Abstract
The development of CETP inhibitors has had a long and difficult course with three compounds failing in phase III clinical trials. Finally, the REVEAL trial has shown that the CETP inhibitor anacetrapib decreased coronary heart disease when added to statin therapy. While the result is different to earlier studies, this is likely related to the size and duration of the trial. The benefit of anacetrapib appears to be largely explained by lowering of non-HDL cholesterol, rather than increases in HDL cholesterol. Although the magnitude of benefit for CHD appeared to be moderate, in part this may have reflected aspects of the trial design. Anacetrapib treatment was associated with a small increase in BP, but was devoid of major side effects and was also associated with a small reduction in diabetes. Treatment with CETP inhibitors, either alone or in combination with statins, could provide another option for patients with coronary disease who require further reduction in LDL and/or non-HDL cholesterol
Keywords: Cholesteryl ester, transfer protein, atherosclerosis, coronary heart disease, LDL, HDL
Subject Terms: Coronary Artery Disease
INTRODUCTION
The development of CETP inhibitors was motivated by the discovery that humans with genetic CETP deficiency have markedly elevated levels of HDL cholesterol (HDL-C), as well as reduced levels of LDL cholesterol (LDL-C), a profile that is typically associated with reduced atherosclerosis.1 CETP inhibitors were subsequently shown to raise HDL-C levels, in some cases quite impressively; in addition the more potent CETP inhibitors lowered LDL-C levels. Based on epidemiological observations, it was expected that this marked increase in HDL would deliver a powerful anti-atherogenic effect. This promise has not been realized in cardiovascular clinical outcome trials of CETP inhibitors. In fact, in the first large trial the CETP inhibitor torcetrapib caused an excess of deaths and cardiovascular disease (Table),2 leading many to conclude that the elevated HDL itself was harmful. The identification of off-target toxic side-effects of torcetrapib2 led to sufficient clinical equipoise to allow further evaluation of this class of drugs. Subsequent trials with the relatively ineffective CETP inhibitor dalcetrapib3 and with the potent inhibitor evacetrapib4 were stopped early for futility (lack of efficacy in reducing CV events). Now results from the largest and longest running trial of a CETP inhibitor, in this case the potent inhibitor anacetrapib, have been published, showing that this drug significantly reduced major coronary events.5 Although the magnitude of risk reduction was moderate, anacetrapib could find a place in the armamentarium of approved non-statin lipid-targeted agents. However, this result leaves many questions unanswered, a few of which include: 1) Why did this trial show benefit when other trials with CETP inhibitors did not? 2) Given the reductions in LDL and non-HDL cholesterol seen with anacetrapib, did the increase in HDL cholesterol contribute to the benefit? This review will attempt to address these questions, while providing a background on the role of CETP in lipoprotein metabolism, emphasizing genetic and human metabolic studies. The reader is referred to earlier reviews for additional background on CETP.6–9
Table.
| TRIAL (drug) |
Patients | Lipoprotein Changes | Duration | Outcome | Comments |
|---|---|---|---|---|---|
| ILLUMINATE (Torcetrapib) | 15,067 Hi CV Risk |
HDL-C↑72% LDL-C ↓* |
1-2 years | ↑CV Events ↑Death ↑ SBP (5mm) |
Electrolyte disturbances, hyeraldosteronism identified as off-target effects *LDL measured indirectly |
| dal-OUTCOMES (Dalcetrapib) | 15,871 Post ACS |
HDL-C↑~30% LDL-C→ |
31 months | CV Events→ ↑SBP(0.6mm) |
Trial stopped early for futility. Possible benefit in a genetic subgroup. |
| ACCELERATE (Evacetrapib) | 12,092 Hi risk vascular disease |
HDL-C↑133% LDL-C↓* |
26 months | CV Events → ↑SBP (1.2mm) |
Trial stopped early for futility ↓Deaths (not pre-specified) *LDL measured indirectly |
| REVEAL (Anacetrapib) | 30,449 Hi risk vascular disease |
HDL-C↑104% LDL-C↓17% |
4.1 years | ↓Coronary Events ↑SBP (0.7mm) |
Trial went to planned completion ↓ new onset diabetes |
A reduction in coronary heart disease with CETP inhibition is revealed
The REVEAL study involved 30,449 patients with atherosclerotic cardiovascular disease who were randomized to receive anacetrapib 100 mg daily or placebo on top of effective statin therapy and followed for a median of 4.1 years. After the failure of CETP inhibitors in three successive clinical trials, expectations were low that anacetrapib, a CETP inhibitor developed by Merck, would meet with success. However, REVEAL demonstrated a highly significant reduction (rate ratio = 0.91, p<.004) in the composite primary endpoint of coronary death, myocardial infarction (MI) or coronary revascularization.5 The individual components of the primary endpoint showed similar rate ratios but were only significant for MI and revascularization. The incidence of the pre-specified outcome of coronary death or MI was significantly lower in the anacetrapib group (rate ratio 0.89, p=.008). The secondary endpoint of major coronary event (MI, coronary death or ischemic stroke) just missed significance (rate ratio = 0.93, p=.052). There was also a significant difference for the secondary outcome of major vascular events favoring anacetrapib. At the trial midpoint, anacetrapib raised HDL-C from 42 to 86 mg/dl (104%) and apoA-I by 36%; in addition, it lowered LDL-C from 63 mg/dl to 53 mg/dl (−17%, as determined by ultracentrifugation), non-HDL-C from 96 to 79 mg/dl (−18%) and Lp(a) by −25%. While the outcome of REVEAL appears inconsistent with the previous negative trials, it is important to assess the differences in CETP inhibitors and trial design (Table). Dalcetrapib is a much less potent CETP inhibitor that raised HDL cholesterol less and did not reduce LDL cholesterol; furthermore, its trial dal-Outcomes was stopped early.3 The ACCELERATE trial was performed with the potent CETP inhibitor evacetrapib which results in lipid changes similar to anacetrapib. However, ACCELERATE involved less than half the number of patients and CVD events, and was stopped early after about 2.2 years for futility.4 In contrast, REVEAL was continued through its planned duration with a median follow-up of 4.1 years. The longer trial duration was likely of key importance because, similar to trials of other lipid lowering drugs, the beneficial effects on CVD were greater after the first year of treatment with anacetrapib. Moreover, an exploratory analysis suggested that the benefit of anacetrapib increased with time as the trial proceeded. Thus, it is highly plausible that anacetrapib succeeded where other CETP inhibitors failed because of relative safety (compared with torcetrapib), potency (compared with dalcetrapib) and a study design that included adequate statistical power and sufficiently long duration to uncover the benefit (compared with evacetrapib).
The demonstration of an overall anti-atherogenic effect of CETP inhibition is buttressed by the majority of animal studies which have demonstrated a pro-atherogenic effect of CETP expression.8 While studies in CETP transgenic mice have produced mixed results in atherosclerosis experiments, inhibition of CETP in rabbits, a species that naturally expresses CETP, have consistently shown reduced atherosclerosis, including in a CETP knockout rabbit which showed reduced aortic and coronary atherosclerosis when fed a high cholesterol diet10. Moreover, multiple large human genetic studies have shown that SNPs in the CETP gene that are associated with increased HDL and reduced LDL cholesterol are associated with reduced CHD.11–13 This includes SNPs that likely reduce the function of the promoter region upstream of the CETP gene,13 and most importantly CETP protein truncating mutations that abrogate the function of CETP.12
How does CETP inhibition affect plasma lipoprotein levels?
Before considering whether the benefit of anacetrapib was related to changes in HDL, LDL or both, it is worth reviewing the mechanisms underlying the effects of CETP inhibition on plasma lipoprotein metabolism. The primary effect of CETP inhibition is a reduced rate of transfer of CE from HDL into triglyceride rich lipoproteins (TRL).14, 15 This leads to an increased content of CE in HDL and the formation of larger HDL particles that are more slowly catabolized than normal. On the other side of the coin, CE is depleted in the TRL including in VLDL, chylomicrons and their remnants.16 There is also a depletion of CE in LDL likely reflecting both diminished direct transfer from HDL, reduced amounts of CE in VLDL being converted into LDL CE and increased LDL particle catabolism (Fig 1). Thus, the major impact of CETP inhibition is an increase in HDL cholesterol and a decrease in non-HDL cholesterol (encompassing both cholesterol in TRL and LDL). Less obviously, the reduced transfer of CE from HDL to TRL also leads to a decrease in VLDL and LDL apoB levels. Careful metabolic studies in mildly hypercholesterolemic subjects treated with anacetrapib have shown that the reductions in VLDL and LDL apoB, as in genetic CETP deficiency, result from an increase in catabolism.17, 18 In contrast, lowering of Lp(a) reflects a decrease in the production rate of apo(a).19 On a background of statin therapy, anacetrapib modestly lowered plasma triglyceride levels, reflecting an increase in VLDL TG catabolism.20
Figure 1.
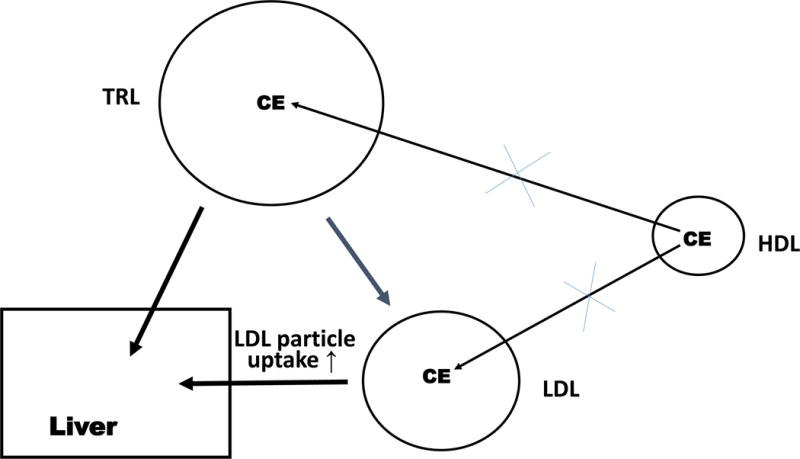
CETP inhibition reduces LDL-C levels by three mechanisms, 1) decreased transfer of HDL CE into triglyceride-rich lipoproteins which are converted into LDL; 2) decreased transfer of HDL CE into LDL; and 3) increased uptake of LDL particles by the hepatic LDL receptor.
Since LDL catabolism is primarily mediated by LDL receptor mediated clearance in the liver, increased LDL catabolism in anacetrapib treated subjects likely reflects an increase in the clearance of LDL particles via the LDLR. This could result from a relative depletion of the regulatory cholesterol pool in the liver resulting from CETP inhibition and leading to an increase in LDLR mRNA (Fig 1B). However, there was no increase in plasma lathosterol, which likely would have been increased as a result of increased sterol biosynthesis, if regulatory cholesterol pools in the liver were depleted.17 Thus, the authors speculated that the increased clearance of apoB could be caused by an increase in the affinity of LDL for its receptor, caused by changes in the properties of LDL particles, such as an increase in the LDL TG/CE ratio or an increase in the size or polydispersity of LDL particles (Fig 1B). However, an increase in the numbers of hepatic LDL receptors cannot be completely excluded since they were not directly measured. Studies in CETP transgenic mice have shown that CETP activity increases the cholesterol content in the liver and lowers the levels of the LDL receptor mRNA and protein, as well as HMGCoA reductase mRNA, consistent with an increased content of the regulatory pool of cholesterol in the liver.21 This may reflect an increased efficiency of cholesterol transfer into hepatocytes resulting from transfer of CE from slowly turning over HDL particles into more rapidly cleared TRL remnants, or decreased channeling of HDL cholesterol into biliary cholesterol bypassing the regulatory cholesterol pool. Whatever the precise mechanism, these studies strongly suggest that increased clearance of apoB lipoproteins via the LDL receptor pathway is occurring in subjects treated with potent CETP inhibitors, an effect that ultimately reflects the reduced rate of transfer of CE from HDL to TRL.
Is the CHD benefit related to increased HDL or decreased LDL/non-HDL cholesterol?
Formally, the only firm conclusion from REVEAL is that CETP inhibition resulted in a reduction in CV events as captured by the primary endpoint,5 and the mechanism of benefit is the subject of speculation. However, the mean reduction of non-HDL cholesterol of 18% in REVEAL fell only slightly above the regression line relating the % reduction in CVD events to the absolute decrease in non-HDL-C, based on earlier studies with LDL lowering drugs, suggesting that the benefit may be largely explained by the reduction in non-HDL cholesterol. However, these point estimates have substantial confidence intervals and also are based on an extrapolation of data that were mostly obtained at much higher levels of non-HDL cholesterol. Moreover, there may have a been a small adverse effect related to BP elevation in REVEAL, perhaps offsetting a beneficial effect of HDL changes. Overall, the conservative interpretation is that the benefit of anacetrapib was mostly or solely due to reduction in atherogenic lipoproteins; however, a contribution of HDL raising cannot be firmly excluded.
After the failure of torcetrapib, studies of HDL functionality were undertaken to exclude an adverse effect of CETP inhibition. These studies showed that in fact HDL from subjects treated with anacetrapib had enhanced ability to promote cholesterol efflux from cholesterol loaded macrophages and preserved anti-inflammatory effects.22 Studies using an assay of HDL cholesterol efflux capacity that had been validated as predicting coronary atherosclerosis and CHD in sizable population studies23, 24 also showed that HDL from subjects treated with evacetrapib had increased cholesterol efflux capacity.25 However, for unknown reasons the magnitude of this increase in subjects concomitantly treated with statins was surprisingly small (total cholesterol efflux capacity was increased by 21%) versus a larger increase in those receiving evacetrapib monotherapy (34 %). Another possibility is that statin use reduces the expression of the ATP binding cassette transporters (ABCA1 and ABCG1) in macrophages, as result of decreased activity of the sterol-activated transcription factor LXR, thereby offsetting the effects of increased HDL levels on macrophage cholesterol efflux. Further studies of HDL functionality in the context of CETP inhibition are warranted.
Is the outcome of REVEAL consistent with insights from human genetics studies?
Mendelian randomization studies of SNPs in multiple genes that are associated with changes in only HDL-C (but not triglycerides or LDL-C), for example in endothelial lipase (LIPG), have shown no association with CHD.26 This has been interpreted as indicating that HDL-C is not in the causal pathway of atherosclerosis,27 and suggests that decreased CHD associated with SNPs that reduce CETP expression or function are likely acting through changes in LDL-C (or non-HDL-C). Consistent with this interpretation, in the study of CETP truncating mutations, the magnitude of the benefit on CHD correlated well with the degree of LDL cholesterol lowering.12 However, variants in HDL-associated genes jointly account for very little of the variance in HDL-C levels and could have pleiotropic effects, weakening the general conclusion that HDL is not in the causal pathway of atherosclerosis. Adding to this complexity, there could be epigenetic effects masking a possible benefit of HDL raising genes. In this regard, a post-hoc analysis of the dal-OUTCOMES trial has identified SNPs in the gene encoding Adenylate Cyclase 9 (ADCY9) as being associated with cardiovascular benefit in a subgroup of patients; a clinical trial (dal-GENE) in which dalcetrapib is being administered to high CV risk patients carrying the putative protective SNPs is ongoing.28, 29 It will be of substantial interest to determine whether carriers of these variants had even better outcomes with anacetrapib in the REVEAL trial. Further analysis of CETP inhibitor clinical trial data, new assays of HDL functionality,30, 31 as well as clinical outcomes studies based on infusion of reconstituted HDL particles that are highly active in promoting cholesterol efflux32 may provide additional insights into the complex relationship of HDL to atherosclerosis.
Is the glass half empty or half full?
Despite achieving its primary endpoint, there may be concern that the benefit demonstrated by REVEAL was moderate and that the reduction in cardiovascular death was not significant. It is worth considering whether CETP inhibition is a mechanism with intrinsically limited benefit, or whether the result may have reflected aspects of the trial design. In general, the relationship between LDL lowering and its impact on CHD has shown that for every 40 mg/dl decrease in LDL cholesterol, there is about a 25% reduction in CHD risk.33 This implies that in a trial of LDL lowering, if patients in both placebo and active treatment groups are treated to very low LDL levels prior to randomization, the % reduction in CHD and the absolute benefit in the active treatment group will be less than they would have been if LDL levels were higher at randomization. This is because the % reduction in LDL-C (or non-HDL-C) will likely be similar at lower or higher starting LDL-C, so that the absolute reduction in LDL-C is greater when the starting LDL-C is higher and the risk reduction is accordingly larger. This supposition is supported by studies performed in a subset of patients in the DEFINE trial which showed a larger absolute reduction in LDL-C by anacetrapib in patients with higher LDL-C at baseline compared to those with lower levels of LDL-C at baseline.34 In REVEAL the mean LDL cholesterol at randomization was very low – 61 mg/dl. In part this reflected the trial goal of achieving a pre-randomization LDL cholesterol < 77 mg/dl by effective use of statins, but also the fact that people with total cholesterol l>155 mg/dl after the statin run-in were intentionally excluded from the study. Notably, patients with non-HDL cholesterol > 101 mg/dl at randomization appeared to benefit more from anacetrapib than patients with non-HDL cholesterol < 85 mg/dl, with reductions in the rate ratio of MI + coronary death of 0.83 versus 0.96.5 In the clinical setting, patients with persistently elevated LDL or non-HDL cholesterol on maximally tolerated statin therapy would be precisely those most likely to be treated with additional lipid-lowering agents. The implication could be that for people who would likely derive the greatest benefit, i.e. with non-HDL cholesterol>100 mg/dl after statin treatment, the clinical impact of anacetrapib could be considerably greater.
It is of interest to consider the results of REVEAL in the light of other recent trials of LDL lowering therapies that met their primary endpoints. There was a 6% reduction (p<.02) in the primary cardiovascular endpoint in IMPROVE-IT after 6 years of treatment with ezetimibe on top of statins35. In this trial the mean LDL cholesterol level in the statin only arm was slightly higher than in REVEAL (69.5 mg/dl) and reductions in LDL cholesterol, non-HDL cholesterol and apoB were comparable to those obtained with atorvastatin. In contrast to evacetrapib, ezetimibe did not substantially increase HDL cholesterol or lower Lp(a) levels. In the FOURIER study in which evolocumab (a PCSK9 mAb) was added to LDL lowering therapy with statins, the reduction in the primary endpoint was about 15%. Compared to anacetrapib, there was a much more dramatic incremental LDL cholesterol lowering of 61%. However, it also should be noted that in FOURIER the LDL cholesterol was 92 mg/dl at entry reflecting the fact that the trial protocol stipulated that patients with already low LDL cholesterol on statins (< 70 mg/dl) were excluded from the study. Thus, the trial was more specifically designed to show a benefit of LDL lowering on CHD. Evolocumab lowered Lp(a) by about 27% and raised HDL cholesterol by 8%. FOURIER was terminated after a mean of only 2.5 years and very likely the beneficial impact on CV events would have been larger had the study been continued for a longer time.
The devil is in the details: Safety and side-effects
In REVEAL, anacetrapib treatment was devoid of major side effects i.e. involving cancer, infectious diseases, cognitive changes, depression etc. Although not observed in a smaller preliminary safety trial,36 there was a 0.7 mm increase in SBP and 0.3 mm in DBP in anacetrapib treated subjects in REVEAL, similar in magnitude to what was observed with evacetrapib and dalcetrapib, but much less than for torcetrapib. Increased BP has not been reported in genetic CETP deficiency. However, this effect has been seen in multiple trials with different structural classes of CETP inhibitors strongly suggesting that it is mechanism-based. The nature of this mechanism is unknown. For torcetrapib, hyperaldosteronism2 and increased responses to endothelin in the vasculature37 were shown, however this occurred even in species that lack a CETP gene. Thus, relevance to subsequent CETP inhibitors is unlikely. Anacetrapib use was associated with a small increase in the proportion of patients with eGFR<60ml/min at the end of the study, but there was no increase in albuminuria or serious adverse events attributed to renal failure. An increase in hemorrhagic stroke, as might be expected from increased BP and also as seen in a recent genetic study of CETP polymorphisms,38 was not observed. The beneficial effect of CETP inhibition was observed across multiple pre-specified subgroups. However, patients taking ACE inhibitors or ARBs appeared to benefit less (p<.01, unadjusted for multiple comparisons). While this could represent a chance finding, in a small study an adverse impact of genetic CETP deficiency on CHD was reduced after adjustment for treatment with non-diuretic anti-hypertensive drugs.39 It is possible that treatment with ACE inhibitors marks a subgroup that had a more marked hypertensive response to CETP inhibition, or that inhibition of the renin-angiotensin system uncovers an adverse effect of CETP inhibition. Further investigation of a possible interaction between the use anti-hypertensive drugs and CETP inhibitors is warranted.
Although several genetic studies have shown an association between CETP polymorphisms and age-related macular degeneration,40, 41 there was no evidence for an increase in the onset or progression of retinal disease in REVEAL. This might reflect the differences between several years of pharmacologic CETP inhibition vs lifelong genetic reduction in CETP deficiency; longer term monitoring will be needed to exclude macular degeneration as a possible adverse effect related to CETP inhibition. Anacetrapib has a very prolonged half-life due to accumulation in adipose tissue,42 a property that appears to be specific to anacetrapib and has not been seen with some other potent CETP inhibitors. Plasma levels of anacetrapib fall substantially after cessation of anacetrapib treatment, but the drug is persistent in adipose tissue for at least several years. While no adverse effect has so far been linked to this property, it will be important to continue to monitor patients in REVEAL for potential consequences.
In REVEAL the incidence of new onset diabetes was reduced by about 10%, and there was a small reduction in HbA1c levels amongst non-diabetics. These benefical effects on diabetes are consistent with previous reports of improvements in glucose/insulin ratios (HOMA-IR) and in HbA1c reported with torcetrapib43 or evacetrapib (but not dalcetrapib)5, as well as the reductions in glucose levels seen in a study of subjects with CETP deficiency.39 The mechanisms underlying the beneficial effects of CETP inhibitors on diabetes are poorly understood, but they contrast with the slight increase in diabetes and HbA1c associated with other mechanisms that lower LDL cholesterol by up-regulating the hepatic LDL receptor pathway, such as statins or genetic factors that reduce PCSK9.44 Since these effects on diabetes are the opposite to those observed with other drugs increasing LDL clearance by the LDLR pathway, it is tempting to speculate that they may be related to the distinctively increased HDL levels resulting from CETP inhibition. Cholesterol accumulation in islet beta-cells is associated with reduced insulin secretion in mice with knockouts of ABCA1/G1 in pancreatic beta-cells likely reflecting decreased HDL-mediated cholesterol efflux.45 However, statins would likely reduce beta cell cholesterol accumulation, so this is not an adequate explanation. There is high expression of CETP in insulin target tissues such as adipose and muscle,46 raising the possibility of a local effect related to insulin sensitization. Finally, anacetrapib treatment on a background of statin therapy causes an increase in TG/apoB ratio of newly secreted TRL, reflecting decreased TG-CE interchange between TRL and HDL, and this TG enrichment of large TRL may increase susceptibility to lipoprotein lipase-mediated lipolysis.20 Enhanced activity of lipoprotein lipase through various mechanisms is associated with decreased risk of both CHD and diabetes47, 48 and this could contribute to the decrease in diabetes associated with use of anacetrapib.5
Is there a light at the end of the tunnel?
The results of the REVEAL trial suggest that CETP inhibitors could represent a useful addition to the armamentarium of drugs currently being used to treat high risk subjects intolerant to or not adequately treated with statins. The clinical use of evolocumab in patients who might benefit from this treatment has so far proven to be less than anticipated, in large part reflecting resistance from third party payers, and possibly from patients due to the need for subcutaneous injections. Thus, cost and convenience will likely be a major factor in the uptake of lipid lowering therapies with incremental benefits over generic statins. As noted above, the side effect profile for anacetrapib is distinct from statins and PCSK9 deficiency, in particular the decrease in diabetes risk (although small) may be viewed beneficially by patients and physicians. Although the increase in mean BP is small, in individual patients it may be larger and BP would need to be closely monitored. Interestingly, CETP inhibitors appear to be more effective as monotherapy than when used with statins. The impact on both reducing apoB levels and on increasing cholesterol efflux capacity is substantially more (about 1.5-2.0 fold) when potent CETP inhibitors are used alone versus when they are added to statins.49, 50 In earlier trials (Table) the degree of LDL-C lowering by CETP inhibitors was overestimated likely reflecting changes in apoB particle composition34 and as a practical matter non-HDL-C should be used in the future to assess the effects of CETP inhibition.51 As monotherapy, the decreases in LDL-C and apoB are similar to the large effects on centrifugally separated LDL cholesterol (−40%) and apoB (−35%) that were observed in complete genetic CETP deficiency.1 This likely reflects the fact that CETP inhibition and statins both act in the same pathway to lower LDL apoB, i.e. by increasing activity of the LDLR, limiting the incremental benefit of the CETP inhibitor. Thus, CETP inhibitors should be further evaluated for use as monotherapy, for example in statin intolerant individuals. In summary, in a trial much larger and more than twice as long as previous trials, the CETP inhibitor anacetrapib was convincingly found to reduce coronary events. While ongoing monitoring and a careful review of safety is essential, anacetrapib could find its way to clinical use as another option for patients with coronary disease who require further reduction in LDL and/or non-HDL cholesterol.
Acknowledgments
SOURCES OF FUNDING
ART was supported by NIH grant HL107653 and DJR by NIH grant HL111398.
Nonstandard Abbreviations and Acronyms
- CETP
cholesteryl ester transfer protein
- REVEAL
Randomized Evaluation of the Effects of Anacetrapib through Lipid modification
- FOURIER
Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk
- ACCELERATE
Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition with Evacetrapib in Patients with a High Risk for Vascular Outcomes
- IMPROVE-IT
The Improved Reduction of Outcomes: Vytorin Efficacy International Trial
- DEFINE
Determining the Efficacy and tolerability of CETP inhibition with anacetrapib
Footnotes
DISCLOSURES
Alan Tall: Consulting: Dalcor, Amgen, CSL, Equity: Staten Biotechnology.
Dan Rader: Consulting: Alnylam, Dalcor, Novartis, Pfizer, Equity: VascularStrategies, Staten Biotechnology.
References
- 1.Inazu A, Brown ML, Hesler CB, Agellon LB, Koizumi J, Takata K, Maruhama Y, Mabuchi H, Tall AR. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. The New England journal of medicine. 1990;323:1234–8. doi: 10.1056/NEJM199011013231803. [DOI] [PubMed] [Google Scholar]
- 2.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B, Investigators I Effects of torcetrapib in patients at high risk for coronary events. The New England journal of medicine. 2007;357:2109–22. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS, dal OI. Effects of dalcetrapib in patients with a recent acute coronary syndrome. The New England journal of medicine. 2012;367:2089–99. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 4.Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, Gibson CM, Granger C, Menon V, Montalescot G, Rader D, Tall AR, McErlean E, Wolski K, Ruotolo G, Vangerow B, Weerakkody G, Goodman SG, Conde D, McGuire DK, Nicolau JC, Leiva-Pons JL, Pesant Y, Li W, Kandath D, Kouz S, Tahirkheli N, Mason D, Nissen SE, Investigators A Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. The New England journal of medicine. 2017;376:1933–1942. doi: 10.1056/NEJMoa1609581. [DOI] [PubMed] [Google Scholar]
- 5.Group HTRC. Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, Wiviott SD, Cannon CP, Braunwald E, Sammons E, Landray MJ. Effects of Anacetrapib in Patients with Atherosclerotic Vascular Disease. The New England journal of medicine. 2017;377:1217–1227. doi: 10.1056/NEJMoa1706444. [DOI] [PubMed] [Google Scholar]
- 6.Tall AR. Plasma cholesteryl ester transfer protein. Journal of lipid research. 1993;34:1255–74. [PubMed] [Google Scholar]
- 7.Barter PJ, Nicholls SJ, Kastelein JJ, Rye KA. Is Cholesteryl Ester Transfer Protein Inhibition an Effective Strategy to Reduce Cardiovascular Risk? CETP Inhibition as a Strategy to Reduce Cardiovascular Risk: The Pro Case. Circulation. 2015;132:423–32. doi: 10.1161/CIRCULATIONAHA.114.014025. [DOI] [PubMed] [Google Scholar]
- 8.Barter PJ, Brewer HB, Jr, Chapman MJ, Hennekens CH, Rader DJ, Tall AR. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:160–7. doi: 10.1161/01.atv.0000054658.91146.64. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Lei D, Peng B, Yang M, Zhang L, Art Charles M, Rye KA, Krauss RM, Johns DG, Ren G. Assessing the mechanisms of cholesteryl ester transfer protein inhibitors. Biochimica et biophysica acta. 2017 doi: 10.1016/j.bbalip.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Niimi M, Yang D, Liang J, Xu J, Kimura T, Mathew AV, Guo Y, Fan Y, Zhu T, Song J, Ackermann R, Koike Y, Schwendeman A, Lai L, Pennathur S, Garcia-Barrio M, Fan J, Chen YE. Deficiency of Cholesteryl Ester Transfer Protein Protects Against Atherosclerosis in Rabbits. Arteriosclerosis, thrombosis, and vascular biology. 2017;37:1068–1075. doi: 10.1161/ATVBAHA.117.309114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johannsen TH, Frikke-Schmidt R, Schou J, Nordestgaard BG, Tybjaerg-Hansen A. Genetic inhibition of CETP, ischemic vascular disease and mortality, and possible adverse effects. Journal of the American College of Cardiology. 2012;60:2041–8. doi: 10.1016/j.jacc.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 12.Nomura A, Won HH, Khera AV, Takeuchi F, Ito K, McCarthy S, Emdin CA, Klarin D, Natarajan P, Zekavat SM, Gupta N, Peloso GM, Borecki IB, Teslovich TM, Asselta R, Duga S, Merlini PA, Correa A, Kessler T, Wilson JG, Bown MJ, Hall AS, Braund PS, Carey DJ, Murray MF, Kirchner HL, Leader JB, Lavage DR, Manus JN, Hartze DN, Samani NJ, Schunkert H, Marrugat J, Elosua R, McPherson R, Farrall M, Watkins H, Juang JJ, Hsiung CA, Lin SY, Wang JS, Tada H, Kawashiri MA, Inazu A, Yamagishi M, Katsuya T, Nakashima E, Nakatochi M, Yamamoto K, Yokota M, Momozawa Y, Rotter JI, Lander ES, Rader DJ, Danesh J, Ardissino D, Gabriel S, Willer CJ, Abecasis GR, Saleheen D, Kubo M, Kato N, Ida Chen YD, Dewey FE, Kathiresan S. Protein-Truncating Variants at the Cholesteryl Ester Transfer Protein Gene and Risk for Coronary Heart Disease. Circulation research. 2017;121:81–88. doi: 10.1161/CIRCRESAHA.117.311145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb TR, Erdmann J, Stirrups KE, Stitziel NO, Masca NG, Jansen H, Kanoni S, Nelson CP, Ferrario PG, Konig IR, Eicher JD, Johnson AD, Hamby SE, Betsholtz C, Ruusalepp A, Franzen O, Schadt EE, Bjorkegren JL, Weeke PE, Auer PL, Schick UM, Lu Y, Zhang H, Dube MP, Goel A, Farrall M, Peloso GM, Won HH, Do R, van Iperen E, Kruppa J, Mahajan A, Scott RA, Willenborg C, Braund PS, van Capelleveen JC, Doney AS, Donnelly LA, Asselta R, Merlini PA, Duga S, Marziliano N, Denny JC, Shaffer C, El-Mokhtari NE, Franke A, Heilmann S, Hengstenberg C, Hoffmann P, Holmen OL, Hveem K, Jansson JH, Jockel KH, Kessler T, Kriebel J, Laugwitz KL, Marouli E, Martinelli N, McCarthy MI, Van Zuydam NR, Meisinger C, Esko T, Mihailov E, Escher SA, Alver M, Moebus S, Morris AD, Virtamo J, Nikpay M, Olivieri O, Provost S, AlQarawi A, Robertson NR, Akinsansya KO, Reilly DF, Vogt TF, Yin W, Asselbergs FW, Kooperberg C, Jackson RD, Stahl E, Muller-Nurasyid M, Strauch K, Varga TV, Waldenberger M, Wellcome Trust Case Control C. Zeng L, Chowdhury R, Salomaa V, Ford I, Jukema JW, Amouyel P, Kontto J, Investigators M. Nordestgaard BG, Ferrieres J, Saleheen D, Sattar N, Surendran P, Wagner A, Young R, Howson JM, Butterworth AS, Danesh J, Ardissino D, Bottinger EP, Erbel R, Franks PW, Girelli D, Hall AS, Hovingh GK, Kastrati A, Lieb W, Meitinger T, Kraus WE, Shah SH, McPherson R, Orho-Melander M, Melander O, Metspalu A, Palmer CN, Peters A, Rader DJ, Reilly MP, Loos RJ, Reiner AP, Roden DM, Tardif JC, Thompson JR, Wareham NJ, Watkins H, Willer CJ, Samani NJ, Schunkert H, Deloukas P, Kathiresan S, Myocardial Infarction G and Investigators CAEC Systematic Evaluation of Pleiotropy Identifies 6 Further Loci Associated With Coronary Artery Disease. Journal of the American College of Cardiology. 2017;69:823–836. doi: 10.1016/j.jacc.2016.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitlock ME, Swenson TL, Ramakrishnan R, Leonard MT, Marcel YL, Milne RW, Tall AR. Monoclonal antibody inhibition of cholesteryl ester transfer protein activity in the rabbit. Effects on lipoprotein composition and high density lipoprotein cholesteryl ester metabolism. The Journal of clinical investigation. 1989;84:129–37. doi: 10.1172/JCI114132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikewaki K, Rader DJ, Sakamoto T, Nishiwaki M, Wakimoto N, Schaefer JR, Ishikawa T, Fairwell T, Zech LA, Nakamura H, et al. Delayed catabolism of high density lipoprotein apolipoproteins A-I and A-II in human cholesteryl ester transfer protein deficiency. The Journal of clinical investigation. 1993;92:1650–8. doi: 10.1172/JCI116750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koizumi J, Inazu A, Yagi K, Koizumi I, Uno Y, Kajinami K, Miyamoto S, Moulin P, Tall AR, Mabuchi H, et al. Serum lipoprotein lipid concentration and composition in homozygous and heterozygous patients with cholesteryl ester transfer protein deficiency. Atherosclerosis. 1991;90:189–96. doi: 10.1016/0021-9150(91)90114-i. [DOI] [PubMed] [Google Scholar]
- 17.Millar JS, Reyes-Soffer G, Jumes P, Dunbar RL, deGoma EM, Baer AL, Karmally W, Donovan DS, Rafeek H, Pollan L, Tohyama J, Johnson-Levonas AO, Wagner JA, Holleran S, Obunike J, Liu Y, Ramakrishnan R, Lassman ME, Gutstein DE, Ginsberg HN, Rader DJ. Anacetrapib lowers LDL by increasing ApoB clearance in mildly hypercholesterolemic subjects. The Journal of clinical investigation. 2015;125:2510–22. doi: 10.1172/JCI80025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikewaki K, Nishiwaki M, Sakamoto T, Ishikawa T, Fairwell T, Zech LA, Nagano M, Nakamura H, Brewer HB, Jr, Rader DJ. Increased catabolic rate of low density lipoproteins in humans with cholesteryl ester transfer protein deficiency. The Journal of clinical investigation. 1995;96:1573–81. doi: 10.1172/JCI118196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas T, Zhou H, Karmally W, Ramakrishnan R, Holleran S, Liu Y, Jumes P, Wagner JA, Hubbard B, Previs SF, Roddy T, Johnson-Levonas AO, Gutstein DE, Marcovina SM, Rader DJ, Ginsberg HN, Millar JS, Reyes-Soffer G. CETP (Cholesteryl Ester Transfer Protein) Inhibition With Anacetrapib Decreases Production of Lipoprotein(a) in Mildly Hypercholesterolemic Subjects. Arteriosclerosis, thrombosis, and vascular biology. 2017;37:1770–1775. doi: 10.1161/ATVBAHA.117.309549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millar JS, Lassman ME, Thomas T, Ramakrishnan R, Jumes P, Dunbar RL, deGoma EM, Baer AL, Karmally W, Donovan DS, Rafeek H, Wagner JA, Holleran S, Obunike J, Liu Y, Aoujil S, Standiford T, Gutstein DE, Ginsberg HN, Rader DJ, Reyes-Soffer G. Effects of CETP inhibition with anacetrapib on metabolism of VLDL-TG and plasma apolipoproteins C-II, C-III, and E. Journal of lipid research. 2017;58:1214–1220. doi: 10.1194/jlr.M074880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang XC, Masucci-Magoulas L, Mar J, Lin M, Walsh A, Breslow JL, Tall A. Down-regulation of mRNA for the low density lipoprotein receptor in transgenic mice containing the gene for human cholesteryl ester transfer protein. Mechanism to explain accumulation of lipoprotein B particles. The Journal of biological chemistry. 1993;268:27406–12. [PubMed] [Google Scholar]
- 22.Yvan-Charvet L, Kling J, Pagler T, Li H, Hubbard B, Fisher T, Sparrow CP, Taggart AK, Tall AR. Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:1430–8. doi: 10.1161/ATVBAHA.110.207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. The New England journal of medicine. 2014;371:2383–93. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. The New England journal of medicine. 2011;364:127–35. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholls SJ, Ruotolo G, Brewer HB, Kane JP, Wang MD, Krueger KA, Adelman SJ, Nissen SE, Rader DJ. Cholesterol Efflux Capacity and Pre-Beta-1 HDL Concentrations Are Increased in Dyslipidemic Patients Treated With Evacetrapib. Journal of the American College of Cardiology. 2015;66:2201–10. doi: 10.1016/j.jacc.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Holm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart A, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland-van der Zee AH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, Konig IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schafer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O’Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet (London, England) 2012;380:572–80. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL, Chang HY, Demirkan A, Den Hertog HM, Donnelly LA, Ehret GB, Esko T, Feitosa MF, Ferreira T, Fischer K, Fontanillas P, Fraser RM, Freitag DF, Gurdasani D, Heikkila K, Hypponen E, Isaacs A, Jackson AU, Johansson A, Johnson T, Kaakinen M, Kettunen J, Kleber ME, Li X, Luan J, Lyytikainen LP, Magnusson PK, Mangino M, Mihailov E, Montasser ME, Muller-Nurasyid M, Nolte IM, O’Connell JR, Palmer CD, Perola M, Petersen AK, Sanna S, Saxena R, Service SK, Shah S, Shungin D, Sidore C, Song C, Strawbridge RJ, Surakka I, Tanaka T, Teslovich TM, Thorleifsson G, Van den Herik EG, Voight BF, Volcik KA, Waite LL, Wong A, Wu Y, Zhang W, Absher D, Asiki G, Barroso I, Been LF, Bolton JL, Bonnycastle LL, Brambilla P, Burnett MS, Cesana G, Dimitriou M, Doney AS, Doring A, Elliott P, Epstein SE, Eyjolfsson GI, Gigante B, Goodarzi MO, Grallert H, Gravito ML, Groves CJ, Hallmans G, Hartikainen AL, Hayward C, Hernandez D, Hicks AA, Holm H, Hung YJ, Illig T, Jones MR, Kaleebu P, Kastelein JJ, Khaw KT, Kim E, Klopp N, Komulainen P, Kumari M, Langenberg C, Lehtimaki T, Lin SY, Lindstrom J, Loos RJ, Mach F, McArdle WL, Meisinger C, Mitchell BD, Muller G, Nagaraja R, Narisu N, Nieminen TV, Nsubuga RN, Olafsson I, Ong KK, Palotie A, Papamarkou T, Pomilla C, Pouta A, Rader DJ, Reilly MP, Ridker PM, Rivadeneira F, Rudan I, Ruokonen A, Samani N, Scharnagl H, Seeley J, Silander K, Stancakova A, Stirrups K, Swift AJ, Tiret L, Uitterlinden AG, van Pelt LJ, Vedantam S, Wainwright N, Wijmenga C, Wild SH, Willemsen G, Wilsgaard T, Wilson JF, Young EH, Zhao JH, Adair LS, Arveiler D, Assimes TL, Bandinelli S, Bennett F, Bochud M, Boehm BO, Boomsma DI, Borecki IB, Bornstein SR, Bovet P, Burnier M, Campbell H, Chakravarti A, Chambers JC, Chen YD, Collins FS, Cooper RS, Danesh J, Dedoussis G, de Faire U, Feranil AB, Ferrieres J, Ferrucci L, Freimer NB, Gieger C, Groop LC, Gudnason V, Gyllensten U, Hamsten A, Harris TB, Hingorani A, Hirschhorn JN, Hofman A, Hovingh GK, Hsiung CA, Humphries SE, Hunt SC, Hveem K, Iribarren C, Jarvelin MR, Jula A, Kahonen M, Kaprio J, Kesaniemi A, Kivimaki M, Kooner JS, Koudstaal PJ, Krauss RM, Kuh D, Kuusisto J, Kyvik KO, Laakso M, Lakka TA, Lind L, Lindgren CM, Martin NG, Marz W, McCarthy MI, McKenzie CA, Meneton P, Metspalu A, Moilanen L, Morris AD, Munroe PB, Njolstad I, Pedersen NL, Power C, Pramstaller PP, Price JF, Psaty BM, Quertermous T, Rauramaa R, Saleheen D, Salomaa V, Sanghera DK, Saramies J, Schwarz PE, Sheu WH, Shuldiner AR, Siegbahn A, Spector TD, Stefansson K, Strachan DP, Tayo BO, Tremoli E, Tuomilehto J, Uusitupa M, van Duijn CM, Vollenweider P, Wallentin L, Wareham NJ, Whitfield JB, Wolffenbuttel BH, Altshuler D, Ordovas JM, Boerwinkle E, Palmer CN, Thorsteinsdottir U, Chasman DI, Rotter JI, Franks PW, Ripatti S, Cupples LA, Sandhu MS, Rich SS, Boehnke M, Deloukas P, Mohlke KL, Ingelsson E, Abecasis GR, Daly MJ, Neale BM, Kathiresan S. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nature genetics. 2013;45:1345–52. doi: 10.1038/ng.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messas N, Dube MP, Tardif JC. Pharmacogenetics of Lipid-Lowering Agents: an Update Review on Genotype-Dependent Effects of HDL-Targetingand Statin Therapies. Current atherosclerosis reports. 2017;19:43. doi: 10.1007/s11883-017-0679-5. [DOI] [PubMed] [Google Scholar]
- 29.Tardif JC, Rhainds D, Brodeur M, Feroz Zada Y, Fouodjio R, Provost S, Boule M, Alem S, Gregoire JC, L’Allier PL, Ibrahim R, Guertin MC, Mongrain I, Olsson AG, Schwartz GG, Rheaume E, Dube MP. Genotype-Dependent Effects of Dalcetrapib on Cholesterol Efflux and Inflammation: Concordance With Clinical Outcomes. Circulation Cardiovascular genetics. 2016;9:340–8. doi: 10.1161/CIRCGENETICS.116.001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronsein GE, Heinecke JW. Time to ditch HDL-C as a measure of HDL function? Current opinion in lipidology. 2017;28:414–418. doi: 10.1097/MOL.0000000000000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westerterp M, Wang N, Tall AR. High-Density Lipoproteins, Endothelial Function, and Mendelian Randomization. Circulation research. 2016;119:13–5. doi: 10.1161/CIRCRESAHA.116.309116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Didichenko SA, Navdaev AV, Cukier AM, Gille A, Schuetz P, Spycher MO, Therond P, Chapman MJ, Kontush A, Wright SD. Enhanced HDL Functionality in Small HDL Species Produced Upon Remodeling of HDL by Reconstituted HDL, CSL112: Effects on Cholesterol Efflux, Anti-Inflammatory and Antioxidative Activity. Circulation research. 2016;119:751–63. doi: 10.1161/CIRCRESAHA.116.308685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R, Cholesterol Treatment Trialists C Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet (London, England) 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 34.Davidson M, Liu SX, Barter P, Brinton EA, Cannon CP, Gotto AM, Jr, Leary ET, Shah S, Stepanavage M, Mitchel Y, Dansky HM. Measurement of LDL-C after treatment with the CETP inhibitor anacetrapib. Journal of lipid research. 2013;54:467–72. doi: 10.1194/jlr.M032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cannon CP, Blazing MA, Braunwald E. Ezetimibe plus a Statin after Acute Coronary Syndromes. The New England journal of medicine. 2015;373:1476–7. doi: 10.1056/NEJMc1509363. [DOI] [PubMed] [Google Scholar]
- 36.Cannon CP, Shah S, Dansky HM, Davidson M, Brinton EA, Gotto AM, Stepanavage M, Liu SX, Gibbons P, Ashraf TB, Zafarino J, Mitchel Y, Barter P, Determining the E and Tolerability I Safety of anacetrapib in patients with or at high risk for coronary heart disease. The New England journal of medicine. 2010;363:2406–15. doi: 10.1056/NEJMoa1009744. [DOI] [PubMed] [Google Scholar]
- 37.Simic B, Hermann M, Shaw SG, Bigler L, Stalder U, Dorries C, Besler C, Luscher TF, Ruschitzka F. Torcetrapib impairs endothelial function in hypertension. European heart journal. 2012;33:1615–24. doi: 10.1093/eurheartj/ehr348. [DOI] [PubMed] [Google Scholar]
- 38.Anderson CD, Falcone GJ, Phuah CL, Radmanesh F, Brouwers HB, Battey TW, Biffi A, Peloso GM, Liu DJ, Ayres AM, Goldstein JN, Viswanathan A, Greenberg SM, Selim M, Meschia JF, Brown DL, Worrall BB, Silliman SL, Tirschwell DL, Flaherty ML, Kraft P, Jagiella JM, Schmidt H, Hansen BM, Jimenez-Conde J, Giralt-Steinhauer E, Elosua R, Cuadrado-Godia E, Soriano C, van Nieuwenhuizen KM, Klijn CJ, Rannikmae K, Samarasekera N, Al-Shahi Salman R, Sudlow CL, Deary IJ, Morotti A, Pezzini A, Pera J, Urbanik A, Pichler A, Enzinger C, Norrving B, Montaner J, Fernandez-Cadenas I, Delgado P, Roquer J, Lindgren A, Slowik A, Schmidt R, Kidwell CS, Kittner SJ, Waddy SP, Langefeld CD, Abecasis G, Willer CJ, Kathiresan S, Woo D, Rosand J, Global Lipids Genetics C. International Stroke Genetics C Genetic variants in CETP increase risk of intracerebral hemorrhage. Annals of neurology. 2016;80:730–740. doi: 10.1002/ana.24780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong S, Sharp DS, Grove JS, Bruce C, Yano K, Curb JD, Tall AR. Increased coronary heart disease in Japanese-American men with mutation in the cholesteryl ester transfer protein gene despite increased HDL levels. The Journal of clinical investigation. 1996;97:2917–23. doi: 10.1172/JCI118751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng CY, Yamashiro K, Chen LJ, Ahn J, Huang L, Huang L, Cheung CM, Miyake M, Cackett PD, Yeo IY, Laude A, Mathur R, Pang J, Sim KS, Koh AH, Chen P, Lee SY, Wong D, Chan CM, Loh BK, Sun Y, Davila S, Nakata I, Nakanishi H, Akagi-Kurashige Y, Gotoh N, Tsujikawa A, Matsuda F, Mori K, Yoneya S, Sakurada Y, Iijima H, Iida T, Honda S, Lai TY, Tam PO, Chen H, Tang S, Ding X, Wen F, Lu F, Zhang X, Shi Y, Zhao P, Zhao B, Sang J, Gong B, Dorajoo R, Yuan JM, Koh WP, van Dam RM, Friedlander Y, Lin Y, Hibberd ML, Foo JN, Wang N, Wong CH, Tan GS, Park SJ, Bhargava M, Gopal L, Naing T, Liao J, Ong PG, Mitchell P, Zhou P, Xie X, Liang J, Mei J, Jin X, Saw SM, Ozaki M, Mizoguchi T, Kurimoto Y, Woo SJ, Chung H, Yu HG, Shin JY, Park DH, Kim IT, Chang W, Sagong M, Lee SJ, Kim HW, Lee JE, Li Y, Liu J, Teo YY, Heng CK, Lim TH, Yang SK, Song K, Vithana EN, Aung T, Bei JX, Zeng YX, Tai ES, Li XX, Yang Z, Park KH, Pang CP, Yoshimura N, Wong TY, Khor CC. New loci and coding variants confer risk for age-related macular degeneration in East Asians. Nature communications. 2015;6:6063. doi: 10.1038/ncomms7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen W, Stambolian D, Edwards AO, Branham KE, Othman M, Jakobsdottir J, Tosakulwong N, Pericak-Vance MA, Campochiaro PA, Klein ML, Tan PL, Conley YP, Kanda A, Kopplin L, Li Y, Augustaitis KJ, Karoukis AJ, Scott WK, Agarwal A, Kovach JL, Schwartz SG, Postel EA, Brooks M, Baratz KH, Brown WL, Complications of Age-Related Macular Degeneration Prevention Trial Research G. Brucker AJ, Orlin A, Brown G, Ho A, Regillo C, Donoso L, Tian L, Kaderli B, Hadley D, Hagstrom SA, Peachey NS, Klein R, Klein BE, Gotoh N, Yamashiro K, Ferris F, III, Fagerness JA, Reynolds R, Farrer LA, Kim IK, Miller JW, Corton M, Carracedo A, Sanchez-Salorio M, Pugh EW, Doheny KF, Brion M, Deangelis MM, Weeks DE, Zack DJ, Chew EY, Heckenlively JR, Yoshimura N, Iyengar SK, Francis PJ, Katsanis N, Seddon JM, Haines JL, Gorin MB, Abecasis GR, Swaroop A. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7401–6. doi: 10.1073/pnas.0912702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishna R, Gheyas F, Liu Y, Hagen DR, Walker B, Chawla A, Cote J, Blaustein RO, Gutstein DE. Chronic Administration of Anacetrapib Is Associated With Accumulation in Adipose and Slow Elimination. Clinical pharmacology and therapeutics. 2017 doi: 10.1002/cpt.700. [DOI] [PubMed] [Google Scholar]
- 43.Barter PJ, Rye KA, Tardif JC, Waters DD, Boekholdt SM, Breazna A, Kastelein JJ. Effect of torcetrapib on glucose, insulin, and hemoglobin A1c in subjects in the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) trial. Circulation. 2011;124:555–62. doi: 10.1161/CIRCULATIONAHA.111.018259. [DOI] [PubMed] [Google Scholar]
- 44.Ference BA, Robinson JG, Brook RD, Catapano AL, Chapman MJ, Neff DR, Voros S, Giugliano RP, Davey Smith G, Fazio S, Sabatine MS. Variation in PCSK9 and HMGCR and Risk of Cardiovascular Disease and Diabetes. The New England journal of medicine. 2016;375:2144–2153. doi: 10.1056/NEJMoa1604304. [DOI] [PubMed] [Google Scholar]
- 45.Cochran BJ, Hou L, Manavalan AP, Moore BM, Tabet F, Sultana A, Cuesta Torres L, Tang S, Shrestha S, Senanayake P, Patel M, Ryder WJ, Bongers A, Maraninchi M, Wasinger VC, Westerterp M, Tall AR, Barter PJ, Rye KA. Impact of Perturbed Pancreatic beta-Cell Cholesterol Homeostasis on Adipose Tissue and Skeletal Muscle Metabolism. Diabetes. 2016;65:3610–3620. doi: 10.2337/db16-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang XC, Moulin P, Quinet E, Goldberg IJ, Yacoub LK, Agellon LB, Compton D, Schnitzer-Polokoff R, Tall AR. Mammalian adipose tissue and muscle are major sources of lipid transfer protein mRNA. The Journal of biological chemistry. 1991;266:4631–9. [PubMed] [Google Scholar]
- 47.Lotta LA, Gulati P, Day FR, Payne F, Ongen H, van de Bunt M, Gaulton KJ, Eicher JD, Sharp SJ, Luan J, De Lucia Rolfe E, Stewart ID, Wheeler E, Willems SM, Adams C, Yaghootkar H, Consortium EP-I. Cambridge FC, Forouhi NG, Khaw KT, Johnson AD, Semple RK, Frayling T, Perry JR, Dermitzakis E, McCarthy MI, Barroso I, Wareham NJ, Savage DB, Langenberg C, O’Rahilly S, Scott RA. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nature genetics. 2017;49:17–26. doi: 10.1038/ng.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tall AR. Increasing Lipolysis and Reducing Atherosclerosis. The New England journal of medicine. 2017;377:280–283. doi: 10.1056/NEJMe1706907. [DOI] [PubMed] [Google Scholar]
- 49.Hovingh GK, Kastelein JJ, van Deventer SJ, Round P, Ford J, Saleheen D, Rader DJ, Brewer HB, Barter PJ. Cholesterol ester transfer protein inhibition by TA-8995 in patients with mild dyslipidaemia (TULIP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet (London, England) 2015;386:452–60. doi: 10.1016/S0140-6736(15)60158-1. [DOI] [PubMed] [Google Scholar]
- 50.Nicholls SJ, Brewer HB, Kastelein JJ, Krueger KA, Wang MD, Shao M, Hu B, McErlean E, Nissen SE. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol: a randomized controlled trial. Jama. 2011;306:2099–109. doi: 10.1001/jama.2011.1649. [DOI] [PubMed] [Google Scholar]
- 51.Hegele RA. CETP Inhibitors - A New Inning? The New England journal of medicine. 2017;377:1284–1285. doi: 10.1056/NEJMe1711407. [DOI] [PubMed] [Google Scholar]


