Abstract
The pathogenesis of human obesity is the result of dysregulation of the reciprocal relationship between food intake and energy expenditure (EE), which influences daily energy balance and ultimately leads to weight gain. According to principles of energy homeostasis, a relatively lower EE in a setting of energy balance may lead to weight gain; however, results from different study groups are contradictory and indicate a complex interaction between EE and food intake which may differentially influence weight change in humans. Recently, studies evaluating the adaptive response of one component to perturbations of the other component of energy balance have revealed both the existence of differing metabolic phenotypes (“spendthrift” and “thrifty”) resulting from overeating or underfeeding, as well as energy-sensing mechanisms linking EE to food intake, which might explain the propensity of an individual to weight gain.
The purpose of this review is to debate the role that human EE plays on body weight regulation and to discuss the physiologic mechanisms linking EE and food intake. An increased understanding of the complex interplay between human metabolism and food consumption may provide insight into pathophysiologic mechanisms underlying weight gain, which may eventually lead to prevention and better treatment of human obesity.
Keywords: energy expenditure, adaptive thermogenesis, body weight regulation, metabolic phenotypes, energy sensing
Introduction
The fundamental principle of energy balance applied to humans states that food intake and energy expenditure (EE) are the counterbalancing factors that determine body weight change. For instance, a persistent state of positive energy balance when food intake consistently exceeds EE leads to weight gain and eventually obesity onset, as the surplus energy is stored by the organism as predominantly body fat. Both food intake and EE are likely influenced by environmental and genetic factors; however, while food intake can vary largely from day to day due to psychological and social influences and it is hard to measure due to the episodic nature consisting in discrete feeding episodes, daily sedentary EE is largely determined by body size and composition and can be accurately measured via indirect calorimetry [1,2]. As EE represents the more stable side of the energy balance equation, in the last 40 years it has been extensively studied to assess its effects on future weight change and to gain insight into the pathogenesis of human obesity. This review will focus on the role that EE on body weight regulation in humans. The physiologic mechanisms that may underlie the EE-food intake link will also be presented and discussed.
EE measured in energy balance as predictor of future weight change
Human EE can be continuously and precisely measured via indirect calorimetry methods [3,4]. Typically, 24-h EE is measured inside a whole-room indirect calorimeter (also known as respiratory or metabolic chamber) in sedentary but physiologic conditions while trying to achieve perfect energy balance (i.e., energy equilibrium where EE equals food intake), namely, providing subjects with the exact amount of energy from food they expend over the 24 hours [5,6]. Measuring EE in energy balance and weight stability is crucial as a subject's EE depends on prior fluctuations in body weight and deviations from energy balance which may alter substrate oxidation (e.g., the preference of carbohydrate over fat oxidation), ultimately influencing the measured value of 24-h EE.
Known determinants of 24-h EE include body fat free mass (FFM) and fat mass (FM), age, gender, ethnicity, glucose tolerance [2,1,7-9] but also familial relationships reflecting an underlying genetic background [10-12] (heritability=0.52 [12]). Together, all the physiologic determinants explain more than 80% of the inter-individual variance in EE in a given population [1], leaving the remaining (unexplained) variance in EE after statistical adjustment for subjects' characteristics as a potential predictor of future weight change based on the energy balance equation. Nevertheless, contradictory results on the role of EE on weight change have been reported in different studies. Concordant with the energy balance principles, a relatively low EE is a predictor of long-term weight gain in an American Indians population living in the Southwest of the United States [11,13,14]. Similarly, a reduced EE predicting increased adiposity was also observed in an Italian population [15] and in studies of infants [16] and children [17]. Conversely, the opposite relationship was shown in a Nigerian population where a higher (instead of lower) EE was associated with increases in body weight over time [18]. Lastly, several reports have shown no association between EE and weight change in humans [19-22]. Potential explanations for these contradictory findings from different research groups may include population-specific body habitus, genetic and environmental factors. Variations in the research setting and differing length of the follow-up may also play role. For instance, an intrinsic deficit in daily EE carried for several years can lead to weight gain due to a sustained and persistent positive energy balance, even when daily food intake is set to achieve and maintain weight stability in the short-term period. In fact, in the context of the long-term longitudinal studies carried out in the Native American population living in Phoenix (Arizona, USA) where the large majority of individuals constantly gain weight over time during adulthood [23], a small but persistent positive daily energy balance due to a relatively lower EE may have a significantly greater impact in life-long body weight regulation in this population. By contrast, higher relative EE (as found in the study of Nigerian population) may result in a small negative daily energy balance that in turn may promote overeating in an effort to restore energy equilibrium, thereby inducing weight gain if the greater food intake is sustained over a longer period and consistently overcomes daily EE. Nonetheless, the strength of the association between EE measured during energy balance and future weight change (regardless of the directionality) is very small as it explains very little of the variance in weight change (R2<5%), indicating that food intake is a more potent determinant of daily energy balance thereby strongly influencing body weight change in humans.
Spendthrift and thrifty metabolic phenotypes
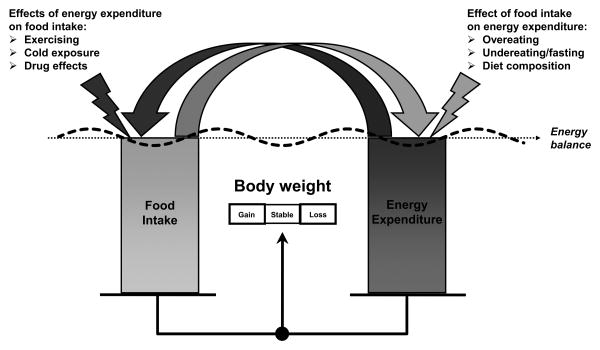
One of the major limitation of measuring EE during energy balance is that this condition is rarely achieved in free-living conditions, mostly because of daily fluctuations in food intake that may constitute a more potent driver of weight gain in humans as compared to EE. As energy balance represents a static and idealistic situation that rarely is achieved in everyday life, researchers have moved on and focused on studying EE in a setting of energy imbalance, pursuing the hypothesis that adaptive changes in EE in a setting of positive/negative energy balance may identify subjects more prone to weight gain/loss compared to others. The reason of this change in focus was justified by the fact the regulation of energy balance is a dynamic process, in which perturbations to one component (food intake or EE) may elicit biological and/or behavioral compensation (or adaptation) in the other component of the system (Figure 1). Given that large fluctuations in daily food intake are commonly experienced from day to day in normal life conditions in the current obesogenic environment, studying the EE response to extreme changes in food intake such as fasting or overfeeding may shed light into the individual susceptibility or resistance of humans to obesity.
Figure 1. Disruption of energy balance due to reciprocal effects of food intake and EE.
Among the daily components of 24-h EE including sleeping metabolic rate (the minimum energy required by a subject to maintain normal body metabolic functions), the cost of being awake and the thermic effect of food (collectively named “awake and fed” thermogenesis [24]) and the energy cost of physical activity [2], the thermic effect of food represents the EE component that underlies the link between food intake and EE. The thermic effect of food, defined as the increase in EE after meals consumption [25] and representing approximately 10% of 24-h EE measured in energy balance [26], is composed of the obligatory costs of metabolizing the ingested nutrients, as well as a proposed variable facultative component that might explain the different response of individuals to excess or reduced food intake that may ultimately determine weight change. In a small pilot cross-over study done in 14 men (7 whites and 7 Native Americans) undergoing 48-hours fasting and overfeeding with a balanced diet twice their energy requirements inside a metabolic chamber, it was shown that on average 24-h EE decreased of about 10% from its baseline value (i.e., during energy balance) with fasting and increased by a similar amount with overfeeding [27]. Very interestingly, in this same study the individual EE responses to fasting and overfeeding both showed a wide inter-subject variability and were closely related to each other, such as the subjects showing larger decreases in EE when fasting tended to have smaller increases in EE when being overfed (i.e., metabolically thrifty individuals) as opposed to subjects showing smaller decreases in EE with fasting and larger increases in EE with overfeeding (i.e., metabolically spendthrift individuals). These results indicated the existence of two human metabolic phenotypes potentially uncovered by dietary manipulations: a more metabolically efficient type that does not expend as much energy when either being overfed or fasting (thrifty) as the type that, even when overeating or fasting, expends energy at comparatively higher metabolic rates (spendthrift). The results from the pilot study identifying these thrifty and spendthrift metabolic phenotypes based on the EE responses to fasting and overfeeding [27] were recently replicated in two other independent studies including subjects from different ethnic groups [28,29]. More importantly, these two longitudinal studies showed for the first time the effects of these intrinsic metabolic characteristics on weight change.
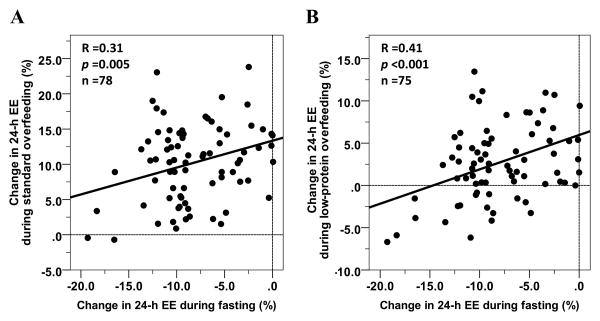
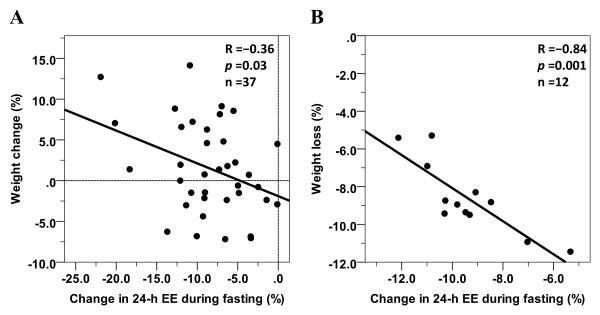
In one of these studies [28] (ClinicalTrials.gov #NCT00523627), after a baseline assessment of 24-h EE, thirty-seven healthy individuals with normal glucose regulation covering a wide range of body sizes underwent in random order 24-h sessions inside a metabolic chamber during fasting and four different overfeeding diets (200% of weight-maintaining energy needs) during a 25-day inpatient stay at the clinical research unit. Concordant with the results from the pilot study, the EE responses to fasting and overfeeding (average of the EE responses across the 4 diets that included: balanced, high-fat, high-carbohydrate and low-protein overfeeding diet) in this cohort of 37 individuals were strongly correlated, such that a larger EE decrease in response to fasting was associated with a smaller EE increase in response to overfeeding [28] (Figure 2). At 6 months following discharge when subjects returned to their usual habits (subjects were not counseled on lifestyle change), a larger decrease in EE with fasting (Figure 3A), a smaller EE increase with low-protein overfeeding and a larger EE response to high-carbohydrate overfeeding all measured during the inpatient stay, each predicted weight gain after 6 months in free-living conditions [28]. Of note, the EE responses to low-protein and high-carbohydrate overfeeding were independent from each other in predicting weight change, whereas the EE response to fasting was dependent on the EE response to low-protein overfeeding [28].
Figure 2. Relationships between the 24-h EE responses to fasting and overfeeding with a standard (Panel A) and with a low-protein (Panel B) diets.
The percentage change in 24-h EE during each dietary intervention was calculated as the difference between the 24-h EE during the dietary intervention and the 24-h EE during energy balance, divided by the 24-h EE during energy balance and expressed as a percentage [28]. Data from the #NCT00523627 study registered at ClinicalTrials.gov.
Figure 3. Inverse relationships between 24-h EE change with fasting and 6-month body weight change in free-living conditions (Panel A, [28]) and weight loss after 6 weeks of caloric restriction on a clinical inpatient unit (Panel B, [29]).
The percentage change in 24-h EE during each dietary intervention was calculated as the difference between the 24-h EE during the dietary intervention and the 24-h EE during energy balance, divided by the 24-h EE during energy balance and expressed as a percentage. Weight change/loss is expressed as absolute change in body weight divided the baseline weight and expressed as a percentage.
In the other independent study [29] (ClinicalTrials.gov #NCT00687115), twelve obese but otherwise healthy individuals had 24-h measures of EE inside a metabolic chamber during energy balance (100% of weight-maintaining energy needs), overfeeding a balanced diet (200% of weight-maintaining energy needs) and fasting in random order, followed by a 6-week 50% caloric restriction period in a carefully monitored inpatient study. More metabolic thrifty individuals (i.e., those subjects with a larger decrease in 24-h EE with fasting at baseline) showed a smaller weight loss after the caloric restriction period as compared to more spendthrift individuals (i.e., those subjects who showed a smaller decrease in EE during fasting at baseline) who instead lost the greatest amount of weight after 6 weeks [29] (Figure 3B).
Taken together, the results from the 3 above-mentioned studies provide supporting evidence about the existence of well-defined human EE phenotypes related to body weight maintenance which exist in both lean and obese individuals: an energy-efficient (“thrifty”) phenotype denoted by a lower 24-h EE, which has more propensity to spontaneous weight gain and/or smaller weight loss during caloric restriction as compared to a more “spendthrift” phenotype.
The physiologic mechanisms underlying the EE responses to dietary intervention which identify these two metabolic profiles found in the human population are still yet to be elucidated but may include percent body fat [26] and fat distribution [30], core body temperature [30], hormonal mediators such as appetitive hormones [26], sympathetic nervous system tone [31] and genetic polymorphisms.
Energy-sensing mechanisms: the putative link between EE and food intake
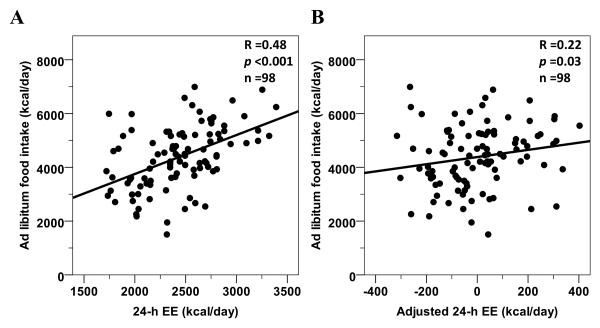
Another approach to study the link between EE and food intake in a setting of energy imbalance is to assume that EE may be the major driver of food intake under the hypothesis that EE, as reflective of body energy demand, may regulate food intake by centrally modulating hunger. From an evolutionary viewpoint, a link between EE, i.e. energy needs, and food intake would ensure that an organism would experience a physiologic drive to find enough food to maintain both life and reproduction. Although studies in overweight Native Americans have consistently found an inverse association between relatively lower EE during energy balance and future body weight change [14,11,13], another study conducted in a leaner Nigerian population has shown that a relatively higher EE predicted future weight gain [18]. It is therefore possible that a relatively higher EE may increase hunger and food-seeking behavior in most individuals, perhaps even leading in some to a greater-than-necessary intake, and eventual weight gain. Supportive of this hypothesis, a study by Caudwell et al. found a direct association between resting EE and food intake as well as hunger sensations measured during a 3-month trial [32]. Similarly, the positive association between EE and food intake was replicated in another study where ad libitum food intake was assessed in an inpatient setting by a computerized vending machine paradigm [33] (Figure 4) (ClinicalTrials.gov #NCT00342732). As EE is mainly determined by body FFM [2], the metabolically active part of the human body, and FFM has been also linked to food intake [34,32,33], it has been debated whether body composition per se or the underlying metabolism may drive food intake. Again, two cross-sectional studies from different study groups [35,36] have demonstrated that EE appears to be the physiologic mediator by which FFM exerts its effect on food intake. These results provided supporting evidence that energy-sensing mechanisms may play a major role in the pathophysiology of weight gain [37,38]. The effects of EE on food intake may act, in part, through central pathways that modulate appetite as FFM has been associated with reduced gray matter volume in regions implicated in energy homeostasis [39]. Alternatively, hormonal signals from the periphery may be potential transmitters of energy needs including adipokines and myokines released from skeletal muscle or other factors released from non-fat tissue. The former include adiponectin and leptin, hormones secreted from adipocytes to indicate the status of energy stores in the body [40-42]. As a fall in leptin levels with calorie restriction or weight loss activates the appetite-stimulating pathways in the body including the melanocortin pathway [43], leptin concentrations might also moderate any EE-food intake link, i.e., by blunting the link if the body already has adequate energy stores or by providing the signal that leads to increased food intake. Concentrations of gastrointestinal hormones known to stimulate hunger, i.e. ghrelin, or act as satiety agents, including glucagon-like peptide 1 (GLP-1), glucagon, insulin, peptide YY (PYY) and pancreatic polypeptide (PP) are also possible mediators of the body's energy sensing. Further, another potential candidate may be the thyroid hormone triiodothyronine (T3), which has been associated with EE [44], increases and decreases with weight gain and weight loss, and changes in free T3 related to weight changes are associated with changes in urinary norepinephrine, resting EE, and 24-h EE [45]. All these hormonal mediators may act in concert to influence the homeostatic link between EE and food intake, reflecting the energy requirements of the organism.
Figure 4. Positive relationships between ad libitum food intake from a vending machine inpatient study and total 24-h EE (Panel A) and 24-h EE after adjustment for its physiological determinants (Panel B).
Ad libitum food intake was the average kcals consumed over 3 days recorded by computerized vending machine systems. Twenty-four-hour EE was measured inside a whole-room calorimeter during energy balance and weight maintenance. Adjusted 24-h EE in Panel B is calculated via linear regression analysis (i.e., residuals) after adjustment for age, gender, ethnicity (Native Americans vs. whites), FM, FFM and spontaneous physical activity. Data from the #NCT00342732 study registered at ClinicalTrials.gov.
Conclusions
Research studies evaluating the adaptive response of one component (EE or food intake) to perturbations of the other component of energy balance have revealed both the existence of differing metabolic phenotypes resulting from overeating or underfeeding, as well as energy-sensing mechanisms linking EE to food intake, which might explain the propensity of an individual to weight gain. An increased understanding of the complex interplay between human metabolism and food consumption may provide insight into pathophysiologic mechanisms underlying weight gain, which may eventually lead to prevention and better treatment of human obesity.
Acknowledgments
Studies #NCT00523627, #NCT00687115 and # NCT00342732 were supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
None of the authors reported a potential conflict of interest.
References
- 1.Weyer C, Snitker S, Rising R, Bogardus C, Ravussin E. Determinants of energy expenditure and fuel utilization in man: effects of body composition, age, sex, ethnicity and glucose tolerance in 916 subjects. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1999;23(7):715–722. doi: 10.1038/sj.ijo.0800910. [DOI] [PubMed] [Google Scholar]
- 2.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. The Journal of clinical investigation. 1986;78(6):1568–1578. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam YY, Ravussin E. Analysis of energy metabolism in humans: A review of methodologies. Molecular metabolism. 2016;5(11):1057–1071. doi: 10.1016/j.molmet.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam YY, Ravussin E. Indirect calorimetry: an indispensable tool to understand and predict obesity. Eur J Clin Nutr. 2017;71(3):318–322. doi: 10.1038/ejcn.2016.220. [DOI] [PubMed] [Google Scholar]
- 5.Lam YY, Redman LM, Smith SR, Bray GA, Greenway FL, Johannsen D, Ravussin E. Determinants of sedentary 24-h energy expenditure: equations for energy prescription and adjustment in a respiratory chamber. The American journal of clinical nutrition. 2014;99(4):834–842. doi: 10.3945/ajcn.113.079566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jonge L, Nguyen T, Smith SR, Zachwieja JJ, Roy HJ, Bray GA. Prediction of energy expenditure in a whole body indirect calorimeter at both low and high levels of physical activity. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2001;25(7):929–934. doi: 10.1038/sj.ijo.0801656. [DOI] [PubMed] [Google Scholar]
- 7.Keys A, Taylor HL, Grande F. Basal metabolism and age of adult man. Metabolism: clinical and experimental. 1973;22(4):579–587. doi: 10.1016/0026-0495(73)90071-1. [DOI] [PubMed] [Google Scholar]
- 8.Bosy-Westphal A, Eichhorn C, Kutzner D, Illner K, Heller M, Muller MJ. The age-related decline in resting energy expenditure in humans is due to the loss of fat-free mass and to alterations in its metabolically active components. J Nutr. 2003;133(7):2356–2362. doi: 10.1093/jn/133.7.2356. [DOI] [PubMed] [Google Scholar]
- 9.Johnstone AM, Murison SD, Duncan JS, Rance KA, Speakman JR. Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. The American journal of clinical nutrition. 2005;82(5):941–948. doi: 10.1093/ajcn/82.5.941. [DOI] [PubMed] [Google Scholar]
- 10.Bogardus C, Lillioja S, Ravussin E, Abbott W, Zawadzki JK, Young A, Knowler WC, Jacobowitz R, Moll PP. Familial dependence of the resting metabolic rate. The New England journal of medicine. 1986;315(2):96–100. doi: 10.1056/NEJM198607103150205. [DOI] [PubMed] [Google Scholar]
- 11.Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WG, Boyce V, Howard BV, Bogardus C. Reduced rate of energy expenditure as a risk factor for body-weight gain. The New England journal of medicine. 1988;318(8):467–472. doi: 10.1056/NEJM198802253180802. [DOI] [PubMed] [Google Scholar]
- 12.Piaggi P, Masindova I, Muller YL, Mercader J, Wiessner GB, Chen P, Consortium STD, Kobes S, Hsueh WC, Mongalo M, Knowler WC, Krakoff J, Hanson RL, Bogardus C, Baier LJ. A Genome-Wide Association Study Using a Custom Genotyping Array Identifies Variants in GPR158 Associated with Reduced Energy Expenditure in American Indians. Diabetes. 2017 doi: 10.2337/db16-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tataranni PA, Harper IT, Snitker S, Del Parigi A, Vozarova B, Bunt J, Bogardus C, Ravussin E. Body weight gain in free-living Pima Indians: effect of energy intake vs expenditure. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2003;27(12):1578–1583. doi: 10.1038/sj.ijo.0802469. [DOI] [PubMed] [Google Scholar]
- 14.Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. The Journal of clinical endocrinology and metabolism. 2013;98(4):E703–707. doi: 10.1210/jc.2012-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buscemi S, Verga S, Caimi G, Cerasola G. Low relative resting metabolic rate and body weight gain in adult Caucasian Italians. International journal of obesity (2005) 2005;29(3):287–291. doi: 10.1038/sj.ijo.0802888. [DOI] [PubMed] [Google Scholar]
- 16.Roberts SB, Savage J, Coward WA, Chew B, Lucas A. Energy expenditure and intake in infants born to lean and overweight mothers. The New England journal of medicine. 1988;318(8):461–466. doi: 10.1056/NEJM198802253180801. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths M, Payne PR, Stunkard AJ, Rivers JP, Cox M. Metabolic rate and physical development in children at risk of obesity. Lancet. 1990;336(8707):76–78. doi: 10.1016/0140-6736(90)91592-x. [DOI] [PubMed] [Google Scholar]
- 18.Luke A, Durazo-Arvizu R, Cao G, Adeyemo A, Tayo B, Cooper R. Positive association between resting energy expenditure and weight gain in a lean adult population. The American journal of clinical nutrition. 2006;83(5):1076–1081. doi: 10.1093/ajcn/83.5.1076. [DOI] [PubMed] [Google Scholar]
- 19.Seidell JC, Muller DC, Sorkin JD, Andres R. Fasting respiratory exchange ratio and resting metabolic rate as predictors of weight gain: the Baltimore Longitudinal Study on Aging. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1992;16(9):667–674. [PubMed] [Google Scholar]
- 20.Weinsier RL, Nelson KM, Hensrud DD, Darnell BE, Hunter GR, Schutz Y. Metabolic predictors of obesity. Contribution of resting energy expenditure, thermic effect of food, and fuel utilization to four-year weight gain of post-obese and never-obese women. The Journal of clinical investigation. 1995;95(3):980–985. doi: 10.1172/JCI117807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anthanont P, Jensen MD. Does basal metabolic rate predict weight gain? The American journal of clinical nutrition. 2016;104(4):959–963. doi: 10.3945/ajcn.116.134965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amatruda JM, Statt MC, Welle SL. Total and resting energy expenditure in obese women reduced to ideal body weight. The Journal of clinical investigation. 1993;92(3):1236–1242. doi: 10.1172/JCI116695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Votruba SB, Thearle MS, Piaggi P, Knowler WC, Hanson RL, Krakoff J. Weight maintenance from young adult weight predicts better health outcomes. Obesity. 2014;22(11):2361–2369. doi: 10.1002/oby.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piaggi P, Krakoff J, Bogardus C, Thearle MS. Lower “awake and fed thermogenesis” predicts future weight gain in subjects with abdominal adiposity. Diabetes. 2013;62(12):4043–4051. doi: 10.2337/db13-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Secor SM. Specific dynamic action: a review of the postprandial metabolic response. Journal of comparative physiology B, Biochemical, systemic, and environmental physiology. 2009;179(1):1–56. doi: 10.1007/s00360-008-0283-7. [DOI] [PubMed] [Google Scholar]
- 26.Thearle MS, Pannacciulli N, Bonfiglio S, Pacak K, Krakoff J. Extent and determinants of thermogenic responses to 24 hours of fasting, energy balance, and five different overfeeding diets in humans. The Journal of clinical endocrinology and metabolism. 2013;98(7):2791–2799. doi: 10.1210/jc.2013-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weyer C, Vozarova B, Ravussin E, Tataranni PA. Changes in energy metabolism in response to 48 h of overfeeding and fasting in Caucasians and Pima Indians. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2001;25(5):593–600. doi: 10.1038/sj.ijo.0801610. [DOI] [PubMed] [Google Scholar]
- 28.Schlogl M, Piaggi P, Pannacciuli N, Bonfiglio SM, Krakoff J, Thearle MS. Energy Expenditure Responses to Fasting and Overfeeding Identify Phenotypes Associated With Weight Change. Diabetes. 2015;64(11):3680–3689. doi: 10.2337/db15-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reinhardt M, Thearle MS, Ibrahim M, Hohenadel MG, Bogardus C, Krakoff J, Votruba SB. A Human Thrifty Phenotype Associated With Less Weight Loss During Caloric Restriction. Diabetes. 2015;64(8):2859–2867. doi: 10.2337/db14-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reinhardt M, Schlogl M, Bonfiglio S, Votruba SB, Krakoff J, Thearle MS. Lower core body temperature and greater body fat are components of a human thrifty phenotype. International journal of obesity (2005) 2016;40(5):754–760. doi: 10.1038/ijo.2015.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vinales KL, Schlogl M, Piaggi P, Hohenadel M, Graham A, Bonfiglio S, Krakoff J, Thearle MS. The Consistency in Macronutrient Oxidation and the Role for Epinephrine in the Response to Fasting and Overfeeding. The Journal of clinical endocrinology and metabolism. 2017;102(1):279–289. doi: 10.1210/jc.2016-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caudwell P, Finlayson G, Gibbons C, Hopkins M, King N, Naslund E, Blundell JE. Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. The American journal of clinical nutrition. 2013;97(1):7–14. doi: 10.3945/ajcn.111.029975. [DOI] [PubMed] [Google Scholar]
- 33.Weise CM, Hohenadel MG, Krakoff J, Votruba SB. Body composition and energy expenditure predict ad-libitum food and macronutrient intake in humans. International journal of obesity (2005) 2014;38(2):243–251. doi: 10.1038/ijo.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blundell JE, Caudwell P, Gibbons C, Hopkins M, Naslund E, King NA, Finlayson G. Body composition and appetite: fat-free mass (but not fat mass or BMI) is positively associated with self-determined meal size and daily energy intake in humans. The British journal of nutrition. 2012;107(3):445–449. doi: 10.1017/S0007114511003138. [DOI] [PubMed] [Google Scholar]
- 35.Piaggi P, Thearle MS, Krakoff J, Votruba SB. Higher Daily Energy Expenditure and Respiratory Quotient, Rather Than Fat-Free Mass, Independently Determine Greater ad Libitum Overeating. The Journal of clinical endocrinology and metabolism. 2015;100(8):3011–3020. doi: 10.1210/jc.2015-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hopkins M, Finlayson G, Duarte C, Whybrow S, Ritz P, Horgan GW, Blundell JE, Stubbs RJ. Modelling the associations between fat-free mass, resting metabolic rate and energy intake in the context of total energy balance. International journal of obesity (2005) 2016;40(2):312–318. doi: 10.1038/ijo.2015.155. [DOI] [PubMed] [Google Scholar]
- 37.Dulloo AG, Jacquet J, Miles-Chan JL, Schutz Y. Passive and active roles of fat-free mass in the control of energy intake and body composition regulation. Eur J Clin Nutr. 2017;71(3):353–357. doi: 10.1038/ejcn.2016.256. [DOI] [PubMed] [Google Scholar]
- 38.Hopkins M, Blundell JE. Energy balance, body composition, sedentariness and appetite regulation: pathways to obesity. Clinical science (London, England : 1979) 2016;130(18):1615–1628. doi: 10.1042/CS20160006. [DOI] [PubMed] [Google Scholar]
- 39.Weise CM, Thiyyagura P, Reiman EM, Chen K, Krakoff J. Fat-free body mass but not fat mass is associated with reduced gray matter volume of cortical brain regions implicated in autonomic and homeostatic regulation. NeuroImage. 2013;64:712–721. doi: 10.1016/j.neuroimage.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 41.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nature medicine. 1995;1(11):1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 42.Rosenbaum M, Leibel RL. Leptin: a molecule integrating somatic energy stores, energy expenditure and fertility. Trends in endocrinology and metabolism: TEM. 1998;9(3):117–124. doi: 10.1016/s1043-2760(98)00028-9. [DOI] [PubMed] [Google Scholar]
- 43.Ceccarini G, Maffei M, Vitti P, Santini F. Fuel homeostasis and locomotor behavior: role of leptin and melanocortin pathways. J Endocrinol Invest. 2015;38(2):125–131. doi: 10.1007/s40618-014-0225-z. [DOI] [PubMed] [Google Scholar]
- 44.Ortega E, Pannacciulli N, Bogardus C, Krakoff J. Plasma concentrations of free triiodothyronine predict weight change in euthyroid persons. The American journal of clinical nutrition. 2007;85(2):440–445. doi: 10.1093/ajcn/85.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenbaum M, Hirsch J, Murphy E, Leibel RL. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. The American journal of clinical nutrition. 2000;71(6):1421–1432. doi: 10.1093/ajcn/71.6.1421. [DOI] [PubMed] [Google Scholar]