Abstract
Objectives
To describe the temporal trends in prevalence of left ventricular systolic dysfunction (LVSD) in individuals without and with heart failure (HF) in the community over a three decade period of observation.
Background
Temporal trends in the prevalence and management of major risk factors may impact the epidemiology of HF.
Methods
We compared the frequency, correlates, and prognosis of LVSD (ejection fraction [EF]<50%) among Framingham Study participants without and with clinical HF in three decades (1985–1994, 1995–2004, 2005–2014).
Results
Among participants without HF (12857 person-observations, mean age 53 years, 56% women), the prevalence of LVSD on echocardiography decreased (1985–1994 vs. 2005–2014: 3.38% vs. 2.2%; p<0.0001) while mean LVEF increased (65% vs. 68%; p<0.001). The elevated risk associated with LVSD (~2- to 4-fold risk of HF or death) remained unchanged over time. Among participants with new-onset HF (N=894, mean age 75 years, 52% women), the frequency of HFpEF (preserved LVEF, ≥50%) increased (1985–1994 vs. 2005–2014: 41.0% vs. 56.2%; p<0.001), HFrEF (reduced LVEF, <40%) decreased (44.1% vs. 31.06%; p =0.002), whereas HFmrEF (mid-range LVEF, 40–<50%) remained unchanged (14.93% vs. 12.77%; p =0.66). Cardiovascular mortality associated with HFrEF declined across decades (hazards ratio, 0.61; 95% CI, 0.39–0.97), but remained unchanged for HFmrEF and HFpEF. Approximately 47% of the observed increase in LVEF among those without HF, and 75% of the rising proportion of HFpEF across decades was attributable to trends in risk factors, especially a decline in the prevalence of coronary heart disease among those with HF.
Conclusions
The profile of HF in the community has changed in recent decades, with a lower prevalence of LVSD and an increased frequency of HFpEF, presumably due to concomitant risk factor trends.
Keywords: heart failure, echocardiography, ejection fraction, epidemiology
Introduction
Advances in the management of coronary heart disease (CHD) and its risk factors have favorably impacted the epidemiology of CHD (1–4). The incidence of post-myocardial infarction (MI) heart failure (HF) has also declined (5–8), although not all reports are consistent (9). Paralleling these observations, investigators have described a rise in the proportion of HF with a preserved left ventricular (LV) ejection fraction (HFpEF) relative to HF with reduced ejection fraction (HFrEF) in recent decades, presumably due to a decline in the incidence of HFrEF (9–11). More recently, reports have described a third entity labeled as HF with a mid-range ejection fraction (HFmrEF) (12–15). Evaluating the relative prevalence of these HF types is challenged by varying data sources, changes in coding practices, differing diagnostic criteria, and a shift towards greater outpatient diagnosis of HF (9,16). Additionally, there are no data regarding trends in the prevalence of asymptomatic left ventricular systolic dysfunction (LVSD, defined as an LVEF<50%), an antecedent of HFrEF.
We investigated if the profile of LVSD and HF in the community has changed over time due to favorable trends in management of CHD/MI and divergent trends in HF risk factors, i.e., with better rates of control of hypertension and dyslipidemia being offset by increasing rates of obesity and diabetes (1). We tested the hypothesis that the prevalence of LVSD in the community is decreasing, and the occurrence of HFpEF is rising in recent decades using data from the Framingham Heart Study (FHS).
Methods
Study samples
The selection criteria and study design of the three FHS cohorts have been described (17–19). Participants who attended routine FHS examinations between 1985 and 2015, and who were under continuous surveillance for the development of HF events, were eligible for the present investigation. The study protocols were approved by the Boston University Medical Center Institutional Review Board and all participants provided written informed consent. The measurement and definitions of key covariates are described under Supplementary Methods, Section A.
Two different samples were used (Supplementary Figure 1).
Sample 1
For studying temporal trends in the epidemiology of LVSD, we used echocardiographic examinations among participants free of overt HF in three successive decades: 1985–1994, 1995–2004, and 2005–2014. Accordingly, Original cohort examination 20, and Offspring cohort examination 4 (N=3901) contributed to the first decade 1985–1994; Offspring cohort examination 6 and Third generation examination 1 (N=6459) contributed to the middle decade 1995–2004; and Offspring examination 8 (N=2497) contributed to the most recent decade, 2005–2014. All covariate data were obtained at the same FHS examination at which echocardiography was performed.
Sample 2
For studying temporal trends in the profile of HF, we evaluated all individuals with a first episode of HF in the three decades (N=894, Supplementary Figure 1). We evaluated LVEF data closest to and within six months after HF onset (based on data from hospitalization records, physician office visits, or FHS) for the categorization of HF type: HFrEF, LVEF<40%; HFmrEF, 40≤LVEF<50%; and HFpEF, LVEF≥50%. Covariate data were obtained from the closest FHS examination antedating the HF episode.
Echocardiographic Measures
At the FHS examinations (sample 1), attendees underwent two-dimensional echocardiography with Doppler color flow imaging (Supplementary Methods, Sections B and C), and M-mode measurements made according to the American Society of Echocardiography guidelines (20). LVEF was calculated using the method of de Simone (21) complemented by the visual assessment of LV systolic function; two-dimensional quantitation of chamber volume was not routinely performed in the first decade. LVEF was categorized as: normal (LVEF≥50%), mildly reduced (LVEF 40–<50%), and moderate or greater impairment (LVEF <40%).
Follow Up and Outcome Events
Information about events during follow up was obtained from medical history and physical examination at the FHS, and review of medical records. All suspected new CVD events were adjudicated by a panel of three experienced investigators, who evaluated pertinent medical records, using previously published criteria (22).
Over the three decades, the diagnosis of HF was made using the same FHS criteria23: the presence of 2 major criteria, or of 1 major criterion and 2 minor criteria (Supplementary Methods, Section D). The sensitivity and specificity of these criteria compare well with other epidemiological criteria for HF (24).
For analyses of prognosis of LVSD, our primary outcome was a composite of new-onset HF or death. For analyses of outcomes in individuals with HF, we assessed all-cause mortality and cause-specific mortality (death due to CVD versus non-CVD causes) (25,26).
Statistical Methods
Sample 1 - without overt HF
We evaluated the distribution of LVEF in each of the 3 decades in individuals without HF who underwent echocardiography at FHS, comparing the distributions using the Kolmogorov-Smirnov test and rank correlations. We estimated the frequency of LVSD (EF<40% and EF mid-range, 40–<50%) and clinical characteristics of the three LVEF groups in each time period. Trends in prevalence of LVSD over time were assessed using logistic regression models adjusting for age and sex. We examined absolute rates of the composite outcome (HF or death) over a follow-up period of 5 years. Using Cox regression (27) models that adjusted for age, sex and cohort type, we estimated the relative risk of the composite outcome in those with LVSD compared with those with a normal LVEF. We confirmed that the assumption of proportionality of hazards was met. We repeated the Cox regression analyses individually for each LVEF category to compare trends over time in the risk of adverse outcomes for participants in that category. Given the modest number of individuals with LVSD and LVEF<40%, we repeated analyses defining LVSD as an LVEF<50%.
Sample 2 - with HF
Among participants with new-onset HF, we assessed the proportions of HFrEF vs. HFmrEF vs. HFpEF within each decade. We compared clinical characteristics associated with each HF type within each decade. Trends in prevalence of reduced LVEF among participants with HF over time were assessed using logistic regression models adjusting for age and sex. We determined the risk of death (all-cause mortality, and death due to CVD and non-CVD causes) for each HF subtype in each decade over a follow-up period of 5 years. We estimated multivariable Cox regression models adjusting for age and sex, comparing the risk of death in participants with HFrEF and HFmrEF with that of HFpEF (referent). For each HF type, we repeated Cox regression models to compare trends in risk of all-cause mortality, and cardiovascular and non-cardiovascular mortality across the three decades. Given the modest number of individuals with HFmrEF, we repeated analyses defining HFrEF as an LVEF<50%.
In additional analyses, we assessed the contributions of trends in correlates of LVEF (linear models) and HFpEF (logistic models) to the trends in LVEF distribution in participants without and with HF, respectively (Supplementary Methods, Sections E and F). A two-sided p value less than 0.05 was considered statistically significant.
Results
Individuals without diagnosed HF
Baseline characteristics of individuals without clinical HF are displayed in Table 1A by decade and according to the three LVEF categories. Participants with moderate or greater LVSD were older than those with normal LVEF, predominantly male, and had higher burden of hypertension, diabetes, CHD, and atrial fibrillation compared to those without LVSD. Participants with LVEF in the mid-range had prevalence of diabetes, smoking, and mean values of lipids that were intermediate between the other two LVEF categories. Across the three decades, the prevalence of CHD and MI rose (by 58–100%) among those with LVEF in midrange, but diminished (by 31–50%) in those with LVEF<40%. Prevalence of atrial fibrillation rose 3 to 5-fold across decades in each LVEF category.
Table 1A.
Characteristics of participants free of heart failure who underwent routine Framingham Study echocardiography (1985–2014)
| 1985–1994 (n=3901 person-observations) | 1995–2004 (n=6459 person-observations) | 2005–2014 (2497 person-observations) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LVEF | <40% | ≥40–<50% | ≥50% | P* | <40% | ≥40–<50% | ≥50% | P* | <40% | ≥40 –<50% |
≥50% | P* |
| N, person obs. | 31 | 101 | 3,769 | 46 | 49 | 6364 | 14 | 41 | 2,442 | |||
| Age, years | 64 (12) | 58 (15) | 55 (13) | <.0001 | 64 (8) | 62 (9) | 47 (13) | <.0001 | 74 (9) | 70 (8) | 66 (9) | <.0001 |
| Men, % | 80.7 | 75.3 | 42.6 | <.0001 | 87.0 | 81.6 | 44.5 | <.0001 | 78.6 | 90.2 | 43.8 | <.0001 |
| Body mass index, kg/m2 | 26.0 (4.0) | 26.9 (4.8) | 26.4 (4.5) | 0.74 | 28.6 (4.6) | 27.1 (4.0) | 26.9 (5.1) | 0.045 | 26.4 (4.8) | 28.6 (4.5) | 28.1 (5.2) | 0.65 |
| Obese, % | 9.7 | 23.8 | 18.1 | 0.16 | 30.4 | 24.5 | 22.6 | 0.42 | 21.4 | 31.7 | 30.2 | 0.76 |
| Systolic BP, mm Hg | 135 (17) | 135 (23) | 129 (21) | 0.002 | 134 (16) | 129 (18) | 121 (17) | <.0001 | 120 (15) | 134 (19) | 128 (17) | 0.99 |
| Diastolic BP, mm Hg | 76 (9) | 79 (11) | 78 (10) | 0.98 | 76 (9) | 74 (10) | 75 (10) | 0.94 | 67 (13) | 74 (16) | 74 (10) | 0.04 |
| HTN, % | 71.0 | 54.5 | 39.7 | <.0001 | 69.6 | 59.2 | 25.5 | <.0001 | 64.3 | 68.3 | 56.0 | 0.24 |
| DM, % | 9.7 | 12.1 | 4.7 | 0.003 | 32.6 | 14.3 | 4.6 | <.0001 | 28.6 | 24.4 | 11.8 | 0.009 |
| Smoker, % | 22.6 | 24.8 | 21.0 | 0.65 | 13.0 | 18.4 | 14.9 | 0.74 | 14.3 | 12.2 | 9.1 | 0.40 |
| Total/HDL cholesterol | 4.87 (1.35) | 4.77(1.72) | 4.49(1.61) | 0.04 | 4.66 (1.27) | 4.21 (1.16) | 3.97 (1.47) | 0.0009 | 3.43 (1.28) | 3.53 (0.93) | 3.46 (1.05) | 0.87 |
| MI, % | 41.9 | 15.8 | 2.1 | <.0001 | 50.0 | 24.5 | 0.74 | <.0001 | 28.6 | 31.7 | 3.2 | <.0001 |
| CHD, % | 71.0 | 27.7 | 6.3 | <.0001 | 65.2 | 44.9 | 2.3 | <.0001 | 35.7 | 43.9 | 8.4 | <.0001 |
| AF, % | 9.7 | 6.9 | 1.5 | <.0001 | 28.3 | 14.3 | 0.93 | <.0001 | 28.6 | 31.7 | 4.8 | <.0001 |
| LVEF,% | 34.3 (3.2) | 46.7 (2.2) | 65.6 (7.1) | <.0001 | 33.2 (4.2) | 45.7 (1.8) | 66.4 (4.3) | <.0001 | 33.9 (4.0) | 45.4 (2.5) | 68.1 (5.2) | <.0001 |
Values are mean (SD) for continuous variables.
P for trend across LVEF categories in a given decade of interest.
AF= atrial fibrillation, BP= blood pressure, CHD = coronary heart disease, DM= diabetes mellitus, FS= fractional shortening, HDL= high density lipoprotein cholesterol, HTN= hypertension, LVEF= left ventricular ejection fraction, MI = myocardial infarction, NA = serum creatinine not available at index examination.
Data on 8883 participants with available values of serum creatinine
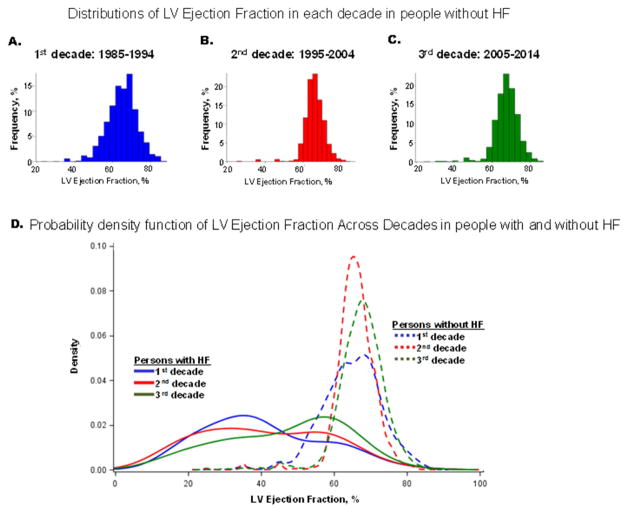
Figure 1 (Panels A–C) displays the distributions of LVEF in the three time periods. The entire LVEF distribution shifted to the right (i.e., higher LVEF) over time, with the median value increasing from 65% in the first decade to 68% in the last decade (Supplementary Figure 2; p<0.0001 for both Kolmogorov-Smirnov test and rank correlations). Table 2 (Part A) shows the prevalence of LVSD decreased from 3.38% in the first decade to 2.2% in the last decade, with a marked decline in the odds of LVSD (Table 2, Part A). Supplementary Figure 3 demonstrates the Kaplan Meier curves for survival free of the composite outcome in individuals without baseline clinical HF by LVEF category in each time period and pooled across the three time periods. Individuals with LVEF in the midrange had a prognosis intermediate between those with LVEF<40% and those with normal LVEF. Supplementary Table 1 (Part A) provides the absolute rates of the composite outcome for the three LVEF categories in each decade. Unadjusted rates were considerably higher for the two lower LVEF categories in each decade relative to those with normal LVEF; event rates were lower in the middle decade in which participants were also younger (due to inclusion of FHS Third Generation participants). Unadjusted absolute events rates for the groups with mid-range LVEF almost doubled in the most recent decade compared with 1985–1994. In Cox regression analyses adjusting for age, sex and cohort type, LVSD conferred a 2- to 4-fold risk of developing the composite outcome (Supplementary Table 2, Part A, data pooled over the decades). Within each LVEF category, the adjusted-risk of developing the composite outcome remained unchanged across the decades (Supplementary Table 2, Part B). Results were unchanged when a single cutpoint (LVEF<50%) was used to define LVSD (Supplementary Table 2, parts C and D).
Figure 1. Distribution of left ventricular ejection fraction by decade in participants free of heart failure (Panels A–C) and in those with heart failure (Panel D).
Panel D displays a smoothed probability density function (Y axis) for values of left ventricular ejection fraction (X axis) by decade in participants free of heart failure (dotted lines) juxtaposed next to those with heart failure (solid lines) for comparison. Data for decade 1985–1994 are indicated in blue, those for decade 1995–2004 in red and for decade 1005–2014 in green. SAS proc sgplot was used to generate the kernel density plots for the EF distribution by time period for the HF and non-HF samples.
Table 2.
Prevalence over time of reduced left ventricular ejection fraction in participants without and with overt heart failure
| Decade | Proportion with LVEF (%)
|
Age-sex-adjusted Odds Ratio (95% CI) for LV systolic dysfunction, LVEF <50% | P | ||
|---|---|---|---|---|---|
| <40% | 40–<50% | ≥50% | |||
|
| |||||
| A. Participants free of HF | |||||
|
| |||||
| 1985–1994 | 0.79 | 2.59 | 96.62 | 1.00 (Referent) | - |
| 1995–2004 | 0.71 | 0.76 | 98.53 | 0.64 (0.49, 0.85) | 0.002 |
| 2005–2014 | 0.56 | 1.64 | 97.80 | 0.36 (0.26, 0.50) | <0.0001 |
| Chi Square P* | <.0001 | ||||
|
| |||||
| B. Participants with HF | |||||
|
| |||||
| 1985–1994 | 44.10 | 14.93 | 40.97 | 1.00 (Referent) | - |
| 1995–2004 | 43.94 | 12.67 | 43.40 | 0.93 (0.67–1.28) | 0.64 |
| 2005–2014 | 31.06 | 12.77 | 56.17 | 0.54 (0.38–0.77) | 0.0007 |
|
| |||||
| P for trend across decades¶ | 0.002 | 0.66 | 0.001 | 0.74 (0.62–0.89) | 0.001† |
Chi-square test was used due to small number of individuals in cells.
comparisons within LVEF category.
p for trend across decades.
HF= heart failure, LVEF = left ventricular ejection fraction.
Individuals with diagnosed HF
Table 1B demonstrates the characteristics of patients with HFrEF, HFmrEF, and HFpEF within each decade. The average age of onset of HFpEF and HFmrEF increased in the most recent decade. Within each time period, clinical characteristics of patients with HFmrEF were intermediate between those with HFrEF and HFpEF. Across time, and in each HF type, there was a rising prevalence of obesity, hypertension, and atrial fibrillation, whereas the prevalence of dyslipidemia, CHD, MI, and smoking declined. Mean levels of blood pressure and the ratio of total to high density lipoprotein cholesterol concentrations decreased across decades, concomitant with rising treatment rates for hypertension and dyslipidemia in each HF subtype. Although the prevalence of CHD declined across decades for all 3 HF subtypes, it was highest in participants with HFmrEF in the last decade.
Table 1B.
Characteristics of participants with clinically diagnosed new-onset heart failure in the Framingham Study (1985–2014)
| 1985–1994 (n=288) | 1995–2004 (n=371) | 2005–2014 (n=235) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LVEF | HFrEF (<40%) | HFmrEF (≥40–<50%) | HFpEF (≥50%) | P* | HFrEF (<40%) | HFmrEF (≥40–<50%) | HFpEF (≥50%) | P* | HFrEF (<40%) | HFmrEF (≥40–<50%) | HFpEF (≥50%) | P* |
| N | 127 | 43 | 118 | 163 | 47 | 161 | 73 | 30 | 132 | |||
| Age, years | 73 (12) | 75 (9) | 74 (15) | 0.71 | 73 (13) | 71 (17) | 77 (12) | 0.02 | 72 (13) | 76 (11) | 78 (12) | 0.002 |
| Men, % | 56.7 | 46.5 | 41.5 | 0.06 | 62.0 | 46.8 | 31.1 | <0.0001 | 64.4 | 53.3 | 40.2 | 0.004 |
| BMI, kg/m2 | 27 (5) | 29 (6) | 28 (5) | 0.47 | 28 (5) | 29 (5) | 29 (6) | 0.12 | 29 (6) | 28 (5) | 29 (7) | 0.78 |
| Obese, % | 23 | 38 | 25 | 0.17 | 26 | 32 | 34 | 0.35 | 38 | 41 | 42 | 0.91 |
| Systolic BP, mm Hg | 147 (26) | 152 (23) | 146 (25) | 0.69 | 140 (21) | 151 (21) | 141 (23) | 0.54 | 135 (19) | 138 (21) | 140 (22) | 0.09 |
| Diastolic BP, mm Hg | 76 (12) | 76 (12) | 76 (11) | 0.66 | 72 (13) | 79 (13) | 71 (12) | 0.57 | 71 (12) | 69 (13) | 70 (11) | 0.43 |
| HTN, % | 78.9 | 81.0 | 79.3 | 0.96 | 76.7 | 85.1 | 75.6 | 0.39 | 74.0 | 76.7 | 86.8 | 0.06 |
| DM, % | 21.4 | 30.8 | 22.2 | 0.47 | 34.7 | 39.4 | 26.3 | 0.25 | 36.8 | 24.0 | 21.2 | 0.07 |
| Smoker, % | 22.4 | 21.4 | 18.6 | 0.76 | 16.8 | 22.2 | 11.2 | 0.13 | 15.1 | 6.9 | 3.0 | 0.006 |
| Tc/HDL | 5.5 (2.0) | 5.7 (2.0) | 5.2 (1.7) | 0.42 | 4.8 (1.9) | 5.1 (1.9) | 4.4 (1.6) | 0.10 | 4.1 (1.5) | 3.7 (1.9) | 3.5 (1.1) | 0.02 |
| MI, % | 58 | 63 | 35 | 0.0002 | 52 | 60 | 24 | <0.0001 | 38 | 33 | 20 | 0.01 |
| CHD, % | 69 | 74 | 50 | 0.0022 | 65 | 70 | 40 | <0.0001 | 49 | 57 | 36 | 0.05 |
| AF, % | 28 | 28 | 41 | 0.07 | 39 | 32 | 55 | 0.003 | 37 | 53 | 52 | 0.11 |
| LVEF (at time of HF), % | 35 (5) | 42 (2) | 58 (5) | <0.0001 | 32 (7) | 42 (2) | 59 (9) | <0.0001 | 28 (8) | 43 (3) | 60 (7) | <0.0001 |
Values are mean (SD) for continuous variables.
P for trend across heart failure categories in a given decade of interest.
AF= atrial fibrillation, BP= blood pressure, CHD= coronary heart disease, DM= diabetes mellitus, FS= fractional shortening, HDL= high density lipoprotein cholesterol, HFmrEF= heart failure with mid-range ejection fraction, HFpEF= Heart failure with preserved ejection fraction, HFrEF= heart failure with reduced ejection fraction, HTN= hypertension, LVEF= left ventricular ejection fraction, MI= myocardial infarction
Figure 1 Panel D shows a rightward shift in the LVEF distribution of participants with new-onset HF across the three decades. Table 2 (Part B) confirms the rising proportion with HFpEF (15% absolute increase) in the most recent decade, paralleled by a decrease in HFrEF prevalence (13% absolute decrease) with the frequency of HFmrEF holding steady at 13–15%. The odds ratio of developing HF with an EF<50% in the most recent decade declined to 0.54.
Figure 2 shows the Kaplan Meier curves for survival following HF onset by LVEF category in each time period and pooled across decades. Median survival time improved for HFrEF but remained same or increased in the other two categories. Supplementary Table 1 (Part B) demonstrates absolute rates of death for the three HF categories in each decade. Participants with HFmrEF demonstrated the best survival in the initial decade relative to the other two HF categories, a pattern that changed with convergence of survival among the groups over the next two decades. There was no significant difference in the risk of death between the three HF subtypes in any of the three decades (Supplementary Table 3). Use of a single cutpoint (LVEF<50%) to define HFrEF yielded essentially similar results (Supplementary Table 3, lower part).
Figure 2. Kaplan Meier curves for survival of participants with HFrEF, HFmrEF and HFpEF in the three decades and pooled across decades.
The horizontal line indicates median survival, and the vertical lines show median survival time for participants with new–onset HF for each subtype of HF.
Analyses of cause-specific mortality within HF type demonstrated a decline in cardiovascular mortality for HFrEF over time, and an increase in non-cardiovascular mortality for HFmrEF (Table 3). Cardiovascular and non-cardiovascular mortality remained unchanged over the decades for HFpEF. Nearly identical results were obtained when cause-specific mortality was evaluated defining HFrEF as an LVEF<50% (Supplementary Table 4).
Table 3.
Temporal trends in cause of death among participants with overt heart failure: Results of age- and sex- adjusted Cox regression
| All-cause mortality | CVD mortality | Non-CVD mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| No. event/no. at risk | Hazards Ratio (95% CI) | P* | No. event/no. at risk | Hazards Ratio (95% CI) | P* | No. event/no. at risk | Hazards Ratio (95% CI) | P* | |
|
| |||||||||
| All participants with Heart Failure | |||||||||
| 1985–1994 | 194/288 | 1.00 (Referent) | 123/288 | 1.00 (Referent) | 71/288 | 1.00 (Referent) | |||
| 1995–2004 | 236/371 | 0.88 (0.73–1.06) | 0.18 | 127/371 | 0.77 (0.60–0.98) | 0.04 | 109/371 | 1.10 (0.81–1.48) | 0.54 |
| 2005–2014 | 149/235 | 0.93 (0.75–1.15) | 0.51 | 64/235 | 0.62 (0.46–0.84) | 0.0018 | 85/235 | 1.44 (1.05–1.98) | 0.02 |
|
| |||||||||
| Participants with HFrEF | |||||||||
|
| |||||||||
| 1985–1994 | 94/127 | 1.00 (Referent) | 71/127 | 1.00 (Referent) | 23/127 | 1.00 (Referent) | |||
| 1995–2004 | 106/163 | 0.78 (0.59–1.03) | 0.08 | 70/163 | 0.70 (0.51–0.98) | 0.04 | 36/163 | 1.00 (0.59–1.69) | 0.99 |
| 2005–2014 | 43/73 | 0.79 (0.55–1.14) | 0.21 | 25/73 | 0.61 (0.39–0.97) | 0.04 | 18/73 | 1.33 (0.71–2.48) | 0.37 |
|
| |||||||||
| Participants with HFmrEF | |||||||||
|
| |||||||||
| 1985–1994 | 25/43 | 1.00 (Referent) | 18/43 | 1.00 (Referent) | 7/43 | 1.00 (Referent) | |||
| 1995–2004 | 30/47 | 1.36 (0.80–2.32) | 0.26 | 15/47 | 0.92 (0.46–1.84) | 0.82 | 15/47 | 2.49 (1.01–6.11) | 0.047 |
| 2005–2014 | 18/30 | 1.11 (0.60–2.04) | 0.74 | 6/30 | 0.51 (0.20–1.29) | 0.16 | 12/30 | 2.66 (1.04–6.81) | 0.04 |
|
| |||||||||
| Participants with HFpEF | |||||||||
|
| |||||||||
| 1985–1994 | 75/118 | 1.00 (Referent) | 34/118 | 1.00 (Referent) | 41/118 | 1.00 (Referent) | |||
| 1995–2004 | 100/161 | 0.88 (0.65–1.20) | 0.42 | 42/161 | 0.81 (0.51–1.27) | 0.36 | 58/161 | 0.95 (0.64–1.42) | 0.81 |
| 2005–2014 | 88/132 | 1.00 (0.73–1.36) | 0.99 | 33/132 | 0.79 (0.49–1.28) | 0.34 | 55/132 | 1.18 (0.78–1.79) | 0.42 |
P for comparison of decades. P values <0.05 are shown in bold.
CVD= cardiovascular disease (coronary disease, stroke, peripheral vascular disease, or heart failure), HFmrEF= heart failure with mid-range ejection fraction, HFpEF= Heart failure with preserved ejection fraction, HFrEF= heart failure with reduced ejection fraction.
Additional analyses: Risk factors
The results of additional analyses relating temporal trends in CVD risk factors to shifts in the mean values of LVEF (in those without HF) and to change in the proportion of HFpEF (among new-onset HF cases) are shown in Supplementary Tables 5 and 6. Increasing rates of treatment for hypertension and decreasing prevalence of CHD/MI were key correlates of declining LVSD prevalence and rising HFpEF frequency, respectively. Approximately 47% of the change in mean LVEF values and 75% of the change in prevalence in HFpEF were attributable to changes in key risk factors for LVSD and HF (Supplemental Methods, Sections E and F).
Discussion
Principal findings
We have characterized concomitant changes in the epidemiology of LVSD and HF subtypes in a large community-based cohort over three decades by analyzing approximately 13,000 echocardiograms and nearly 900 well-phenotyped HF cases over a 30-year time period. We observed a decrease in the prevalence of asymptomatic LVSD, accompanied by a shift in HF phenotype towards a preponderance of HFpEF over HFrEF, with the proportion of HFmrEF remaining unaltered. Participants presenting with a mid-range EF (both without and with HF) were intermediate in terms of their risk factor profile relative to their counterparts with lower or higher EF, confirming other reports (13,27). We demonstrated that the prognosis of asymptomatic LVSD remained essentially unchanged over time, Among HF patients, the prognosis of those with HFrEF improved whereas that of HFmrEF and HFpEF remained unchanged. Use of an LVEF cut point of 50% to define LVSD (in those without HF) or HFrEF (in those with HF) yielded essentially similar results.
Changing epidemiology of asymptomatic LVSD
The rightward shift in the entire LVEF distribution suggests that the decline in prevalence of LVSD was not limited to the lower extreme (LVEF <40%) of the distribution. Temporal trends in risk factors accounted for about 45% of the shift in LVEF distribution. This observation likely reflects the net balance between positive (rising burden of hypertension and obesity, declining rates of smoking and total to HDL cholesterol ratio) and negative correlates of LVSD (increase in prevalence of diabetes (1) and MI). Improved management of MI and decline in the occurrence of ST segment elevation MI (28) may have contributed too. It is important to note that more than half of the change in mean LVEF remained unexplained, suggesting the need for additional studies. We did not observe any change in prognosis of LSVD over time, with a 2 to 4-fold increased risk of the composite outcome, despite availability of evidence-based treatment recommendations for those with LVEF <40% (29).
Changing epidemiology of overt HF
Using the same standardized criteria for HF consistently over a 30-year period, we confirm and extend prior observations made in the Olmsted County from 2000–2010 (10) (using validated ICD9 codes) documenting the increasing predominance of HFpEF over HFrEF. Temporal trends in risk factors for HFrEF versus HFpEF (a lower prevalence of CHD and rising hypertension rates among those with HF) (1) explained about 75% of the shift towards the greater prevalence of HFpEF observed. An increased awareness of HFpEF in recent decades may have contributed to this trend too.
Among HF patients in our investigation, the prognosis of those with HFrEF improved over the last two decades as evidenced by a 30–40% decline in cardiovascular mortality. All-cause and cardiovascular mortality for the HFmrEF and HFpEF groups remained unchanged. The absolute mortality rates in individuals with HFrEF were higher than in the other two groups with HF in the initial decade (1985–1994). In the most recent decade (2005–2014) the mortality rates for all 3 HF subtypes converged.
The overall similar mortality risk for HFpEF versus HFrEF in the last decade of our investigation is consistent with some community-based reports (11,30) and data from two registries of HF patients (31,32). In contrast, two recent large meta-analyses (26,33) that included data from both observational studies and randomized trials reported a lower mortality risk for HFpEF relative to HFrEF. In the latter meta-analysis (33), the difference in mortality risk for HFpEF versus HFrEF narrowed in patients over age 75 years, a threshold that approximates the average age of HF onset in the FHS sample. Disease spectrum bias may also contribute to the similar mortality rates for HFrEF and HFpEF observed in cohort studies compared to randomized trials that have reported a better prognosis for HFpEF (34).
The decline in cardiovascular mortality for HFrEF over the decades suggests the effectiveness of evidence-based management strategies. In comparison, the prognosis of HFmrEF and HFpEF remained unchanged, underscoring the importance of ongoing trials of HFpEF patients (35). Data from the Olmsted County also suggest a trend towards decreasing mortality for HFrEF but not for HFpEF (11). The trends towards increasing non-CVD mortality in participants with HFmrEF in our investigation requires confirmation in larger samples.
Strengths and Limitations
The conjoint analysis comparing and linking trends in LVEF in participants without and with HF over a 30-year time period, the large community-based sample undergoing routine serial echocardiography and the use of the same criteria for diagnosis of HF across these decades, and the parsing of the epidemiology of HFmrEF from that of HFrEF and HFpEF are key strengths of our investigation. Nonetheless, several limitations warrant consideration. These include unavoidable biases due to differential missingness of echocardiographic data in those free of HF (sample 1), and possible misclassification of LVEF due to changes in the echocardiographic equipment over time and potential intra-reader temporal drifts (36). Individuals with missing echocardiograms often have higher risk (37). We implemented several quality control procedures in our echocardiography laboratory (Supplemental Methods, Section C) to minimize drifts in echocardiographic measurements. Tissue Doppler-based echocardiographic measures provide important information about LV diastolic function, and are a component of criteria for the diagnosis of HFpEF. However, the lack of availability of these measures and of plasma natriuretic peptide levels in the first two decades was an unavoidable limitation. The small sample sizes for HFmrEF in each of the three decades is an unavoidable limitation, given the overall lower prevalence of the condition (12–15% of all HF). Therefore, findings for this condition must be interpreted with caution and replicated in larger samples. Additionally, we were unable to evaluate the reasons for the unchanged prognosis of LVSD (without HF) over the decades in our sample. Lastly, our study sample included middle-aged to elderly white individuals of European ancestry, limiting the generalizability of our findings.
Conclusions
Our observations over the last three decades suggest that secular trends in CVD risk factors may be resulting in a change in the profile of HF in the community, marked by a decline in the prevalence of asymptomatic LVSD paralleled by a concomitant increase in the prevalence of HFpEF. The cardiovascular mortality of HFrEF has declined over the last three decades, reflecting the impact of major clinical trials. The unchanged prognosis of asymptomatic LVSD and of HFmrEF and HFpEF indicate unmet needs of patients with these conditions.
Supplementary Material
Perspectives.
Competency in Medical Knowledge
Serial observations in our large community-based cohort over the last three decades suggest that temporal trends in CVD risk factors may be altering the profile of HF in the community, characterized by a decline in the prevalence of asymptomatic LVSD with a concomitant increase in the prevalence of HFpEF. The cardiovascular mortality of HFrEF has declined over the last three decades, reflecting the impact of major clinical trials. The unchanged prognosis of asymptomatic LVSD and of HFmrEF and HFpEF indicate unmet needs of patients with these conditions.
Translational Outlook
It is important that our findings be explored and replicated in multi-ethnic samples, and future studies are warranted to elucidate the additional factors that may have contributed to the changing profile of LVSD and HF in the general population. The unchanged prognosis of LVSD in the absence of clinical HF, despite the availability of evidence-based treatment, underscores the need for strategies to better implement guidelines-based care for this condition. The prognosis of HFmrEF and HFpEF remain largely unchanged over the 30-year period, identifying major areas for improvement. Evidence-based management of patients with HFmrEF is challenged by the fact that they have not been consistently targeted in clinical trials and by the overall modest prevalence of the condition among HF patients (12–15%). Meta-analysis of data from controlled clinical trials of HF that enrolled patients with LVEF in the range 40–50% may inform future guidelines for managing these patients, and future clinical trials could consider pre-specifying this subgroup for analyses.
Acknowledgments
This work was supported by the National Heart, Lung and Blood Institute (contracts NO1-HC-25195 and HHSN268201500001I; both to RSV); grants from the NIH/NHLBI K23HL118529 (CWT), R01-HL131532 (SC), R01-HL134168 (SC), and R01HL080124 (RSV); Harvard Medical School Fellowship (CWT); 1R01HL128914 and 2R01 HL092577 (EJB); Evans Scholar award and Jay and Louise Coffman endowment, Department of Medicine, Boston University School of Medicine (RSV). There are no relations with or funding by industry.
Abbreviations
- EF
ejection fraction
- HF
HF
- HFpEF
HF with preserved LVEF
- HFrEF
HF with reduced LVEF
- HFmrEF
HF with mid-range LVEF
- LVSD
left ventricular systolic dysfunction
Footnotes
Declaration of Conflicts of interests: We declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 3.Luepker RV. Cardiovascular disease: rise, fall, and future prospects. Annu Rev Public Health. 2011;32:1–3. doi: 10.1146/annurev-publhealth-112810-151726. [DOI] [PubMed] [Google Scholar]
- 4.Navar AM, Peterson ED, Wojdyla D, et al. Temporal Changes in the Association Between Modifiable Risk Factors and Coronary Heart Disease Incidence. JAMA. 2016;316:2041–2043. doi: 10.1001/jama.2016.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerber Y, Weston SA, Berardi C, et al. Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: a community study. Am J Epidemiol. 2013;178:1272–1280. doi: 10.1093/aje/kwt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gjesing A, Gislason GH, Kober L, et al. Nationwide trends in development of heart failure and mortality after first-time myocardial infarction 1997–2010: A Danish cohort study. Eur J Intern Med. 2014;25:731–738. doi: 10.1016/j.ejim.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Hung J, Teng TH, Finn J, et al. Trends from 1996 to 2007 in incidence and mortality outcomes of heart failure after acute myocardial infarction: a population-based study of 20,812 patients with first acute myocardial infarction in Western Australia. J Am Heart Assoc. 2013;2:e000172. doi: 10.1161/JAHA.113.000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McManus DD, Piacentine SM, Lessard D, et al. Thirty-Year Trends in the Incidence Rates, Clinical Features, Treatment Practices, and Short-term Outcomes of Patients < 55 Years of Age Hospitalized with an Initial Acute Myocardial Infarction. Am J Cardiol. 2011;108:477–482. doi: 10.1016/j.amjcard.2011.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roger VL. Epidemiology of heart failure. Circ Res. 2013;113:646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber Y, Weston SA, Redfield MM, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996–1004. doi: 10.1001/jamainternmed.2015.0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 12.Lam CS, Solomon SD. The middle child in heart failure: heart failure with mid-range ejection fraction (40–50%) Eur J Heart Fail. 2014;16:1049–1055. doi: 10.1002/ejhf.159. [DOI] [PubMed] [Google Scholar]
- 13.Lam CS, Teng TH. Understanding Heart Failure With Mid-Range Ejection Fraction. JACC Heart Fail. 2016;4:473–476. doi: 10.1016/j.jchf.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Lund LH. Heart Failure With “Mid-Range” Ejection Fraction-New Opportunities. J Card Fail. 2016;22:769–771. doi: 10.1016/j.cardfail.2016.07.439. [DOI] [PubMed] [Google Scholar]
- 15.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 16.Ezekowitz JA, Kaul P, Bakal JA, Quan H, McAlister FA. Trends in heart failure care: has the incident diagnosis of heart failure shifted from the hospital to the emergency department and outpatient clinics? Eur J Heart Fail. 2011;13:142–147. doi: 10.1093/eurjhf/hfq185. [DOI] [PubMed] [Google Scholar]
- 17.Dawber TR, Meadors GF, Moore FE. Epidemiologic approaches to heart disease: the Framingham Study. Am J Public Health. 1951;41:279–286. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families.The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 19.Splansky GL, Corey D, Yang Q, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 20.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 21.de SG, Devereux RB, Ganau A, et al. Estimation of left ventricular chamber and stroke volume by limited M-mode echocardiography and validation by two-dimensional and Doppler echocardiography. Am J Cardiol. 1996;78:801–807. doi: 10.1016/s0002-9149(96)00425-0. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, Wolf PA, Garrison RJ, editors. Framingham Heart Study, 30 year follow-up. Bethesda, MD: US Department of Health and Human Services; 1987. Section 34: Some risk factors related to the annual incidence of cardiovascular disease and death in pooled repeated biennial measurements. [Google Scholar]
- 23.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 24.Mosterd A, Deckers JW, Hoes AW, et al. Classification of heart failure in population based research: an assessment of six heart failure scores. Eur J Epidemiol. 1997;13:491–502. doi: 10.1023/a:1007383914444. [DOI] [PubMed] [Google Scholar]
- 25.Lee DS, Gona P, Albano I, et al. A systematic assessment of causes of death after heart failure onset in the community: impact of age at death, time period, and left ventricular systolic dysfunction. Circ Heart Fail. 2011;4:36–43. doi: 10.1161/CIRCHEARTFAILURE.110.957480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Somaratne JB, Berry C, McMurray JJV, Poppe KK, Doughty RN, Whalley GA. The prognostic significance of heart failure with preserved left ventricular ejection fraction: a literature-based meta-analysis. European Journal of Heart Failure. 2009;11:855–862. doi: 10.1093/eurjhf/hfp103. [DOI] [PubMed] [Google Scholar]
- 27.Cox DR. Regression models and life tables (with discussion) J Royal Stat Soc. 1972;34(Series B):187–220. [Google Scholar]
- 28.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 29.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 30.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 31.Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 32.Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in Patients Hospitalized With Heart Failure and Preserved Left Ventricular Ejection Fraction. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 33.The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012;33:1750–1757. doi: 10.1093/eurheartj/ehr254. [DOI] [PubMed] [Google Scholar]
- 34.Burkhoff D. Mortality in heart failure with preserved ejection fraction: an unacceptably high rate. Eur Heart J. 2012;33:1718–1720. doi: 10.1093/eurheartj/ehr339. [DOI] [PubMed] [Google Scholar]
- 35.Senni M, Paulus WJ, Gavazzi A, et al. New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. Eur Heart J. 2014;35:2797–2815. doi: 10.1093/eurheartj/ehu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gardin JM. How reliable are serial echocardiographic measurements in detecting regression in left ventricular hypertrophy and changes in function? J Am Coll Cardiol. 1999;34:1633–1636. doi: 10.1016/s0735-1097(99)00399-x. [DOI] [PubMed] [Google Scholar]
- 37.Poppe KK, Squire IB, Whalley GA, et al. Known and missing left ventricular ejection fraction and survival in patients with heart failure: a MAGGIC meta-analysis report. Eur J Heart Fail. 2013;15:1220–7. doi: 10.1093/eurjhf/hft101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.