Abstract
Objective
We aimed to determine updated conversion factors (k-factors) enabling accurate estimation of radiation effective dose (ED) for coronary computed tomographic angiography (CCTA) and calcium scoring performed on 12 contemporary scanner models and current clinical cardiac protocols, and compare these to the standard chest k-factor of 0.014mSv·mGy−1cm−1.
Background
Accurate estimation of ED from cardiac CT scans is essential to meaningfully compare the benefits and risks of different cardiac imaging strategies, and optimize test and protocol selection. Presently, ED from cardiac CT is generally estimated by multiplying a scanner-reported parameter, the dose-length product (DLP), by a k-factor which was determined for non-cardiac chest CT using single-slice scanners and a superseded definition of ED.
Methods
Metal-oxide-semiconductor field-effect transistor radiation detectors were positioned in organs of anthropomorphic phantoms, which were scanned using all cardiac protocols–120 clinical protocols in total–on 12 CT scanners representing the spectrum of scanners for 5 manufacturers (GE, Hitachi, Philips, Siemens, Toshiba). Organ doses were determined for each protocol, and ED calculated as defined in International Commission on Radiological Protection Publication 103. EDs and scanner-reported DLPs were used to determine k-factors for each scanner model and protocol.
Results
k-factors averaged 0.026 mSv·mGy−1cm−1 (95% confidence interval: 0.0258–0.0266) and ranged between 0.020–0.035mSv·mGy−1cm−1. The standard chest k-factor underestimates ED by an average of 46%, ranging from 30–60%, depending on scanner, mode, and tube potential. Factors were higher for prospective axial vs. retrospective helical scan modes, calcium scoring vs. CCTA, and higher (100–120kV) vs. lower (80 kV) tube potential, and varied between scanner models (range of average k-factors 0.0229–0.0277mSv·mGy−1cm−1).
Conclusions
Cardiac k-factors for all scanners and protocols are considerably higher than the currently used value, suggesting that radiation doses from cardiac CT have been significantly and systematically underestimated. Using cardiac-specific factors can more accurately inform the benefit-risk calculus of cardiac imaging strategies.
Keywords: Cardiac computed tomography, radiation dose, conversion factors
Introduction
Cardiac CT has experienced tremendous advances in the past decade. A growing evidence base supports the role of coronary artery calcium scoring for risk stratification, and some guidelines now recommend it as a reasonable test to perform for asymptomatic adults at intermediate risk (1). Coronary CT angiography (CCTA) has demonstrated high accuracy for diagnosing obstructive coronary artery disease (2), the ability to improve prognostication (3), and in some settings, capability to more rapidly and cost-effectively diagnose chest pain patients (4). In many clinical contexts, CCTA now stands as an option that can be selected to guide optimal patient management and incorporated into clinical pathways.(5, 6)
Each cardiac imaging modality has strengths and weaknesses, and optimizing management requires a weighting of these features for each option in the context of the patient and clinical question. One particular concern for CCTA is its associated radiation burden. While initial studies found high radiation dose and risk (7), numerous technical advances such as prospectively-triggered axial scan modes, lower tube potentials, and iterative image reconstruction now enable, in the best-case scenario, for CTA to be performed with extremely low radiation burden, comparable to that of several chest x-rays (8). However, such low CCTA doses require a confluence of several factors: availability of these technical advances which are not all implemented on entry-level scanners, operator expertise, favorable patient heart rate/rhythm and habitus, and willingness to tolerate some image noise and limitation in the number of phases of the cardiac cycle available for interpretation. Thus, despite some patients receiving extremely low doses, many still receive considerably higher doses. Indeed, contemporary CTA practice is characterized by a wide range of radiation doses between laboratories and between patients (9), and thus the benefit-risk calculus of CTA and its comparison to other modalities may vary depending on the particular radiation dose. In particular, when taking care of patients with chest pain, the physician’s choice between CTA and nuclear myocardial perfusion imaging may depend in part on radiation burden. Such comparison is predicated on accurate radiation dosimetry for both exams.
The single parameter most commonly used to compare ionizing radiation burden between different imaging modalities, scanners, and protocols is the effective dose (ED), in units of millisieverts (mSv). ED characterizes whole-body exposure from a non-uniform radiation exposure as a weighted average of organ absorbed doses. It is presently defined in accordance with a formulation specified by the International Commission on Radiological Protection (ICRP) in its Publication 103 (10), as the sum over all specified organs of doubly weighted organ absorbed doses, where weights reflect both the relative sensitivity of each organ to radiation, and the radiation source. ED is not without limitations (11, 12); for example, the organ weights are averages for all ages and both genders, thus precluding a gender-specific ED, and ED is not patient-size dependent. Accordingly, ED is not designed for patient-specific radiation risk assessment. Nevertheless it remains the only metric that can be easily used to compare whole body radiation exposure across modalities and protocols. This has led to its great popularity in the clinical literature and clinical practice. ICRP Publication 103 (10) updated the radiation weighting factors for each organ, based on a comprehensive, updated review of the radioepidemiologic and radiobiologic evidence, and refined methodology, in comparison to the previous specification of ED in ICRP Publication 60 (13).
By far, the simplest and most commonly used method to estimate the ED for CT scans is by multiplying another radiation parameter, the dose-length-product (DLP), by a conversion factor, often referred to as a k-factor. DLP, which is limited to CT, is reported on the scanner console after each CT scan, and reflects both the intensity of the radiation exposure (in mGy) and the craniocaudal length irradiated (in cm). The k-factor that is conventionally used for cardiac scans, 0.014 mSv·mGy−1·cm−1, was introduced in European Commission guidelines for chest CT scans (14) and later adopted by the American Association of Physicists in Medicine (15). Using this chest k-factor to estimate ED from cardiac CT has numerous limitations that potentially compromise the accuracy of ED estimates. The chest k-factor was: i) never designed for cardiac studies, but rather for thoracic CT; ii) based on the older, now superseded ICRP 60 definition of ED; and iii) determined using three single-slice scanners, which technologically are markedly different from the CT scanners currently in use for cardiac CT. Moreover, the European Commission guidelines document (14) had in fact provided two different chest k-factors: 0.019 mSv·mGy−1·cm−1 in its Appendix A and 0.014 mSv·mGy−1·cm−1 in its Appendix C.
Thus, updated dosimetry is essential to ensure accurate estimation of ED from cardiac CT. Heretofore, there has been no systematic attempt to determine k-factors for the diversity of scanner models and protocols used in cardiac CT practice, and the k-factors in the literature covering a limited combination of scanners and modes have not been widely adopted. In this study, we systematically determined k-factors for all contemporary cardiac CTA scanner designs and protocols, to provide a single source of data that can be used to more accurately estimate ED of cardiac CT. Our approach was to estimate EDs from measurements performed using solid-state metal-oxide–semiconductor field-effect transistor (MOSFET) dosimeters placed in a physical anthropomorphic phantom, and to determine k-factors relating these EDs to scanner-reported DLPs.
Methods
Phantom
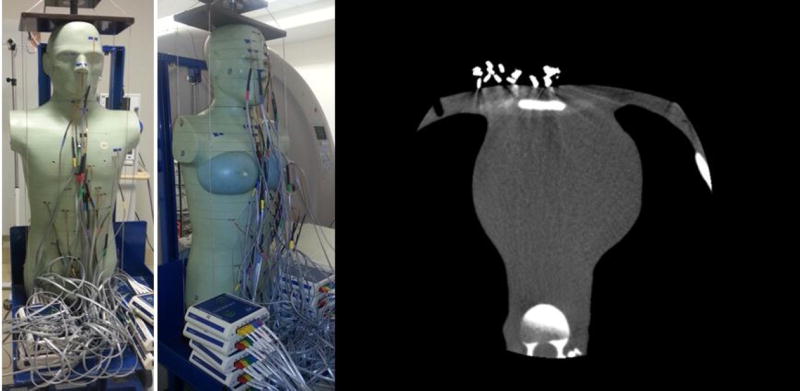
A whole-body adult anthropomorphic dosimetry verification phantom (Figure 1) was used for all experiments (ATOM 701; CIRS, Norfolk, VA). The phantom weighs 73 kg with thoracic dimensions of 23×32 cm without breasts. It is constructed from tissue-equivalent resins and polymers that represent the body’s anatomy and radiation attenuation characteristics at diagnostic photon energies, and thus it not only physically but also radiographically simulates an adult patient. The phantom is composed of a stack of 25-mm-thick contiguous transverse sections, each containing several 5-mm-diameter holes through which detectors can be placed for organ dose measurements. In these holes, tissue-equivalent MOSFET holders were employed to place the MOSFET detectors. Holes in which MOSFETs were not placed were filled with tissue-equivalent plugs. For female scans, medium-sized tissue-equivalent breast phantoms were constructed from actual CT data of a female lying in the supine position and affixed to the body phantom. Further methodologic details for all sections are provided in the Online Appendix.
Figure 1. Anthropomorphic phantom and axial CT image sample obtained in a cardiac CT scan.
Anthropomorphic phantom assembled with MOSFETs in place and an axial image sample obtained in a cardiac CT scan of the phantom.
Left: Male phantom; Middle: Female phantom; Right: cardiac CT image.
MOSFET Dosimeters and Organ Dose Determination
Organ dosimetry was performed using a mobileMOSFET dose verification system (TN-RD-70W; Best Medical, Ottawa, Canada), associated with high-sensitivity MOSFETs (TN-1002RD-H; Best Medical, Ottawa, Canada). Voltage (in mV) readings were translated to dose (in mGy) by calibration of the MOSFETs using an ion chamber (10×6–3CT; Radcal, Monrovia, California) with a control unit (Accu-Dose 2186; Radcal), and a standard 32cm diameter cylinder polymethylmethacrylate phantom (West Physics Consulting, Atlanta, GA), according to the calibration scheme of Trattner et al. (16) Separate calibration factors were determined for each x-ray tube potential used for cardiac scan modes, due to MOSFET sensitivity to energy spectrum. MOSFETs were positioned within the phantom in all 27 internal organs contributing to ED determination (10). The MOSFET voltage reading Xtissue in a given tissue was translated to dose Dtissue in that tissue by
where the calibration factor, CF, is in units of mGy/mV, and ftissue is a scaling factor which converts dose-in-air to dose-in-tissue at the effective energy Eeff of x-rays used and is defined as:
i.e. the ratio of the mass energy-absorption coefficient µen/ρ of the specific tissue to that of air (15). Mass energy-absorption coefficients were obtained from data tabulated by the National Institute of Standards and Technology at the appropriate effective energy which was determined based on information obtained from the CT manufacturers (17), or if not available, simulations using the Monte Carlo radiation transport program MCNP/MCNPX (Los Alamos National Laboratory) to obtain effective energies. These simulations were validated with known values of effective energy.
To characterize doses to the 27 organs, we used 44 and 41 MOSFETs for female and male phantoms, respectively. Doses in larger or highly radiosensitive organs such as lungs and female breasts were determined based on measurements in multiple MOSFETs (Table 2) and an average was used to estimate the organ absorbed dose. For lung, a weighted average was taken, in which the weight for each MOSFET reading was determined by the percentage of the lung’s volume surrounding the relevant MOSFET. Bone surface and bone marrow doses were measured in eight different MOSFET locations and a weighted average was determined according to their mass as specified by Eckerman et al (18).
Table 2.
Organs and assigned MOSFETs for organ dosimetry in the phantom.
| Organ* | Number of MOSFETs |
Weighting factor ICRP Publication 103 |
Weighting factor ICRP Publication 60 |
|---|---|---|---|
| Brain | 6 | 0.01 | In remainder |
| Salivary gland | 1 | 0.01 | - |
| Red Bone Marrow (RBM) | 5 | 0.12 | 0.12 |
| Bone surface | 5 | 0.01 | 0.01 |
| Thyroid | 1 | 0.04 | 0.05 |
| Lung | 5 | 0.12 | 0.12 |
| Esophagus | 3 | 0.04 | 0.05 |
| Breast | Male:1 Female:2 | 0.12 | 0.05 |
| Stomach | 1 | 0.12 | 0.12 |
| Liver | 3 | 0.04 | 0.05 |
| Colon | 3 | 0.12 | 0.12 |
| Bladder | 1 | 0.04 | 0.05 |
| Gonads | 1 | 0.08 | 0.20 |
| Remainder† | 11 | 0.12 | 0.05 |
Organ list of the adult anthropomorphic phantom, with number of MOSFETs used for dosimetry experiments and tissue (organ) weighting factor as defined in ICRP Publication 103 and in ICRP Publication 60.
Skin was not included.
Remainder organs in ICRP 103 definition of effective dose: adrenals, extrathoracic region, gall bladder, heart, kidneys, oral mucosa, pancreas, prostate, small intestine, spleen, thymus, uterus/cervix, without lymphatic nodes and muscle (each remainder organ with tissue weight of 0.12/11= 0.0109).
Remainder organs in ICRP 60 definition of effective dose: adrenals, brain, upper large intestine, small intestine, kidneys, muscle, pancreas, spleen, thymus and uterus.
CT Scanners
Twelve contemporary CT scanner models representing all five major CT manufacturers were studied. These scanner models were chosen based on their use in the PROspective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial, a 193 site, pragmatic comparative effectiveness trial which randomized outpatients with chest pain to initial testing with either CCTA or functional testing (5), performed in local laboratories, and thus the CT scanner models used are reflective of those used in current clinical practice. All models have either single or dual x-ray sources, and between 32 and 320 detector-rows available for cardiac imaging. We performed medical physics experiments in physical anthropomorphic phantoms using one scanner of each model. Experiments were performed on multiple scanners at New York-Presbyterian Hospital/Columbia University Medical Center and at the Cleveland Clinic, in Cleveland, Ohio, and on single scanners at several additional facilities (Table 3).
Table 3.
Scanners used for deriving k-factors
| Manufacturer | Scanner model | Detector Rows for Cardiac Scanning |
Source | Location |
|---|---|---|---|---|
| GE | LightSpeed VCT XTe | 64 | Single | New York-Presbyterian Hospital |
| GE | Discovery CT750 HD | 64 | Single | New York-Presbyterian Hospital |
| Hitachi | Scenaria | 64 | Single | Ocean Radiology, New York |
| Philips | Brilliance 64 | 64 | Single | SUNY Downstate Medical Center, New York |
| Philips | Brilliance iCT 256 | 128 | Single | Cleveland Clinic, Cleveland |
| Siemens | SOMATOM Sensation 64 | 32 | Single | Cleveland Clinic, Cleveland |
| Siemens | SOMATOM Definition AS+ | 64 | Single | Cleveland Clinic, Cleveland |
| Siemens | SOMATOM Definition | 2×32 | Dual | Cleveland Clinic, Cleveland |
| Siemens | SOMATOM Definition FLASH | 2×64 | Dual | Cleveland Clinic, Cleveland |
| Siemens | SOMATOM Force* | 2×96 | Dual | NYU Langone Medical Center, New York |
| Toshiba | Aquilion 64 | 64 | Single | New York Radiology Partners, New York |
| Toshiba | Aquilion Prime | 80 | Single | Carnegie Hill Radiology, New York |
| Toshiba | Aquilion ONE | 320 | Single | New York-Presbyterian Hospital |
List of the scanners used in this study, including details of the manufacturer, model, and number of detector rows of each scanner and the location where dosimetry experiments took place for deriving the k-factors.
Used only for 70 kVp protocols (Online Supplemental Table 1).
Protocols scanned
A variety of protocols are used for cardiac imaging in contemporary scanners, with the particular protocol options differing among scanners. Components of a protocol include the scan mode, tube potential, and other scan parameters. Almost all scanners have a retrospectively-gated low-pitch helical mode, generally with an option for ECG-controlled tube current modulation which lowers the x-ray tube current to ≤20% of its maximum except during a designated portion of the cardiac cycle. Most newer and all higher-end scanners have in addition at least one prospectively-triggered scan mode which turns the x-ray beam off except during a designated portion of the cardiac cycle, most commonly diastasis. Additional “padding” of x-ray exposure time may be performed enabling reconstruction of additional phases of the cardiac cycle. Prospectively ECG-triggered scanning is most commonly axial, but may be helical, sometimes with a high pitch, or a volume scan with no movement of the patient couch.
Typical protocols for each cardiac mode were determined for each scanner studied, based on discussion with physicists and applications specialists from the vendor, as well as experienced radiologists, cardiologists, and technologists at each collaborating site. Since k-factors may vary based on photon energy (19), scans were generally performed for each scanner and scan mode at tube potentials of 80, 100, and 120 kVp, unless a tube potential was not available on the scanner or the scan mode was not typically used at a particular tube potential. In addition, for a few scanners, scans for k-factor determination were performed using less-commonly used tube potentials of 70, 135, and 140 kVp. In some cases, so as to optimize MOSFET statistics, scans were performed at a tube current higher than that which would be used in clinical practice. The choice of tube current does not affect k-factors since both DLP and MOSFET voltages scale linearly with tube current. In all other respects, scans were performed with parameters mimicking those typically used clinically for that protocol. In addition to CCTA protocols, coronary artery calcium scoring scans were performed for most scanners. A simulator was used to generate the ECG signal (“chicken heart”) for all studies. Most scans were performed using a signal simulating normal sinus rhythm at 60bpm; in a few cases, where a protocol is intended for patients with higher heart rates, the simulated heart rate was increased to 80bpm.
Dosimetry measurements were performed for the female and male phantoms, separately. For each protocol, MOSFET readings were recorded for multiple scans, equally divided between male and female phantoms, with the phantom in the identical position for each repetition. The number of scans performed was determined according to the approach of Trattner et al (20) to ensure that the ED estimate was within ±10% of its true value with >90% confidence. The number of scans ranged from 4 to 10; for most protocols (84/120; 70%) ten scans were performed. Scan numbers as well as additional details for each protocol are found in Online Supplemental Table 1.
ED and Conversion Coefficient Calculation
We determined ED for each combination of scanner, scan mode, and voltage according to the ICRP Publication 103 definition (10) as
where and are the average absorbed doses determined for each organ or tissue, T, for male and female phantoms, respectively. These averages were obtained over the repeated scans for each protocol. We also determined ED according to the superseded ICRP Publication 60 definition (13) which included fewer organs and some differences in tissue weightings. Actual DLP values, as reported on the scanner console, were recorded after each scan performed. For each combination of scanner and protocol, all DLPs of repeated scans for both female and male were averaged. Each k-factor was determined as the ratio of the ED to the averaged DLP.
Results
Cardiac k-factors of 12 scanners and 120 cardiac protocols (each protocol incorporating a scan mode, tube potential, and other parameter selections), calculated using the up-to-date (10) definition of ED, are presented in Table 4. A detailed description of each protocol is available in the Supplemental Table 1, as are additional protocols and k-factors at 70, 135, and 140 kVp tube potential. K-factor mean and median was 0.026 mSv·mGy−1 cm−1 ranging between 0.020–0.035mSv·mGy−1 cm−1 (95% confidence interval: 0.0258–0.0266; coefficient of variation 8.9%). Thus, using the European Guidelines chest k-factor of 0.014 mSv·mGy−1cm−1 underestimates ED by 46%, in comparison to using an average cardiac k-factor, and by 30%-60%, in comparison to using a scanner- and protocol-specific cardiac k-factor.
Table 4.
Conversion factors relating effective dose to dose-length product, using current definition of effective dose
| ICRP 103 | Prospective Axial | Prospective Axial padding | Prospective Helical | Retrospective Helical | Retrospective Helical TCM* | Calcium | Average | CV [%] |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scanner / kVp | 80 | 100 | 120 | 80 | 100 | 120 | 80 | 100 | 120 | 80 | 100 | 120 | 80 | 100 | 120 | 120 | ||
| GE Lightspeed VCT XTe | 0.0259 | 0.0298 | 0.0295 | 0.0263 | 0.0283 | 0.0290 | × | × | × | 0.0242 | 0.0267 | 0.0264 | 0.0243 | 0.0259 | 0.0269 | 0.0306† | 0.0275 | 8.0 |
| GE Discovery CT750 HD | × | 0.0302 | × | 0.0262‡ | 0.0278‡ | 0.0283‡ | × | × | × | × | 0.0284 | 0.0267 | × | 0.0268 | 0.0269 | 0.0285 | 0.0277 | 4.4 |
| Hitachi Scenaria | 0.0254 | 0.0272 | 0.0277 | 0.0260 | 0.0268 | 0.0281 | × | × | × | 0.0258 | 0.0272 | 0.0281 | × | × | × | 0.0265 | 0.0269 | 3.5 |
| Philips Brilliance 64 | × | × | × | × | × | × | × | × | × | 0.0249 | 0.0260 | 0.0261 | 0.0246 | 0.0260 | 0.0267 | 0.0285 | 0.0261 | 4.9 |
| Philips Brilliance iCT 256 | 0.0301 | 0.0314 | 0.0316 | 0.0279 | 0.0295 | 0.0303 | × | × | × | 0.0242§ | 0.0254§ | 0.0254§ | 0.0231 | 0.0248 | 0.0249 | × | 0.0274 | 11.2 |
| Siemens SOMATOM Sensation 64 | 0.0264 | 0.0268 | 0.0266 | × | × | × | × | × | × | 0.02503 | 0.0249 | 0.0249 | 0.0256 | 0.0253 | 0.0251 | × | 0.0256 | 3.0 |
| Siemens SOMATOM Definition AS+ | 0.0271 | 0.0275 | 0.0273 | 0.0256 | × | 0.0257 | × | × | × | 0.0243 | 0.0251 | 0.0246 | 0.0244 | 0.0252 | 0.0250 | × | 0.0256 | 4.6 |
| Siemens SOMATOM Definition | 0.0288 | 0.0288 | 0.0274 | × | × | × | × | × | × | 0.0259 | 0.0286 | 0.0262 | 0.0271 | 0.0287 | 0.0281 | × | 0.0277 | 4.1 |
| Siemens SOMATOM Definition FLASH | 0.0253 | 0.0266 | 0.0254 | 0.0240 | 0.0252 | 0.0256 | 0.0263 | 0.0268 | 0.0266 | 0.0203 | 0.0256 | 0.0256 | 0.0230 | 0.0243 | 0.0248 | × | 0.0250 | 6.7 |
| Toshiba Aquilion 64 | × | × | × | × | × | × | × | × | × | × | 0.0241 | 0.0252 | × | 0.0231 | 0.0236 | 0.0349 | 0.0262 | 18.8 |
| Toshiba Aquilion Prime | × | × | × | × | × | × | 0.0209 | 0.0229 | 0.0242 | 0.0208 | 0.0231 | 0.0232 | 0.0212 | 0.0233 | 0.0234 | 0.0256 | 0.0229 | 6.7 |
| Toshiba Aquilion ONE | × | 0.0227║ | 0.0250║ | 0.0229# | 0.0259# | 0.0261# | × | × | × | × | × | × | × | × | × | 0.02574 | 0.0247 | 6.2 |
| Average per protocol type & kV | 0.0270 | 0.0279 | 0.0276 | 0.0256 | 0.0273 | 0.0277 | 0.0236 | 0.0248 | 0.0254 | 0.0239 | 0.0259 | 0.0257 | 0.0242 | 0.0253 | 0.0256 | 0.0289 | ||
| CV | 6.8 | 9.2 | 7.8 | 6.1 | 5.4 | 6.2 | 16.0 | 10.9 | 6.6 | 8.6 | 6.6 | 4.9 | 7.3 | 6.6 | 6.0 | 10.9 | ||
| Average per protocol type, all kV | 0.0275 | 0.0269 | 0.0246 | 0.0253 | 0.0251 | |||||||||||||
| CV | 7.9 | 6.6 | 9.6 | 7.3 | 6.8 | |||||||||||||
| Average all Prospective axial, all Retrospective | 0.0272 | 0.0252 | ||||||||||||||||
| CV | 7.3 | 7.0 | ||||||||||||||||
| Average | 0.0262 | |||||||||||||||||
| CV | 8.9 | |||||||||||||||||
Tube current modulation
Average of two beams with 20 and 40 mm widths
HR 80 bpm (all others are 60 bpm)
Average of high and standard resolution modes
Target mode volume scan
Continuous volume scan with exposure throughout a full cardiac cycle
Scanner- and protocol-specific k-factors, in units of mSv·mGy−1cm−1, calculated for cardiac CT using ICRP Publication 103 definition of effective dose, for 12 scanners and 120 scan protocols with standard tube potentials of 80, 100, and 120 kVp. K-factors here are summarized in 5 categories of axial and helical CT angiography protocol types, and calcium score. Averages of the k-factors across protocol types and energy levels, and per scanner, are displayed along with the coefficient of variation, CV, (ratio of standard deviation to mean in percentage values). Scan length for all scans is 13.3–14.2 cm. Heart rate is 60 bpm for all scans except where otherwise noted. A detailed description of each protocol here is also provided in the Online Supplemental Table 1, as are additional k-factors and detailed descriptions for very low (70 kVp) and high (135 or 140 kVp) tube potential protocols for selected scanners.
The average k-factor for prospectively ECG-triggered axial CCTA protocols was 0.0272 mSv·mGy−1cm−1, slightly higher than the average k-factor of retrospectively ECG-gated helical CCTA protocols of 0.0252 mSv·mGy−1cm−1. Calcium scoring scans had an average k-factor of 0.0289 mSv·mGy−1cm−1, higher than that for CCTAs which averaged 0.0260 mSv·mGy−1cm−1. CCTA 80 kVp protocols averaged 0.0250 mSv·mGy−1cm−1, lower than 100–120 kVp protocols, which averaged 0.0264 mSv·mGy−1cm−1. Average k-factors for scanner models varied between 0.0229–0.0277 mSv·mGy−1cm−1, a range of 20%. As seen in Supplemental Table 1, 72% of the k-factors determined had 5% precision at a 95% confidence level, whereas 98% had 10% precision at this level. At a 90% confidence level, 80% of k-factors had 5% precision and all had 10% precision.
K-factors based on the older ICRP 60 definition of ED (13) are shown in Online Supplemental Table 2, with an average k-factor of 0.021 mSv·mGy−1cm−1. Thus, even when the same superseded definition of ED is used, calculation of ED with the chest k-factor of 0.014 mSv·mGy−1cm−1 underestimates ED by 33% in comparison to using the average cardiac k-factor.
Discussion
The proposed cardiac k-factors determined for 12 contemporary scanners and over 120 contemporary cardiac CT protocols, using the current definition of ED, are all greater than the chest k-factor that is widely used to estimate ED from cardiac scans, and is incorporated into professional society guidelines (14, 15). Use of this chest k-factor to estimate ED results in an underestimation of ED by 46% compared to the average cardiac k-factor we determined and by 30–60%, depending on the specific scanner and protocol.
Our findings are consistent with recent findings from several other studies, each investigating a limited number of protocols (Table 1). All studies, including one (21) led by a member of the European Commission group which introduced the chest k-factor of 0.014 mSv·mGy−1·cm−1, found considerably higher k-factors, also varying between scanners and protocols and ranging from 0.020 to 0.043 mSv·mGy−1·cm−1. Given our findings, together with this supportive data, we believe that the use of the European Commission chest k-factor to estimate ED in cardiac CT, a practice never endorsed by the European Commission or American Association of Physicists in Medicine, should be reconsidered. For a better estimation of ED we propose that ideally, a scanner- and protocol-specific factor be used, and if one is not easily available, then we recommend use of our mean (as well as median) k-factor of 0.026 mSv·mGy−1·cm−1. Several factors contribute to this difference between cardiac and chest k-factors. One is a fundamental distinction between cardiac scans, which typically involve approximately 12–14 cm of craniocaudal coverage, and thoracic scans covering the entire chest, which spans approximately 27 cm craniocaudally. While all or most of the breast tissue is typically irradiated in both cardiac and chest CT scans, chest scans extend both cranially and caudally to include areas without breast tissue, and thus there is more breast irradiation per length scanned in cardiac CT. Since the breasts are a highly radiosensitive organ, one should expect a higher k-factor for a cardiac scan(22, 23). Additionally, most vendors of CT scanners used in this study report using different bow-tie filters for cardiac and chest scans, which is another factor which contributes to the difference between cardiac and chest k-factors.
Table 1.
Cardiac k-factors in the literature
| Scanner used | Cardiac Protocols | Scan Length [cm] |
k-factor ICRP 103 [mSv•mGy−1 cm−1] |
k-factor ICRP 60 [mSv•mGy−1 cm−1] |
Methodology | Ref |
|---|---|---|---|---|---|---|
| GE LightSpeed VCT | Retrospectively ECG-gated helical 80, 100, 120, 140 kVp | 16 | 0.0204–0.0268 | n/s | Monte Carlo dosimetry* | Huda et al. (22) |
| GE LightSpeed VCT, GE HD750 | Prospectively ECG-triggered axial 100, 120, 140 kVp | n/s | Median 0.028 | n/s | Monte Carlo dosimetry* | Gosling et al. (25, 26) |
| Siemens SOMATOM Sensation 64 | Retrospectively ECG-gated helical 80, 100, 120, 140 kVp | 16 | 0.0264–0.0274 | n/s | Monte Carlo dosimetry* | Huda et al. (22) |
| Siemens SOMATOM Definition AS+ | Retrospectively ECG-gated helical, Prospectively ECG-triggered axial 100, 120kVp | 15 | 0.032 | 0.024 | Physical dosimetry | Fink et al. (27) |
| Siemens SOMATOM Definition AS+ | Retrospectively ECGgated helical 80, 100, 120, 140 kVp | 16 | 0.0217–0.0282 | n/s | MonteCarlo dosimetry* | Huda et al. (22) |
| Siemens SOMATOM Definition AS+ | Retrospective ECG-gated helical 100, 120, 140 kVp | 12.5 | 0.031–0.032 | n/s | Monte Carlo dosimetry | Christner et al (28) |
| Siemens SOMATOM Definition | Retrospectively ECG-gated helical, Prospectively ECG-triggered axial 100, 120 kVp | 15 | 0.028 | 0.021 | Physical dosimetry† | Fink et al. (27) |
| Siemens SOMATOM Definition Flash | Retrospectively ECG-gated helical, Prospectively ECG-triggered axial, Prospectively ECG-triggered helical 100, 120 kVp | 13.5, 15 | 0.034 | 0.028 | Physical dosimetry | Goetti et al. (29) |
| Siemens SOMATOM Definition Flash | Retrospectively ECG-gated helical, Prospectively ECG-triggered axial, Prospectively ECG-triggered helical 100, 120 kVp | 15, 16.8 | 0.023 | 0.018 | Physical dosimetry | Fink et al.(27) |
| Toshiba Aquilion 64 | Retrospectively ECG-gated helical 120 kVp | n/s | 0.019–0.043 Mean 0.030 | 0.017–0.030 Mean 0.024 | Monte Carlo dosimetry‡ | Geleijns et al. (21) |
| Toshiba Aquilion 64 | Retrospectively ECG-gated helical 120 kVp | n/s | 0.026 | 0.020 | Monte Carlo dosimetry§ | Geleijns et al. (21) |
| Toshiba Aquilion ONE | Retrospectively ECG-gated helical, Prospectively ECG-triggered helical, Prospectively ECG-triggered volume 100, 120 kVp | 14 | 0.027–0.034 | 0.020–0.024 | Physical dosimetry | Einstein et al. (19) |
| Toshiba Aquilion ONE | Retrospectively ECG-gated helical, Prospectively ECG-triggered volume 120 kVp | 14 | (Retro) 0.025 (Pro) 0.022 | (Retro) 0.020 (Pro) 0.017 | Physical dosimetry | Seguchi et al. (30) |
Cardiac k-factors for adults reported in the literature by different groups, calculated using either physical dosimetry (various phantoms and dosimeters) or using a computational approach (simulations). These k-factors were for different scanners, scan modes, and parameters as tabulated.
ImPACT- CT Patient Dosimetry Calculator (version 1.0, ImPACT 2009), use of the NRPB Monte Carlo dose data
ImPACT- CT Patient Dosimetry Calculator (version 0.9x, ImPACT 2006), use of the NRPB Monte Carlo dose data
Algorithm based on the Electron Gamma Shower V4 (EGS4) code in combination with low-energy photon-scattering expansion that was developed by the National Laboratory for High Energy Physics (Japan)
ImPACT- version number was not reported
Another contributor to the difference between our cardiac k-factors and the European chest scan k-factor is the definition of ED used. The older definition resulted, for cardiac scans, in a k-factor that is 21% lower than in the current ED definition. The primary driver of this difference is the updated tissue weighting factors, determining each organ’s contribution to the whole-body ED, which are incorporated in the ICRP 103 ED definition, to better reflect the current state of radiation epidemiologic data. In particular, the tissue weighting factor for the breast increased from 0.05 to 0.12. Since the breast is directly irradiated by the x-ray beam in cardiac scans, it has a high organ radiation dose, and together with lung dose is the main organ contributing to the ED from cardiac CT. Additionally, in the ICRP 103 formulation of ED, the heart is included among the “remainder organs”, whereas previously the heart had not been assigned a tissue weighting factor and thus did not contribute to ED. The update in the tissue weighting factors, which is not reflected in the European Commission chest k-factor, is another source for ED underestimation using this factor.
An additional limitation of the European guidelines chest conversion factor of 0.014 mSv·mGy−1·cm−1 is that it was determined based on modeling three now-antiquated single-slice scanners, none of which were capable of performing CCTA (Siemens DRH, GE 9800, and Philips LX). The use of these old scanners for contemporary cardiac CT dosimetry should no longer be considered applicable. Moreover, as noted above, the very same European guidelines document, in another appendix which considered some more recent scanners (up to 16-slice), already suggested a higher (non-cardiac) chest conversion factor of 0.019 mSv·mGy−1·cm−1.
Limitations
Our study has a few potential limitations. There are several experimental and computational components to the determination of a k-factor, each with associated uncertainty. These include the scanner-reported DLP, effective energy calculation, energy dependent absorption coefficients, and MOSFET measurement and calibration (16). However, we performed repeated measurements to ensure that ED determination had high precision with high confidence, using the scheme of Trattner et al (20). Additionally, we performed most scans with a simulated heart rate of 60 bpm without heart rate variability. Fluctuations in heart rate or higher rates that cannot be controlled have the potential to alter data acquisition and impact the k-factor. However, in a recent MOSFET study in pediatric cardiac CT, Trattner et al (23) found no impact of heart rate on k-factor. The use of up to 46 MOSFETs simultaneously raises a question of a potential impact of the wires on the measured dose levels and hence on the k-factors. Yet, we have tested such impact using a pediatric phantom with 50 MOSFETs which were more densely placed than in the adult phantom here, and found that individual k-factors typically varied by only ±0.001 mSv·mGy−1·cm−1 depending on whether all 50 MOSFETs were placed simultaneously or not (23). The effective energy values used to determine f-factors above refer to the energy just upon entrance to the phantom’s body and not at the exact location of the MOSFET. However, experiments we performed using various protocols in one scanner demonstrated the difference in simulated effective energy in the exact MOSFET location vs. simulated effective energy upon entrance to the phantom body was approximately 1%, a sufficiently low error to justify the use of body-entrance effective energy values. Finally, ED is more formally defined computationally, and our approach was largely experimental. Our motivation was to avoid the need to make assumptions regarding proprietary aspects of scanner design and protocols, which would have been required for Monte Carlo simulation. Even so, for a single scanner we have compared MOSFET to Monte Carlo estimation of effective dose and found outstanding agreement (24).
Conclusion
In conclusion, we determined cardiac-specific conversion factors for contemporary scanners and routinely-used clinical protocols to enable a more accurate estimation of ED, given a scanner-reported DLP, as compared to the commonly-used factor of 0.014 mSv·mGy−1·cm−1. While mentioned in current guidelines, this latter factor was determined for chest rather than cardiac CT, based on now-obsolete single-slice scanners, and using a now-outdated definition of ED. The cardiac-specific factors we determined are, for all 12 scanners and 120 scan protocols used, considerably higher than the chest conversion factor, suggesting that radiation doses from cardiac CT have been significantly and systematically underestimated. We suggest that ideally, a scanner- and protocol-specific conversion factor should be used for estimating ED from cardiac CT, or if scanning information is unavailable then one should use our mean and median conversion factor of 0.026 mSv·mGy−1·cm−1. The use of cardiac-specific factors is critical to ensure more accurate dosimetry to inform the benefit-risk calculus of cardiac imaging strategies, and optimize radiation safety of patients.
Supplementary Material
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE
New methodology introduced here provides more accurate tools to estimate radiation effective dose from cardiac CT scans.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS
Updated methodology for determining radiation dose from cardiac CT should be used to enhance the benefit-risk calculus of cardiac imaging strategies and optimize test and protocol selection.
TRANSLATIONAL OUTLOOK
The use of more accurate methodology for estimating radiation dose from CT may affect the balance of benefits and risks of cardiac imaging strategies. Additional studies are needed, incorporating this methodology as well as updated dosimetry methodology for other modalities, to re-assess the comparative effectiveness of strategies for managing patients with chest pain and other clinical scenarios requiring cardiovascular evaluation.
Acknowledgments
The authors thank Richard Maldonado, Sheldon Herbert, Ted Pozniakoff, Ketan Bhatia, Amsale Robi, Tony Zapata, Carlos DeOleo, and Jose Casado (New York-Presbyterian Hospital), Michael Manning (Cleveland Clinic), Donna McKenzie and Peter Daniels (SUNY Downstate Medical Center), Carol Murphy (NYU Langone Medical Center), Steven Polk (Ocean Radiology), and Steven Wolff (Carnegie Hill Radiology), for their administrative and technical assistance in scanning. We thank Ray Filipkowski (GE), Donald Boshela (Hitachi), Peter Prinsen and Tom Morton (Philips), Tom O’Donnell (Siemens), and Di Zhang and Steve Grant (Toshiba) for technical information regarding scanners, Abdelbasset Hallil (Best Medical) for information regarding and technical support for MOSFETs, and Pamela Douglas (Duke), Udo Hoffmann (MGH), and Brian Ghoshhajra (MGH) for helpful comments on the manuscript.
Grants and Disclosures: This work was supported by NIH-NHLBI R01 HL109711, by a Herbert Irving Associate Professorship, and as a Victoria and Esther Aboodi Cardiology Researcher (Dr. Einstein). Dr. Halliburton has received institutional research grants for other investigator-initiated studies from Philips Healthcare and Siemens Healthcare; she is presently an employee of Philips Healthcare. Dr. Peters has received institutional research grants for other investigator-initiated studies from, and served on the speakers’ bureau of Toshiba America Medical Systems. Dr. Einstein has received institutional research grants for other investigator-initiated studies from GE Healthcare, Philips Healthcare, and Toshiba America Medical Systems. Other authors have no disclosures. No funding sources had access to the data or involvement in its analysis.
Abbreviations list
- CCTA
coronary computed tomographic angiography
- CT
computed tomography
- CTA
CT angiography
- DLP
dose-length-product
- ED
effective dose
- ICRP
International Commission on Radiological Protection
- kVp
kilovolt peak
- MOSFET
metal-oxide semiconductor field-effect transistor
- mSv
millisieverts
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Budoff MJ, Nakazato R, Mancini GB, et al. CT angiography for the prediction of hemodynamic significance in intermediate and severe lesions: head-to-head comparison with quantitative coronary angiography using fractional flow reserve as the reference standard. JACC Cardiovasc Imaging. 2016;9:559–564. doi: 10.1016/j.jcmg.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 3.Hadamitzky M, Achenbach S, Al-Mallah M, et al. Optimized prognostic score for coronary computed tomographic angiography: results from the CONFIRM registry (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter Registry) J Am Coll Cardiol. 2013;62:468–76. doi: 10.1016/j.jacc.2013.04.064. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein JA, Chinnaiyan KM, Abidov A, et al. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58:1414–22. doi: 10.1016/j.jacc.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 5.Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of Anatomical versus Functional Testing for Coronary Artery Disease. N Engl J Med. 2015;372:1291–1300. doi: 10.1056/NEJMoa1415516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385(9985):2383–2391. doi: 10.1016/S0140-6736(15)60291-4. [DOI] [PubMed] [Google Scholar]
- 7.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298:317–23. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 8.Schuhbaeck A, Achenbach S, Layritz C, et al. Image quality of ultra-low radiation exposure coronary CT angiography with an effective dose <0.1 mSv using high-pitch spiral acquisition and raw data-based iterative reconstruction. Eur Radiol. 2013;23:597–606. doi: 10.1007/s00330-012-2656-2. [DOI] [PubMed] [Google Scholar]
- 9.Lu MT, Douglas PS, Udelson JE, et al. Safety of coronary CT angiography and functional testing for stable chest pain: a randomized comparison of test complications, incidental findings and radiation dose. doi: 10.1016/j.jcct.2017.08.005. Submitted 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ICRP Publication 103. The 2007 Recommendations of the International Commission on Radiological Protection Ann ICRP. 2007;37:1–332. doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Martin CJ. Effective dose: how should it be applied to medical exposures? Br J Radiol. 2007;80:639–47. doi: 10.1259/bjr/25922439. [DOI] [PubMed] [Google Scholar]
- 12.McCollough CH, Christner JA, Kofler JM. How effective is effective dose as a predictor of radiation risk? AJR Am J Roentgenol. 2010;194:890–6. doi: 10.2214/AJR.09.4179. [DOI] [PubMed] [Google Scholar]
- 13.1990 Recommendations of the International Commission on Radiological Protection. ICRP Publication 60. Ann ICRP. 1991;21:1. [PubMed] [Google Scholar]
- 14.Bongartz G, Golding SJ, Jurik AG, et al. European Guidelines for Multislice Computed Tomography. European Commission. 2004 [Google Scholar]
- 15.The American Association of Physicists in Medicine. The measurement, reporting, and management of radiation dose in CT, AAPM Report No. 96. 2008 [Google Scholar]
- 16.Trattner S, Prinsen P, Wiegert J, et al. Calibration and Error Analysis of Metal-Oxide-Semiconductor Field-Effect Transistor Dosimeters for Computed Tomography Radiation Dosimetry. submitted to Med Phys. 2016 doi: 10.1002/mp.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bushberg JT, Seibert JA, Leidholdt EM, Boone JM. The Essential Physics of Medical Imaging. third. Philadelphia: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 18.Eckerman KF, Bolch WE, Zankl M, Petoussi-Henss N. Response functions for computing absorbed dose to skeletal tissues from photon irradiation. Radiat Prot Dosimetry. 2007;127:187–91. doi: 10.1093/rpd/ncm468. [DOI] [PubMed] [Google Scholar]
- 19.Einstein AJ, Elliston CD, Arai AE, et al. Radiation dose from single-heartbeat coronary CT angiography performed with a 320-detector row volume scanner. Radiology. 2010;254:698–706. doi: 10.1148/radiol.09090779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trattner S, Cheng B, Pieniazek RL, Hoffmann U, Douglas PS, Einstein AJ. Sample size requirements for estimating effective dose from computed tomography using solid-state metal-oxide-semiconductor field-effect transistor dosimetry. Med Phys. 2014;41:042102. doi: 10.1118/1.4868693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geleijns J, Joemai RM, Dewey M, et al. Radiation exposure to patients in a multicenter coronary angiography trial (CORE 64) AJR Am J Roentgenol. 2011;196:1126–32. doi: 10.2214/AJR.09.3983. [DOI] [PubMed] [Google Scholar]
- 22.Huda W, Tipnis S, Sterzik A, Schoepf UJ. Computing effective dose in cardiac CT. Phys Med Biol. 2010;55:3675–84. doi: 10.1088/0031-9155/55/13/007. [DOI] [PubMed] [Google Scholar]
- 23.Trattner S, Chelliah A, Ruzal-Shapiro CB, et al. Estimating Effective Dose of Radiation from Pediatric Cardiac CT Angiography Using a 64-Slice Scanner: New Conversion Factors Relating Dose-Length Product to Effective Dose. AJR Am J Roentgenol. 2017;208:585–594. doi: 10.2214/AJR.15.15908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prinsen P, Wiegert J, Trattner S, et al. High correlation between CT radiation dose estimates obtained by fast monte carlo computation and solid-state metal-oxide semiconductor field-effect transistor measurements in physical anthropomorphic phantoms. European Congress of Radiology Abstracts. 2014:0245. doi: 10.1002/mp.13780. [DOI] [PubMed] [Google Scholar]
- 25.Gosling O, Loader R, Venables P, Rowles N, Morgan-Hughes G, Roobottom C. Cardiac CT: are we underestimating the dose? A radiation dose study utilizing the 2007 ICRP tissue weighting factors and a cardiac specific scan volume. Clin Radiol. 2010;65:1013–7. doi: 10.1016/j.crad.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Gosling OE, Roobottom CA. Radiation exposure from cardiac computed tomography. JACC Cardiovasc Imaging. 2010;3:1201–2. doi: 10.1016/j.jcmg.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Fink C, Krissak R, Henzler T, et al. Radiation dose at coronary CT angiography: second-generation dual-source CT versus single-source 64-MDCT and first-generation dual-source CT. AJR Am J Roentgenol. 2011;196:W550–7. doi: 10.2214/AJR.10.5153. [DOI] [PubMed] [Google Scholar]
- 28.Christner JA, Kofler JM, McCollough CH. Estimating effective dose for CT using dose-length product compared with using organ doses: consequences of adopting International Commission on Radiological Protection publication 103 or dual-energy scanning. AJR Am J Roentgenol. 2010;194:881–9. doi: 10.2214/AJR.09.3462. [DOI] [PubMed] [Google Scholar]
- 29.Goetti R, Leschka S, Boschung M, et al. Radiation doses from phantom measurements at high-pitch dual-source computed tomography coronary angiography. Eur J Radiol. 2012;81:773–9. doi: 10.1016/j.ejrad.2011.01.068. [DOI] [PubMed] [Google Scholar]
- 30.Seguchi S, Aoyama T, Koyama S, Fujii K, Yamauchi-Kawaura C. Patient radiation dose in prospectively gated axial CT coronary angiography and retrospectively gated helical technique with a 320-detector row CT scanner. Med Phys. 2010;37:5579–85. doi: 10.1118/1.3496985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.