Kugel Desmoulin S, Cherian C, Polin L, White K, Kushner J, Fulterer A, Chang MH, Mitchell-Ryan S, Stout M, Romero MF, Hou Z, Matherly LH, Gangjee A. Synthesis, biological, and antitumor activity of a highly potent 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate inhibitor with proton-coupled folate transporter and folate receptor selectivity over the reduced folate carrier that inhibits beta-glycinamide ribonucleotide formyltransferase. J Med Chem. 2011;54(20):7150–7164. doi: 10.1021/jm200739e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Cherian C, Kugel Desmoulin S, Mitchell-Ryan S, Hou Z, Matherly LH, Gangjee A. Synthesis and biological activity of 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl regioisomers as inhibitors of de novo purine biosynthesis with selectivity for cellular uptake by high affinity folate receptors and the proton-coupled folate transporter over the reduced folate carrier. J Med Chem. 2012;55(4):1758–1770. doi: 10.1021/jm201688n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cherian C, Kugel Desmoulin S, Wang L, Polin L, White K, Kushner J, Stout M, Hou Z, Gangjee A, Matherly LH. Therapeutic targeting malignant mesothelioma with a novel 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate via its selective uptake by the proton-coupled folate transporter. Cancer Chemother Pharmacol. 2013;71(4):999–1011. doi: 10.1007/s00280-013-2094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golani LK, George C, Zhao S, Raghavan S, Orr S, Wallace A, Wilson MR, Hou Z, Matherly LH, Gangjee A. Structure-activity profiles of novel 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolates with modified amino acids for cellular uptake by folate receptors alpha and beta and the proton-coupled folate transporter. J Med Chem. 2014;57:8152–8166. doi: 10.1021/jm501113m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Wallace A, Raghavan S, Deis SM, Wilson MR, Yang S, Polin L, White K, Kushner J, Orr S, George C, O’Connor C, Hou Z, Mitchell-Ryan S, Dann CE, 3rd, Matherly LH, Gangjee A. 6-Substituted Pyrrolo[2,3-d]pyrimidine Thienoyl Regioisomers as Targeted Antifolates for Folate Receptor alpha and the Proton-Coupled Folate Transporter in Human Tumors. J Med Chem. 2015;58(17):6938–6959. doi: 10.1021/acs.jmedchem.5b00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Golani LK, Wallace-Povirk A, Deis SM, Wong J, Ke J, Gu X, Raghavan S, Wilson MR, Li X, Polin L, de Waal PW, White K, Kushner J, O’Connor C, Hou Z, Xu HE, Melcher K, Dann CE, 3rd, Matherly LH, Gangjee A. Tumor targeting with novel 6-substituted pyrrolo [2,3-d]pyrimidine antifolates with heteroatom bridge substitutions via cellular uptake by folate receptor alpha and the proton-coupled folate transporter and inhibition of de novo purine nucleotide biosynthesis. J Med Chem. 2016;59(17):7856–7876. doi: 10.1021/acs.jmedchem.6b00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson MR, Hou Z, Yang S, Polin L, Kushner J, White K, Huang J, Ratnam M, Gangjee A, Matherly LH. Targeting nonsquamous nonsmall cell lung cancer via the proton-coupled folate transporter with 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolates. Mol Pharmacol. 2016;89(4):425–434. doi: 10.1124/mol.115.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hou Z, Gattoc L, O’Connor C, Yang S, Wallace-Povirk A, George C, Orr S, Polin L, White K, Kushner J, Morris RT, Gangjee A, Matherly LH. Dual targeting of epithelial ovarian cancer via folate receptor alpha and the proton-coupled folate transporter with 6-substituted pyrrolo[2,3-d]pyrimidine antifolates. Molecular cancer therapeutics. 2017;16:819–830. doi: 10.1158/1535-7163.MCT-16-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Avallone A, Di Gennaro E, Silvestro L, Iaffaioli VR, Budillon A. Targeting thymidylate synthase in colorectal cancer: critical re-evaluation and emerging therapeutic role of raltitrexed. Expert Opin Drug Saf. 2014;13(1):113–129. doi: 10.1517/14740338.2014.845167. [DOI] [PubMed] [Google Scholar]
- 43.Hazarika M, White RM, Johnson JR, Pazdur R. FDA drug approval summaries: pemetrexed (Alimta) Oncologist. 2004;9(5):482–488. doi: 10.1634/theoncologist.9-5-482. [DOI] [PubMed] [Google Scholar]
- 44.Cohen MH, Justice R, Pazdur R. Approval summary: pemetrexed in the initial treatment of advanced/metastatic non-small cell lung cancer. Oncologist. 2009;14(9):930–935. doi: 10.1634/theoncologist.2009-0092. [DOI] [PubMed] [Google Scholar]
- 45.Thompson CA. FDA approves pralatrexate for treatment of rare lymphoma. Am J Health Syst Pharm. 2009;66(21):1890. doi: 10.2146/news090080. [DOI] [PubMed] [Google Scholar]
- 46.Zain J, O’Connor O. Pralatrexate: basic understanding and clinical development. Expert Opin Pharmacother. 2010;11(10):1705–1714. doi: 10.1517/14656566.2010.489552. [DOI] [PubMed] [Google Scholar]
- 47.Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Molecular cancer therapeutics. 2007;6(2):404–417. doi: 10.1158/1535-7163.MCT-06-0343. 6/2/404 [pii] [DOI] [PubMed] [Google Scholar]
- 48.Shih C, Thornton DE. Preclinical pharmacology studies and the clinical development of a novel multitargeted antifolate, MTA (LY231514) In: Jackman AL, editor. Anticancer Drug Development Guide: Antifolate Drugs in Cancer Therapy. Humana Press, Inc; Totowa, NJ: 1999. pp. 183–201. [Google Scholar]
- 49.Racanelli AC, Rothbart SB, Heyer CL, Moran RG. Therapeutics by cytotoxic metabolite accumulation: pemetrexed causes ZMP accumulation, AMPK activation, and mammalian target of rapamycin inhibition. Cancer Res. 2009;69(13):5467–5474. doi: 10.1158/0008-5472.CAN-08-4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothbart SB, Racanelli AC, Moran RG. Pemetrexed indirectly activates the metabolic kinase AMPK in human carcinomas. Cancer Res. 2010;70(24):10299–10309. doi: 10.1158/0008-5472.CAN-10-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agarwal S, Bell CM, Rothbart SB, Moran RG. AMP-activated Protein Kinase (AMPK) Control of mTORC1 Is p53- and TSC2-independent in Pemetrexed-treated Carcinoma Cells. J Biol Chem. 2015;290(46):27473–27486. doi: 10.1074/jbc.M115.665133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beardsley GP, Moroson BA, Taylor EC, Moran RG. A new folate antimetabolite, 5,10-dideaza-5,6,7,8-tetrahydrofolate is a potent inhibitor of de novo purine synthesis. J Biol Chem. 1989;264(1):328–333. [PubMed] [Google Scholar]
- 53.Mendelsohn LG, Worzalla JF, Walling JM. Preclinical and clinical evaluation of the glycinamide ribonucleotide formyltransferase inhibitors lometrexol and LY309887. In: Jackman AL, editor. Anticancer Drug Development Guide: Antifolate Drugs in Cancer Therapy. Humana Press, Inc; Totowa, NJ: 1999. pp. 261–280. [Google Scholar]
- 54.Moran RG, Baldwin SW, Taylor EC, Shih C. The 6S- and 6R-diastereomers of 5, 10-dideaza-5, 6, 7, 8-tetrahydrofolate are equiactive inhibitors of de novo purine synthesis. J Biol Chem. 1989;264(35):21047–21051. [PubMed] [Google Scholar]
- 55.Ray MS, Muggia FM, Leichman CG, Grunberg SM, Nelson RL, Dyke RW, Moran RG. Phase I study of (6R)-5,10-dideazatetrahydrofolate: a folate antimetabolite inhibitory to de novo purine synthesis. J Natl Cancer Inst. 1993;85(14):1154–1159. doi: 10.1093/jnci/85.14.1154. [DOI] [PubMed] [Google Scholar]
- 56.Boritzki TJ, Barlett CA, Zhang C, Howland EF. AG2034: a novel inhibitor of glycinamide ribonucleotide formyltransferase. Investigational new drugs. 1996;14(3):295–303. doi: 10.1007/BF00194533. [DOI] [PubMed] [Google Scholar]
- 57.Bissett D, McLeod HL, Sheedy B, Collier M, Pithavala Y, Paradiso L, Pitsiladis M, Cassidy J. Phase I dose-escalation and pharmacokinetic study of a novel folate analogue AG2034. Br J Cancer. 2001;84(3):308–312. doi: 10.1054/bjoc.2000.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Budman DR, Johnson R, Barile B, Bowsher RR, Vinciguerra V, Allen SL, Kolitz J, Ernest CS, 2nd, Kreis W, Zervos P, Walling J. Phase I and pharmacokinetic study of LY309887: a specific inhibitor of purine biosynthesis. Cancer Chemother Pharmacol. 2001;47(6):525–531. doi: 10.1007/s002800000272. [DOI] [PubMed] [Google Scholar]
- 59.Bronder JL, Moran RG. Antifolates targeting purine synthesis allow entry of tumor cells into S phase regardless of p53 function. Cancer Res. 2002;62(18):5236–5241. [PubMed] [Google Scholar]
- 60.Bronder JL, Moran RG. A defect in the p53 response pathway induced by de novo purine synthesis inhibition. J Biol Chem. 2003;278(49):48861–48871. doi: 10.1074/jbc.M304844200. [DOI] [PubMed] [Google Scholar]
- 61.Hori H, Tran P, Carrera CJ, Hori Y, Rosenbach MD, Carson DA, Nobori T. Methylthioadenosine phosphorylase cDNA transfection alters sensitivity to depletion of purine and methionine in A549 lung cancer cells. Cancer Res. 1996;56(24):5653–5658. [PubMed] [Google Scholar]
- 62.Lubin M, Lubin A. Selective killing of tumors deficient in methylthioadenosine phosphorylase: a novel strategy. PLoS One. 2009;4(5):e5735. doi: 10.1371/journal.pone.0005735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao R, Goldman ID. Resistance to antifolates. Oncogene. 2003;22(47):7431–7457. doi: 10.1038/sj.onc.1206946. [DOI] [PubMed] [Google Scholar]
- 64.Gonen N, Assaraf YG. Antifolates in cancer therapy: structure, activity and mechanisms of drug resistance. Drug Resist Updat. 2012;15(4):183–210. doi: 10.1016/j.drup.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 65.Matherly LH, Angeles SM, McGuire JJ. Determinants of the disparate antitumor activities of (6R)-5,10-dideaza-5,6,7,8-tetrahydrofolate and methotrexate toward human lymphoblastic leukemia cells, characterized by severely impaired antifolate membrane transport. Biochem Pharmacol. 1993;46(12):2185–2195. doi: 10.1016/0006-2952(93)90608-y. [DOI] [PubMed] [Google Scholar]
- 66.Qiu A, Min SH, Jansen M, Malhotra U, Tsai E, Cabelof DC, Matherly LH, Zhao R, Akabas MH, Goldman ID. Rodent intestinal folate transporters (SLC46A1): secondary structure, functional properties, and response to dietary folate restriction. American journal of physiology Cell physiology. 2007;293(5):C1669–1678. doi: 10.1152/ajpcell.00202.2007. [DOI] [PubMed] [Google Scholar]
- 67.Inoue K, Nakai Y, Ueda S, Kamigaso S, Ohta KY, Hatakeyama M, Hayashi Y, Otagiri M, Yuasa H. Functional characterization of PCFT/HCP1 as the molecular entity of the carrier-mediated intestinal folate transport system in the rat model. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G660–668. doi: 10.1152/ajpgi.00309.2007. 00309.2007 [pii] [DOI] [PubMed] [Google Scholar]
- 68.Wollack JB, Makori B, Ahlawat S, Koneru R, Picinich SC, Smith A, Goldman ID, Qiu A, Cole PD, Glod J, Kamen B. Characterization of folate uptake by choroid plexus epithelial cells in a rat primary culture model. J Neurochem. 2008;104(6):1494–1503. doi: 10.1111/j.1471-4159.2007.05095.x. JNC5095 [pii] [DOI] [PubMed] [Google Scholar]
- 69.Unal ES, Zhao R, Qiu A, Goldman ID. N-linked glycosylation and its impact on the electrophoretic mobility and function of the human proton-coupled folate transporter (HsPCFT) Biochim Biophys Acta. 2008;1778(6):1407–1414. doi: 10.1016/j.bbamem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giovannetti EZP, Assaraf YG, Funel N, Gemelli M, Stark M, Thunnissen E, Hou Z, Muller IB, Perrino M, Jansen G, Matherly LH, Peters GJ. Role of proton-coupled folate transporter in pemetrexed-resistance of mesothelioma: clinical evidence and new pharmacological tools. Ann Oncol. 2017 doi: 10.1093/annonc/mdx499. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Advanced drug delivery reviews. 2004;56(8):1067–1084. doi: 10.1016/j.addr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 72.Zhao R, Goldman ID. Folate and thiamine transporters mediated by facilitative carriers (SLC19A1-3 and SLC46A1) and folate receptors. Mol Aspects Med. 2013;34(2–3):373–385. doi: 10.1016/j.mam.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Assaraf YG, Leamon CP, Reddy JA. The folate receptor as a rational therapeutic target for personalized cancer treatment. Drug Resist Updat. 2014;17(4–6):89–95. doi: 10.1016/j.drup.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 74.Gibbs DD, Theti DS, Wood N, Green M, Raynaud F, Valenti M, Forster MD, Mitchell F, Bavetsias V, Henderson E, Jackman AL. BGC 945, a novel tumor-selective thymidylate synthase inhibitor targeted to alpha-folate receptor-overexpressing tumors. Cancer Res. 2005;65(24):11721–11728. doi: 10.1158/0008-5472.CAN-05-2034. [DOI] [PubMed] [Google Scholar]
- 75.Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC, Frazer DM, Anderson GJ, Vulpe CD, Simpson RJ, McKie AT. Identification of an intestinal heme transporter. Cell. 2005;122(5):789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 76.Hou Z, Matherly LH. Biology of the Major Facilitative Folate Transporters SLC19A1 and SLC46A1. Current topics in membranes. 2014;73:175–204. doi: 10.1016/B978-0-12-800223-0.00004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hou Z, Kugel Desmoulin S, Etnyre E, Olive M, Hsiung B, Cherian C, Wloszczynski PA, Moin K, Matherly LH. Identification and functional impact of homo-oligomers of the human proton-coupled folate transporter. J Biol Chem. 2012;287(7):4982–4995. doi: 10.1074/jbc.M111.306860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilson MR, Kugel S, Huang J, Wilson LJ, Wloszczynski PA, Ye J, Matherly LH, Hou Z. Structural determinants of human proton-coupled folate transporter oligomerization: role of GXXXG motifs and identification of oligomeric interfaces at transmembrane domains 3 and 6. Biochem J. 2015;469(1):33–44. doi: 10.1042/BJ20150169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chattopadhyay S, Tamari R, Min SH, Zhao R, Tsai E, Goldman ID. Commentary: a case for minimizing folate supplementation in clinical regimens with pemetrexed based on the marked sensitivity of the drug to folate availability. Oncologist. 2007;12(7):808–815. doi: 10.1634/theoncologist.12-7-808. [DOI] [PubMed] [Google Scholar]
- 80.Deng Y, Zhou X, Kugel Desmoulin S, Wu J, Cherian C, Hou Z, Matherly LH, Gangjee A. Synthesis and biological activity of a novel series of 6-substituted thieno[2,3-d]pyrimidine antifolate inhibitors of purine biosynthesis with selectivity for high affinity folate receptors over the reduced folate carrier and proton-coupled folate transporter for cellular entry. J Med Chem. 2009;52(9):2940–2951. doi: 10.1021/jm8011323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gallagher FA, Kettunen MI, Day SE, Hu DE, Ardenkjaer-Larsen JH, Zandt R, Jensen PR, Karlsson M, Golman K, Lerche MH, Brindle KM. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature. 2008;453(7197):940–943. doi: 10.1038/nature07017. [DOI] [PubMed] [Google Scholar]
- 82.Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nature reviews Cancer. 2011;11(9):671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 83.Mitchell-Ryan S, Wang Y, Raghavan S, Ravindra MP, Hales E, Orr S, Cherian C, Hou Z, Matherly LH, Gangjee A. Discovery of 5-Substituted Pyrrolo[2,3-d]pyrimidine Antifolates as Dual-Acting Inhibitors of Glycinamide Ribonucleotide Formyltransferase and 5-Aminoimidazole-4-carboxamide Ribonucleotide Formyltransferase in De Novo Purine Nucleotide Biosynthesis: Implications of Inhibiting 5-Aminoimidazole-4-carboxamide Ribonucleotide Formyltransferase to AMPK Activation and Antitumor Activity. J Med Chem. 2013;56(24):10016–10032. doi: 10.1021/jm401328u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gangjee A, Zeng Y, McGuire JJ, Kisliuk RL. Synthesis of classical, four-carbon bridged 5-substituted furo[2,3-d]pyrimidine and 6-substituted pyrrolo[2,3-d]pyrimidine analogues as antifolates. J Med Chem. 2005;48(16):5329–5336. doi: 10.1021/jm058213s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gangjee A, Zeng Y, McGuire JJ, Mehraein F, Kisliuk RL. Synthesis of classical, three-carbon-bridged 5-substituted furo[2,3-d]pyrimidine and 6-substituted pyrrolo[2,3-d]pyrimidine analogues as antifolates. J Med Chem. 2004;47(27):6893–6901. doi: 10.1021/jm040123k. [DOI] [PubMed] [Google Scholar]
- 86.Deng Y, Wang Y, Cherian C, Hou Z, Buck SA, Matherly LH, Gangjee A. Synthesis and discovery of high affinity folate receptor-specific glycinamide ribonucleotide formyltransferase inhibitors with antitumor activity. J Med Chem. 2008;51(16):5052–5063. doi: 10.1021/jm8003366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kugel Desmoulin S, Wang L, Polin L, White K, Kushner J, Stout M, Hou Z, Cherian C, Gangjee A, Matherly LH. Functional loss of the reduced folate carrier enhances the antitumor activities of novel antifolates with selective uptake by the proton-coupled folate transporter. Mol Pharmacol. 2012;82(4):591–600. doi: 10.1124/mol.112.079004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deis SM, Doshi A, Hou Z, Matherly LH, Gangjee A, Dann CE., 3rd Structural and enzymatic analysis of tumor-targeted antifolates that inhibit glycinamide ribonucleotide formyltransferase. Biochemistry. 2016;55(32):4574–4582. doi: 10.1021/acs.biochem.6b00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gates SB, Worzalla JF, Shih C, Grindey GB, Mendelsohn LG. Dietary folate and folylpolyglutamate synthetase activity in normal and neoplastic murine tissues and human tumor xenografts. Biochem Pharmacol. 1996;52(9):1477–1479. doi: 10.1016/s0006-2952(96)00554-0. [DOI] [PubMed] [Google Scholar]
- 90.Ifergan I, Jansen G, Assaraf YG. Cytoplasmic confinement of breast cancer resistance protein (BCRP/ABCG2) as a novel mechanism of adaptation to short-term folate deprivation. Mol Pharmacol. 2005;67(4):1349–1359. doi: 10.1124/mol.104.008250. [DOI] [PubMed] [Google Scholar]
- 91.Howell SB, Mansfield SJ, Taetle R. Thymidine and hypoxanthine requirements of normal and malignant human cells for protection against methotrexate cytotoxicity. Cancer Res. 1981;41(3):945–950. [PubMed] [Google Scholar]
- 92.Jackson RC, Harkrader RJ. The contributions of de-novo and salvage pathways of nucleotide biosynthesis in normal and malignant cells. In: Tattersall MHN, Fox RM, editors. Nucleosides and Cancer Treatment. Academic Press; Sydney: 1981. pp. 18–31. [Google Scholar]
- 93.Issaeva N, Thomas HD, Djureinovic T, Jaspers JE, Stoimenov I, Kyle S, Pedley N, Gottipati P, Zur R, Sleeth K, Chatzakos V, Mulligan EA, Lundin C, Gubanova E, Kersbergen A, Harris AL, Sharma RA, Rottenberg S, Curtin NJ, Helleday T. 6-thioguanine selectively kills BRCA2-defective tumors and overcomes PARP inhibitor resistance. Cancer Res. 2010;70(15):6268–6276. doi: 10.1158/0008-5472.CAN-09-3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4(11):891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 95.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 96.Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008;49(Suppl 2):24S–42S. doi: 10.2967/jnumed.107.047258. [DOI] [PubMed] [Google Scholar]
- 97.Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nat Rev Cancer. 2008;8(1):56–61. doi: 10.1038/nrc2255. [DOI] [PubMed] [Google Scholar]
- 98.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chiche J, Ilc K, Laferriere J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouyssegur J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69(1):358–368. doi: 10.1158/0008-5472.CAN-08-2470. [DOI] [PubMed] [Google Scholar]
- 100.Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, Renner K, Timischl B, Mackensen A, Kunz-Schughart L, Andreesen R, Krause SW, Kreutz M. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109(9):3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 101.Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A, Kreutz M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107(5):2013–2021. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]
- 102.Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL Tumour Angiogenesis Research G. Lactate dehydrogenase 5 expression in operable colorectal cancer: strong association with survival and activated vascular endothelial growth factor pathway--a report of the Tumour Angiogenesis Research Group. J Clin Oncol. 2006;24(26):4301–4308. doi: 10.1200/JCO.2006.05.9501. [DOI] [PubMed] [Google Scholar]
- 103.Chang Q, Jurisica I, Do T, Hedley DW. Hypoxia predicts aggressive growth and spontaneous metastasis formation from orthotopically grown primary xenografts of human pancreatic cancer. Cancer Res. 2011;71(8):3110–3120. doi: 10.1158/0008-5472.CAN-10-4049. [DOI] [PubMed] [Google Scholar]
- 104.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3(4):347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 105.Yotnda P, Wu D, Swanson AM. Hypoxic tumors and their effect on immune cells and cancer therapy. Methods Mol Biol. 2010;651:1–29. doi: 10.1007/978-1-60761-786-0_1. [DOI] [PubMed] [Google Scholar]
- 106.Durand RE. The influence of microenvironmental factors during cancer therapy. In Vivo. 1994;8(5):691–702. [PubMed] [Google Scholar]
- 107.Tannock IF. Conventional cancer therapy: promise broken or promise delayed? Lancet. 1998;351(Suppl 2):SII9–16. doi: 10.1016/s0140-6736(98)90327-0. [DOI] [PubMed] [Google Scholar]
- 108.Tannock IF. The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br J Cancer. 1968;22(2):258–273. doi: 10.1038/bjc.1968.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379(6560):88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 110.Yuan J, Glazer PM. Mutagenesis induced by the tumor microenvironment. Mutat Res. 1998;400(1–2):439–446. doi: 10.1016/s0027-5107(98)00042-6. [DOI] [PubMed] [Google Scholar]
- 111.Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1(6):401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 112.Vaupel P, Hockel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxidants & redox signaling. 2007;9(8):1221–1235. doi: 10.1089/ars.2007.1628. [DOI] [PubMed] [Google Scholar]
- 113.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2(1):38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 114.Rofstad EK. Microenvironment-induced cancer metastasis. Int J Radiat Biol. 2000;76(5):589–605. doi: 10.1080/095530000138259. [DOI] [PubMed] [Google Scholar]
- 115.Blancher C, Harris AL. The molecular basis of the hypoxia response pathway: tumour hypoxia as a therapy target. Cancer Metastasis Rev. 1998;17(2):187–194. doi: 10.1023/a:1006002419244. [DOI] [PubMed] [Google Scholar]
- 116.Semenza GL. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol. 2000;35(2):71–103. doi: 10.1080/10409230091169186. [DOI] [PubMed] [Google Scholar]
- 117.Wang Y, Ohh M. Oxygen-mediated endocytosis in cancer. J Cell Mol Med. 2010;14(3):496–503. doi: 10.1111/j.1582-4934.2010.01016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8(3):180–192. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 119.Raz S, Sheban D, Gonen N, Stark M, Berman B, Assaraf YG. Severe hypoxia induces complete antifolate resistance in carcinoma cells due to cell cycle arrest. Cell death & disease. 2014;5:e1067. doi: 10.1038/cddis.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McKeown SR. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br J Radiol. 2014;87(1035):20130676. doi: 10.1259/bjr.20130676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brown JM. Tumor hypoxia in cancer therapy. Methods Enzymol. 2007;435:297–321. doi: 10.1016/S0076-6879(07)35015-5. [DOI] [PubMed] [Google Scholar]
- 122.Kimura H, Braun RD, Ong ET, Hsu R, Secomb TW, Papahadjopoulos D, Hong K, Dewhirst MW. Fluctuations in red cell flux in tumor microvessels can lead to transient hypoxia and reoxygenation in tumor parenchyma. Cancer Res. 1996;56(23):5522–5528. [PubMed] [Google Scholar]
- 123.Brurberg KG, Thuen M, Ruud EB, Rofstad EK. Fluctuations in pO2 in irradiated human melanoma xenografts. Radiat Res. 2006;165(1):16–25. doi: 10.1667/rr3491.1. [DOI] [PubMed] [Google Scholar]
- 124.Lohse I, Rasowski J, Cao P, Pintilie M, Do T, Tsao MS, Hill RP, Hedley DW. Targeting hypoxic microenvironment of pancreatic xenografts with the hypoxia-activated prodrug TH-302. Oncotarget. 2016;7(23):33571–33580. doi: 10.18632/oncotarget.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun JD, Liu Q, Ahluwalia D, Li W, Meng F, Wang Y, Bhupathi D, Ruprell AS, Hart CP. Efficacy and safety of the hypoxia-activated prodrug TH-302 in combination with gemcitabine and nab-paclitaxel in human tumor xenograft models of pancreatic cancer. Cancer Biol Ther. 2015;16(3):438–449. doi: 10.1080/15384047.2014.1003005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 127.Jain M, Nilsson R, Sharma S, Madhusudhan N, Kitami T, Souza AL, Kafri R, Kirschner MW, Clish CB, Mootha VK. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336(6084):1040–1044. doi: 10.1126/science.1218595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim D, Fiske BP, Birsoy K, Freinkman E, Kami K, Possemato RL, Chudnovsky Y, Pacold ME, Chen WW, Cantor JR, Shelton LM, Gui DY, Kwon M, Ramkissoon SH, Ligon KL, Kang SW, Snuderl M, Vander Heiden MG, Sabatini DM. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature. 2015;520(7547):363–367. doi: 10.1038/nature14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee GY, Haverty PM, Li L, Kljavin NM, Bourgon R, Lee J, Stern H, Modrusan Z, Seshagiri S, Zhang Z, Davis D, Stokoe D, Settleman J, de Sauvage FJ, Neve RM. Comparative oncogenomics identifies PSMB4 and SHMT2 as potential cancer driver genes. Cancer Res. 2014;74(11):3114–3126. doi: 10.1158/0008-5472.CAN-13-2683. [DOI] [PubMed] [Google Scholar]