Abstract
The mouse strain SKH1 is widely used in skin research due to its hairless phenotype and intact immune system. Due to the complex nature of aryl hydrocarbon receptor (AHR) function in the skin, the development of additional in vivo models is necessary to study its role in cutaneous homeostasis and pathology. Variants of the Ah allele, exist among different mouse strains. The Ahb-1 and Ahd alleles express high and low affinity ligand binding forms of the AHR, respectively. The outbred SKH1 mice express the Ahb-2 and/or Ahd alleles. SKH1 mice were crossed with C57BL/6J mice, which harbor the Ahb-1 allele, to create useful models for studying endogenous AHR function. SKH1 mice were bred to be homozygous for either the Ahb-1 or Ahd allele to establish strains for use in comparative studies of the effects of differential ligand-mediated activation through gene expression changes upon UVB exposure. Ahb-1 or Ahd allelic status was confirmed by DNA sequence analysis. We tested the hypothesis that SKH1-Ahb-1 mice would display enhanced inflammatory signaling upon UVB exposure compared to SKH1-Ahd mice. Differential basal AHR activation between the strains was determined by assessing Cyp1a1 expression levels in the small intestine, liver, and skin of the SKH1-Ahb-1 mice compared to SKH1-Ahd mice. To determine whether SKH1-Ahb-1 mice are more prone to a pro-inflammatory phenotype in response to UVB, gene expression of inflammatory mediators was analyzed. SKH1-Ahb-1 mice expressed enhanced gene expression of the chemotactic factors Cxcl5, Cxcl1, and Ccl20, as well as the inflammatory signaling factors S100a9 and Ptgs2, compared to SKH1-Ahd mice in skin. These data supports a role for AHR activation and enhanced inflammatory signaling in skin.
Keywords: Ah receptor, AHR, UVB, SKH1
1. Introduction
The aryl hydrocarbon receptor (AHR) is the only member of the family of bHLH/PAS (basic Helix-Loop-Helix/Per-Arnt-Sim) transcription factors that is activated by low molecular weight ligands. The primary pathway through which AHR influence gene expression involves translocation of the AHR from the cytoplasm to the nucleus upon ligand binding, followed by heterodimerization with the aryl hydrocarbon receptor nuclear translocator (ARNT), which is required for DNA binding to dioxin response elements (DREs) (Beischlag et al. 2008). The AHR was initially identified as a low abundance protein that bound 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) with high affinity and subsequently determined to be the principal mediator of TCDD and other halogenated aromatic hydrocarbon-induced toxicities. Importantly, a number of physiologically relevant endogenous and dietary AHR ligands have been identified, with many of them representing tryptophan derivatives (e.g. indoxyl sulfate, kynurenine).(Hubbard et al. 2015) There is considerable interest in the diverse physiological functions of the AHR, including the modulation of innate and adaptive immunity (Stockinger et al. 2014). Additionally, recent studies in skin demonstrate important roles for the AHR in modulating keratinocyte differentiation and establishing barrier function (Haas et al. 2016; Magiatis et al. 2013; Sutter et al. 2011; van den Bogaard et al. 2013). The development and availability of various agonists, antagonists, and selective modulators of the AHR, in conjunction with the influence of AHR upon skin biology, suggests the AHR may represent a viable therapeutic target for skin disorders (Tigges et al. 2014; van den Bogaard et al. 2015).
Due to ease of topical application and observation of skin phenotypes, the mouse strain SKH1 (Crl: SKH1-hr) is the most common hairless line used in dermatological research, including UV exposure, wound healing and carcinogenesis studies. The hairless, unpigmented phenotype of this strain is a consequence of an autosomal recessive mutation in the hairless (Hr) gene, which encodes a transcriptional co-repressor of thyroid, vitamin D and retinoic acid-related receptor-mediated gene expression. However, despite its hairless and immune competent phenotype, the SKH1 strain suffers from an ill-defined outbred genetic background, which complicates a mechanistic assessment of its biology (Benavides et al. 2009). Hairless mouse strains, including the HRS/J, have been utilized to examine outcomes (e.g. chloracne and tumor promotion) associated with toxicological exposure to potent environmental AHR ligands such as TCDD (Knutson and Poland 1982; Poland et al. 1984; Puhvel et al. 1982; Vos et al. 1982),(Poland et al. 1982). . In contrast, hairless mice have not been used to assess the impact of AHR allelic variants upon UV-mediated inflammatory responses.
Four alleles encoding different forms of the AHR are known to exist among laboratory mouse strains. The Ahb-1, Ahb-2 and Ahb-3 alleles encode an AHR which binds aromatic hydrocarbons with high affinity, while the Ahd allele encodes an AHR with low-affinity binding. The difference in ligand-binding affinity between the high and low affinity-binding alleles was determined to be due to the substitution of a valine residue in the Ahd allele for the alanine found in all of the Ahb allele forms (Poland et al. 1994). Thus, experimental comparison of mouse strains expressing the high- and low-affinity binding alleles of the AHR are useful for determining whether AHR-mediated effects are dependent on ligand-induced functions of the receptor. The human AHR exhibits high affinity for a number of endogenous AHR ligands and thus the Ahb-1 mice are the most relevant from the formation of AHR ligands during UV exposure viewpoint (Hubbard et al. 2015).
Preliminary studies in our laboratory indicate that the wild-type SKH1 strain expresses an AHR species with an apparent molecular weight greater than the high affinity Ahb-1 allele expressed by inbred C57Bl/6J mice, historically the predominant strain utilized to examine AHR-mediated toxicity and physiology (Poland and Glover 1990). These data imply that SKH1 mice harbor either one of the Ahb-2, Ahb-3 or Ahd allelic variants, all of which exhibit similar molecular weights. Therefore, we proceeded to develop SKH1 hairless mouse models harboring the extensively characterized high and low affinity alleles, Ahb-1 or Ahd respectively, to effectively examine the potential role of AHR in UVB-mediated inflammatory responses in skin. The generation of such models will allow a further determination of the importance of endogenous AHR ligand-mediated signaling in skin disorders including atopic dermatitis, psoriasis, and UV-mediated or chemical carcinogenesis, where the AHR is reported to contribute to their etiology and pathology.
2. Material and methods
2.1. Animals and husbandry
All mouse lines were bred in-house after acquisition. C57BL/6J mice and Ahd congenic mice on a C57BL/6J background were originally purchased from the Jackson Laboratory (Bar Harbor, ME). Wild-type SKH1 mice were a kind gift from Dr. Jeffrey M. Peters (The Pennsylvania State University). Animals were housed in specific pathogen-free conditions under a 12 h light/dark cycle with ad libitum access to standard chow and water in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University. All mouse experiments were repeated twice to ensure reproducibility.
2.2. Genotyping of hairless mouse strains
SKH1-Ahb-1 mice were generated in house by mating wild-type SKH1 mice with C57Bl/6J mice. Resulting heterozygous pups were mated with wild-type SKH1 mice for five generations to select for a hairless phenotype. Heterozygote F5 littermates were mated, and resulting SKH1-Ahb-1 homozygotes were selected and mated, followed by colony expansion. Mice were selected for breeding pairs based on Western blot analysis for AHR and PCR based genotyping using the following primers: OL4064: 5′ –CAGTGGGAATAAGGCAAGAGTGA –3′ and OL4066: 5′– AGGGAGATGAAGTATGTGTATGTA– 3′, which dependent on the allelic form of the AHR yield a different product size.
2.3. Sequencing of hairless mouse strains
RNA was isolated from livers and converted to cDNA to be used for Ah allele determination. Liver samples from the BALB/c mice were a kind gift from Dr. Robert Paulson (The Pennsylvania State University). cDNA was further amplified through additional PCR cycles using the following primer set: F-5' - AAGAAAGGGAAGGACGGAGC-3' and R-5' - GGCTTGAAGGAGGACACAGA-3'. Sequencing of DNA from all strains of mice was performed at the Penn State Genomics Core Facility (University Park, PA), using the primers listed above. Sequencing results were translated using the online ExPASy Translate Tool (http://web.expasy.org/translate/) (Swiss Institute of Bioinformatics) and compared to amino acid sequences for Ahb-1, Ahb-2, Ahb-3, and Ahd mice previously described (Poland et al. 1994).
2.4. Topical AHR ligand exposure
Age-matched male mice were topically exposed to 20% DMSO in acetone (100 µl) on the top of the left ear and 100 ng of the AHR agonist formylindolo(3,2-b)carbazole in acetone (FICZ) (Enzo Life Sciences, Farmingdale, NY) on the top of the right ear. The treatments were reapplied every 8 h for a total of 3 applications. Mice were sacrificed by CO2 asphyxiation 24 h after the initial dose and their ears were dissected, snap-frozen in liquid nitrogen and stored at −80°C until further analysis.
2.5. UV irradiation time course
Age-matched male mice were anesthetized through i.p. administration of avertin prior to irradiation to immobilize each mouse to guarantee that each receives the same UV dose. The mice were subsequently exposed to UV with a dose of 3.6 kJ/m2 from UV bulbs (American Ultraviolet Light Co., Lebanon, IN) covered with cellulose triacetate membrane to allow only UV wavelengths between 280 and 320 nm. Output of the UV bulbs was measured using a UVX radiometer (UVP, Upland, CA). Mice were sacrificed by CO2 asphyxiation at 6, 12, 24, or 48 h after initial UV exposure. Full thickness dorsal skin samples were dissected, snap-frozen in liquid nitrogen, and stored at −80°C until further analysis.
2.6. RNA isolation and quantitative reverse transcription PCR
Total RNA was isolated from tissue samples using TRI Reagent (Sigma-Aldrich, St. Louis, MO), and then converted to cDNA using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Thermofisher). Quantitative real-time PCR was conducted using PerfeCTa SYBR Green Supermix for iQ (Quanta Biosciences, Beverly, MA) on a CFX Connect Real-Time PCR Detection System (Bio-Rad, Hercules, CA). Primers used for real-time PCR are listed in Table S1. Ribosomal protein, large, P0 (Rplp0) was used to normalize gene expression.
2.7. Western blot analysis
Skin samples were excised for protein analysis and homogenized in MENG buffer (20 mM MOPS, 2 mM EDTA, 0.02% sodium azide, 10% Glycerol, pH 7.4), supplemented with 20 mM molybdate, 500 mM NaCl, and cOmplete Mini protease inhibitors cocktail (Roche Diagnostics, Mannheim, Germany). Samples were resolved by 8% tricine-SDS-PAGE, and transferred to polyvinylidene difluoride membrane. Rpt1 antibody was used to probe for AHR (Perdew et al. 1995) and B-actin was detected with B-actin (C4) antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Biotin-conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA) and 125I-streptavidin were used for detection and radioactivity was visualized using BioMax film. 125I-streptavidin was prepared as described previously (Narayanan et al. 2012).
2.8. Statistical analyses
GraphPad Prism 5 software was used to conduct all statistical analyses. Data were analyzed using unpaired, two-tailed Student’s t-test unless otherwise indicated in the figure legend.
3. Results
3.1. Outbred SKH1 mice exhibit a mixed genetic background expressing the Ahd and/or Ahb-2 alleles
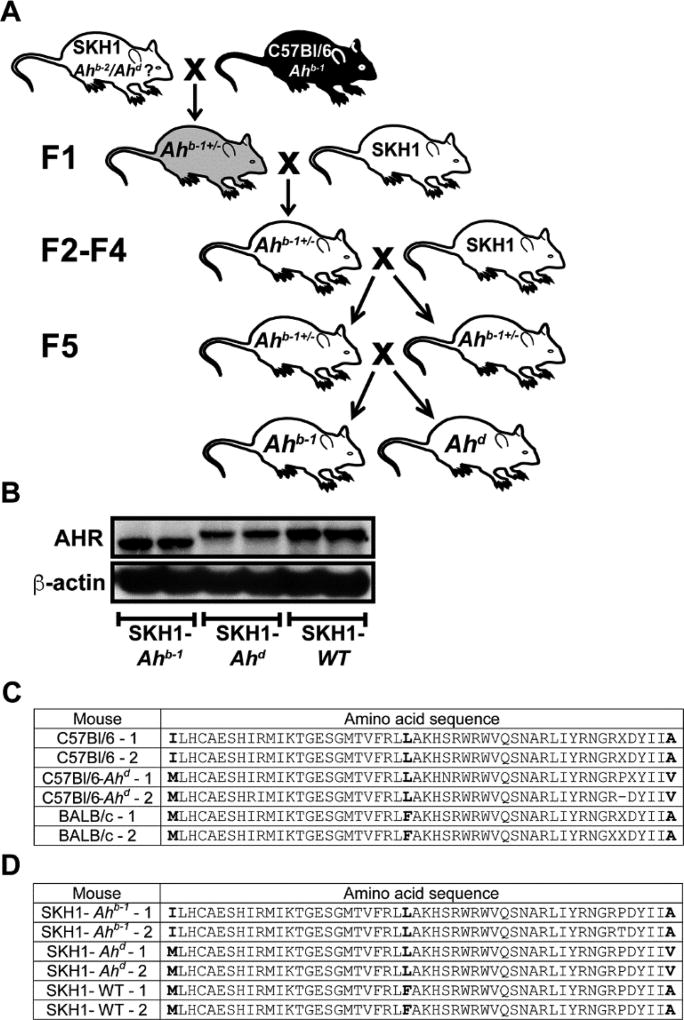
Inbred C57BL/6J mice, harboring the Ahb-1 allele, and outcrossed SKH1 mice were crossbred to generate a mouse strain expressing the high affinity Ahb-1 allele on a hairless, immunocompetent background. Heterozygous littermates from the F5 generation were mated and homozygote Ahb-1 allele offspring were selected and bred to colony status. Mice homozygous for the Ahd allele found in the SKH1 mice were also selected and bred to colony status to use as a strain that would exhibit reduced AHR activation potential (Fig. 1A). Due to the outbred status of the SKH1 mice, we will refer to the SKH1 mice expressing the Ahb-1 allele as SKH1-Ahb-1 mice, and the SKH1 mice expressing the Ahd allele as SKH1-Ahd mice. Ah allelic status did not impact overt cutaneous phenotype, as no gross pathological conditions were observed, and all strains exhibited similar levels of health and breeding capabilities.
Fig. 1.
Selective crossbreeding of C57Bl/6J and SKH1 mice results in hairless mice expressing either the Ahb-1 or Ahd allele. A, Schematic diagram depicting the breeding protocol used to produce mouse strains expressing either the Ahb-1 or Ahd allele on an SKH1 background. B, Western blot analysis of AHR in the skin of SKH1-Ahb-1, SKH1-Ahd, and SKH1-WT mice. (n=2 mice/genotype). C, Table of the amino acid sequence of the AHR between amino acid residues 324–375 of three strains of mice. D, Amino acid translation derived from cDNA sequencing of the variable region in the AHR in three established SKH1 mouse lines. Point mutations are highlighted with bold type. Because the SKH1 outcrossed strain carries two alleles of the Ahr, the WT SKH1 mice sequenced here were homozygous for the Ahb-2 allele.
Western blot analysis of AHR expression in the skin of the generated hairless mouse strains demonstrated that the hairless mice selectively bred to express the Ahb-1 allele express an AHR species with a lower relative molecular weight than that expressed by the selectively bred controls. The SKH1-Ahd line was characterized as expressing an AHR species with a relative molecular weight similar to the original outbred SKH1 founder line used to establish these strains (hereafter referred to as SKH1 wild-type (WT) mice) (Fig. 1B). It is important to note that the level of AHR expression is lower in the SKH1-Ahd mouse line. These observations are thus consistent with previous studies characterizing the Ahb-1 allelic variant as a 95 kDa protein, whereas the other Ah alleles encode higher molecular weight species; the Ahd and Ahb-2 allelic variants each encode a 104 kDa protein, while the Ahb-3 allelic variant encodes a 105 kDa protein (Poland and Glover 1990).
To definitively establish the Ah allelic status of the derived SKH1-WT, SKH1-Ahb-1 and SKH1-Ahd lines, DNA sequencing analyses were performed upon cDNA derived from liver RNA isolated from two mice of each line along with established inbred mouse strains known to harbor defined Ah alleles [C57BL/6J mice (Ahb-1), C57BL/6J mice congenic for the Ahd allele, and BALB/c mice (Ahb-2)]. Sequence data for each line were translated to amino acid sequences and compared to those previously established for each allelic variant of AHR.(Poland et al. 1994) Analysis of the SKH1-Ahb-1 and defined C57BL/6J lines identified the presence of amino acid residues I324, L348 and A375, consistent with the reported Ahb-1 sequence, thus confirming establishment of the high affinity SKH1-Ahb-1 line. Analysis of the SKH1-Ahd and congenic C57BL/6J-Ahd lines identified the presence of amino acid residues M324, L348 and V375, consistent with the reported Ahd sequence, thus confirming the establishment of the reduced affinity SKH1-Ahd line. Analysis of the parental SKH1 WT and BALB/c lines identified the presence of amino acid residues M324, F348 and A375, consistent with the reported Ahb-2 sequence, thus confirming that the parental line harbors the Ahb-2 allelic variant (Fig. 1C, D). The generation of SKH1-Ahb-1 and SKH1-Ahd allelic variants thus facilitated an examination of AHR function within the context of a hairless and immune-competent phenotype.
3.2. SKH1-Ahb-1 and SKH1-Ahd mice exhibit differential basal Cyp1a1 expression within the small intestine, liver, and skin
With the notable exception of (3Z)-3-[(3,5-dimethyl-1H-pyrrol-2-yl) methylidene]-1,3-dihydro-2H-indol-2-one (SU5416), the AHRb-1 and AHRd allelic variants appear to exhibit differential ligand binding capacity at least with regard to certain AHR ligands, such as TCDD or benzo(a)pyrene (Mezrich et al. 2012). The ability of the AHRb-1 to bind ligands with higher affinity relative to the AHRd may lead to elevated basal expression of AHR gene targets. To investigate this possibility, the basal expression of the prototypical AHR target gene, Cyp1a1 was examined in the SKH1-Ahb-1 and SKH1-Ahd lines (Fig. 2A). Data obtained reveal a significant 3-fold reduction in basal Cyp1a1 within ileal tissue of SKH1-Ahd mice, compared to SKH1-Ahb-1 mice. Reduced basal Cyp1a1 expression within duodenal tissue was similarly observed in SKH1-Ahd compared to SKH1-Ahb-1, although this proved to be insignificant. In contrast to gastrointestinal tissue, basal Cyp1a1 expression within liver and skin were significantly elevated in SKH1-Ahd compared to SKH1-Ahb-1 mice. These data suggest differential levels of basal AHR activity within the SKH1-Ahb-1 and SKH1-Ahd lines.
Fig. 2.
Basal and induced levels of Cyp1a1 differ in SKH1-Ahb-1 and SKH1-Ahd hairless mice. A, Duodenum, ileum, liver and skin tissues were collected from 17–18-week old SKH1-Ahb-1 (black) and SKH1-Ahd (white) mice and Cyp1a1 expression levels were assessed by qRT-PCR and normalized to Rplp0. n = 5 mice/genotype. ns = not significant A, 17-week old SKH1-Ahb-1 (black) and SKH1-Ahd (white) mice were topically treated with either DMSO or FICZ in acetone as described in Materials and Methods. B, Cyp1a1 expression was determined by qRT-PCR and normalized to Rplp0. n = 4 mice/genotype. Statistical significance was determined using one-way ANOVA followed by Tukey’s multiple comparison test. Letters indicate compared columns with asterisks indicating statistical significance. Data represent mean ± S.E.M., with p-value ≤ 0.05 (*), and p-value ≤ 0.001 (***).
3.3. SKH1-Ahb-1 mice express elevated levels of Cyp1a1 in the skin upon topical exposure to an AHR agonist
Due to the differences in basal levels of Cyp1a1 expression in the tissues of SKH1-Ahb-1 and SKH1-Ahd mice, we wanted to determine if local exposure to a potent AHR agonist on the skin would lead to differential Cyp1a1 induction. The potent AHR agonist FICZ was topically applied to the ears of mice from both strains and levels of Cyp1a1 induction after exposure were compared. The SKH1-Ahb-1 mice exhibited a 3.3-fold induction of Cyp1a1 levels in the skin after FICZ exposure compared to vehicle, whereas SKH1-Ahd mice exhibited a 1.7-fold induction of Cyp1a1 (Fig. 2B). This result demonstrates that the high affinity allele-expressing SKH1-Ahb-1 mice are more sensitive to FICZ exposure locally.
3.4. SKH1-Ahb-1 mice exhibit enhanced inflammatory gene expression in response to acute UVB exposure
AHR and inflammatory gene expression are known to be enhanced following UV exposure. Evidence suggests that enhanced local and systemic AHR activity is a consequence of UV-mediated photo-oxidation of tryptophan, generating the potent AHR agonist FICZ.(Rannug et al. 1987) Evidence also exists implicating the AHR as a modulator of inflammatory signaling in response to various stimuli, suggesting that AHR activity may play a role in the UV-mediated skin inflammatory response. To examine if the differential basal AHR activity exhibited by SKH1-Ahb-1 and SKH1-Ahd allelic variants contributes to basal and UV-mediated inflammatory signaling within the skin, gene expression analyses were performed (Fig. 3 and 4). S100a9, Cxcl5, and Ptgs2 gene expression is significantly lower in SKH1-Ahd strain; the latter two genes are directly regulated by the AHR. Mice were exposed to UVB and pro-inflammatory gene expression assessed at 6, 12, 24, and 48 h after initial exposure. In order to examine changes in acute inflammatory gene expression, it was necessary to choose a dose of UVB at or above the minimal erythemal dose (MED). Previous studies established a dose of at least 2.24 kJ/m2 for SKH1 mice, so we chose a dose of 3.6 kJ/m2 for a UVB time course study (Ravindran, et al. 2014; Wilgus, et al. 2003). Members of the CXC subfamily of chemokines are important in the initial response of the skin to UVB exposure due to their role in neutrophil chemotaxis to sites of injury or infection. Since AHR was previously shown to regulate Cxcl5 under pro-inflammatory conditions, we wanted to determine if Ah allelic status influenced the expression of this family of chemokines (Tauchi et al. 2005).
Fig. 3.
SKH1-Ahb-1 mice exhibit greater induction of chemokine gene expression after UVB exposure compared to SKH1-Ahd mice. 20–22-week old SKH1-Ahb-1 (black) and SKH1-Ahd (white) mice were sham or UVB irradiated and sacrificed at 6, 12, 24, or 48 h post-UVB exposure (n=4–5 mice/genotype/time point). After sacrifice, back skin was excised, used for RNA isolation, and converted to cDNA. Relative gene expression levels of the chemokines Cxcl5 (A), Cxcl1 (B), Cxcl2 (C), and Ccl20 (D) were determined by qRT-PCR and normalized to the internal control gene Rplp0. Bar graphs depict gene expression levels of sham irradiated mice. ns = not significant. Significance was determined by two-way ANOVA. Data represent mean ± S.E.M., with p-value ≤ 0.01 (**), or p-value ≤ 0.0001 (****).
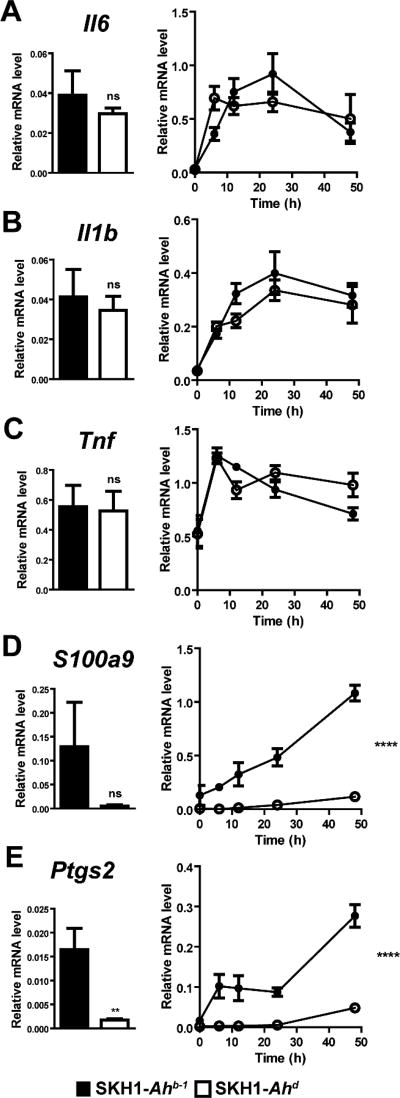
Fig. 4.
SKH1-Ahb-1 mice exhibit greater induction of inflammatory gene expression after UVB exposure compared to SKH1-Ahd mice. 20–22-week old SKH1-Ahb-1 (black) and SKH1-Ahd (white) mice were sham or UVB irradiated and sacrificed at 6, 12, 24, or 48 h post-UVB exposure (n=4–5 mice/genotype/time point). After sacrifice, back skin was excised, used for RNA isolation, and converted to cDNA. Relative gene expression levels of the inflammatory mediators Il6 (A), Il1b (B), Tnf (C), S100a9 (D), and Ptgs2 (E) were determined by qRT-PCR and normalized to the internal control gene Rplp0. Bar graphs depict gene expression levels of sham irradiated mice. Note: At the 6 h and 12 h time points for S100a9 and Ptgs2, n = 2–3 values for SKH1-Ahd mouse results due to lack of amplification of the additional samples. ns = not significant. Significance was determined by two-way ANOVA. Data represent mean ± S.E.M., with p-value ≤ 0.0001 (****).
Acute UVB exposure prompted an increase in expression of the CXC subfamily chemokines Cxcl1, Cxcl2, and Cxcl5 in both strains compared to sham irradiated mice, with Cxcl5 expression achieving a maximum at 48 h while Cxcl1 and Cxcl2 reached maximal expression at 24 h (Fig. 3a–c, right panels). However, SKH1-Ahb-1 mice exhibit a significantly enhanced response to UVB compared to SKH1-Ahd with regard to the magnitude of Cxcl5 and Cxcl1 expression. Following UV exposure Cxcl5 expression increases approximately 12.5-fold at 6 h, 7-fold at 12 h, and 3-fold at both the 24 h and 48 h time points in SKH1-Ahb-1 over SKH1-Ahd mice (Fig. 3A, right panel). In the absence of the inflammatory stimulus provided by UVB, SKH1-Ahb-1 mice exhibit elevated basal expression of Cxcl5, with a 3.5-fold induction compared to SKH1-Ahd mice (Fig. 3A, left panel). UV-mediated induction of Cxcl1 exhibits a similarly differential increase, with approximately 3-fold at 6 h, and 3.5-fold at 12 h and 24 h in SKH1-Ahb-1 mice compared to SKH1-Ahd mice (Fig. 3B, right panel). However, no significant difference in Cxcl2 induction is observed between the two genotypes (Fig. 3C, right panel). Ccl20, a member of the CC subfamily of chemokines, exhibits a 2-fold increase at both the 6 h and 24 h time points in the SKH1-Ahb-1 mice compared to SKH1-Ahd mice (Fig. 3D, right panel). No significant basal difference in Cxcl1, Cxcl2, or Ccl20 is observed between the two strains (Fig. 3B, C, and D, left panels).
The difference observed in chemokine expression levels following UVB exposure led to additional studies comparing the two mouse strains with regard to the expression of additional inflammatory mediators, which influence chemokine regulation. Indeed, Il6 (Fig. 4A) and Il1b (Fig. 4B) are elevated in the skin following UVB exposure in both mouse strains (right panels) with maximal Il6 induction at 24 h and 6 h in the SKH1-Ahb-1 and SKH1-Ahd mice respectively. Both strains exhibit maximal Il1b induction at 24 h. Strain differences were observed with Il6 displaying a significant 2-fold increase in the SKH1- Ahd mice compared to the SKH1-Ahb -1 mice at 6 h (Fig. 4A, right panel). Tnf expression exhibits maximal induction in both strains at 6 h with SKH1-Ahb-1 displaying a significant 1.2-fold increase over SKH1-Ahd at 12 h (Fig. 4C, right panel). No significant differences were observed with regard to the basal expression of Il6, Il1b or Tnf between the two strains.
The levels of the inflammatory mediators S100 calcium binding protein A9 (calgranulin B) (S100a9) and prostaglandin-endoperoxide synthase 2 (Ptgs2) (also known as cyclooxygenase-2 or COX2) were also assessed in the skin of the strains. UVB exposure prompts an increase in S100a9 expression in both strains with maximum induction evident at 48 h (Fig. 4D, right panel). SKH1-Ahb-1 mice exhibit a greater induction of S100a9 at all time points after UVB exposure compared to SKH1-Ahd mice. Ptgs2 was elevated in both strains after UVB exposure, with a peak at 48 h in both, and levels were significantly different between the two strains at every time point after UVB exposure, with a 32–fold difference at both 6 h and 12 h, a 15–fold difference at 24 h, and a 5–fold difference at 48 h (Fig. 4E, right panel). S100a9 and Ptgs2 levels were basally elevated in SKH1-Ahb-1 mice compared to SKH1-Ahd mice, but only Ptgs2 was statistically significant, with a 9.5-fold difference (Figure 4E, left panel). These data demonstrate that the SKH1-Ahb-1 mice exhibit a more pro-inflammatory phenotype following UVB exposure as measured by gene expression levels of inflammatory mediators.
4. Discussion
The primary aim of this study was to generate a hairless, immunocompetent mouse model harboring the high affinity Ahb-1 allelic variant to facilitate the examination of the role of the AHR within the skin. In particular, whether the AHR participates in the expression of genes encoding acute inflammatory mediators in response to UVB exposure as an initial characterization of these models. In addition, this model allows the indirect examination as to whether AHR activation and subsequent occupation of promoters in certain inflammatory genes occurs after UV exposure, likely due to the formation of endogenous ligands. Preliminary protein analyses, based upon relative molecular weight, indicated that the outbred SKH1 strain harbors an allelic variant that encodes an AHR species with a higher molecular weight than the high affinity Ahb-1 allele and were assumed to have the Ahd allele. To introduce the Ahb-1 allele, SKH1 mice were crossed with C57BL/6J mice. The resultant F1 offspring, heterozygous for the intended Ahb-1 allele, were backcrossed onto the SKH1 background for five generations while selecting for expression of the Ahb-1 allele in combination with the hairless phenotype. Upon acquisition of the hairless and Ahb-1 positive phenotype, sibling matings were performed to obtain SKH1 hairless mice homozygous for the Ahb-1 allele. Sequencing of these offspring confirmed the generation of the desired Ahb-1 homozygous line, which was expanded to colony status. Furthermore, we also identified SKH1 hairless offspring homozygous for Ahd, indicating that the parental SKH1 line was either homozygous Ahd or of a mixed Ahd/Ahb-2/Ahb-3 nature and that Ahd was inadvertently selected for during our mating scheme. The serendipitous generation of homozygous Ahd rather than Ahb-2 or Ahb-3 allowed for direct comparison between SKH1 lines expressing AHR species at opposite ends of the ligand binding affinity spectrum with regard to their influence upon skin responses. The level of expression of the AHR in each strain of mice differs, with the Ahd SKH1 mice exhibiting a lower level of expression in skin. Thus, there are likely two factors that decrease the responsiveness of the Ahd mice to ligand exposure, a 10-fold lower affinity for most ligands and a lower level of AHR expression, when compared to the Ahb mice. It is important to point out that studies in these mouse strains cannot exclude the possibility that during the segregation of the Ahb versus Ahd locus other co-segregated loci linked to these alleles may influence the results obtained.
Functional confirmation of the SKH1 lines with regard to AHR activity was obtained through mRNA expression analysis of Cyp1a1 under basal and induced conditions. The data demonstrate reduced basal Cyp1a1 in gastrointestinal tissues from the reduced affinity SKH1-Ahd line, consistent with a diminished potential for AHRd to be activated by dietary and microbial ligands known to be a major route to AHR activation. In contrast, Cyp1a1 expression within liver and skin exhibited enhanced levels in SKH1-Ahd when compared to the high affinity SKH1-Ahb-1 line. The contradictory elevated hepatic and skin Cyp1a1 observed within the SKH1-Ahd line may be due to a lack of intestinal metabolic clearance by CYP1A1 leading to AHR activation in other tissues, although we have no data to directly support this possibility. However, support for this scenario can be found in a study using mice with an intestinal-specific ablation of ARNT, which would result in a loss of AHR signaling; these mice show constitutive up-regulation of AHR activity in other tissues (Ito et al. 2007). Recent studies have suggested that dietary AHR ligand availability influences skin barrier integrity with ligand deficiency associating with increased impairment of the epidermal barrier. Supporting this observation, Ahr−/− mice similarly exhibit decreased skin barrier function (Haas et al. 2016). Although not the focus of the current study, it remains to be determined whether SKH1-Ahd display altered skin barrier function over SKH1-Ahb-1 due to increased systemic AHR ligand availability or the converse is true due to the greater activation potential of the Ahb-1 allelic variant. In contrast, the SKH1-Ahb-1 mice were more sensitive to Cyp1a1 induction compared to the SKH1-Ahd mice when local exposure to the high affinity AHR agonist FICZ occurred on the skin. This demonstrates the potential for the SKH1-Ahb-1 mice to be more sensitive to ligands produced in or on the skin, including those generated during UVB exposure or through metabolism by commensal microorganisms. These data also highlight the difference in potential induction of AHR-induced gene expression between endogenously and exogenously derived ligands.
The development of the SKH1-Ahb-1 and SKH1-Ahd lines allowed us to examine the hypothesis that mice harboring the high affinity Ahb-1 allelic variant are more sensitive to UVB-mediated inflammatory signaling. The high affinity agonist FICZ and other potential AHR agonists generated during UVB exposure are more likely to activate the AHRb-1 in SKH1-Ahb-1, leading to the enhanced expression of AHR regulated pro-inflammatory mediators. The targets examined in this study were chosen either because of direct transcriptional regulation by the AHR, or because they are enhanced in the skin after acute UVB exposure; some of the targets analyzed fit both criteria. Cxcl5 is induced by AHR in the context of pro-inflammatory conditions, but is also a mediator of UVB-induced pain in the skin (Dawes et al. 2011; Tauchi et al. 2005). We have recently demonstrated that Cxcl5 promoter region contains 7 overlapping consensus DREs and co-treatment of primary mouse keratinocytes with cytokines and AHR ligands leads to synergistic induction of Cxcl5 mRNA (Smith et al. 2017). Ptgs2 (also known as Cox2) contributes to inflammation through the production of PGE2 and the development of UVB-induced skin cancer in vivo (Rundhaug et al. 2007). Additionally, the AHR contributes to Ptgs2 expression through functional DRE in the promoter region, as Ahr−/− mice exposed to UVB fail to induce COX2 protein expression (Fritsche et al. 2007). AHR also contributes to the expression of Ccl20, Cxcl1, Il6, Il1b, and Tnf (Connor et al. 1994; Hollingshead et al. 2008; Lahoti et al. 2015; Tamaki et al. 2004; Vogel et al. 2007). Importantly, both Ccl20 and Il6 genes harbor multiple DREs in their respective promoters that in combination with inflammatory signaling leads to synergistic increases in Ccl20 or Il6 mRNA. These results have led to the proposal to refer to cytokine/chemokine genes that are subject to combinatorial regulation by the AHR and transcription factors activated by inflammatory signaling as “xenokines” (Smith et al. 2017). Previous studies have identified Il6, Ccl20, S100a9, Ptgs2, Cxcl5, Cxcl1, and Cxcl2 as UV-responsive targets in human skin (Dawes et al. 2014). Keratinocytes secrete IL1B in response to UVB, and TNF is produced in epidermal cells in response to UV (Feldmeyer et al. 2007; Schwarz and Luger 1989). The gene expression results support the hypothesis because the SKH1-Ahb-1 mice exhibit elevated levels of inflammatory genes in response to UVB, particularly Cxcl5, Cxcl1, Ccl20, S100a9, and Ptgs2.
UV light induces expression of the prototypical AHR response genes, CYP1A1 and CYP1B1 in human epidermis and AHH activity was induced in response to UVB in neonatal rat skin (Katiyar et al. 2000; Mukhtar et al. 1986). One possible explanation for this observed induction is that photoproducts generated from exposure to UV act as AHR agonists. Potential AHR ligands formed after UVB exposure include tryptophan-derived photoproducts such as FICZ and photo-oxidized skin surface lipids (Kostyuk et al. 2012; Rannug et al. 1987). Yet another class of AHR ligands that may be produced in an inflammatory microenvironment includes certain eicosanoids (Chiaro et al. 2008a; Chiaro et al. 2008b). While FICZ is a potent inducer of AHR, its effects are transient compared to other ligands such as TCDD, with a maximum induction in CYP1A1 levels at 3 h in a human keratinocyte cell line (Wei et al. 1998). If the UVB-induced levels of Cxcl5, Cxcl1, Ccl20, S100a9, and Ptgs2 observed in the skin of SKH1-Ahb-1 mice are due to the generation of an endogenously derived ligand, FICZ is most likely not the only mediator over the entire 48 h time course due to its rapid metabolism. Additionally, though there is evidence that FICZ could be generated in vivo, whether it is produced under physiologically relevant conditions in the skin and whether sufficient cellular tryptophan levels exist to enable its synthesis are questions requiring further study (Nguyen and Bradfield 2008; Wincent et al. 2009). An additional complicating factor is that IL1B represses Cyp1a1 and Cyp1b1 expression, so these genes may not always represent ideal indicators of endogenous AHR activation under conditions of injury, infection, or stress (Barker et al. 1992).
The complicated nature of determining if and how endogenous AHR ligands activate the receptor in vivo under basal and pathological conditions means that the hairless mouse model characterized here may prove valuable due to the expression of the high affinity Ahb-1 allele. Although an SKH1-Ahr−/− mouse has been developed, a benefit of our model compared to a complete knockout is that the effects of in vivo activation of AHR by endogenous ligands can be studied without the negative health consequences and developmental problems found in the Ahr−/− mouse (Frauenstein et al. 2013). Due to the hairless phenotype of the SKH1-Ahb-1 mouse, inflammation caused by hair removal will not impact experimental results. This mouse is an ideal model for the study of UV-mediated effects, as this study demonstrates, including photocarcinogenesis, as well as chemical carcinogenesis. In addition, the SKH1-Ahb-1 and SKH1- Ahd mouse models will be useful for studies involving pharmacological manipulation of the AHR by topically applied therapeutic agents, as well as studies involving the further elucidation of the role of AHR in a variety of skin processes and diseases, including; keratinocyte differentiation, the response to UVB- and microbe-derived ligands, psoriasis, and carcinogenesis.
Supplementary Material
Highlights.
Outcrossed SKH1 mice contain several alleles of the Ahr.
SKH1 mice were backcrossed with C57BL6/J mice to obtain the Ahb-1 allele.
SKH1 mouse lines with either the Ahb-1 or Ahd allele were established.
After UV exposure Ccl20, Cxcl5, and Ptgs2 exhibit greater expression in SKH1-Ahb-1 mice.
Acknowledgments
This work was also supported by NIH grants ES004869, ES019964, and ES028244 to Gary H. Perdew, and the T32 training grant AI074551 to Kayla J. Smith. We would like to acknowledge Angela Alnemri and Shana Santarelli for their contribution to the data generation. We would like to thank Kelly Wagner for her assistance with PCR-based genotyping and Marcia H. Perdew for editorial assistance. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barker CW, Fagan JB, Pasco DS. Interleukin-1 beta suppresses the induction of P4501A1 and P4501A2 mRNAs in isolated hepatocytes. J Biol Chem. 1992;267:8050–5. [PubMed] [Google Scholar]
- Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18:207–50. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides F, Oberyszyn TM, VanBuskirk AM, Reeve VE, Kusewitt DF. The hairless mouse in skin research. J Dermatol Sci. 2009;53:10–8. doi: 10.1016/j.jdermsci.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaro CR, Morales JL, Prabhu KS, Perdew GH. Leukotriene A4 metabolites are endogenous ligands for the Ah receptor. Biochemistry. 2008a;47:8445–55. doi: 10.1021/bi800712f. [DOI] [PubMed] [Google Scholar]
- Chiaro CR, Patel RD, Perdew GH. 12(R)-Hydroxy-5(Z),8(Z),10(E),14(Z)-eicosatetraenoic acid [12(R)-HETE], an arachidonic acid derivative, is an activator of the aryl hydrocarbon receptor. Mol Pharmacol. 2008b;74:1649–56. doi: 10.1124/mol.108.049379. [DOI] [PubMed] [Google Scholar]
- Connor MJ, Nanthur J, Puhvel SM. Influence of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on TNF-alpha levels in the skin of congenic haired and hairless mice. Toxicol Appl Pharmacol. 1994;129:12–5. doi: 10.1006/taap.1994.1223. [DOI] [PubMed] [Google Scholar]
- Dawes JM, Antunes-Martins A, Perkins JR, Paterson KJ, Sisignano M, Schmid R, Rust W, Hildebrandt T, Geisslinger G, Orengo C, Bennett DL, McMahon SB. Genome-wide transcriptional profiling of skin and dorsal root ganglia after ultraviolet-B-induced inflammation. PLoS One. 2014;9:e93338. doi: 10.1371/journal.pone.0093338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes JM, Calvo M, Perkins JR, Paterson KJ, Kiesewetter H, Hobbs C, Kaan TK, Orengo C, Bennett DL, McMahon SB. CXCL5 mediates UVB irradiation-induced pain. Sci Transl Med. 2011;3:90ra60. doi: 10.1126/scitranslmed.3002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer L, Keller M, Niklaus G, Hohl D, Werner S, Beer HD. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol. 2007;17:1140–5. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- Frauenstein K, Sydlik U, Tigges J, Majora M, Wiek C, Hanenberg H, Abel J, Esser C, Fritsche E, Krutmann J, Haarmann-Stemmann T. Evidence for a novel anti-apoptotic pathway in human keratinocytes involving the aryl hydrocarbon receptor, E2F1, and checkpoint kinase 1. Cell Death Differ. 2013;20:1425–34. doi: 10.1038/cdd.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche E, Schafer C, Calles C, Bernsmann T, Bernshausen T, Wurm M, Hubenthal U, Cline JE, Hajimiragha H, Schroeder P, Klotz LO, Rannug A, Furst P, Hanenberg H, Abel J, Krutmann J. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci U S A. 2007;104:8851–6. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas K, Weighardt H, Deenen R, Kohrer K, Clausen B, Zahner S, Boukamp P, Bloch W, Krutmann J, Esser C. Aryl Hydrocarbon Receptor in Keratinocytes Is Essential for Murine Skin Barrier Integrity. J Invest Dermatol. 2016;136:2260–2269. doi: 10.1016/j.jid.2016.06.627. [DOI] [PubMed] [Google Scholar]
- Hollingshead BD, Beischlag TV, Dinatale BC, Ramadoss P, Perdew GH. Inflammatory signaling and aryl hydrocarbon receptor mediate synergistic induction of interleukin 6 in MCF-7 cells. Cancer Res. 2008;68:3609–17. doi: 10.1158/0008-5472.CAN-07-6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard TD, Murray IA, Perdew GH. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab Dispos. 2015;43:1522–35. doi: 10.1124/dmd.115.064246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Chen C, Satoh J, Yim S, Gonzalez FJ. Dietary phytochemicals regulate whole-body CYP1A1 expression through an arylhydrocarbon receptor nuclear translocator-dependent system in gut. J Clin Invest. 2007;117:1940–50. doi: 10.1172/JCI31647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar SK, Matsui MS, Mukhtar H. Ultraviolet-B exposure of human skin induces cytochromes P450 1A1 and 1B1. J Invest Dermatol. 2000;114:328–33. doi: 10.1046/j.1523-1747.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- Knutson JC, Poland A. Response of murine epidermis to 2,3,7,8-tetrachlorodibenzo-p-dioxin: interaction of the ah and hr loci. Cell. 1982;30:225–34. doi: 10.1016/0092-8674(82)90028-9. [DOI] [PubMed] [Google Scholar]
- Kostyuk V, Potapovich A, Stancato A, De Luca C, Lulli D, Pastore S, Korkina L. Photo-oxidation products of skin surface squalene mediate metabolic and inflammatory responses to solar UV in human keratinocytes. PLoS One. 2012;7:e44472. doi: 10.1371/journal.pone.0044472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoti TS, Boyer JA, Kusnadi A, Muku GE, Murray IA, Perdew GH. Aryl Hydrocarbon Receptor Activation Synergistically Induces Lipopolysaccharide-Mediated Expression of Proinflammatory Chemokine (c-c motif) Ligand 20. Toxicol Sci. 2015;148:229–40. doi: 10.1093/toxsci/kfv178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magiatis P, Pappas P, Gaitanis G, Mexia N, Melliou E, Galanou M, Vlachos C, Stathopoulou K, Skaltsounis AL, Marselos M, Velegraki A, Denison MS, Bassukas ID. Malassezia yeasts produce a collection of exceptionally potent activators of the Ah (dioxin) receptor detected in diseased human skin. J Invest Dermatol. 2013;133:2023–30. doi: 10.1038/jid.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezrich JD, Nguyen LP, Kennedy G, Nukaya M, Fechner JH, Zhang X, Xing Y, Bradfield CA. SU5416, a VEGF receptor inhibitor and ligand of the AHR, represents a new alternative for immunomodulation. PLoS One. 2012;7:e44547. doi: 10.1371/journal.pone.0044547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtar H, DelTito BJ, Jr, Matgouranis PM, Das M, Asokan P, Bickers DR. Additive effects of ultraviolet B and crude coal tar on cutaneous carcinogen metabolism: possible relevance to the tumorigenicity of the Goeckerman regimen. J Invest Dermatol. 1986;87:348–53. doi: 10.1111/1523-1747.ep12524446. [DOI] [PubMed] [Google Scholar]
- Narayanan GA, Murray IA, Krishnegowda G, Amin S, Perdew GH. Selective aryl hydrocarbon receptor modulator-mediated repression of CD55 expression induced by cytokine exposure. J Pharmacol Exp Ther. 2012;342:345–55. doi: 10.1124/jpet.112.193482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–16. doi: 10.1021/tx7001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdew GH, Abbott B, Stanker LH. Production and characterization of monoclonal antibodies directed against the Ah receptor. Hybridoma. 1995;14:279–83. doi: 10.1089/hyb.1995.14.279. [DOI] [PubMed] [Google Scholar]
- Poland A, Glover E. Characterization and strain distribution pattern of the murine Ah receptor specified by the Ahd and Ahb-3 alleles. Mol Pharmacol. 1990;38:306–12. [PubMed] [Google Scholar]
- Poland A, Knutson JC, Glover E. Histologic changes produced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in the skin of mice carrying mutations that affect the integument. J Invest Dermatol. 1984;83:454–9. doi: 10.1111/1523-1747.ep12273574. [DOI] [PubMed] [Google Scholar]
- Poland A, Palen D, Glover E. Tumour promotion by TCDD in skin of HRS/J hairless mice. Nature. 1982;300:271–3. doi: 10.1038/300271a0. [DOI] [PubMed] [Google Scholar]
- Poland A, Palen D, Glover E. Analysis of the four alleles of the murine aryl hydrocarbon receptor. Mol Pharmacol. 1994;46:915–21. [PubMed] [Google Scholar]
- Puhvel SM, Sakamoto M, Ertl DC, Reisner RM. Hairless mice as models for chloracne: a study of cutaneous changes induced by topical application of established chloracnegens. Toxicol Appl Pharmacol. 1982;64:492–503. doi: 10.1016/0041-008x(82)90247-2. [DOI] [PubMed] [Google Scholar]
- Rannug A, Rannug U, Rosenkranz HS, Winqvist L, Westerholm R, Agurell E, Grafstrom AK. Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances. J Biol Chem. 1987;262:15422–7. [PubMed] [Google Scholar]
- Ravindran A, Mohammed J, Gunderson AJ, Cui X, Glick AB. Tumor-promoting role of TGFbeta1 signaling in ultraviolet B-induced skin carcinogenesis is associated with cutaneous inflammation and lymph node migration of dermal dendritic cells. Carcinogenesis. 2014;35:959–66. doi: 10.1093/carcin/bgt486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundhaug JE, Mikulec C, Pavone A, Fischer SM. A role for cyclooxygenase-2 in ultraviolet light-induced skin carcinogenesis. Mol Carcinog. 2007;46:692–8. doi: 10.1002/mc.20329. [DOI] [PubMed] [Google Scholar]
- Schwarz T, Luger TA. Effect of UV irradiation on epidermal cell cytokine production. J Photochem Photobiol B. 1989;4:1–13. doi: 10.1016/1011-1344(89)80097-1. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Boyer JA, Muku GE, Murray IA, Gowda K, Desai D, Amin SG, Glick AB, Perdew GH. Ah receptor activation potentiates neutrophil chemoattractant (C-X-C motif) ligand 5 expression in keratinocytes and skin. Toxicol Sci. 2017;160:83–94. doi: 10.1093/toxsci/kfx160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, Di Meglio P, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol. 2014;32:403–32. doi: 10.1146/annurev-immunol-032713-120245. [DOI] [PubMed] [Google Scholar]
- Sutter CH, Bodreddigari S, Campion C, Wible RS, Sutter TR. 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases the expression of genes in the human epidermal differentiation complex and accelerates epidermal barrier formation. Toxicol Sci. 2011;124:128–37. doi: 10.1093/toxsci/kfr205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki A, Hayashi H, Nakajima H, Takii T, Katagiri D, Miyazawa K, Hirose K, Onozaki K. Polycyclic aromatic hydrocarbon increases mRNA level for interleukin 1 beta in human fibroblast-like synoviocyte line via aryl hydrocarbon receptor. Biol Pharm Bull. 2004;27:407–10. doi: 10.1248/bpb.27.407. [DOI] [PubMed] [Google Scholar]
- Tauchi M, Hida A, Negishi T, Katsuoka F, Noda S, Mimura J, Hosoya T, Yanaka A, Aburatani H, Fujii-Kuriyama Y, Motohashi H, Yamamoto M. Constitutive expression of aryl hydrocarbon receptor in keratinocytes causes inflammatory skin lesions. Mol Cell Biol. 2005;25:9360–8. doi: 10.1128/MCB.25.21.9360-9368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges J, Haarmann-Stemmann T, Vogel CF, Grindel A, Hubenthal U, Brenden H, Grether-Beck S, Vielhaber G, Johncock W, Krutmann J, Fritsche E. The new aryl hydrocarbon receptor antagonist E/Z-2-benzylindene-5,6-dimethoxy-3,3-dimethylindan-1-one protects against UVB-induced signal transduction. J Invest Dermatol. 2014;134:556–9. doi: 10.1038/jid.2013.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bogaard EH, Bergboer JG, Vonk-Bergers M, van Vlijmen-Willems IM, Hato SV, van der Valk PG, Schroder JM, Joosten I, Zeeuwen PL, Schalkwijk J. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest. 2013;123:917–27. doi: 10.1172/JCI65642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bogaard EH, Podolsky MA, Smits JP, Cui X, John C, Gowda K, Desai D, Amin SG, Schalkwijk J, Perdew GH, Glick AB. Genetic and pharmacological analysis identifies a physiological role for the AHR in epidermal differentiation. J Invest Dermatol. 2015;135:1320–8. doi: 10.1038/jid.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CF, Nishimura N, Sciullo E, Wong P, Li W, Matsumura F. Modulation of the chemokines KC and MCP-1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice. Arch Biochem Biophys. 2007;461:169–75. doi: 10.1016/j.abb.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Vos JG, Van Leeuwen FX, de Jong P. Acnegenic activity of 3-methylcholanthrene and benzo[a]pyrene, and a comparative study with 2,3,7,8-tetrachlorodibenzo-p-dioxin in the rabbit and hairless mouse. Toxicology. 1982;23:187–96. doi: 10.1016/0300-483x(82)90097-x. [DOI] [PubMed] [Google Scholar]
- Wei YD, Helleberg H, Rannug U, Rannug A. Rapid and transient induction of CYP1A1 gene expression in human cells by the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole. Chem Biol Interact. 1998;110:39–55. doi: 10.1016/s0009-2797(97)00111-7. [DOI] [PubMed] [Google Scholar]
- Wilgus TA, Koki AT, Zweifel BS, Rubal PA, Oberyszyn TM. Chemotherapeutic efficacy of topical celecoxib in a murine model of ultraviolet light B-induced skin cancer. Mol Carcinog. 2003;38:33–9. doi: 10.1002/mc.10142. [DOI] [PubMed] [Google Scholar]
- Wincent E, Amini N, Luecke S, Glatt H, Bergman J, Crescenzi C, Rannug A, Rannug U. The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans. J Biol Chem. 2009;284:2690–6. doi: 10.1074/jbc.M808321200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.