Abstract
The neuropsychological effects of naturally occurring psychoactive chemicals have been recognized for millennia. Hallucinogens, which include naturally occurring chemicals such as mescaline and psilocybin, as well as synthetic compounds, such as lysergic acid diethylamide (LSD), induce profound alterations of human consciousness, emotion, and cognition. The discovery of the hallucinogenic effects of LSD and the observations that LSD and the endogenous ligand serotonin share chemical and pharmacological profiles led to the suggestion that biogenic amines like serotonin were involved in the psychosis of mental disorders such as schizophrenia. Although they bind other G protein-coupled receptor (GPCR) subtypes, studies indicate that several effects of hallucinogens involve agonist activity at the serotonin 5-HT2A receptor. In this chapter, we review recent advances in understanding hallucinogen drug action through characterization of structure, neuroanatomical location, and function of the 5-HT2A receptor.
Keywords: Serotonin, 5-HT2A receptor, G protein-coupled receptor (GPCR), Lysergic acid diethylamide (LSD), Psilocin, Psilocybin, Mescaline, Schizophrenia, Psychosis, Hallucinogen, Psychedelics, Antipsychotics
1 Introduction to Receptor Pharmacology
The concept of cellular receptors as we currently know was originally conceived at the end of the nineteenth century and the beginning of the twentieth century by pioneering physiologists such as Langley (1909) and Erlich (1913). At that time, it was speculated that “something” located either at the cell surface or within cells could “read” the chemical information contained in substances present in the environment in such a way that this information is finally converted into observable physiological effects. Later in the 1930s, the British pharmacologist Alfred Joseph Clark introduced the receptor theory as a quantitative discipline. He proposed that the data obtained in experimental assays were the result of unimolecular interactions between the evaluated drug and a type of “substance” found at the cell surface corresponding to what we know now as receptor (Clark 1933).
Currently, it is widely accepted that receptors exist in several forms, including proteins localized at the cell surface, such as enzymes, ion channels, and transporters, as well as nuclear and cytosolic proteins. These receptors are organized in superfamilies according to their protein structure; the largest of these groups includes the seven transmembrane (7TM) receptors, also known as G protein-coupled receptors (GPCRs) because their final response is essentially generated by the activation of heterotrimeric G proteins. From a structural point of view, these receptors are peptides that cross the lipid bilayer through seven trans-membrane domains in such a way that they are embedded in the plasma membrane with the amino terminus facing the extracellular space and the carboxyl terminus orientated towards the cytoplasm. At the same time, three extracellular and three intracellular loops interconnect these domains (Katritch et al. 2013). The recent explosion in GPCR crystal structures confirms these biophysical features (Rosenbaum et al. 2009; Audet and Bouvier 2012).
The most extensively characterized mechanism of action of these receptors resides in their coupling to G proteins after binding to a specific activating element known in pharmacology as an agonist. G proteins are heterotrimeric complexes found at the plasma membrane and they are constituted by monomeric Gα subunits and Gβγ dimeric subunits (Oldham and Hamm 2008). Following their interaction with an activated receptor, Gα monomeric proteins dissociate from Gβγ subunits and exchange GDP guanine nucleotide for GTP. Subsequently, the Gα subunits bound to GTP initiate different second messenger cascades at the intracellular level by interacting with other proteins such as adenylyl cyclases or phospholipases. The sequential activation or inhibition of these different elements in a cascade fashion constitutes the biological process known as cell signaling, which may be initiated at the cell surface by the activation of receptors and often concludes in the cell nucleus with modulated transcription of different genes (Ferguson 2001).
All of these cell signaling processes, initiated upon the activation of GPCRs, can be terminated at different points. At the G protein level, bound GTP is hydrolyzed to GDP through the GTPase activity of the Gα subunit, permitting its association with Gβγ subunits to reassemble the heterotrimeric complex, allowing the signaling process to commence again. In the case of GPCRs, receptor activation may be terminated by phosphorylation of different amino acids in the intracellular loops or in the carboxy terminus by specific receptor kinases known as GPCR kinases (GRKs), which leads to receptor desensitization with respect to its G protein coupling. At the same time, phosphorylated receptors are susceptible to interact with β-arrestins, a family of cytosolic proteins involved in the endocytosis of receptors after their activation by agonist molecules (Luttrell 2008). Once receptors are internalized within the different intracellular compartments, the endosomes may either divert to lysosomes by following different degradation pathways, or alternatively may recycle back to the plasma membrane where the receptors will be restored in a state that is susceptible to activation once again by an agonist ligand.
Receptor internalization can be considered as another mechanism that completes the cell signaling processes initiated by receptor activation (Hanyaloglu and von Zastrow 2008). This general paradigm has been widely accepted until the present time and it is based, for the most part, on the investigations carried out over several decades by Robert Lefkowitz and his collaborators who used the β2 adrenoceptor as an experimental model (Pierce et al. 2002). Nevertheless, new findings discovered in more recent investigations have contributed to a conceptual expanding of this general mechanistic model. Specifically, some recent reports suggest that β-arrestins play new roles in addition to those originally linked to receptor endocytosis. One of these new functions includes the participation of β-arrestins as scaffolding elements that help to facilitate the transmission of signaling from receptors to diverse intracellular effectors—indicating that G proteins, although considered as the canonical pathway for the signal transmission in GPCRs, are not the exclusive elements connecting these receptors to cell signaling pathways (Luttrell and Lefkowitz 2002). Similarly, recent investigations conducted with β2 adrenoceptors have revealed their capacity to couple to Gα subunits from the lumen of endosomes, meaning that the activation of intracellular effectors continues following endocytosis (Irannejad et al. 2013). Taken together, this intricate amalgam of interacting proteins demonstrates the extreme complexity of the molecular mechanisms involved in the function of these receptors at the cellular level, and makes patent the large number of unanswered questions remaining in this field of knowledge. As evidence of their biological relevance, approximately 900 different types of GPCRs have been identified in the human genome; these receptors participate in the majority of the physiological processes including sense perceptions, as well as neuropsychological, cardiovascular, and endocrine functions, and are the therapeutic target of nearly half of the total number of medicines that are currently prescribed for the treatment of diseases (Overington et al. 2006).
2 Serotonin and Serotonin Receptors: A Historical Perspective
2.1 Serotonin
The discovery of serotonin (5-hydroxytryptamine, 5-HT) dates back to the middle of the nineteenth century when early experimenters recognized that a substance contained in serum was capable of inducing the contraction of smooth muscle. Later in the first third of the twentieth century, Italian scientists extracted a substance from enterochromaffin cells in the gastrointestinal tract, named for this reason “enteramine”, that also caused smooth muscle contraction, particularly in stomach and uterus (Mohammad-Zadeh et al. 2008). Nearly at the same time, during the 1940s, Maurice Rapport isolated and characterized a substance from blood that acted as a vasoconstrictor; they called the compound serotonin due to these peculiarities, i.e., serum and tonic (Rapport et al. 1948). The similar chemical structures observed for enteramine and serotonin, basically defined by the presence of an indole entity, led to conjecture that both substances corresponded essentially to the same compound; this point was corroborated later when serotonin was first synthesized and found to have the same properties as the substances obtained from natural sources, i.e., enterochromaffin cells and serum (Reid and Rand 1952).
The intimate relationship between 5-HT signaling and hallucinogens was envisaged during the period of intense research activity that coincided with the discovery of this biogenic amine. In 1943, the Swiss chemist Albert Hoffman (1906–2008) serendipitously discovered the hallucinogenic properties of lysergic acid diethylamide (LSD) (Hofmann 1979; Nichols 2004). In 1951, John Gaddum at the University of Edinburgh reported the antagonistic action of LSD on the effects induced by 5-HT in rat uterus and rabbit ear preparations (see Green 2008). These results, together with the demonstration in 1968 by George Aghajanian at Yale University that LSD modulates the activity of midbrain neurons containing 5-HT (Aghajanian et al. 1968; Aghajanian 2009), led to conjecture that the psychoactive effects of LSD were mediated by its interaction with the serotonergic system in the CNS.
Currently, it is common knowledge that 5-HT is present in all animal organisms where it participates in numerous physiological functions by acting either as a hormone or as a neurotransmitter. 5-HT can be found in the body at the peripheral level in platelets, mastocytes, and enterochromaffin cells, and in the central nervous system (CNS) in serotonergic neurons located preferentially in the brainstem, which only contains 1–2% of the total amount of 5-HT present in the whole organism (Hannon and Hoyer 2008; Berger et al. 2009).
5-HT is synthesized from tryptophan, an essential amino acid that is obtained from food, following two biochemical reactions. First, tryptophan is hydroxylated to 5-hydroxytryptophan by the enzyme tryptophan hydroxylase. Second, 5-hydroxytryptophan is decarboxylated by an amino decarboxylase, resulting finally in 5-HT. Tryptophan hydroxylase is the rate-limiting step in this sequential reaction. Control of the synthesis of 5-HT is also determined by the availability of oxygen, the heterocyclic compound pteridine, and by the amount of tryptophan present in the bloodstream. The principal route of degradation of 5-HT is by deamination, which is performed by monoamine oxidase (MAO) enzymes. The deamination of 5-HT results in the formation of 5-hydroxyindolacetaldehyde, which in turn is oxidized to the final metabolite 5-hydoxyindolacetic acid or 5-HIAA (Mohammad-Zadeh et al. 2008).
In the CNS, 5-HT plays a fundamental role as a neurotransmitter. However, 5-HT is not able to cross the blood–brain barrier, which means that it must be synthesized within the brain from tryptophan. This synthesis takes place in a cluster of specific neurons with their soma restrictively located in the different raphe nuclei of the brainstem (Dahlström and Fuxe 1964). Axons originating from these neurons innervate practically the entire brain, projecting their terminals to either the fore-brain (upper raphe nuclei) or to the spinal cord (lower raphe nuclei) (Tork 1990). 5-HT is released from terminals into the synaptic cleft where it is liberated to interact with specific receptors located mainly in postsynaptic neurons. Once neurotransmission occurs, 5-HT is taken back up into serotonergic neurons by specific transporters found on the plasma membrane of presynaptic terminals and is either stored for future synaptic release or metabolized by MAO enzymes.
5-HT performs its physiological functions by binding to specific cell membrane receptors. Currently, 14 different serotonin receptors, classified into 7 subfamilies according to their primary structure and functional properties, have been described. Excluding 5-HT3, which belongs to the ion channel receptor superfamily, the remainder of the 5-HT receptors are GPCRs (Hoyer et al. 1994).
2.2 Serotonin Receptors
Despite the fact that the existence of at least two different subtypes of 5-HT receptors (initially referred as D and M) had been reported in the 1950s (Gaddum and Picarelli 1957), it was not until the mid-1970s when different 5-HT receptor subtypes were pharmacologically characterized in mammalian brain homogenates using radioligand binding techniques newly developed during that period. Several radioligands developed during that period (such as [3H]5-HT, [3H]LSD and [3H] spiperone) were found to bind to sites suspected to be 5-HT receptors (Peroutka and Snyder 1979). During the course of those early investigations, it was observed that radioligands differentiated between two classes of 5-HT sites; binding sites with high affinity for [3H]5-HT were designated as 5-HT1 receptors, whereas a second population of sites with high affinity for [3H]spiperone and low affinity for [3H] 5-HT were designated as 5-HT2 receptors. In this way, by the mid-1980s up to five different 5-HT receptors were described based on their pharmacological profile. The extensive development of new molecular biology techniques that occurred at the end of the 1980s through the beginning of the 1990s permitted cloning of the 14 5-HT receptor subtypes that are currently known. Thus, the contemporary classification of 5-HT receptors has been made according to genetic homologies; consequently, the classification of 5-HT receptors is based on their primary structure, as determined by their amino acid sequence, which in turn is responsible for the functional properties of these cell membrane proteins (for reviews, see Hannon and Hoyer 2008; Hoyer et al. 1994; Millan et al. 2008; Nichols and Nichols 2008; Raymond et al. 2001; Barnes and Sharp 1999).
The 5-HT2 receptor subfamily comprises three different subtypes, namely 5-HT2A, 5-HT2B and 5-HT2C, which are grouped together due to their high structural homology (their genetic sequences are about 50% identical). After interaction with 5-HT or another agonist, 5-HT2 receptors couple preferentially to Gq/11 proteins, promoting the subsequent activation of phospholipase C (PLC). In turn, PLC activation promotes the generation of intracellular inositol phosphates (IP) and promotes the mobilization of intracellular calcium. The 5-HT2A receptor corresponds to the D-type 5-HT receptor described by Gaddum and Picarelli and later characterized by Peroutka and Snyder as a site exhibiting high affinity for [3H] spiperone. There is extensive evidence that the 5-HT2A receptor is responsible for the neuropsychological effects of serotonergic hallucinogens in animal models used for experimentation as well as in human subjects (Halberstadt 2015). This chapter is focused on the biochemical and pharmacological properties of the 5-HT2A receptor in relation to its role in the effects of hallucinogenic drugs of abuse, such as LSD, mescaline, and psilocybin.
3 Chemical Neuroanatomy of 5-HT2A Receptors
The major physiological effects induced by hallucinogens, in particular when evaluated in human subjects, are related to altered states of consciousness, including changes in cognition, mood, and perception. It is widely accepted at the present time that these effects are generated mostly by the interaction of hallucinogens with 5-HT2A receptors as agonists (Halberstadt 2015; Vollenweider et al. 1998; Gonzalez-Maeso et al. 2007; Schmid et al. 2015). Although hallucinogens do not bind exclusively to 5-HT2A receptors (LSD binds to most 5-HT receptor sub-types as well as to dopaminergic and adrenergic receptors), it has been evidenced in both humans and experimental animals that the activation of 5-HT2A receptors is necessary to generate hallucinogenesis and a related behavioral response in animals (Vollenweider et al. 1998; Gonzalez-Maeso et al. 2007). Therefore, the study of the anatomic distribution of 5-HT2A receptor in the CNS is essential to elucidate what brain structures are implicated in the neuropsychological effects elicited by hallucinogenic compounds. As noted above, the development of radiochemicals based on the isotopic labeling of particular ligands permitted the characterization of receptors as binding sites specifically recognized by these radioligands. In addition, by exposure to sensitive autoradiographic films, these radioligands can be used to visualize the distribution of their binding sites in histological sections obtained from the human brain postmortem. The initial receptor autoradiography studies investigating 5-HT2A receptor localization were conducted using either antagonist ([3H] spiperone and [3H]ketanserin) or agonist ([3H]LSD and [125I]DOI) radioligands (Mengod et al. 2006). Although all of these radioligands have high affinity for 5-HT2A receptors, they are not completely selective because they bind to sites corresponding to dopamine receptors ([3H]spiperone), adrenergic receptors ([3H] ketanserin), or to other 5-HT2 receptor subtypes ([125I]DOI). Because of this nonspecific binding, blockers for those undesired binding sites must be used in order to obtain a specific signal corresponding to 5-HT2A receptors. Another issue that must be taken into consideration when conducting quantitative receptor autoradiography experiments is the pharmacological nature of the radioligand used (in terms of being an agonist or an antagonist). According to the ternary complex model of drug receptor interactions (see below), antagonist radioligands label the entire population of receptors (inactive [R] and active [R*] states), whereas agonist radioligands selectively bind to the receptors present in their active conformation (R*)—therefore detecting only a fraction of the total receptor population. The development of [3H]MDL100,907, a highly selective 5-HT2A receptor antagonist, in the mid-1990s, addressed many of the problems associated with other 5-HT2A receptor radioligands (Johnson et al. 1996), particularly in receptor autoradiography studies where [3H]MDL100,907 displayed a remarkably specific signal devoid of nonspecific binding (Lopez-Gimenez et al. 1997, 1998).
The anatomic localization of 5-HT2A receptors in primate brain visualized with [3H]MDL100,907 showed a heterogeneous and wide-ranging distribution throughout different brain areas, and was comparable to that observed in other mammalian species (Lopez-Gimenez et al. 2001). The region containing the highest density of 5-HT2A binding sites was the neocortex, where the autoradiographic signal displayed a banded pattern corresponding predominantly to pyramidal neurons distributed according to the cytoarchitectural organization of different cortical areas. Other regions where 5-HT2A receptors were detected included structures of the hippocampus, thalamic nuclei, the mammillary bodies in the hypothalamus, and different nuclei of the midbrain.
The corpus striatum is a brain structure where 5-HT2A receptors distribution is paradoxical when comparing different mammalian species. Initial investigations of 5-HT2A receptor distribution, performed using [3H]ketanserin as the radioligand, described a homogeneous pattern of labeling throughout caudate and putamen nuclei in both rat and human brain samples, with a high component of nonspecific binding (Pazos et al. 1985, 1987). The nonspecific binding was attributed to the vesicular monoamine transporter since it could be blocked by tetrabenazine. Interestingly, when equivalent histological sections were incubated with agonist radioligands such as [3H]LSD or [125I]DOI, the autoradiographic signal did not show the abundance of nonspecific binding observed with [3H]ketanserin, and the pattern of distribution was markedly heterogeneous (Appel et al. 1990; Waeber and Palacios 1994). Binding sites were particularly enriched in the nucleus accumbens and in the posterior portion of the caudate-putamen in rat brain, whereas in human samples the distribution displayed a characteristic patchy pattern in both caudate and putamen nuclei corresponding to striosomes.
Striosomes were originally discovered by acetylcholinesterase histochemistry and are anatomical structures differentiated from the rest of the striatum or matrix. In terms of neuronal connections, striosomes and matrix can be differentiated based on their input–output systems (Gerfen 1992). Afferents reaching the matrix originate predominantly from areas related to sensorimotor processing, whereas striosomes receive inputs essentially from limbic regions. On the other hand, the caudate and putamen matrix projects mainly to the pallidum and the substantia nigra pars reticulata, whereas striosomal afferents target dopaminergic nigral neurons. Other radioligands derived from psychoactive compounds, such as benzodiazepines and opioids, also displayed a patchy distribution of labeling in human striatum; suggesting, therefore, the participation of this anatomic structure in neuropsychological effects produced by these types of drugs (Graybiel 1990). Although it was originally speculated that 5-HT2A binding sites in striosomes may correspond to the active conformation of these receptors, where agonist drugs with hallucinogenic effects (such as [3H]LSD and [125I]DOI) bind, this possibility was later disproved by experiments performed with the antagonist radioligand [3H]MDL100,907, which produced the same patchy distribution of striatal labeling that was observed in consecutive histological sections treated with [125I]DOI (Lopez-Gimenez et al. 1999) (Fig. 1).
Fig. 1.
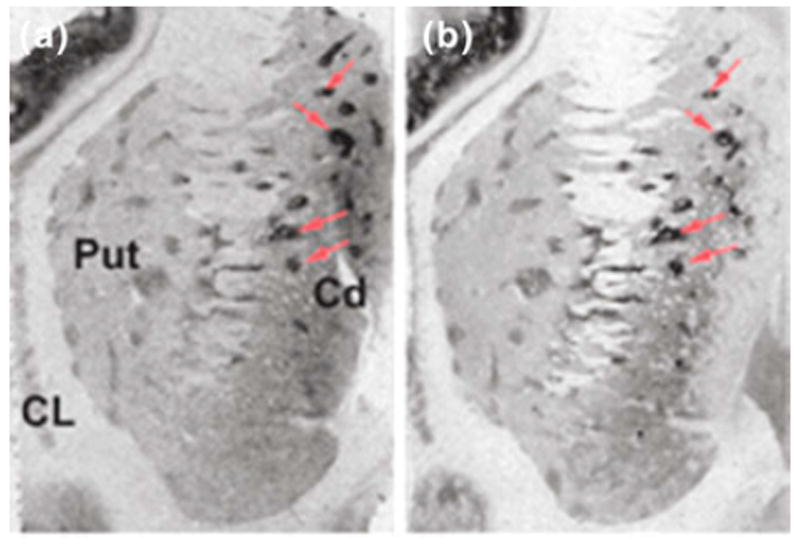
Consecutive histological sections of human striatum showing the distribution of binding sites labeled by [125I]DOI (a) and [3H]MDL100,907 (b). Arrow heads indicate some of the striosomes visualized with these radioligands. Cd, caudate; Put, putamen; CL, claustrum
The peculiar compartmentalized distribution of 5-HT2A receptors in the human corpus striatum is not found in all mammalian species. [125I]DOI labeling of 5-HT2A receptors in striosomes has been clearly observed in human, mouse, and guinea pig brain. By contrast, no striosome labeling has been detected in rat, cat, pig, cow, or monkey brain (Waeber and Palacios 1994). The results reported for cow and monkey are especially remarkable: the autoradiographic signal in cow striatum is high, specific, and homogeneous, whereas only sparse 5-HT2A receptor labeling is detected in monkey caudate, putamen, and accumbens. The cause of these cross-species differences remains to be elucidated. A phylogenetic explanation can be excluded because species closely related in evolutionary terms, such as primates (human and monkey) and rodents (rat, mouse, and guinea pig), often exhibit substantial differences with regard to the chemical neuroanatomy of their striatal 5-HT2A receptors. These differences could have fundamental functional consequences and should be taken into account when evaluating drugs that interact with 5-HT2A receptors, particularly when interpreting and comparing results obtained from behavioral pharmacology studies conducted using common laboratory species such as rat and mouse, or when extrapolating those results to human subjects.
4. Hallucinogenic and Non-Hallucinogenic 5-HT2A Receptor Agonists
The mechanism of action of hallucinogens has attracted the attention of pharmacologists and neuroscientists for decades (Nichols 2004; Aghajanian and Marek 1999; Fantegrossi et al. 2008). These compounds elicit profound alterations of cognition, perception, and mood, and have been used by most human cultures for millennia (Hanks and Gonzalez-Maeso 2013). The role of the 5-HT2A receptor in the mechanism of action of hallucinogens was first proposed by Richard Glennon, Milt Titeler and their teams (Glennon et al. 1984, 1986). However, it was not until the development of 5-HT2A knockout mice in 2003 that the fundamental role of 5-HT2A receptor-dependent signaling in the cellular and behavioral effects of hallucinogens was verified conclusively (Gonzalez-Maeso et al. 2003 2007). These findings in rodent models are further supported by studies conducted by Franz Vollenweider and his collaborators, which demonstrate that the psychosis-like effects of LSD and psilocybin in healthy volunteers are reversed by the 5-HT2A receptor antagonist ketanserin (Vollenweider et al. 1998; Schmid et al. 2015; Preller et al. 2016, 2017).
From a basic pharmacological perspective, it is particularly interesting that whereas all hallucinogens (such as LSD, mescaline, and psilocin) bind with high affinity and activate the serotonin 5-HT2A receptor, certain closely related 5-HT2A receptor agonists, such as lisuride and ergotamine, do not behave as hallucinogens in humans (Marona-Lewicka et al. 2002; Silbergeld and Hruska 1979; Mokler et al. 1983; Adams and Geyer 1985); indeed, these non-hallucinogenic 5-HT2A receptor agonists are used as therapeutic drugs in the treatment of diseases such as migraine and Parkinson’s disease (Fig. 2). Hallucinogenic and non-hallucinogenic 5-HT2A receptor agonists, therefore, serve as attractive pharmacological tools to investigate the molecular and signaling mechanisms that allow chemically related agonists to target the same receptor molecule but induce different neuropsychological effects. What might explain the differences between the neuropsychological effects of hallucinogenic and non-hallucinogenic 5-HT2A agonists?
Fig. 2.
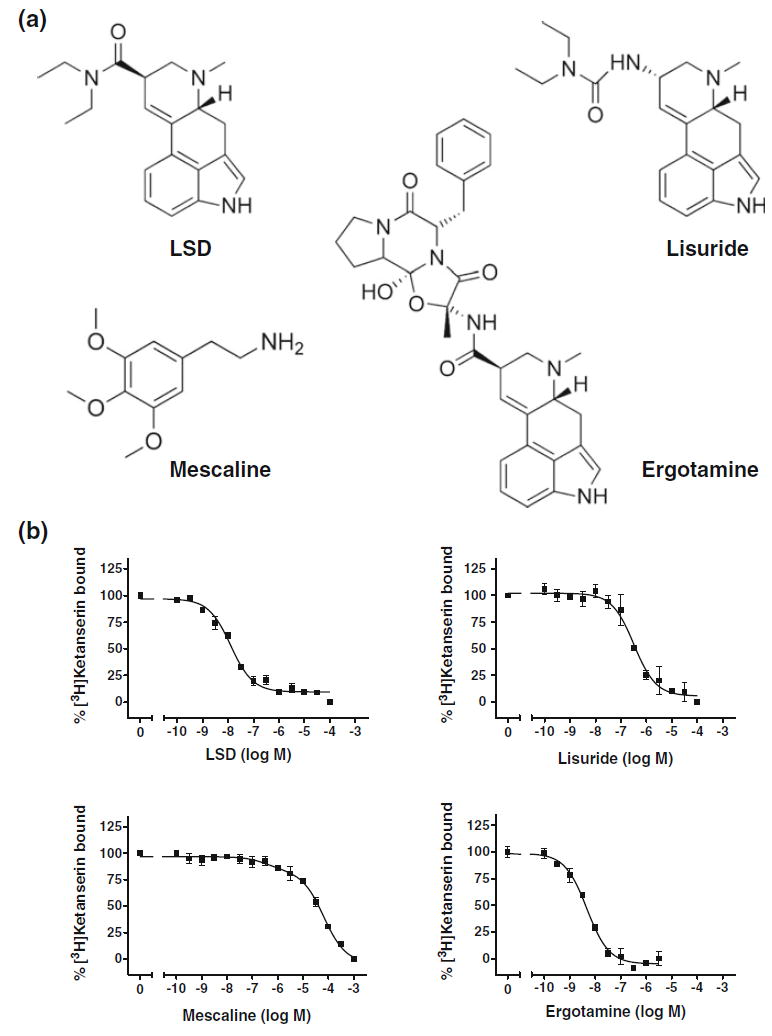
a Chemical structures of hallucinogen (LSD and mescaline) and non-hallucinogen (lisuride and ergotamine) 5-HT2A receptor agonists. b [3H]Ketanserin competition curves by LSD, mescaline, lisuride and ergotamine in mouse somatosensory cortex plasma membrane preparations (see Gonzalez-Maeso et al. 2007). Note that the affinity of the ligand does not correlate with its psychoactive potential
Individual GPCRs can couple to multiple signal transduction pathways (Millan et al. 2008; Raymond et al. 2001; Barnes and Sharp 1999; Hoyer et al. 2002). It has been proposed that agonists can stabilize distinct active conformational receptor states (Urban et al. 2007; Kenakin 1995; Kenakin 1997). These active states can differ in their propensity to activate the various signaling proteins coupled to the receptor. This phenomenon of “biased agonism” explains how agonists acting at the same receptor target can elicit different patterns of cellular signaling responses.
As briefly discussed above, the “ternary complex model” (Kenakin 2002; Strange 1998) is the most widely accepted model of GPCR signaling. This model proposes that the receptor is in a dynamic equilibrium between the inactive (R) and the active (R*) conformational states. Based upon this model, neutral antagonists have identical affinities for the inactive and the active conformational states, whereas agonists exhibit higher affinity for the active state (Fig. 3). Because agonists have higher affinity for the active conformation of the receptor, agonist binding stabilizes GPCRs in their active state, shifting the dynamic equilibrium from R to R*. The ternary complex model was first proposed by Robert Lefkowitz and his group (De Lean et al. 1980; Lefkowitz et al. 1993) and was based on radioligand binding assays in tissue culture plasma membrane preparations in which they showed that the β2-adrenergic receptor agonist hydroxybenzylisoproterenol displaced the β2-adrenergic receptor antagonist [3H]dihydroalprenolol with a biphasic profile, with high-affinity (KH) and low-affinity (KL) binding sites (De Lean et al. 1980). They also demonstrated that the fraction of binding sites with high affinity gradually decreased in the presence of increasing concentrations of the non-hydrolyzable GTP analog Gpp(NH)p (De Lean et al. 1980). This model provided a general scheme for the agonist-induced activation of GPCRs and their effectors that has been widely used in pharmacology and drug development (Fig. 3).
Fig. 3.
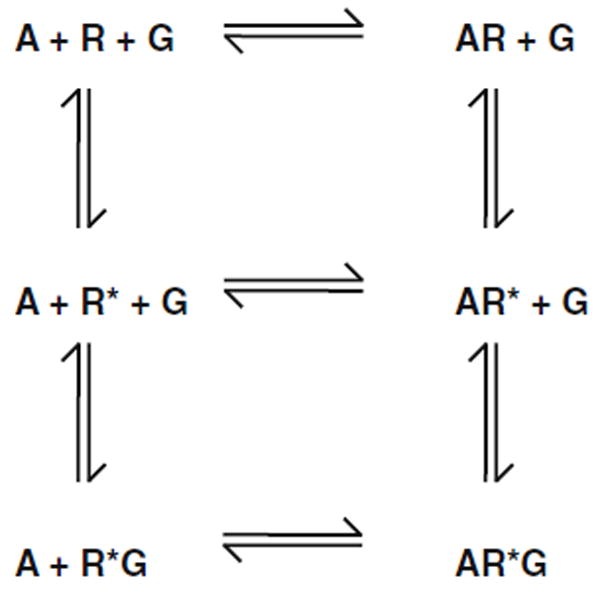
The ternary complex model. According to this model, the receptor in its inactive state (R) undergoes a conformational transition, which leads to the formation of an active state (R*). This active state in turn interacts with heterotrimeric G proteins (G). Note that according to this model, the relative degree of activation of each effector pathway by the tested agonists must be the same
More recently, the theoretical pharmacologist Terry Kenakin proposed a model where GPCRs adopt multiple conformational states (Kenakin 1995). According to this model, different agonists show a preference (in terms of binding affinity) for a subset of the receptor conformational states. Once these conformational states are stabilized by an agonist, the receptor modulates the activity of some, but not all, of the signaling pathways coupled to the receptor, consequently inducing an agonist-specific functional outcome (Fig. 4). This concept, first termed “agonist-directed trafficking of receptor signaling” and now known as “biased agonism”, raised an enormous amount of interest in the molecular pharmacology field because it should theoretically be possible to design new drugs that specifically affect signaling pathways involved in the therapeutic response without inducing unwanted side effects (Urban et al. 2007).
Fig. 4.

Model of biased agonism. Selective agonists stabilize a subset of receptor conformations that selectively activates some but not all signaling pathways. Recent findings suggest that biased agonism is involved in the psychoactive differences between hallucinogen and closely related non-hallucinogen 5-HT2A receptor agonists
With this background, Kelly Berg, William Clarke, and their team tested the signal transduction pathways activated by a battery of serotonin 5-HT2A receptor agonists in CHO-K1 cells (Berg et al. 1998). Importantly, their findings provided the first demonstration in heterologous expression systems that the relative efficacies of agonists can differ depending upon the signal transduction pathway that is measured (Berg et al. 1998). For example, relative to 5-HT, some agonists, such as 3-trifluoromethylphenylpiperazine (TFMPP), preferentially activate the PLC-IP pathway, whereas other agonists, such as LSD, showed a preference for the PLA2-AA pathway. These findings provided the basis for further in silico, in cellulo and in vivo investigations indicating that the different neuropsychological effects of hallucinogenic and non-hallucinogenic 5-HT2A receptor agonists are likely linked to differences in the 5-HT2A receptor-dependent signaling responses that they elicit.
5 Biased Agonism at the 5-HT2A Receptor
The best characterized 5-HT2A receptor-coupled signaling pathway is Gq/11-mediated activation of PLC, leading to the formation of inositol phosphates and diacylglycerol, followed by Ca2+ release from the endoplasmic reticulum (Hannon and Hoyer 2008; Nichols and Nichols 2008; Raymond et al. 2001; Barnes and Sharp 1999). However, whether this pathway plays a role in mediating the effects of hallucinogens is uncertain. Hallucinogens stimulate PLC-IP signaling with low efficacy, and there is no correlation between behavioral activity and the efficacy of the ligands in vitro and in cellulo (Marona-Lewicka et al. 2002; Porter et al. 1999; Egan et al. 1998). It has been demonstrated that head-twitch behavior induced by hallucinogens is absent in 5-HT2A knockout mice (Gonzalez-Maeso et al. 2007) (see also Canal 2012). Importantly, however, it has been shown that the head-twitch response induced by the hallucinogen DOI is only slightly reduced in Gαq knockout mice compared to wild-type controls (Garcia et al. 2007). Together, these findings suggest that although Gq-dependent signaling may contribute to the behavioral effects of hallucinogens in rodents, the Gq pathway is not the only signaling cascade that is responsible for the hallucinogen-induced behavioral response. In addition to Gq/11-mediated PLC-IP signaling, studies in heterologous expression systems identified several other signaling transduction pathways that are coupled to the 5-HT2A receptor, including phospholipase A2 (PLA2) (Kurrasch-Orbaugh et al. 2003), PLA2-mediated arachidonic acid (AA) release (Berg et al. 1998), and Gi/o-dependent Gβγ-associated activation of ERK1/2 (Kurrasch-Orbaugh et al. 2003). The role of Gi/o protein in hallucinogen-specific signaling is further supported by recent findings in heterologous expression systems and in mouse models.
Most signaling pathways ultimately modulate gene expression in response to extracellular stimuli (Hill et al. 2001). It has been shown that GPCR activation can induce concentration-dependent changes in the level of expression of specific genes (Yuen et al. 2002; Wurmbach et al. 2001). To test the hypothesis that hallucinogenic and non-hallucinogenic 5-HT2A receptor agonists modulate specific signaling pathways that are responsible for their behavioral effects, we relied on the quantification of changes in gene expression as a read-out for multidimensional neuronal signaling. This approach was tested in HEK293 cells and in mouse somatosensory cortex, a region that has been implicated in the cellular and behavioral responses induced by hallucinogenic drugs. Importantly, each agonist studied elicited a reproducible and unique response in this signaling assay. Two transcripts (c-Fos and IκBα) were induced at a similar level by both hallucinogenic and non-hallucinogenic drugs (Gonzalez-Maeso et al. 2003, 2007). However, the transcripts Egr-1 and Egr-2 were consistently activated by hallucinogens (DOI, DOM, DOB, mescaline, LSD, and psilocin), but expression of these two genes was unaffected by non-hallucinogenic agonists (R-lisuride, S-lisuride and ergotamine) (Gonzalez-Maeso et al. 2003, 2007). Additionally, with the exception of IκBα, the entire transcriptome fingerprint induced by the hallucinogenic and non-hallucinogenic agonists was abolished in somatosensory cortex of 5-HT2A knock-out mice (Gonzalez-Maeso et al. 2003, 2007). Together, these results indicate that both hallucinogenic and non-hallucinogenic agonists modulate neuronal signaling in somatosensory cortex via the 5-HT2A receptor. Furthermore, the ability to predict behavioral activity based on intrinsic reporter profiles supports the existence of distinct 5-HT2A receptor-dependent signaling responses that characterize the effects of hallucinogens in the brain.
This hypothesis was tested in mouse cortical primary cultures in order to ascertain whether hallucinogens modulate specific 5-HT2A receptor-dependent signaling pathways (Gonzalez-Maeso et al. 2007). Interestingly, it was demonstrated that while hallucinogenic and non-hallucinogenic 5-HT2A receptor agonists activate 5-HT2A receptors that are coupled to PLC, hallucinogen-dependent responses also involve pertussis toxin (PTX)-sensitive heterotrimeric Gi/o proteins (Gonzalez-Maeso et al. 2007). These findings revealed that hallucinogen-characteristic transcriptome fingerprint depends on modulation of both Gq/11 and Gi/o, and are consistent with the biased agonism model as it explains how distinct neurophysiological responses could be produced by hallucinogenic and non-hallucinogenic 5-HT2A receptor agonists that target the same population of cortical pyramidal 5-HT2A receptors.
Notably, this hypothesis has been recently validated using a quantitative phosphoproteomic approach (Karaki et al. 2014). Thus, it has been demonstrated that hallucinogenic and non-hallucinogenic 5-HT2A receptor agonists induce a distinct pattern of protein phosphorylation in HEK293 cells. Using this heterologous expression system, the authors also demonstrated that pretreatment with PTX abolishes ERK1,2 phosphorylation induced by the hallucinogens DOI and LSD, whereas PTX treatment did not affect the responses induced by the non-hallucinogenic 5-HT2A receptor agonists lisuride and ergotamine (Karaki et al. 2014). Together with previous findings (Gonzalez-Maeso et al. 2007), these observations indicate that hallucinogens selectively activate Gi/o-dependent signaling in vitro and in vivo, whereas non-hallucinogenic 5-HT2A receptor agonists do not activate Gi/o (Karaki et al. 2014).
These findings that were based upon the transcriptome fingerprint induced by hallucinogenic and non-hallucinogenic 5-HT2A receptor agonists are highly relevant to the development of more specific therapeutic drugs (Gonzalez-Maeso et al. 2003, 2007). Indeed, induction of c-Fos, Egr-1, and Egr-1 in cortical regions is now widely used to investigate signaling events induced by hallucinogenic 5-HT2A agonists in rodents (Canal et al. 2010; Chiu et al. 2014; Santini et al. 2013; Malkova et al. 2014; Lee et al. 2014; Abbas et al. 2009).
Although heterotrimeric G proteins represent one of the main mechanisms involved in GPCR-dependent responses, it has been demonstrated that G protein-independent mechanisms related to β-arrestin can also play a key role in their signaling properties (see above). The role of β-arrestins in GPCR function is further supported by the recent crystal structure of active β-arrestin-1 bound to a -derived carboxyl terminal (Shukla et al. 2013). Additionally, using the 5-HT precursor 5-hydroxytryptophan (5-HTP) and the hallucinogen DOI, Laura Bohn and others working in her laboratory demonstrated that 5-HT induces the head-twitch response in mice by a β-arrestin-2-dependent mechanism, whereas the effect of DOI on this behavior is independent of β-arrestin-2 (Schmid et al. 2008). They also showed that 5-HT, but not DOI, activates a signaling cascade composed of β-arrestin-2, phosphoinositide 3-kinease, Src, and Akt that is responsible for head-twitch behavior (Schmid and Bohn 2010). Interestingly, more recent findings by the same laboratory suggested that the atypical antipsychotic clozapine induces antipsychotic-like behavioral effects in mice by acting as a 5-HT2A receptor agonist through a β-arrestin-2-independent activation of Akt (Schmid et al. 2014). Further work is needed to delineate the roles of Akt and Src in the behavioral effects of 5-HTP, hallucinogens, and clozapine.
6 Additional Signaling Pathways Modulated by Activation of the 5-HT2A Receptor
Recent findings have elucidated additional neuronal signaling pathways downstream from the 5-HT2A receptor that is potentially involved in the unique behavioral effects induced by hallucinogens (Halberstadt 2015; Hanks and Gonzalez-Maeso 2013). It has been shown that the last four amino acids (VSCV) of the carboxyl terminus of the 5-HT2A receptor constitute a canonical Type I PDZ-binding domain (X-Ser/Thr-X-ϕ) (Kornau et al. 1995; Xia 2003; Xia et al. 2003). PDZ-binding domains are known to physically interact with PSD-95/ Discs-large/ZO-1 (PDZ) domain-containing proteins such as postsynaptic density 95 (PSD-95), which is a prototypic member. Co-immunoprecipitation studies suggested that the wild-type 5-HT2A receptor, but not a mutant lacking the last four amino acids of the carboxyl terminus, interacts directly with PSD-95 (Xia et al. 2003). It was also demonstrated that the association with PSD-95 enhanced 5-HT2A receptor-dependent Gq/11-coupling, and that this augmentation was accompanied by inhibition of agonist-induced 5-HT2A receptor internalization (Xia et al. 2003). 5-HT2A receptor-mediated head-twitch behavior is reduced and the 5-HT2A receptor-dependent induction of genes such as c-Fos, Egr-1 and Period-1 is disrupted in PSD-95 knockout mice (Abbas et al. 2009). These results suggest that PSD-95 is essential for hallucinogen actions at the 5-HT2A receptor. Additionally, findings based on a proteomic approach that combined affinity chromatography using an immobilized synthetic PDZ ligand with mass spectrometry demonstrated that the 5-HT2A receptor carboxyl terminus interacts with specific PDZ proteins in vitro and in vivo (Becamel et al. 2001, 2002a, 2002b, 2004). These results indicate that the 5-HT2A receptor is associated with protein networks that are important for its synaptic localization and coupling to signaling machinery.
Cellular responses induced by cytokines and growth factors are mediated by the evolutionary conserved Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathway (Kiu and Nicholson 2012). In addition to this integral component of the signaling system, it has been reported that desen-sitization of 5-HT2A receptor-dependent signaling is accompanied by activation of STAT3 and an increase in RGS7 protein in rat frontal cortex (Singh et al. 2009). It was also found that the 5-HT2A receptor and STAT3 co-precipitate with JAK2, indicating that they are part of the same protein complex (Singh et al. 2009). Because the hallucinogen DOI activates both the MEK-ERK1/2 and JAK2-STAT3 intracellular signaling pathways (Oufkir et al. 2010), it is tempting to speculate that these signal transduction pathways play a role in regulating the effects induced by hallucinogen 5-HT2A agonists. The tyrosine kinase inhibitor genistein (5 μM) decreased the potency of DOI-induced contraction of rat aorta, whereas this effect did not occur with daidzein (5 μM), which is the inactive isomer of genistein (Florian and Watts 1998). In cultured aortic smooth muscle cells, activation of the 5-HT2A receptor stimulated tyrosine-phosphorylation of ERK, and this effect was reduced by the MAPK inhibitor PD098059 (10 μM) (Florian and Watts 1998). Together, these results suggest that hallucinogens cause rat aortic contraction via a pathway that is at least partially independent of the pathways classically associated with the 5-HT2A receptor.
A specific conserved motif, NPxxY, which is found at the junction between TM7 and the carboxyl terminal domains of a number of rhodopsin-like GPCRs, including the 5-HT2A receptor, has been implicated as a determinant of ADP-ribosylation factor (ARF)-mediated signaling because wild-type receptors with an alternative DPxxY motif show selective defects in this pathway (Robertson et al. 2003; Mitchell et al. 1998). Although as described above, the 5-HT2A receptor is known to activate PLC via the heterotrimeric G protein Gq/11, previous findings have convincingly demonstrated that the 5-HT2A receptor can also signal through the PLD pathway independent of Gq/11 in an ARF-dependent manner. Both co-immunoprecipitation assays and the effect of negative mutant ARF constructs on 5-HT2A receptor-induced PLD activation demonstrate that ARF1 plays a key role in the function of this receptor (Robertson et al. 2003). The N376PxxY motif in TM7 was shown to be essential for ARF-dependent PLD signaling and co-immunoprecipitation with ARF1. In addition, ARF1 rather that ARF6 participates in this mechanism through a GTP-dependent interaction with the carboxyl terminus of the 5-HT2A receptor (Robertson et al. 2003). It has also been reported that the spatial coordination of the 5-HT2A receptor with transducer and effector proteins into a physical complex is likely to reinforce the impact of receptor activation on G protein-independent signaling pathway (Barclay et al. 2011).
By visualizing GFP-tagged 5-HT2A receptors in living cells, it has been shown that activation of protein kinase C (PKC) by its specific activator phorbol 12-myristate 13-acetate leads to internalization of the receptor in the absence of 5-HT (Bhattacharyya et al. 2002, 2010). Additionally, inhibition of PKC with sphingosine prevents internalization of the 5-HT2A receptor by 5-HT. Because receptors that had been internalized by phorbol 12-myristate 13-acetate exposure in the absence of 5-HT also recycle to the cell surface with a time-course comparable to that seen after activation of the 5-HT2A receptor by 5-HT, these findings suggest that 5-HT2A receptors internalize and return to the cell surface in response to both 5-HT and PKC (Bhattacharyya et al. 2002, 2010). Although the human and rat 5-HT2A receptors differ by only a few amino acids, the human receptor takes longer to recycle to the cell surface after internalization (Bhattacharya et al. 2010). Further investigation based upon the comparison of the primary sequences of human and rat 5-HT2A receptors demonstrated that replacing serine 457 in the carboxyl terminus of the human isoform with alanine resulted in faster recycling (Bhattacharya et al. 2010). By extension, this study also indicates that extrapolating results from non-human receptor isoforms may sometimes lead to misinterpretations.
It is well accepted that protein kinases mediate many of the downstream actions of both ionotropic and metabotropic receptors. Interestingly, relatively recent findings suggest that genetic deletion of p90 ribosomal S6 kinase 2 (RSK2) potentiates 5-HT2A receptor-dependent signaling (Sheffler et al. 2006). Thus, studies of 5-HT2A receptor signaling in fibroblasts obtained from wild-type and RSK2 knockout mice demonstrated that 5-HT2A receptor-dependent phosphoinositide hydrolysis is augmented in RSK2 knockout fibroblasts.
Several lines of evidence have shown that 5-HT1A and 5-HT2A receptors, which are co-expressed in cortical pyramidal neurons (Martin-Ruiz et al. 2001), often show opposite effects on common signaling pathways. It has been demonstrated that activation of 5-HT1A receptors suppresses NMDA receptor function in frontal cortex pyramidal neurons (Yuen et al. 2008). Most importantly, activation of 5-HT2A receptors by hallucinogens significantly attenuates the effect of 5-HT1A receptor on NMDA receptor currents and microtubule depolymerization. Inhibition of the β-arrestin/Src/dynamin signaling was shown to block 5-HT2A receptor-dependent activation of ERK and the counteractive effect of 5-HT2A on 5-HT1A-dependent regulation of NMDA receptor currents. These findings could be important for cognitive control—a function known to be heavily influenced by hallucinogens and the 5-HT2A receptor.
DARPP-32 is a key regulator of kinase-phosphatase signaling cascades modulated by serotonergic, dopaminergic, and glutamatergic neurotransmission (Svenningsson et al. 2004). Four distinct phosphorylation sites determine the function of DARPP-32. Phosphorylation at Thr34 converts DARPP-32 into a potent inhibitor of protein phosphatase-1 (PP1) (Svenningsson et al. 2003). Phosphorylated Ser97 increases the ability of protein kinase A (PKA) to phosphorylate DARPP-32 at Thr34. Phosphorylation of Ser130 prevents the dephos-phorylation of Thr34 by protein phosphatase-2B (PP2B). Phosphorylation of Thr75 converts DARPP-32 into an inhibitor of PKA (Svenningsson et al. 2003). Dopaminergic agonists, such as (+)-amphetamine, serotonergic 5-HT2A agonists such as LSD, and glutamatergic antagonists such as phencyclidine (PCP), have all been shown to induce phosphorylation or dephosphorylation of DARPP-32 at three sites in a pattern predicted to cause a synergistic inhibition of protein PP1 and concomitant regulation of its downstream effector proteins GSK-3, cAMP response element-binding protein (CREB), and c-Fos (Svenningsson et al. 2003). Notably, in mice with point mutations at DARPP-32 phosphorylation sites, the effects of (+)amphetamine, LSD, and PCP on sensorimotor gating and repetitive movements are strongly attenuated. Thus, three pathways that regulate the state of phosphorylation of Thr34-, Thr75-, and Ser130-DARPP-32 inhibit PP1, which leads to increased phosphorylation of various PP1 substrates (Svenningsson et al. 2003). Further work will be needed to identify the precise PP1 substrates involved in the psychoactive behavioral effects of these drugs.
Brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) are members of the neurotrophin family, a small family of secreted proteins that also includes nerve growth factor (NGF), neurotrophin 3 (NT-3), and neurotrophin 4 (NT-4) (Griebel and Holsboer 2012). Administration of DOI induces a differential regulation BDNF mRNA expression in rat hippocampus and neocortex, with downregulation in hippocampus and upregulation in neocortex (Vaidya et al. 1997). This interesting effect is blocked by selective 5-HT2A antagonists but is unaffected by selective 5-HT2C antagonists. Additionally, the mGlu2/3 receptor agonist LY354740 dose-dependently represses the ability of DOI to induce upregulation of BDNF mRNA expression in rat frontal cortex, whereas the effect of DOI is enhanced by the mGlu2/3 receptor antagonist LY341495 (Gewirtz et al. 2002). Immobilization stress also decreases the expression of BDNF mRNA in rat hippocampus, an effect that is blocked by the 5-HT2A receptor antagonist MDL100,907 (Vaidya et al. 1997). These results suggest that 5-HT2A receptor-dependent signaling is involved in the stress-induced regulation of BDNF expression in the rat hippocampus. Given that the mGlu2/3 receptor agonist LY354740 suppresses the effect of immobilization stress on BDNF mRNA expression (Lee et al. 2006), these results are consistent with the hypothesis that mGlu2/3 receptor agonists may modulate 5-HT2A receptor-dependent stress-induced behaviors.
7 Role of mGlu2 Receptor in Hallucinogen Action
Although it is generally accepted that the 5-HT2A receptor expressed in frontal cortical pyramidal neurons represents the main molecular target responsible for the cellular, electrophysiological and behavioral effects of hallucinogens in rodents (Gonzalez-Maeso et al. 2007; Beique et al. 2007; Puig et al. 2003; Celada et al. 2008), effects on sub-cortical regions such as thalamocortical projections may also contribute (Marek et al. 2001; Scruggs et al. 2000). Relatively recent findings demonstrate that the mGlu2/3 receptor agonist LY354740 antagonizes 5-HT2A receptor-induced excitatory postsynaptic potential/currents (EPSPs/EPSCs) in pyramidal neurons (Marek et al. 2000). It was also shown that LY354740 suppresses the head-twitch behavior induced by the hallucinogen DOI (Gewirtz and Marek 2000). Similar effects have been observed with mGlu2/3 receptor agonists such as LY379268 and LY404030 (Aghajanian and Marek 2000). These findings led to the conclusion that activation of mGlu2 autoreceptors mediates the presynaptic effects of mGlu2/3 agonists in suppressing the electrophysiological effects of hallucinogenic 5-HT2A receptor agonists recorded in cortical pyramidal neurons (Aghajanian and Marek 2000). An alternative (although not mutually exclusive) explanation for the crosstalk between 5-HT2A and mGlu2 receptors is that these two receptors may be expressed in close physical proximity in the postsynaptic density of cortical pyramidal neurons.
Traditionally, GPCRs were thought to function as monomers. The monomeric function of GPCRs is supported by assays that measured agonist binding and G protein coupling of purified receptors reconstituted into a lipid bilayer (including rhodopsin, β2-adrenergic and μ-opioid receptors) (Whorton et al. 2007, 2008; Kuszak et al. 2009). Nevertheless, many instances of homomerization and heteromerization (macromolecular complexes formed by non-covalent interactions between GPCRs) have recently been reported (Gonzalez-Maeso 2011; Milligan 2013; Ferre et al. 2014). The existence of GPCR heteromers is further suggested by the recent explosion of research elucidating the crystal structures of GPCRs; for example, four crystal structures that were recently reported (CXCR4, μ-opioid, κ-opioid, and β1-adrenergic receptors) contained receptor dimers (Huang et al. 2013; Manglik et al. 2012; Wu et al. 2010, 2012). Interestingly, previous findings suggest that the Gq/11-coupled 5-HT2A receptor and the Gi/o-coupled mGlu2 receptor form a specific GPCR heteromeric complex in heterologous expression systems, as well as in mouse and human frontal cortex (Gonzalez-Maeso et al. 2008; Moreno et al. 2012; Fribourg et al. 2011); these results have been independently confirmed by other groups (Rives et al. 2009). The close molecular proximity between 5-HT2A and mGlu2 receptors does not occur with the closely related Gi/o-coupled mGlu3 receptor, and is either rescued or disrupted with different mGlu2/mGlu3 chimeric constructs (Gonzalez-Maeso et al. 2008; Moreno et al. 2012, 2016; Fribourg et al. 2011; Baki et al. 2016). The conclusion that 5-HT2A and mGlu2 receptors are co-expressed as a GPCR heteromeric complex in frontal cortex is supported by observations that demonstrate the co-expression and co-immunoprecipitation of these receptors in mouse and human frontal cortex (Gonzalez-Maeso et al. 2008; Fribourg et al. 2011; Moreno et al. 2016), as well as by the close physical proximity of 5-HT2A and mGlu2 receptors at cortical synaptic junctions at the electron microscopy level (Moreno et al. 2012) (Fig. 5).
Fig. 5.
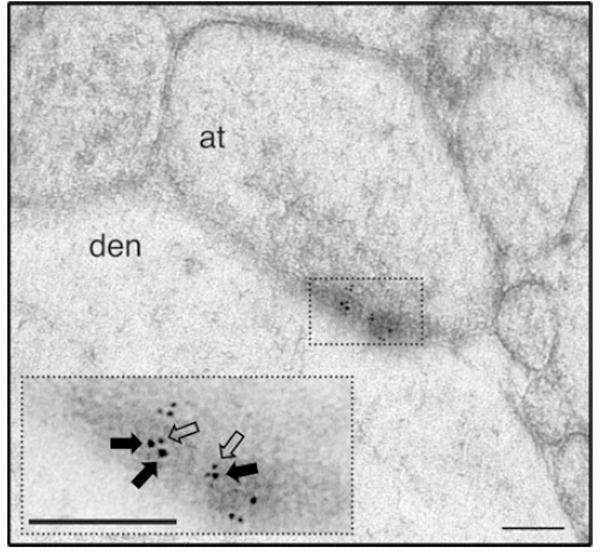
Immunogold labeling for 5-HT2A and mGlu2 receptors in mouse cortical neurons. Note that the 10-nm gold particles (filled arrows) and the 6-nm gold particles (open arrows) are located in very close proximity at the synaptic junction (see Moreno et al. 2012). Inset, high magnification view of region delineated in boxed area. Scale bars, 100 nm. (den, dendrite; at, axon terminal)
The role of mGlu2 in the psychoactive effects induced by hallucinogens is supported by the impaired ability of the hallucinogens DOI and LSD to induce head-twitch behavior in mGlu2 knockout mice compared to wild-type littermates (Figs. 6 and 7) (Moreno et al. 2011). It has been demonstrated that the ability of DOI to induce head-twitch behavior in mGlu2 knockout mice is rescued by over-expressing mGlu2 in frontal cortex using a viral (HSV)-mediated transgene expression approach (Moreno et al. 2012). Because DOI-induced head-twitch behavior is not rescued in mGlu2 knockout mice over-expressing mGlu2∆TM4N [a mGlu2/mGlu3 chimeric construct that does not form heteromers with the 5-HT2A receptor (Gonzalez-Maeso et al. 2008; Fribourg et al. 2011)] in frontal cortex (Moreno et al. 2012), these findings suggest that the 5-HT2A-mGlu2 receptor complex is critical for the hallucinogen-like behaviors induced by 5-HT2A receptor agonists (Figs. 6 and 7). The translational potential of these findings is suggested by the alterations in the expression of 5-HT2A and mGlu2 receptors in the frontal cortex of schizophrenic subjects postmortem (Gonzalez-Maeso et al. 2008; Moreno et al. 2012, 2016; Muguruza et al. 2013). However, further investigation of this heteromeric receptor complex is definitely necessary because the functional significance of GPCR homo- and heteromerization remains a controversial topic (Bouvier and Hebert 2014; Lambert and Javitch 2014) [in addition, see: Delille et al. (2012), Frederick et al. (2015)].
Fig. 6.
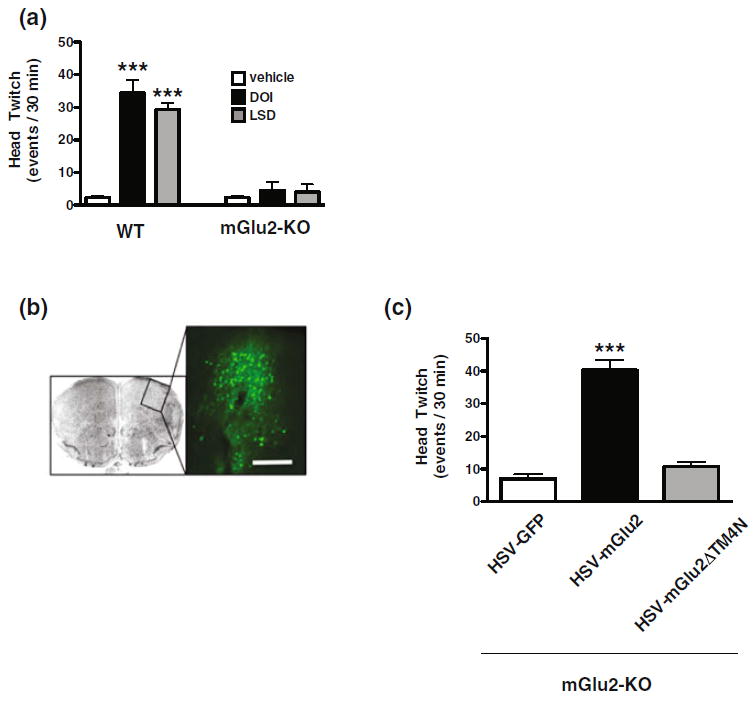
a Head-twitch behavior induced by DOI and LSD is absent in mGlu2 knockout mice (see Moreno et al. 2011). b, c Expression of 5-HT2A and mGlu2 as a GPCR heteromer is necessary for head-twitch psychosis-like behavior induced by hallucinogenic 5-HT2A agonists in mice (see Moreno et al. 2012). Representative image of HSV-mediated transgene expression in mouse frontal cortex. Scale bar, 200 μm (b). Virally mediated over-expression of wild-type mGlu2, but not the mGlu2/mGlu3 chimeric construct mGlu2∆TM4 N that does not form the 5-HT2A-mGlu2 heteromeric receptor complex, rescues the head-twitch behavior induced by the hallucinogenic 5-HT2A receptor agonist DOI (c)
Fig. 7.
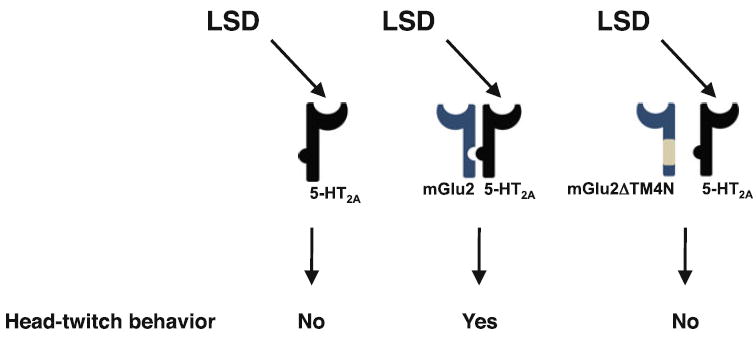
Model of the mechanism underlying hallucinogen-induced head-twitch behavioral response
8 Future Directions
In conclusion, recent work suggests that hallucinogens may be unique in their ability to modulate the activity of specific 5-HT2A receptor-linked signaling pathways. Hallucinogens are used recreationally; hence, elucidating their biophysical and molecular mechanism of action is an important objective in drug abuse research (Nutt et al. 2013). However, clinical studies also suggest that hallucinogens, when administered under medical supervision, may serve as therapeutic drugs that can be used for the treatment of severe psychiatric and neurological disorders such as alcoholism (Krebs and Johansen 2012), obsessive compulsive disorder (Moreno et al. 2006), and cluster headache (Sewell et al. 2006). Further basic and translational research is therefore warranted to better define the specific signaling and neuronal circuit mechanisms responsible for their psychoactive effects.
Acknowledgments
The preparation of this review article has been supported at least in part by the National Institutes of Health R01MH084894 and R01MH111940 (J.G.M.) and the Spanish Government SAF2010-15663 (J.F.L.G.).
Contributor Information
Juan F. López-Giménez, Instituto de Biomedicina y Biotecnología de Cantabria IBBTEC-CSIC, 39011 Santander, Cantabria, Spain
Javier González-Maeso, Department of Physiology and Biophysics, School of Medicine, Virginia Commonwealth University, Richmond, VA 23298, USA.
References
- Abbas AI, Yadav PN, Yao W-D, Arbuckle MI, Grant SGN, Caron MG, Roth BL. PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J Neurosci. 2009;29:7124–7136. doi: 10.1523/JNEUROSCI.1090-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams LM, Geyer MA. Patterns of exploration in rats distinguish lisuride from lysergic acid diethylamide. Pharmacol Biochem Behav. 1985;23:461–468. doi: 10.1016/0091-3057(85)90022-x. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK. Modeling, “psychosis” in vitro by inducing disordered neuronal network activity in cortical brain slices. Psychopharmacology. 2009;206:575–585. doi: 10.1007/s00213-009-1484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin and hallucinogens. Neuropsychopharmacology. 1999;21:16S–23S. doi: 10.1016/S0893-133X(98)00135-3. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Brain Res Rev. 2000;31:302–312. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Foote WE, Sheard MH. Lysergic acid diethylamide: sensitive neuronal units in the midbrain raphe. Science. 1968;161:706–708. doi: 10.1126/science.161.3842.706. [DOI] [PubMed] [Google Scholar]
- Appel NM, Mitchell WM, Garlick RK, Glennon RA, Teitler M, De Souza EB. Autoradiographic characterization of (+-)-1-(2,5-dimethoxy-4-[125I] iodophenyl)-2-aminopropane ([125I]DOI) binding to 5-HT2 and 5-HT1c receptors in rat brain. J Pharmacol Exp Ther. 1990;255:843–857. [PubMed] [Google Scholar]
- Audet M, Bouvier M. Restructuring G-protein-coupled receptor activation. Cell. 2012;151:14–23. doi: 10.1016/j.cell.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Baki L, Fribourg M, Younkin J, Eltit JM, Moreno JL, Park G, Vysotskaya Z, Narahari A, Sealfon SC, Gonzalez-Maeso J, Logothetis DE. Cross-signaling in metabotropic glutamate 2 and serotonin 2A receptor heteromers in mammalian cells. Pflugers Arch. 2016;468:775–793. doi: 10.1007/s00424-015-1780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay Z, Dickson L, Robertson DN, Johnson MS, Holland PJ, Rosie R, Sun L, Fleetwood-Walker S, Lutz EM, Mitchell R. 5-HT2A receptor signalling through phospholipase D1 associated with its C-terminal tail. Biochem J. 2011;436:651–660. doi: 10.1042/BJ20101844. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Becamel C, Figge A, Poliak S, Dumuis A, Peles E, Bockaert J, Lubbert H, Ullmer C. Interaction of serotonin 5-hydroxytryptamine type 2C receptors with PDZ10 of the multi-PDZ domain protein MUPP1. J Biol Chem. 2001;276:12974–12982. doi: 10.1074/jbc.M008089200. [DOI] [PubMed] [Google Scholar]
- Becamel C, Alonso G, Galeotti N, Demey E, Jouin P, Ullmer C, Dumuis A, Bockaert J, Marin P. Synaptic multiprotein complexes associated with 5-HT(2C) receptors: a proteomic approach. EMBO J. 2002a;21:2332–2342. doi: 10.1093/emboj/21.10.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becamel C, Galeotti N, Poncet J, Jouin P, Dumuis A, Bockaert J, Marin P. A proteomic approach based on peptide affinity chromatography, 2-dimensional electrophoresis and mass spectrometry to identify multiprotein complexes interacting with membrane-bound receptors. Biol Proced Online. 2002b;4:94–104. doi: 10.1251/bpo39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becamel C, Gavarini S, Chanrion B, Alonso G, Galeotti N, Dumuis A, Bockaert J, Marin P. The serotonin 5-HT2A and 5-HT2C receptors interact with specific sets of PDZ proteins. J Biol Chem. 2004;279:20257–20266. doi: 10.1074/jbc.M312106200. [DOI] [PubMed] [Google Scholar]
- Beique JC, Imad M, Mladenovic L, Gingrich JA, Andrade R. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc Natl Acad Sci U S A. 2007;104:9870–9875. doi: 10.1073/pnas.0700436104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol. 1998;54:94–104. [PubMed] [Google Scholar]
- Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Sankar S, Panicker MM. Differences in the C-terminus contribute to variations in trafficking between rat and human 5-HT(2A) receptor isoforms: identification of a primate-specific tripeptide ASK motif that confers GRK-2 and beta arrestin-2 interactions. J Neurochem. 2010;112:723–732. doi: 10.1111/j.1471-4159.2009.06493.x. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Puri S, Miledi R, Panicker MM. Internalization and recycling of 5-HT2A receptors activated by serotonin and protein kinase C-mediated mechanisms. Proc Natl Acad Sci U S A. 2002;99:14470–14475. doi: 10.1073/pnas.212517999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier M, Hebert TE. CrossTalk proposal: weighing the evidence for Class A GPCR dimers, the evidence favours dimers. J Physiol. 2014;592:2439–2441. doi: 10.1113/jphysiol.2014.272252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE. Head-twitch response in rodents induced by the hallucinogen 2, 5-dimethoxy-4- iodoamphetamine: a comprehensive history, a refievaluation of mechanisms, and its …. Drug Testing and Analysis. 2012 doi: 10.1002/dta.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Olaghere da Silva UB, Gresch PJ, Watt EE, Sanders-Bush E, Airey DC. The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology. 2010;209:163–174. doi: 10.1007/s00213-010-1784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Diaz-Mataix L, Artigas F. The hallucinogen DOI reduces low-frequency oscillations in rat prefrontal cortex: reversal by antipsychotic drugs. Biol Psychiatry. 2008;64:392–400. doi: 10.1016/j.biopsych.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Chiu HY, Chan MH, Lee MY, Chen ST, Zhan ZY, Chen HH. Long-lasting alterations in 5-HT2A receptor after a binge regimen of methamphetamine in mice. Int J Neuropsychopharmacol. 2014:1–12. doi: 10.1017/S1461145714000455. [DOI] [PubMed] [Google Scholar]
- Clark AJ. The mode of action of drugs on cells 1933 [Google Scholar]
- Dahlström A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand Suppl SUPPL. 1964;232:231–255. [PubMed] [Google Scholar]
- De Lean A, Stadel JM, Lefkowitz RJ. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem. 1980;255:7108–7117. [PubMed] [Google Scholar]
- Delille HK, Becker JM, Burkhardt S, Bleher B, Terstappen GC, Schmidt M, Meyer AH, Unger L, Marek GJ, Mezler M. Heterocomplex formation of 5-HT(2A)-mGlu(2) and its relevance for cellular signaling cascades. Neuropharmacology. 2012:1–8. doi: 10.1016/j.neuropharm.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Egan CT, Herrick-Davis K, Miller K, Glennon RA, Teitler M. Agonist activity of LSD and lisuride at cloned 5HT2A and 5HT2C receptors. Psychopharmacology. 1998;136:409–414. doi: 10.1007/s002130050585. [DOI] [PubMed] [Google Scholar]
- Erhlich P. Chemotherapeutics: scientific principles, methods and results. Lancet. 1913;2:445–451. [Google Scholar]
- Fantegrossi WE, Murnane KS, Reissig CJ. The behavioral pharmacology of hallucinogens. Biochem Pharmacol. 2008;75:17–33. doi: 10.1016/j.bcp.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- Ferre S, Casado V, Devi LA, Filizola M, Jockers R, Lohse MJ, Milligan G, Pin JP, Guitart X. G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol Rev. 2014;66:413–434. doi: 10.1124/pr.113.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian JA, Watts SW. Integration of mitogen-activated protein kinase kinase activation in vascular 5-hydroxytryptamine2A receptor signal transduction. J Pharmacol Exp Ther. 1998;284:346–355. [PubMed] [Google Scholar]
- Frederick AL, Yano H, Trifilieff P, Vishwasrao HD, Biezonski D, Meszaros J, Urizar E, Sibley DR, Kellendonk C, Sonntag KC, Graham DL, Colbran RJ, Stanwood GD, Javitch JA. Evidence against dopamine D1/D2 receptor heteromers. Mol Psychiatry. 2015 doi: 10.1038/mp.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fribourg M, Moreno JL, Holloway T, Provasi D, Baki L, Mahajan R, Park G, Adney SK, Hatcher C, Eltit JM, Ruta JD, Albizu L, Li Z, Umali A, Shim J, Fabiato A, Mackerell AD, Jr, Brezina V, Sealfon SC, Filizola M, Gonzalez-Maeso J, Logothetis DE. Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell. 2011;147:1011–1023. doi: 10.1016/j.cell.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddum JH, Picarelli ZP. Two kinds of tryptamine receptor. Br J Pharmacol Chemother. 1957;12:323–328. doi: 10.1111/j.1476-5381.1957.tb00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia EE, Smith RL, Sanders-Bush E. Role of G(q) protein in behavioral effects of the hallucinogenic drug 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane. Neuropharmacology. 2007;52:1671–1677. doi: 10.1016/j.neuropharm.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992;15:133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Marek GJ. Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology. 2000;23:569–576. doi: 10.1016/S0893-133X(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Chen AC, Terwilliger R, Duman RC, Marek GJ. Modulation of DOI-induced increases in cortical BDNF expression by group II mGlu receptors. Pharmacol Biochem Behav. 2002;73:317–326. doi: 10.1016/s0091-3057(02)00844-4. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984;35:2505–2511. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, Young R. Structure-activity relationships and mechanism of action of hallucinogenic agents based on drug discrimination and radioligand binding studies. Psychopharmacol Bull. 1986;22:953–958. [PubMed] [Google Scholar]
- Gonzalez-Maeso J. GPCR oligomers in pharmacology and signaling. Mol Brain. 2011;4:20. doi: 10.1186/1756-6606-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, Weisstaub N, Hen R, Gingrich JA, Sealfon SC. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci. 2003;23:8836–8843. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT (2A) Receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- Green AR. Gaddum and LSD: the birth and growth of experimental and clinical neuropharmacology research on 5-HT in the UK. Br J Pharmacol. 2008;154:1583–1599. doi: 10.1038/bjp.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Holsboer F. Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning? Nat Rev Drug Discov. 2012;11:462–478. doi: 10.1038/nrd3702. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL. Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav Brain Res. 2015;277C:99–120. doi: 10.1016/j.bbr.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks JB, Gonzalez-Maeso J. Animal models of serotonergic psychedelics. ACS Chem Neurosci. 2013;4:33–42. doi: 10.1021/cn300138m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- Hill SJ, Baker JG, Rees S. Reporter-gene systems for the study of G-protein-coupled receptors. Curr Opin Pharmacol. 2001;1:526–532. doi: 10.1016/s1471-4892(01)00091-1. [DOI] [PubMed] [Google Scholar]
- Hofmann A. How LSD originated. J Psychedelic Drugs. 1979;11:53–60. doi: 10.1080/02791072.1979.10472092. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Huang J, Chen S, Zhang JJ, Huang XY. Crystal structure of oligomeric beta1-adrenergic G protein-coupled receptors in ligand-free basal state. Nat Struct Mol Biol. 2013;20:419–425. doi: 10.1038/nsmb.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R, Tomshine JC, Tomshine JR, Chevalier M, Mahoney JP, Steyaert J, Rasmussen SG, Sunahara RK, El-Samad H, Huang B, von Zastrow M. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–538. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MP, Siegel BW, Carr AA. [3H]MDL 100,907: a novel selective 5-HT2A receptor ligand. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:205–209. doi: 10.1007/BF00178722. [DOI] [PubMed] [Google Scholar]
- Karaki S, Becamel C, Murat S, Mannoury la Cour C, Millan MJ, Prezeau L, Bockaert J, Marin P, Vandermoere F. Quantitative phosphoproteomics unravels biased phosphorylation of serotonin 2A receptor at Ser280 by hallucinogenic versus nonhallucinogenic agonists. Mol Cell Proteomics. 2014;13:1273–1285. doi: 10.1074/mcp.M113.036558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katritch V, Cherezov V, Stevens RC. Structure-function of the G protein-coupled receptor superfamily. Annu Rev Pharmacol Toxicol. 2013;53:531–556. doi: 10.1146/annurev-pharmtox-032112-135923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends Pharmacol Sci. 1995;16:232–238. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Agonist-specific receptor conformations. Trends Pharmacol Sci. 1997;18:416–417. doi: 10.1016/s0165-6147(97)01127-9. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Drug efficacy at G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2002;42:349–379. doi: 10.1146/annurev.pharmtox.42.091401.113012. [DOI] [PubMed] [Google Scholar]
- Kiu H, Nicholson SE. Biology and signi3cance of the JAK/STAT signalling pathways. Growth Factors. 2012;30:88–106. doi: 10.3109/08977194.2012.660936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Krebs TS, Johansen PO. Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials. J Psychopharmacol. 2012;26:994–1002. doi: 10.1177/0269881112439253. [DOI] [PubMed] [Google Scholar]
- Kurrasch-Orbaugh DM, Watts VJ, Barker EL, Nichols DE. Serotonin 5-hydroxytryptamine 2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J Pharmacol Exp Ther. 2003a;304:229–237. doi: 10.1124/jpet.102.042184. [DOI] [PubMed] [Google Scholar]
- Kurrasch-Orbaugh DM, Parrish JC, Watts VJ, Nichols DE. A complex signaling cascade links the serotonin2A receptor to phospholipase A2 activation: the involvement of MAP kinases. J Neurochem. 2003b;86:980–991. doi: 10.1046/j.1471-4159.2003.01921.x. [DOI] [PubMed] [Google Scholar]
- Kuszak AJ, Pitchiaya S, Anand JP, Mosberg HI, Walter NG, Sunahara RK. Purification and functional reconstitution of monomeric mu-opioid receptors: allosteric modulation of agonist binding by Gi2. J Biol Chem. 2009;284:26732–26741. doi: 10.1074/jbc.M109.026922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NA, Javitch JA. CrossTalk opposing view: weighing the evidence for class A GPCR dimers, the jury is still out. J Physiol. 2014;592:2443–2445. doi: 10.1113/jphysiol.2014.272997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley JN. On the contraction of muscle, chiefly in relation to the presence of ‘receptive’ substances. J Physiol. 1909;39:235–295. doi: 10.1113/jphysiol.1909.sp001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Duman RS, Marek GJ. The mGlu2/3 receptor agonist LY354740 suppresses immobilization stress-induced increase in rat prefrontal cortical BDNF mRNA expression. Neurosci Lett. 2006;398:328–332. doi: 10.1016/j.neulet.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Lee MY, Chiang CC, Chiu HY, Chan MH, Chen HH. N-acetylcysteine modulates hallucinogenic 5-HT receptor agonist-mediated responses: behavioral, molecular, and electro-physiological studies. Neuropharmacology. 2014 doi: 10.1016/j.neuropharm.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Cotecchia S, Samama P, Costa T. Constitutive activity of receptors coupled to guanine nucleotide regulatory proteins. Trends Pharmacol Sci. 1993;14:303–307. doi: 10.1016/0165-6147(93)90048-O. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Mengod G, Palacios JM, Vilaro MT. Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100,907. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:446–454. doi: 10.1007/pl00005075. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Vilaro MT, Palacios JM, Mengod G. [3H]MDL 100,907 labels 5-HT2A serotonin receptors selectively in primate brain. Neuropharmacology. 1998;37:1147–1158. doi: 10.1016/s0028-3908(98)00102-6. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Mengod G, Palacios JM, Vilaro MT. Human striosomes are enriched in 5-HT2A receptors: autoradiographical visualization with [3H]MDL100,907,[125I](±)DOI and [3H]ketanserin. Eur J Neurosci. 1999;11:3761–3765. doi: 10.1046/j.1460-9568.1999.00827.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Vilaro MT, Palacios JM, Mengod G. Mapping of 5-HT2A receptors and their mRNA in monkey brain: [3H]MDL100,907 autoradiography and in situ hybridization studies. J Comp Neurol. 2001;429:571–589. doi: 10.1002/1096-9861(20010122)429:4<571::aid-cne5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Luttrell LM. Reviews in molecular biology and biotechnology: transmembrane signaling by G protein-coupled receptors. Mol Biotechnol. 2008;39:239–264. doi: 10.1007/s12033-008-9031-1. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- Malkova NV, Gallagher JJ, Yu CZ, Jacobs RE, Patterson PH. Manganese-enhanced magnetic resonance imaging reveals increased DOI-induced brain activity in a mouse model of schizophrenia. Proc Natl Acad Sci U S A. 2014;111:E2492–2500. doi: 10.1073/pnas.1323287111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, Granier S. Crystal structure of the micro-opioid receptor bound to a morphinan antagonist. Nature. 2012;485:321–326. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther. 2000;292:76–87. [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Gewirtz JC, Schoepp DD. A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neuroscience. 2001;105:379–392. doi: 10.1016/s0306-4522(01)00199-3. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Kurrasch-Orbaugh DM, Selken JR, Cumbay MG, Lisnicchia JG, Nichols DE. Re-evaluation of lisuride pharmacology: 5-hydroxytryptamine1A receptor-mediated behavioral effects overlap its other properties in rats. Psychopharmacology. 2002;164:93–107. doi: 10.1007/s00213-002-1141-z. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz R, Puig MV, Celada P, Shapiro DA, Roth BL, Mengod G, Artigas F. Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J Neurosci. 2001;21:9856–9866. doi: 10.1523/JNEUROSCI.21-24-09856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengod G, Vilaro MT, Cortes R, Lopez-Gimenez JF, Raurich A, Palacios JM. Chemical neuroanatomy of 5-HT receptor subtypes in the mammalian brain. In: Roth BL, editor. serotonin T, and therapeutics rFmpth. Humana Press; 2006. pp. 319–364. [Google Scholar]
- Millan MJ, Marin P, Bockaert J, la Cour CM. Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol Sci. 2008;29:454–464. doi: 10.1016/j.tips.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Milligan G. The prevalence, maintenance and relevance of GPCR oligomerization. Mol Pharmacol. 2013:158–169. doi: 10.1124/mol.113.084780. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R, McCulloch D, Lutz E, Johnson M, MacKenzie C, Fennell M, Fink G, Zhou W, Sealfon SC. Rhodopsin-family receptors associate with small G proteins to activate phospholipase D. Nature. 1998;392:411–414. doi: 10.1038/32937. [DOI] [PubMed] [Google Scholar]
- Mohammad-Zadeh LF, Moses L, Gwaltney-Brant SM. Serotonin: a review. J Vet Pharmacol Ther. 2008;31:187–199. doi: 10.1111/j.1365-2885.2008.00944.x. [DOI] [PubMed] [Google Scholar]
- Mokler DJ, Commissaris RL, Warner MR, Rech RH. Blockade of the behavioral effects of lysergic acid diethylamide, 2,5-dimethoxy-4-methylamphetamine, quipazine and lisuride by 5-hydroxytryptamine antagonists. J Pharmacol Exp Ther. 1983;227:557–562. [PubMed] [Google Scholar]
- Moreno FA, Wiegand CB, Taitano EK, Delgado PL. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry. 2006;67:1735–1740. doi: 10.4088/jcp.v67n1110. [DOI] [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Albizu L, Sealfon SC, Gonzalez-Maeso J. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett. 2011;493:76–79. doi: 10.1016/j.neulet.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Muguruza C, Umali A, Mortillo S, Holloway T, Pilar-Cuellar F, Mocci G, Seto J, Callado LF, Neve RL, Milligan G, Sealfon SC, Lopez-Gimenez JF, Meana JJ, Benson DL, Gonzalez-Maeso J. Identification of three residues essential for 5-hydroxytryptamine 2A-metabotropic glutamate 2 (5-HT2A.mGlu2) receptor heteromerization and its psychoactive behavioral function. J Biol Chem. 2012;287:44301–44319. doi: 10.1074/jbc.M112.413161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Miranda-Azpiazu P, Garcia-Bea A, Younkin J, Cui M, Kozlenkov A, Ben-Ezra A, Voloudakis G, Fakira AK, Baki L, Ge Y, Georgakopoulos A, Moron JA, Milligan G, Lopez-Gimenez JF, Robakis NK, Logothetis DE, Meana JJ, Gonzalez-Maeso J. Allosteric signaling through an mGlu2 and 5-HT2A heteromeric receptor complex and its potential contribution to schizophrenia. Sci Signal. 2016;9:ra5. doi: 10.1126/scisignal.aab0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muguruza C, Moreno JL, Umali A, Callado LF, Meana JJ, Gonzalez-Maeso J. Dysregulated 5-HT(2A) receptor binding in postmortem frontal cortex of schizophrenic subjects. Eur Neuropsychopharmacol. 2013;23:852–864. doi: 10.1016/j.euroneuro.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Nichols CD. Serotonin receptors. Chem Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, King LA, Nichols DE. Effects of Schedule I drug laws on neuroscience research and treatment innovation. Nat Rev Neurosci. 2013;14:577–585. doi: 10.1038/nrn3530. [DOI] [PubMed] [Google Scholar]
- Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- Oufkir T, Arseneault M, Sanderson JT, Vaillancourt C. The 5-HT 2A serotonin receptor enhances cell viability, affects cell cycle progression and activates MEK-ERK1/2 and JAK2-STAT3 signalling pathways in human choriocarcinoma cell lines. Placenta. 2010;31:439–447. doi: 10.1016/j.placenta.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- Pazos A, Cortes R, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res. 1985;346:231–249. doi: 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain–IV. Autoradiographic mapping of serotonin-2 receptors. Neuroscience. 1987;21:123–139. doi: 10.1016/0306-4522(87)90327-7. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Snyder SH. Multiple serotonin receptors: differential binding of [3H] 5-hydroxytryptamine, [3H]lysergic acid diethylamide and [3H]spiroperidol. Mol Pharmacol. 1979;16:687–699. [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol. 1999;128:13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preller KH, Pokorny T, Hock A, Kraehenmann R, Stampfli P, Seifritz E, Scheidegger M, Vollenweider FX. Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc Natl Acad Sci U S A. 2016;113:5119–5124. doi: 10.1073/pnas.1524187113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preller KH, Herdener M, Pokorny T, Planzer A, Kraehenmann R, Stampfli P, Liechti ME, Seifritz E, Vollenweider FX. The fabric of meaning and subjective effects in lsd-induced states depend on serotonin 2a receptor activation. Curr Biol. 2017;27:451–457. doi: 10.1016/j.cub.2016.12.030. [DOI] [PubMed] [Google Scholar]
- Puig MV, Celada P, Diaz-Mataix L, Artigas F. In vivo modulation of the activity of pyramidal neurons in the rat medial prefrontal cortex by 5-HT2A receptors: relationship to thalamocortical afferents. Cereb Cortex. 2003;13:870–882. doi: 10.1093/cercor/13.8.870. [DOI] [PubMed] [Google Scholar]
- Rapport MM, Green AA, Page IH. Serum vasoconstrictor, serotonin; isolation and characterization. J Biol Chem. 1948;176:1243–1251. [PubMed] [Google Scholar]
- Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, Garnovskaya MN. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther. 2001;92:179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- Reid G, Rand M. Pharmacological actions of synthetic 5-hydroxytryptamine (serotonin, thrombocytin) Nature. 1952;169:801–802. doi: 10.1038/169801a0. [DOI] [PubMed] [Google Scholar]
- Rives ML, Vol C, Fukazawa Y, Tinel N, Trinquet E, Ayoub MA, Shigemoto R, Pin JP, Prezeau L. Crosstalk between GABAB and mGlu1a receptors reveals new insight into GPCR signal integration. EMBO J. 2009;28:2195–2208. doi: 10.1038/emboj.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DN, Johnson MS, Moggach LO, Holland PJ, Lutz EM, Mitchell R. Selective interaction of ARF1 with the carboxy-terminal tail domain of the 5-HT2A receptor. Mol Pharmacol. 2003;64:1239–1250. doi: 10.1124/mol.64.5.1239. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini MA, Ratner C, Aznar S, Klein AB, Knudsen GM, Mikkelsen JD. Enhanced prefrontal serotonin 2A receptor signaling in the subchronic phencyclidine mouse model of schizophrenia. J Neurosci Res. 2013;91:634–641. doi: 10.1002/jnr.23198. [DOI] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a ß-arrestin2/Src/Akt signaling complex in vivo. J Neurosci official J Soc Neurosci. 2010;30:13513–13524. doi: 10.1523/JNEUROSCI.1665-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Raehal KM, Bohn LM. Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc Natl Acad Sci U S A. 2008;105:1079–1084. doi: 10.1073/pnas.0708862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Streicher JM, Meltzer HY, Bohn LM. Clozapine acts as an agonist at serotonin 2A receptors to counter MK-801-induced behaviors through a betaarrestin2-independent activation of Akt. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid Y, Enzler F, Gasser P, Grouzmann E, Preller KH, Vollenweider FX, Brenneisen R, Muller F, Borgwardt S, Liechti ME. Acute effects of lysergic acid diethylamide in healthy subjects. Biol Psychiatry. 2015;78:544–553. doi: 10.1016/j.biopsych.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Scruggs JL, Patel S, Bubser M, Deutch AY. DOI-Induced activation of the cortex: dependence on 5-HT2A heteroceptors on thalamocortical glutamatergic neurons. J Neurosci. 2000;20:8846–8852. doi: 10.1523/JNEUROSCI.20-23-08846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell RA, Halpern JH, Pope HG., Jr Response of cluster headache to psilocybin and LSD. Neurology. 2006;66:1920–1922. doi: 10.1212/01.wnl.0000219761.05466.43. [DOI] [PubMed] [Google Scholar]
- Sheffler DJ, Kroeze WK, Garcia BG, Deutch AY, Hufeisen SJ, Leahy P, Bruning JC, Roth BL. p90 ribosomal S6 kinase 2 exerts a tonic brake on G protein-coupled receptor signaling. Proc Natl Acad Sci U S A. 2006;103:4717–4722. doi: 10.1073/pnas.0600585103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla AK, Manglik A, Kruse AC, Xiao K, Reis RI, Tseng WC, Staus DP, Hilger D, Uysal S, Huang LY, Paduch M, Tripathi-Shukla P, Koide A, Koide S, Weis WI, Kossiakoff AA, Kobilka BK, Lefkowitz RJ. Structure of active beta-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature. 2013;497:137–141. doi: 10.1038/nature12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbergeld EK, Hruska RE. Lisuride and LSD: dopaminergic and serotonergic interactions in the “serotonin syndrome”. Psychopharmacology. 1979;65:233–237. doi: 10.1007/BF00492209. [DOI] [PubMed] [Google Scholar]
- Singh RK, Dai Y, Staudinger JL, Muma NA. Activation of the JAK-STAT pathway is necessary for desensitization of 5-HT2A receptor-stimulated phospholipase C signalling by olanzapine, clozapine and MDL 100907. Int J Neuropsychopharmacol. 2009;12:651–665. doi: 10.1017/S1461145708009590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange PG. Three-state and two-state models. Trends Pharmacol Sci. 1998;19:85–86. doi: 10.1016/s0165-6147(98)01175-4. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Carruthers R, Rachleff I, Wattler S, Nehls M, McKinzie DL, Fienberg AA, Nomikos GG, Greengard P. Diverse psychotomimetics act through a common signaling pathway. Science (New York, NY) 2003;302:1412–1415. doi: 10.1126/science.1089681. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Tork I. Anatomy of the serotonergic system. Ann N Y Acad Sci. 1990;600:9–34. doi: 10.1111/j.1749-6632.1990.tb16870.x. discussion 34–35. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Marek GJ, Aghajanian GK, Duman RS. 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci. 1997;17:2785–2795. doi: 10.1523/JNEUROSCI.17-08-02785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Waeber C, Palacios JM. Binding sites for 5-hydroxytryptamine-2 receptor agonists are predominantly located in striosomes in the human basal ganglia. Brain Res Mol Brain Res. 1994;24:199–209. doi: 10.1016/0169-328x(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Whorton MR, Bokoch MP, Rasmussen SG, Huang B, Zare RN, Kobilka B, Sunahara RK. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle ef3ciently activates its G protein. Proc Natl Acad Sci U S A. 2007;104:7682–7687. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorton MR, Jastrzebska B, Park PS, Fotiadis D, Engel A, Palczewski K, Sunahara RK. Efficient coupling of transducin to monomeric rhodopsin in a phospholipid bilayer. J Biol Chem. 2008;283:4387–4394. doi: 10.1074/jbc.M703346200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Chien EY, Mol CD, Fenalti G, Liu W, Katritch V, Abagyan R, Brooun A, Wells P, Bi FC, Hamel DJ, Kuhn P, Handel TM, Cherezov V, Stevens RC. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330:1066–1071. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, Liu W, Thompson AA, Huang XP, Carroll FI, Mascarella SW, Westkaemper RB, Mosier PD, Roth BL, Cherezov V, Stevens RC. Structure of the human kappa-opioid receptor in complex with JDTic. Nature. 2012;485 doi: 10.1038/nature10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurmbach E, Yuen T, Ebersole BJ, Sealfon SC. Gonadotropin-releasing hormone receptor-coupled gene network organization. J Biol Chem. 2001;276:47195–47201. doi: 10.1074/jbc.M108716200. [DOI] [PubMed] [Google Scholar]
- Xia Z. The PDZ-binding domain is essential for the dendritic targeting of 5-HT2A serotonin receptors in cortical pyramidal neurons in vitro. Neuroscience. 2003;122:907–920. doi: 10.1016/s0306-4522(03)00589-x. [DOI] [PubMed] [Google Scholar]
- Xia Z, Gray JA, Compton-Toth BA, Roth BL. A direct interaction of PSD-95 with 5-HT2A serotonin receptors regulates receptor trafficking and signal transduction. J Biol Chem. 2003;278:21901–21908. doi: 10.1074/jbc.M301905200. [DOI] [PubMed] [Google Scholar]
- Yuen T, Zhang W, Ebersole BJ, Sealfon SC. Monitoring G-protein-coupled receptor signaling with DNA microarrays and real-time polymerase chain reaction. Methods Enzymol. 2002;345:556–569. doi: 10.1016/s0076-6879(02)45047-1. [DOI] [PubMed] [Google Scholar]
- Yuen E, Jiang Q, Chen P, Feng J, Yan Z. Activation of 5-HT2A/C receptors counteracts 5-HT1A regulation of NMDAR channels in pyramidal neurons of prefrontal cortex. J Biol Chem. 2008 doi: 10.1074/jbc.M801713200. [DOI] [PMC free article] [PubMed] [Google Scholar]


