Abstract
Impaired clinical insight (CI) is a common symptom of psychotic disorders and a promising treatment target. However, to date, our understanding of how variability in CI is tied to underlying brain dysfunction in the clinical high-risk period is limited. Developing a stronger conception of this link will be a vital first step for efforts to determine if CI can serve as a useful prognostic indicator. The current study investigated whether variability in CI is related to major brain networks in adolescents and young adults at ultra high-risk (UHR) of developing psychosis. Thirty-five UHR youth were administered structured clinical interviews as well as an assessment for CI and underwent resting-state magnetic resonance imaging scans. Functional connectivity was calculated in the default mode network (DMN) and fronto-parietal network (FPN), two major networks that are dysfunctional in psychosis and are hypothesized to affect insight. Greater DMN connectivity between the posterior cingulate/precuneus and ventromedial prefrontal cortex (DMN) was related to poorer CI (R2 = .399). There were no significant relationships between insight and the FPN. This is the first study to relate a major brain network to clinical insight before the onset of psychosis. Findings are consistent with evidence if a hyperconnected DMN in schizophrenia and UHR, and similar to a previous study of insight and connectivity in schizophrenia. Results suggest that a strongly connected DMN may be related to poor self-awareness of subthreshold psychotic symptoms in UHR adolescents and young adults.
Keywords: Insight, Prodrome, Ultra High-Risk, Default Mode Network
1. INTRODUCTION
Clinical insight (CI) in schizophrenia refers to an individual’s awareness of having the disorder (Amador and Kronengold, 2004). It is estimated that approximately 50% of those diagnosed with schizophrenia are unaware of their illness (Arango and Amador, 2011) and CI may predict treatment adherence and outcomes (Lincoln et al., 2007). Insight deficits are likely related to neurological dysfunction (Larøi et al., 2004), and many studies have associated distributed brain regions with CI in schizophrenia (Gerretsen et al., 2014a; Liemburg et al., 2012; Morgan et al., 2010; Shad and Keshavan, 2015). However, little is known about how variability in CI is related to brain function in youth exhibiting a prodromal syndrome (i.e., those at ultra high-risk for psychosis; UHR). Further, investigating CI prior to psychosis onset may reveal whether it is a good prognostic or diagnostic indicator and also yield important clues that can inform intervention. Thus, the aim of this study was to investigate relationships between CI and function of major brain networks in UHR youth.
In psychotic disorders, degree of CI is considered to lie on a continuum and to consist of multiple dimensions on which an individual may be impaired (Amador et al., 1993; Beck et al., 2004; Birchwood et al., 1994; David, 1990; Gerretsen et al., 2014c; Marková et al., 2003; McEvoy et al., 1981). Insight is relatively stable (Arango and Amador, 2011), but may fluctuate with symptom severity (Parellada et al., 2011; Quee et al., 2014). However, symptoms alone do not predict CI (Mintz et al., 2003; van der Meer et al., 2013). In addition, insight tends to predict treatment adherence in schizophrenia and may predict general function or specific social or work capacities (Lincoln et al., 2007).
Poor CI may be a risk factor for psychotic episodes because it appears to worsen with acute episodes and improve with treatment (Gerretsen et al., 2014b). Despite evidence linking insight impairment to acute episodes, there is little research on CI before psychosis onset. The only study of CI in UHR thus far found it to be moderately impaired, but less impaired than in first episode psychosis (Lappin et al., 2007). CI was not correlated with symptom severity in the UHR group, suggesting that it accounts for unique variance in the UHR phenotype. A retrospective chart review study of patients diagnosed with schizophrenia revealed that insight assessed by emergency department physicians declined leading up to a first psychotic episode and better insight at baseline predicted fewer and shorter hospitalizations as well as better treatment compliance (Bota et al., 2006). Investigating CI and related brain networks during the psychosis prodrome may offer valuable information regarding prognosis and diagnosis.
Most researchers agree that insight deficits are associated, at least in part, with neurological dysfunction (Larøi et al., 2004). Structural studies suggest that impaired CI is largely associated with reduced frontal gray matter, as well as reduced gray matter in temporal, parietal, and subcortical regions (Bergé et al., 2011; Buchy et al., 2011; Cooke et al., 2008; Flashman et al., 2001; Gerretsen et al., 2013; Morgan et al., 2010b; Sapara et al., 2007; Shad et al., 2004; Spalletta et al., 2014). However, methods vary widely and some studies have found no gray matter differences between those with poor and good CI (Bassitt et al., 2007; McFarland et al., 2013; Rossell et al., 2003). Task-based functional studies of insight and self-reflection have illustrated an important role for the medial prefrontal cortex, and have implicated lateral frontal and parietal regions as well (Ćurčić-Blake et al., 2015; Shad et al., 2012; Shad and Keshavan, 2015).
Structural and functional findings that CI is associated with widespread regions suggest that networks of brain regions may work together to influence insight. Resting-state functional connectivity (rsFC) studies have illustrated that CI is associated with connectivity of the default mode network (DMN) and self-referential regions, including areas in the medial and lateral frontal and parietal lobes (Gerretsen et al., 2014a; Liemburg et al., 2012). These two studies presented conflicting findings, however, so it is still unclear how CI variation is related to connectivity of brain networks in psychotic disorders.
Nodes of the DMN have been associated with self-referential processing and social cognition, including theory of mind and distinguishing self from others (Li et al., 2014; Northoff et al., 2006; Qin and Northoff, 2011; Schilbach et al., 2008). It appears to be dysfunctional in disorders marked by social cognitive deficits, such as autism, schizophrenia, and attention deficit/hyperactivity disorder (Broyd et al., 2009; Di Martino et al., 2009; Whitfield-Gabrieli and Ford, 2012). In schizophrenia, many studies have shown hyperconnectivity of the DMN and abnormal rsFC of the dlPFC (the primary node of the fronto-parietal control network (FPN)), though the literature is mixed (Sheffield and Barch, 2016; Whitfield-Gabrieli and Ford, 2012).
Function of the DMN and FPN may contribute to CI deficits (Shad et al., 2007) through challenges in distinguishing self from other and making decisions in regards to oneself, as well as inflexible and disorganized thinking (Diamond, 2013; Gilleen et al., 2011; Nekovarova et al., 2014; Northoff et al., 2006; Northoff and Qin, 2011; Shad et al., 2007). The DMN and FPN and related cognitive processes appear to be dysfunctional in UHR, as well (Bora and Murray, 2014; Fusar-Poli et al., 2012; Nelson et al., 2012; Schmidt et al., 2014; Shim et al., 2010; Wotruba et al., 2013). Because schizophrenia is considered a neurodevelopmental disconnection disorder (Satterthwaite and Baker, 2015), investigating rsFC in UHR populations and related phenotypes such as insight may reveal important characteristics of the psychotic disorder continuum as well as potential treatment options.
Thus far, all insight neuroimaging studies have been performed with first-episode psychosis or chronic schizophrenia populations. Studying insight and associated brain networks in a UHR sample may help to inform about characteristics of insight across the continuum of psychotic disorders, particularly before onset of a psychotic disorder (Gerretsen et al., 2014a). Because CI may predict psychotic episodes and outcomes, understanding it in the prodromal population may help clinicians to design specific early interventions that may reduce duration of untreated psychosis or prevent psychosis onset. Previous research has indicated that large-scale networks are associated with psychotic disorders and CI. Therefore, we aimed to investigate the relationship between CI and rsFC of the DMN and FPN in a UHR sample. We first hypothesized that poorer CI would be associated with stronger DMN connectivity and weaker FPN connectivity. We also hypothesized that poorer clinical insight would be associated with weaker anticorrelations between the DMN and FPN (i.e. negative relationship).
2. METHODS
2.1 Procedures
2.1.1 Participants
Participants were recruited at the Adolescent Development and Preventative Treatment (ADAPT) research program at the University of Colorado, Boulder under direction of Dr. Mittal. The sample consisted of 35 adolescents and young adults, ages 15–22. Adolescents and young adults were identified as UHR with the Structured Interview of Prodromal Syndromes (SIPS) by an advanced doctoral student or clinical psychologist (Miller et al., 1999). Per the SIPS, participants were considered UHR if they had moderate (score of 3 on scale of 0–6) to severe but not psychotic (score of 5) positive symptoms or a decline in functioning accompanied by schizotypal traits and/or a family history of schizophrenia. Five participants had a first-degree relative with a psychotic disorder and a decline in functioning without positive symptoms. No participants had pure schizotypal presentation. Exclusion criteria for the UHR group included history of head injury, diagnosis of an Axis I psychotic disorder, neurological disorder, or MRI contraindication. To assess these criteria, a trained advanced doctoral student or clinical psychologist administered the Structured Clinical Interview for DSM-IV-TR Disorders (SCID-IV) (First et al., 1997). Exclusion criteria for this imaging study further included current substance dependence and in-scanner head motion greater than 3 mm in any direction. All participants had an IQ above 70, so no participants were excluded for this reason.
2.1.2. Clinical Insight measure
CI was assessed with the Scale to Assess Unawareness of Mental Disorder (SUMD), a clinician-rated measure (Amador et al., 1993). The SUMD includes three general items: awareness of having a mental disorder, awareness of the effects of medication, and awareness of social consequences of the disorder. It also includes items for awareness and attribution of specific symptoms (these were not assessed). Each item is rated on a scale of 0 – 5, with 0 indicating “not applicable” (full awareness) and 5 indicating “not at all aware”. The general items load on a single factor with good internal consistency (Boyer et al., 2012; Michel et al., 2013), so in the present study, these items were summed to create a score for current clinical insight, with higher scores representing poorer insight. We chose to sum the scores so that our insight measure would encompass these three important facets of CI and allow us to investigate individual differences in overall CI in a highly variable population. In addition, the awareness of illness item refers to general awareness of having a disorder, so is not limited to awareness of positive symptoms; for this reason we did not restrict our analysis to only participants with positive symptoms.
2.1.3. Scanning
Participants underwent both structural and functional magnetic resonance imaging (MRI) scans on a 3T Siemens Magnetom TrioTim scanner. Structural images were acquired with a T1-weighted 3D magnetization prepared rapid gradient multi-echo sequence (sagittal MPRAGE; repetition time [TR] = 2,530 ms; echo times [TE] = 1.64 ms, 3.5 ms, 5.36 ms, 7.22 ms, 9.08 ms; GRAPPA parallel imaging factor 2; 1 mm3 voxels, 192 interleaved slices; FOV = 256 mm; flip angle 57°). A 5 min 34 s functional resting state blood-oxygen-level-dependent (BOLD) scan was acquired with a T2*-weighted echo-planar functional protocol (number of volumes = 165; TR = 2,000 ms; TE = 29 ms; matrix size = 64 × 64 × 33; FA = 75°; 3.8 × 3.× 3 3.5 mm3 voxels; 33 interleaved slices; FOV = 240 mm). During the resting state scan, participants were instructed to relax and close their eyes.
2.1.4. Preprocessing
Data were preprocessed using the Data Processing Assistant for Resting State fMRI, Advanced Edition (DPARSFA) (Yan and Zang, 2010). The first four time points were removed, and then scans were slice-timing corrected, motion corrected, and coregistered to the T1 image. The images were then normalized to MNI space using unified segmentation and smoothed with a 6 mm Gaussian kernel. After smoothing, images were temporally filtered (0.01 – 0.08 Hz). Finally, nuisance covariates were regressed out: white matter and cerebrospinal fluid signals, head motion scrubbing regressors (framewise displacement (FD) > 0.5, two volumes before, and one volume after the bad time point), and 12 motion parameters.
2.2. Statistical Analyses
2.2.1. Functional connectivity
Seed-based connectivity was calculated in DPARSFA with seeds in the DMN and FPN. The DMN was defined by two 10 mm spheres centered on the posterior cingulate cortex (PCC)/precuneus; MNI coordinates −11, −57, 13) and the ventromedial prefrontal cortex (vmPFC; MNI coordinates 1, 31, −2), as specified in previous studies (Orr et al., 2014). The FPN was defined by 10 mm spheres centered on the right dorsolateral prefrontal cortex (dlPFC; MNI coordinates 43, 22, 34) and right inferior parietal lobule (IPL; MNI coordinates 51, −47, 42). We restricted our analyses to the right hemisphere because previous research has suggested that insight deficits may be related to right hemisphere dysfunction (Gerretsen et al., 2015, 2013; Gerretsen et al., 2014a). We also wanted to reduce the risk of Type I error. Additionally, a control seed was included in the primary visual cortex to specify that any results obtained were indeed related to insight and not a generally dysfunctional brain (MNI coordinates −7, −83, 2).
DPARSFA calculated correlations between seed regions of interest (ROIs) and all voxels in the brain (seed-to-voxel approach) and between ROIs (ROI-to-ROI approach). For seed-to-voxel calculations, the correlations were converted into z maps with Fisher’s r to z transformation. For ROI-to-ROI calculations, DPARSFA created a matrix of Fisher’s z scores for each participant, representing connectivity between pairs of ROIs. We investigated connectivity between ROIs in the networks of interest, between the PCC/precuneus and right dlPFC (DMN and FPN), and between the primary visual cortex and PCC/precuneus. We also performed seed-to-voxel connectivity analyses for the PCC/precuneus and right dlPFC.
2.2.2. Connectivity and clinical insight analyses
SPSS version 23 was used to perform four hierarchical linear regression analyses with ROI-to-ROI connectivity as the outcome variables. FD, a measure of average head motion, was entered in the first step, because head motion can bias connectivity analyses (Power et al., 2014). CI was entered in the second step. Models were considered significant if they passed Bonferroni correction for multiple comparisons, with p < .0125 (.05/4 analyses). Post-hoc analyses were performed with positive and disorganized symptoms included as covariates and excluding individuals on neuroleptics.
For exploratory seed-to-voxel analyses, SPM8 was used to perform voxel-wise linear regressions on the rsFC maps of the PCC and right dlPFC seeds. Multiple linear regression was specified, with clinical insight as a predictor and FD as a covariate. A cluster-forming threshold of p < .001 was employed at the voxel level, and significant clusters passed family-wise error (FWE) correction for multiple comparisons.
3. RESULTS
3.1. Participants
Demographic and clinical characteristics of participants are presented in Table 1. A total of 16 participants were rated as “somewhat aware” to “unaware” (score of 3 – 5) on the awareness of illness item, indicating some level of insight impairment. Mean FD for this group was 0.195 mm (SD = 0.093). Current CI was correlated with positive symptoms (r(35) = .566) and disorganized symptoms (r(35) = .564). No other demographic or clinical factors correlated with clinical insight.
Table 1.
Participant Characteristics (N = 35).
| Mean | Standard Deviation | Range | |
|---|---|---|---|
| Demographic Characteristics | |||
| Age (Years) | 18.80 | 1.73 | 15 – 22 |
| Gender (M/F) | 23/12 | ||
| Handedness (R/L) | 27/1 | ||
| Race (White/Non-White) | 23/11 | ||
| Parental Education (Years) | 15.32 | 1.97 | 12 – 18 |
| WRAT Sum IQ | 111.31 | 12.55 | 87 – 145 |
| Clinical Characteristics | |||
| Antipsychotic Medications (Y/N) | 8/27 | ||
| Chlorpromazine Equivalents (N=8) | 119.79 | 74.79 | 50 – 250 |
| Positive Symptoms | 12.23 | 5.80 | 0 – 23 |
| Negative Symptoms | 9.17 | 7.12 | 0 – 24 |
| Disorganized Symptoms | 5.63 | 4.07 | 0 – 12 |
| Clinical Insight (SUMD) | |||
| Awareness of Mental Disorder | 2.14 | 1.42 | 0 – 5 |
| Awareness of Medication Effects | 1.00 | 1.39 | 0 – 4 |
| Awareness of Social Consequences | 1.74 | 1.56 | 0 – 4 |
| Current Clinical Insight | 4.89 | 3.13 | 0 – 12 |
Note, WRAT, Wide Range Achievement Test; SUMD, Scale to Assess Unawareness of Mental Disorder; Symptom severity was assessed with the Structured Interview of Prodromal Syndromes; Chlorpromazine equivalents were computed only for those individuals taking antipsychotics
3.1.1. Connectivity and clinical insight
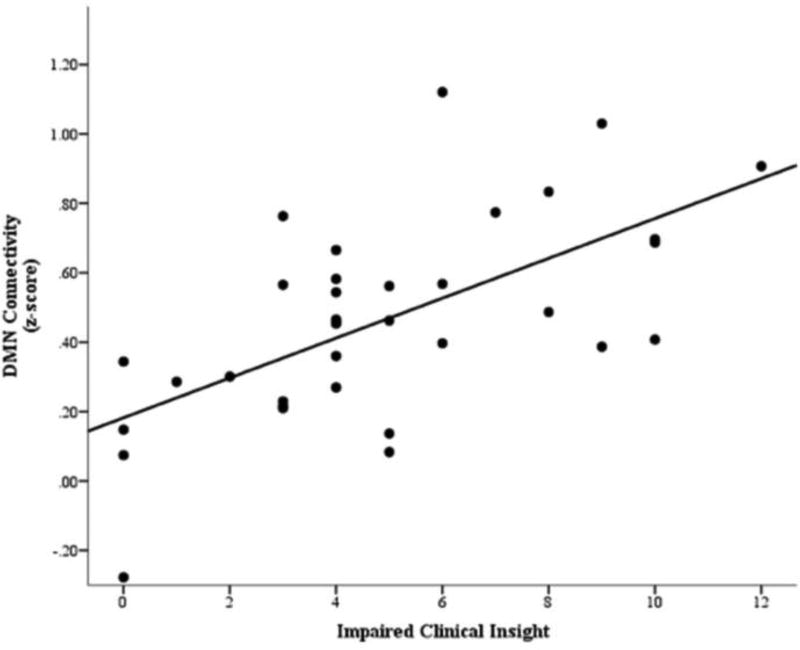
Hierarchical regression indicated a significant main effect of clinical insight on DMN (PCC/precuneus – vmPFC) connectivity. Poorer clinical insight predicted significantly stronger connectivity within the DMN (Figure 1), and clinical insight accounted for 39.9% of the variance in DMN connectivity. Because clinical insight was correlated with positive and disorganized symptoms, a post-hoc analysis was done with positive and disorganized symptom severity as covariates and the model remained significant (adjusted R2 = .346, F(4, 30) = 5.50, p = .002). A further analysis excluded 8 participants taking antipsychotics, and the model remained significant as well (adjusted R2 = .282, F(2, 24) = 6.10, p = .007). The regression model predicting connectivity within the FPN was not significant, and the models predicting connectivity between the DMN and FPN and between the visual cortex and DMN were not significant after correcting for multiple comparisons. The only significant predictor in the latter two models was FD. These models remained nonsignificant after covarying for positive and disorganized symptoms. Results are presented in Table 2.
Figure 1.
Scatterplot of default mode network (DMN) connectivity and clinical insight impairment measured with the Scale to Assess Unawareness of Mental Disorder (SUMD), illustrating the main effect of impaired clinical insight on DMN connectivity. Higher scores reflect poorer clinical insight.
Table 2.
Hierarchical regressions for clinical insight predicting default mode network (DMN) and fronto-parietal network (FPN) connectivity.
| Predictor | β | t | p | ΔR2 | F(model) | p(model) |
|---|---|---|---|---|---|---|
| Analysis 1: DMN Connectivity (PCC/Precuneus – vmPFC) | ||||||
| Step 1 | .016 | .554 | .462 | |||
| FD | .128 | .744 | .462 | |||
| Step 2 | .399 | 11.38 | .000 | |||
| FD | .166 | 1.23 | .229 | |||
| Impaired Clinical Insight* | .633 | 4.68 | .000 | |||
| Analysis 2: FPN Connectivity (Right dlPFC – IPL) | ||||||
| Step 1 | .000 | .002 | .962 | |||
| FD | −.008 | −.047 | .962 | |||
| Step 2 | .033 | .552 | .581 | |||
| FD | −.019 | −.110 | .913 | |||
| Impaired Clinical Insight | −.183 | −1.05 | .302 | |||
| Analysis 3: DMN – FPN Connectivity (PCC/Precuneus – dlPFC) | ||||||
| Step 1 | .107 | 3.95 | .055 | |||
| FD | .327 | 1.99 | .055 | |||
| Step 2 | .073 | 3.50 | .042 | |||
| FD* | .343 | 2.13 | .040 | |||
| Impaired Clinical Insight | .270 | 1.68 | .102 | |||
| Analysis 4: Primary Visual Cortex – DMN Connectivity (Primary Visual Cortex – PCC/Precuneus) | ||||||
| Step 1 | .170 | 6.77 | .014 | |||
| FD | .412 | 2.60 | .014 | |||
| Step 2 | .004 | 3.37 | .047 | |||
| FD* | .409 | 2.54 | .016 | |||
| Impaired Clinical Insight | −.063 | −.390 | .699 | |||
| Analysis 5: DMN Connectivity Controlling for Symptom Severity | ||||||
| Step 1 | .016 | .554 | .462 | |||
| FD | .128 | .744 | .462 | |||
| Step 2 | .122 | 1.66 | .196 | |||
| FD | .076 | .452 | .654 | |||
| Positive Symptoms | .173 | .840 | .407 | |||
| Disorganized Symptoms | .223 | 1.08 | .288 | |||
| Step 3 | .285 | 5.50 | .002 | |||
| FD | .186 | 1.30 | .203 | |||
| Positive Symptoms | −.087 | −.473 | .639 | |||
| Disorganized Symptoms | −.038 | −.205 | .839 | |||
| Impaired Clinical Insight* | .705 | 3.85 | .001 | |||
Note, FD, framewise displacement; PCC, posterior cingulate cortex; vmPFC, ventromedial prefrontal cortex; IPL, inferior parietal lobule
Predictor variable significantly predicts outcome variable
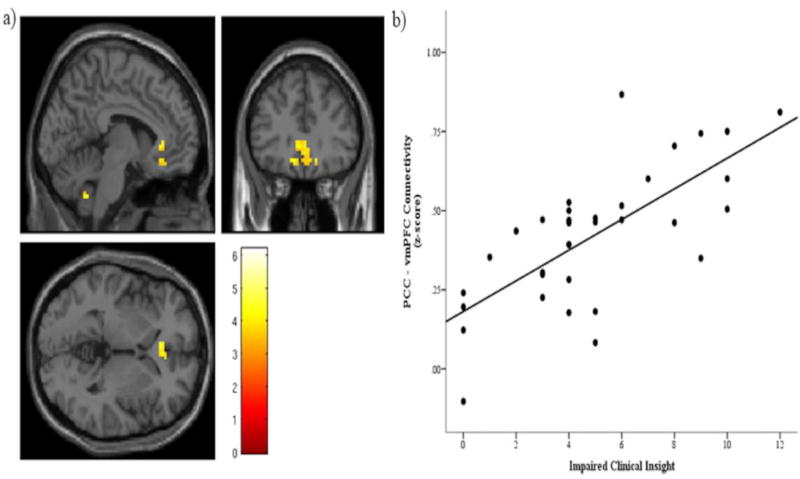
Seed-to-voxel analysis for clinical insight showed a significant main effect of clinical insight on connectivity between the PCC and vmPFC. The significant cluster was centered at MNI coordinates (−6, 30, 0) with a peak T value of 4.78, pFWE-corr = .011 (see Figure 2 and Table 3). When positive and disorganized symptoms were entered into the mdoel, a similar cluster was still present at the uncorrected p-value, but was no longer significant at the familywise error-corrected level. No significant effects emerged for seed-to-voxel connectivity from the right dlPFC.
Figure 2.
Significant main effect of clinical insight predicting connectivity between the posterior cingulate cortex (PCC) and ventromedial prefrontal cortex (vmPFC). a) Significant cluster centered at MNI coordinates (−6, 30, 0), with a cluster extent of 113 voxels. A cluster forming threshold of p < .001 was applied, and this cluster was significant at the cluster level with a familywise-corrected significance of pFWE-corrected = .011. The color bar shows T values. Note, the cerebellar cluster was nonsignificant. b) Scatterplot of individual connectivity values (Fisher’s z scores) extracted from the significant cluster displayed in a), plotted against impaired clinical insight.
Table 3.
Seed-to-voxel results. Models were specified in Statistical Parametric Mapping (SPM) using the flexible factorial approach, including framewise displacement (FD) as a covariate of no interest. A cluster-forming threshold of p < .001 was applied, and clusters were considered significant if they passed cluster FWE-corrected p < .05.
| Region | # Voxels | MNI Coordinates (x, y, z) |
Brodmann Area |
T value |
pFWE-corrected (cluster level) |
|---|---|---|---|---|---|
| PCC Seed | |||||
| L Olfactory | 113 | −6, 30, 0 | 11 | 4.78 | .011 |
| L Rectus | −12, 30, −12 | 11 | 4.37 | ||
| Medial Orbital | 3, 42, −12 | 11 | 4.32 | ||
| R dlPFC Seed | |||||
| No significant clusters |
Note, PCC, posterior cingulate cortex; dlPFC dorsolateral prefrontal cortex
4. DISCUSSION
This study is the first to investigate clinical insight (CI) and resting-state functional connectivity (rsFC) during the psychosis prodrome. Poorer CI was associated with greater connectivity between the ventromedial prefrontal cortex (vmPFC) and posterior cingulate/precuneus (PCC/precuneus), nodes of the default mode network (DMN). Results illustrate similar relationships between CI and rsFC to previous studies in schizophrenia, and suggest that CI may be a good prognostic indicator for UHR youth.
This study is the first to find that stronger DMN connectivity is associated with poorer CI in youth with prodromal syndromes, and it was shown in both ROI-to-ROI and seed-to-voxel analyses. In addition, results were not accounted for by symptom severity or neuroleptic use. CI appears to account for unique variance in DMN connectivity and may add to describing the clinical profile of these individuals or identifying risk or resilience factors; however, since there are no longitudinal studies of CI in UHR, we cannot draw any firm conclusions on whether insight is a unique marker for conversion. These findings are in agreement with past research indicating hyperconnectivity and hyperactivity within the DMN in UHR (Shim et al., 2010) and schizophrenia (Whitfield-Gabrieli and Ford, 2012) compared to healthy controls. In addition, a previous study linked stronger DMN connectivity to impaired CI in schizophrenia (Gerretsen et al., 2014a), though between the PCC/precuneus and left angular gyrus and not between PCC/precuneus and vmPFC. In contrast, the other resting-state connectivity and CI study in schizophrenia indicated lower connectivity in the PCC and ACC in those with impaired insight; however, this study used a different connectivity method, did not measure connectivity between nodes, and dichotomized clinical insight (Liemburg et al., 2012).
The DMN, and especially midline structures, are thought to underlie self-reflection (Northoff et al., 2006). A review by Qin and Northoff (2011) revealed that nodes of the DMN are active during self-and other-reflection tasks, as well as during rest. Studies employing self-reflection tasks have also implicated DMN structures in both self-reflection and insight, indicating a relationship between these two constructs (Ćurčić-Blake et al., 2015; Modinos et al., 2011; van der Meer et al., 2013). One prior study implicated PCC and vmPFC activity during a clinical insight task that asked mental-illness related questions (Raij et al., 2012). Another found stronger connectivity between the PCC and vmPFC during self-reflection in schizophrenia patients with poor clinical insight (Ćurčić-Blake et al., 2015). In addition, a higher psychosis score in healthy individuals with psychosis-prone traits was associated with higher activity of the vmPFC (Modinos et al., 2011). Taken together, these studies indicate that an overactive or hyperconnected DMN may reflect poor self-awareness, such as inability to disengage from internally-focused thought (Sass, 2014), overactive “tagging” of stimuli for self-relevance (Ćurčić-Blake et al., 2015), and difficulty in retrieving autobiographical memories (van der Meer et al., 2010).
While the PCC may be more involved in other-reflection and the vmPFC may be more involved in self-reflection (Qin and Northoff, 2011), it is possible that the communication between the two is problematic in those with poor insight or self-reflective abilities. Since our study did not use a task and used a measure of connectivity rather than activity, we speculate that strong connectivity between these areas may be related to poor self-reflection because these individuals are overly self-focused and less able to evaluate external stimuli. It is possible that these connections do not disengage properly when attention is supposed to be directed outward, as has been shown in the schizophrenia literature (Whitfield-Gabrieli and Ford, 2012).
Disruption of self-reflective processes may result in a distorted view of the self in relation to others or in relation to psychotic symptoms. The current study suggests that these processes may be disrupted across the psychosis continuum. In fact, self-disturbance has been described in UHR populations, and even predicted transition to psychosis (Nelson et al., 2012). If UHR individuals experience a breakdown in self-monitoring (Garety et al., 2001; Nelson et al., 2012; Sass, 2014), it is possible that this breakdown is related to a hyperconnected DMN and influences awareness of having a prodromal syndrome. Further research is required to determine if self-disturbance is directly associated with both insight and the DMN.
While we present compelling evidence that the DMN is associated CI in UHR, hypotheses involving the FPN were not supported, suggesting that CI in UHR is more closely related to self-awareness than executive function. Gerretsen et al. (2014a) also did not find within-network connectivity of the FPN to be associated with CI in a schizophrenia sample. Despite many structural studies showing a relationship between the PFC and CI (Bergé et al., 2011; Buchy et al., 2011; Cooke et al., 2008; Flashman et al., 2001; Gerretsen et al., 2013; Morgan et al., 2010b; Sapara et al., 2007; Shad et al., 2004; Spalletta et al., 2014) and functional studies showing aberrant dlPFC connectivity to be associated with executive function difficulties in schizophrenia (Sheffield and Barch, 2016), it is unlikely that the FPN has a direct relationship with CI. It may be that insight is not directly associated with executive function networks, and rather, executive functioning influences CI through self-monitoring or metacognition (Shad et al., 2007). Future investigations should parse apart self-monitoring and metacognitive abilities in relation to CI.
Findings support the dysconnection hypothesis of schizophrenia (Friston et al., 2016), and provide further evidence that these dysconnectivity processes may be occurring in at-risk individuals. Thus, dysconnectivity in schizophrenia appears to have a neurodevelopmental component (Satterthwaite and Baker, 2015). More longitudinal research is needed to elucidate the neurodevelopmental trajectories of major networks such as the DMN and associated phenotypes. Linking phenotypes such as poor insight to aberrant connectivity is an important step in understanding the psychosis continuum. CI appears to have both trait-like and state-like qualities related to both premorbid factors and phase and severity of illness (Gerretsen et al., 2014b; Quee et al., 2011). Therefore, it will be important to investigate whether prodromal CI predicts later CI or other phenotypes, and to what degree clinical insight can change.
This study does not come without limitations. By nature of this sample, there was a limited range of CI compared to studies of schizophrenia because individuals with severely impaired clinical insight would likely be diagnosed with schizophrenia. Further, psychometrics of the SUMD were previously assessed in schizophrenia populations (Amador et al., 1993; Boyer et al., 2012; Michel et al., 2013), but internal consistency was low in this sample. While variability in this population is expected, it would be beneficial to investigate validity of the SUMD in a larger UHR sample. Because the SUMD assesses insight specifically in populations with psychotic disorders, we were unable to include a healthy control group. In addition, a minority of participants were on antipsychotic medications, and it is unknown how these medications may have affected their insight. However, the significant regression model remained significant when these participants were excluded, suggesting that neuroleptics did not influence findings. It is also important to note that awareness of medication effects is included in the CI measure, so these indviduals’ perception of medication was taken into account when they were included in the model. Regarding neuroimaging, there are potential signal confounds inherent in fMRI (Weinberger & Radulescu, 2015); also, during resting-state scans, the participants’ mental states at the time of scanning may vary widely (Buckner et al., 2013). Further, connectivity analyses are limited to those participants who were able to be scanned with minimal head motion, to avoid biasing connectivity measures. Finally, methods used in this study cannot infer causation or directionality of connectivity, as it was a cross-sectional study.
In the future, because this study is an ongoing longitudinal study, we will be able to follow up with those who developed psychotic disorders and investigate whether CI and DMN connectivity predict later transition to psychosis. We may also be able to investigate any demographic or clinical characteristics clinical insight may be associated with pre- and post-conversion (e.g., symptomatology, cognition, treatment compliance). It will be important for future studies with more power to replicate our results and investigate right and left hemisphere differences in this population. Future neuroimaging studies may also wish to investigate relations between CI and metacognitive and self-reflective abilities. Finally, psychiatric control groups will be essential in future work to elucidate CI characteristics in UHR samples in comparison to psychosis spectrum disorders.
Acknowledgments
Thank you to the Adolescent Development and Preventative Treatment program in Boulder, CO for collecting and sharing the data, and thank you to the Imaging Genetics and Informatics lab at GSU for help with data analysis.
Role of funding source
This work was supported by the National Institute of Mental Health grants RO1MH094650 and R21/R33MH103231 to V.A.M, and by the Georgia State University Neurogenomics 2CI Fellowship to S.V.C. The funders had no further role in study design, in the collection, analysis and interpretation of data, nor in the writing of the report or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
S.V.C. designed the study, analyzed data, and prepared the manuscript; V.A.M. collected data and edited the manuscript; J.A.B. collected data and edited the manuscript; A.A. assisted with data analysis; T.Z.K. assisted with study design and edited the manuscript; J.A.T. assisted with study design and data analysis and edited the manuscript. All authors approved the manuscript for submission.
Conflict of interest
V.A.M is a consultant to Takeda Pharmaceuticals.
References
- Amador XF, Kronengold H. Understanding and assessing insight. In: Amador XF, David AS, editors. Insight and Psychosis. Oxford Univ Press; New York City: 2004. pp. 3–30. [Google Scholar]
- Amador XF, Strauss DH, Yale SA, Flaum M, Endicott J, Gorman JM. Assessment of insight in psychosis. Am. J. Psychiatry. 1993 doi: 10.1176/ajp.150.6.873. [DOI] [PubMed] [Google Scholar]
- Arango C, Amador X. Lessons learned about poor insight. Schizophr. Bull. 2011;37:27–28. doi: 10.1093/schbul/sbq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassitt DP, Neto MRL, De Castro CC, Busatto GF. Insight and regional brain volumes in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2007;257:58–62. doi: 10.1007/s00406-006-0685-z. [DOI] [PubMed] [Google Scholar]
- Beck A, Baruch E, Balter J, Steer R, Warman D. A new instrument for measuring insight: The Beck Cognitive Insight Scale. Schizophr. Res. 2004;68:319–329. doi: 10.1016/S0920-9964(03)00189-0. [DOI] [PubMed] [Google Scholar]
- Bergé D, Carmona S, Rovira M, Bulbena a, Salgado P, Vilarroya O. Gray matter volume deficits and correlation with insight and negative symptoms in first-psychotic-episode subjects. Acta Psychiatr Scand. 2011;123:431–9. doi: 10.1111/j.1600-0447.2010.01635.x. [DOI] [PubMed] [Google Scholar]
- Birchwood M, Smith J, Drury V, Healy J, Macmillan F, Slade M. A self-report insight scale for psychosis: Reliability, validity and sensitivity to change. Acta Psychiatr. Scand. 1994:62–67. doi: 10.1111/j.1600-0447.1994.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Bora E, Murray RM. Meta-analysis of Cognitive Deficits in Ultra-high Risk to Psychosis and First-Episode Psychosis: Do the Cognitive Deficits Progress Over, or After, the Onset of Psychosis? Schizophr. Bull. 2014;40:744–755. doi: 10.1093/schbul/sbt085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bota RG, Munro JS, Ricci WF, Bota The Dynamics of Insight in the Prodrome of Schizophrenia. CNS Spectr. 2006;11:355–362. doi: 10.1017/s1092852900014486. [DOI] [PubMed] [Google Scholar]
- Boyer L, Aghababian V, Richieri R, Loundou A, Padovani R, Simeoni MC, Auquier P, Lançon C. Insight into illness, neurocognition and quality of life in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;36:271–276. doi: 10.1016/j.pnpbp.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJS. Default-mode brain dysfunction in mental disorders: A systematic review. Neurosci. Biobehav. Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Buchy L, Ad-Dab’bagh Y, Malla A, Lepage C, Bodnar M, Joober R, Sergerie K, Evans A, Lepage M. Cortical thickness is associated with poor insight in first-episode psychosis. J. Psychiatr. Res. 2011;45:781–7. doi: 10.1016/j.jpsychires.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Yeo BTT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat. Neurosci. 2013;16:832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- Cooke MA, Fannon D, Kuipers E, Peters E, Williams SC, Kumari V. Neurological basis of poor insight in psychosis: a voxel-based MRI study. Schizophr. Res. 2008;103:40–51. doi: 10.1016/j.schres.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ćurčić-Blake B, van der Meer L, Pijnenborg GHM, David AS, Aleman A. Insight and psychosis: Functional and anatomical brain connectivity and self-reflection in Schizophrenia. Hum. Brain Mapp. 2015 doi: 10.1002/hbm.22955. 00, /a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David A. Insight and Psychosis. Br. J. Psychiatry. 1990;156:798–808. doi: 10.1192/bjp.156.6.798. [DOI] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional Brain Correlates of Social and Nonsocial Processes in Autism Spectrum Disorders: An Activation Likelihood Estimation Meta-Analysis. Biol. Psychiatry, Perception, Empathy, and Reward in Attention-Deficit/Hyperactivity Disorder and Autism. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders: Clinician Version. SCID-CV 1997 [Google Scholar]
- Flashman LA, McAllister TW, Johnson SC, Rick JH, Green RL, Saykin AJ. Specific frontal lobe subregions correlated with unawareness of illness in schizophrenia: a preliminary study. J. Neuropsychiatry Clin. Neurosci. 2001;13:255–257. doi: 10.1176/appi.neuropsych.13.2.255. [DOI] [PubMed] [Google Scholar]
- Friston K, Brown HR, Siemerkus J, Stephan KE. The dysconnection hypothesis (2016) Schizophr. Res. 2016;176:83–94. doi: 10.1016/j.schres.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Deste G, Smieskova R, Barlati S, Yung AR, Howes O, Stieglitz R-D, Vita A, McGuire P, Borgwardt S. Cognitive Functioning in Prodromal Psychosis: A Meta-analysis. Arch. Gen. Psychiatry. 2012;69:562–571. doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- Garety PA, Kuipers E, Fowler D, Freeman D, Bebbington PE. A cognitive model of the positive symptoms of psychosis. Psychol. Med. 2001;31:189–195. doi: 10.1017/S0033291701003312. [DOI] [PubMed] [Google Scholar]
- Gerretsen P, Chakravarty MM, Mamo D, Menon M, Pollock BG, Rajji TK, Graff-Guerrero A. Frontotemporoparietal asymmetry and lack of illness awareness in schizophrenia. Hum. Brain Mapp. 2013;34:1035–43. doi: 10.1002/hbm.21490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerretsen P, Menon M, Chakravarty MM, Lerch JP, Mamo DC, Remington G, Pollock BG, Graff-Guerrero A. Illness denial in schizophrenia spectrum disorders: A function of left hemisphere dominance. Hum. Brain Mapp. 2015;36:213–225. doi: 10.1002/hbm.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerretsen P, Menon M, Mamo DC, Fervaha G, Remington G, Pollock BG, Graff-Guerrero A. Impaired insight into illness and cognitive insight in schizophrenia spectrum disorders: Resting state functional connectivity. Schizophr. Res. 2014a doi: 10.1016/j.schres.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerretsen P, Plitman E, Rajji TK, Graff-Guerrero A. The effects of aging on insight into illness in schizophrenia: a review. Int. J. Geriatr. Psychiatry. 2014b;29:1145–1161. doi: 10.1002/gps.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerretsen P, Remington G, Borlido C, Quilty L, Hassan S, Polsinelli G, Teo C, Mar W, Simon R, Menon M, Pothier D, Nakajima S, Caravaggio F, Mamo D, Rajji T, Mulsant B, Deluca V, Ganguli R, Pollock B, Graf-Guerrero A. The VAGUS insight into psychosis scale - Self-report & clinician-rated versions. Psychiatry Res. 2014;220:184–189. doi: 10.1016/j.psychres.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleen J, Greenwood K, David AS. Domains of Awareness in Schizophrenia. Schizophr. Bull. 2011;37:61–72. doi: 10.1093/schbul/sbq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin JM, Morgan KD, Valmaggia LR, Broome MR, Woolley JB, Johns LC, Tabraham P, Bramon E, McGuire PK. Insight in individuals with an At Risk Mental State. Schizophr. Res. 2007;90:238–244. doi: 10.1016/j.schres.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Larøi F, Barr WB, Keefe RSE. The neuropsychology of insight in psychiatric and neurological disorders. In: Amador XF, David AS, editors. Insight and Psychosis. Oxford Univ Press; New York City: 2004. pp. 119–156. [Google Scholar]
- Liemburg EJ, van der Meer L, Swart M, Curcic-Blake B, Bruggeman R, Knegtering H, Aleman A. Reduced connectivity in the self-processing network of schizophrenia patients with poor insight. PloS One. 2012;7:e42707–e42707. doi: 10.1371/journal.pone.0042707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln T, Lullmann E, Rief W. Correlates and long-term consequences of poor insight in patients with schizophrenia: A systematic review. Schizophr. Bull. 2007;33:1324–1342. doi: 10.1093/schbul/sbm002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Mai X, Liu C. The default mode network and social understanding of others: what do brain connectivity studies tell us. Front. Hum. Neurosci. 2014:8. doi: 10.3389/fnhum.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marková IS, Roberts KH, Gallagher C, Boos H, McKenna PJ, Berrios GE. Assessment of insight in psychosis: a re-standardization of a new scale. Psychiatry Res. 2003;119:81–88. doi: 10.1016/S0165-1781(03)00101-X. [DOI] [PubMed] [Google Scholar]
- McEvoy J, Aland J, Wilson W, Guy W, Hawkins L. Measuring chronic schizophrenic patients’ attitudes toward their illness and treatment. Hosp. Community Psychiatry. 1981;32:856–858. doi: 10.1176/ps.32.12.856. [DOI] [PubMed] [Google Scholar]
- McFarland J, Cannon DM, Schmidt H, Ahmed M, Hehir S, Emsell L, Barker G, McCarthy P, Elliott Ma, McDonald C. Association of grey matter volume deviation with insight impairment in first-episode affective and non-affective psychosis. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263:133–41. doi: 10.1007/s00406-012-0333-8. [DOI] [PubMed] [Google Scholar]
- Michel P, Baumstarck K, Auquier P, Amador X, Dumas R, Fernandez J, Lancon C, Boyer L. Psychometric properties of the abbreviated version of the Scale to Assess Unawareness in Mental Disorder in schizophrenia. BMC Psychiatry. 2013;13:229–229. doi: 10.1186/1471-244X-13-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Hoffman R, Davidson L. Symptom assessment in schizophrenic prodromal states. Psychiatr. Q. 1999;70:273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- Mintz AR, Dobson KS, Romney DM. Insight in schizophrenia: a meta-analysis. Schizophr. Res. 2003;61:75–88. doi: 10.1016/S0920-9964(02)00316-X. [DOI] [PubMed] [Google Scholar]
- Modinos G, Renken R, Ormel J, Aleman A. Self-reflection and the psychosis-prone brain: an fMRI study. Neuropsychology. 2011;25:295–305. doi: 10.1037/a0021747. [DOI] [PubMed] [Google Scholar]
- Morgan KD, Dazzan P, Morgan C, Lappin J, Hutchinson G, Suckling J, Fearon P, Jones PB, Leff J, Murray RM, David AS. Insight, grey matter and cognitive function in first-onset psychosis. Br. J. Psychiatry. 2010a;197:141–148. doi: 10.1192/bjp.bp.109.070888. [DOI] [PubMed] [Google Scholar]
- Morgan KD, Dazzan P, Morgan C, Lappin J, Hutchinson G, Suckling J, Fearon P, Jones PB, Leff J, Murray RM, David AS. Insight, grey matter and cognitive function in first-onset psychosis. Br. J. Psychiatry. 2010b;197:141–148. doi: 10.1192/bjp.bp.109.070888. [DOI] [PubMed] [Google Scholar]
- Nekovarova T, Fajnerova I, Horacek J, Spaniel F. Bridging disparate symptoms of schizophrenia: a triple network dysfunction theory. Behav. Neurosci. Front. 2014;8:1–10. doi: 10.3389/fnbeh.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B, Thompson A, Yung AR. Basic self-disturbance predicts psychosis onset in the ultra high risk for psychosis “prodromal” population. Schizophr. Bull. 2012;38:1277–1287. doi: 10.1093/schbul/sbs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Northoff G, Qin P. How can the brain’s resting state activity generate hallucinations? A “resting state hypothesis” of auditory verbal hallucinations. Schizophr. Res. 2011;127:202–214. doi: 10.1016/j.schres.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Orr JM, Turner Ja, Mittal Va. Widespread brain dysconnectivity associated with psychotic-like experiences in the general population. NeuroImage Clin. 2014;4:343–351. doi: 10.1016/j.nicl.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parellada M, Boada L, Fraguas D, Reig S, Castro-Fornieles J, Moreno D, Gonzalez-Pinto A, Otero S, Rapado-Castro M, Graell M, Baeza I, Arango C. Trait and state attributes of insight in first episodes of early-onset schizophrenia and other psychoses: a 2-year longitudinal study. Schizophr. Bull. 2011;37:38–51. doi: 10.1093/schbul/sbq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Northoff G. How is our self related to midline regions and the default-mode network? NeuroImage. 2011;57:1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Quee PJ, van der Meer L, Bruggeman R, de Haan L, Krabbendam L, Cahn W, Mulder NCL, Wiersma D, Aleman A. Insight in Psychosis: Relationship With Neurocognition, Social Cognition and Clinical Symptoms Depends on Phase of Illness. Schizophr. Bull. 2011;37:29–37. doi: 10.1093/schbul/sbq133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quee PJ, van der Meer L, Krabbendam L, de Haan L, Cahn W, Wiersma D, van Beveren N, Pijnenborg GHM, Mulder CL, Bruggeman R, Aleman a. Insight change in psychosis: Relationship with neurocognition, social cognition, clinical symptoms and phase of illness. Acta Psychiatr. Scand. 2014;129:126–133. doi: 10.1111/acps.12138. [DOI] [PubMed] [Google Scholar]
- Raij TT, Riekki TJJ, Hari R. Association of poor insight in schizophrenia with structure and function of cortical midline structures and frontopolar cortex. Schizophr. Res. 2012;139:27–32. doi: 10.1016/j.schres.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Rossell S, Coakes J, Shapleske J, Woodruff P, David A. Insight: Its relationship with cognitive function, brain volume and symptoms in schizophrenia. Psychol. Med. 2003;33:111–119. doi: 10.1017/s0033291702006803. [DOI] [PubMed] [Google Scholar]
- Sapara A, Cooke M, Fannon D, Francis A, Buchanan RW, Anilkumar APP, Barkataki I, Aasen I, Kuipers E, Kumari V. Prefrontal cortex and insight in schizophrenia: a volumetric MRI study. Schizophr. Res. 2007;89:22–34. doi: 10.1016/j.schres.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Sass La. Self-disturbance and schizophrenia: Structure, specificity, pathogenesis (Current issues, New directions) Schizophr. Res. 2014;152:5–11. doi: 10.1016/j.schres.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T, Baker JT. How can studies of resting-state functional connectivity help us understand psychosis as a disorder of brain development? Curr. Opin. Neurobiol. 2015;30:85–91. doi: 10.1016/j.conb.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious. Cogn., Social Cognition, Emotion, and Self-Consciousness. 2008;17:457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Smieskova R, Simon A, Allen P, Fusar-Poli P, McGuire PK, Bendfeldt K, Aston J, Lang UE, Walter M, Radue E-W, Riecher-Rössler A, Borgwardt SJ. Abnormal effective connectivity and psychopathological symptoms in the psychosis high-risk state. J. Psychiatry Neurosci. JPN. 2014;39:239–48. doi: 10.1503/jpn.130102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shad M, Keshavan MS. Neurobiology of insight deficits in schizophrenia: An fMRI study. Schizophr. Res. 2015;165:220–226. doi: 10.1016/j.schres.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shad M, Keshavan MS, Steinberg JL, Mihalakos P, Thomas BP, Motes Ma, Soares JC, Tamminga Ca. Neurobiology of self-awareness in schizophrenia: an fMRI study. Schizophr. Res. 2012;138:113–9. doi: 10.1016/j.schres.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shad M, Keshavan MS, Tamminga Ca, Cullum CM, David A. Neurobiological underpinnings of insight deficits in schizophrenia. Int. Rev. Psychiatry Abingdon Engl. 2007;19:437–446. doi: 10.1080/09540260701486324. [DOI] [PubMed] [Google Scholar]
- Shad MU, Muddasani S, Prasad K, Sweeney Ja, Keshavan MS. Insight and prefrontal cortex in first-episode Schizophrenia. NeuroImage. 2004;22:1315–1320. doi: 10.1016/j.neuroimage.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Sheffield JM, Barch DM. Cognition and resting-state functional connectivity in schizophrenia. Neurosci. Biobehav. Rev. 2016;61:108–120. doi: 10.1016/j.neubiorev.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim G, Oh JS, Jung WH, Jang JH, Choi C-H, Kim E, Park H-Y, Choi J-S, Jung MH, Kwon JS. Altered resting-state connectivity in subjects at ultra-high risk for psychosis: an fMRI study. Behav. Brain Funct. BBF. 2010;6:58–58. doi: 10.1186/1744-9081-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalletta G, Piras F, Piras F, Caltagirone C, Orfei MD. The structural neuroanatomy of metacognitive insight in schizophrenia and its psychopathological and neuropsychological correlates. Hum. Brain Mapp. 2014;35:4729–40. doi: 10.1002/hbm.22507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: A theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci. Biobehav. Rev. 2010;34:935–946. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- van der Meer L, de Vos AE, Stiekema APM, Pijnenborg GHM, van Tol M-J, Nolen Wa, David AS, Aleman A. Insight in schizophrenia: involvement of self-reflection networks? Schizophr. Bull. 2013;39:1288–95. doi: 10.1093/schbul/sbs122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Radulescu E. Finding the Elusive Psychiatric “Lesion” With 21st-Century Neuroanatomy: A Note of Caution. Am. J. Psychiatry. 2015;173:27–33. doi: 10.1176/appi.ajp.2015.15060753. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default Mode Network Activity and Connectivity in Psychopathology. Annu. Rev. Clin. Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Wotruba D, Michels L, Buechler R, Metzler S, Theodoridou a, Gerstenberg M, Walitza S, Kollias S, Rossler W, Heekeren K. Aberrant Coupling Within and Across the Default Mode, Task-Positive, and Salience Network in Subjects at Risk for Psychosis. Schizophr. Bull. 2013;40:1–10. doi: 10.1093/schbul/sbt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C-G, Zang Y-F. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Front. Syst. Neurosci. 2010;4:1–7. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]