CASE
A 77-year-old woman was treated with ipilimumab and nivolumab for stage 4 melanoma. Five months after treatment initiation, she developed severe generalized weakness. Lab test results revealed low free T4 of 0.7 ng/dL (0.9–1.7 ng/dL) and an inappropriately normal thyroid-stimulating hormone (TSH) of 0.62 μIU/mL (0.50–5.00 μIU/mL). Brain magnetic resonance imaging (MRI) for evaluation of central hypothyroidism showed diffuse pituitary enlargement and stalk thickening, consistent with hypophysitis (Figs. 1B, D, 2B, D), with mass effect on the optic chiasm. Baseline brain MRI had shown a normal pituitary (Figs. 1A, C, 2A, C). Levothyroxine and steroids were initiated to treat the hypothyroidism and reduce the mass effect on the optic chiasm, respectively. Hypophysitis is one of the most common endocrinopathies associated with checkpoint inhibitor therapy,1 particularly with ipilimumab, and can result in transient or permanent disruption of pituitary function.2 Hypophysitis commonly presents with headache, fatigue, weakness, and other nonspecific symptoms. The diagnosis is based on evidence of new anterior and/or posterior pituitary dysfunction. Reversible pituitary enlargement may be seen on brain MRI, but importantly, brain MRI may be normal in patients with hypophysitis. Patients should be evaluated for hypoadrenalism and hypothyroidism when hypophysitis is suspected. Treatment is supportive and includes hormone replacement.
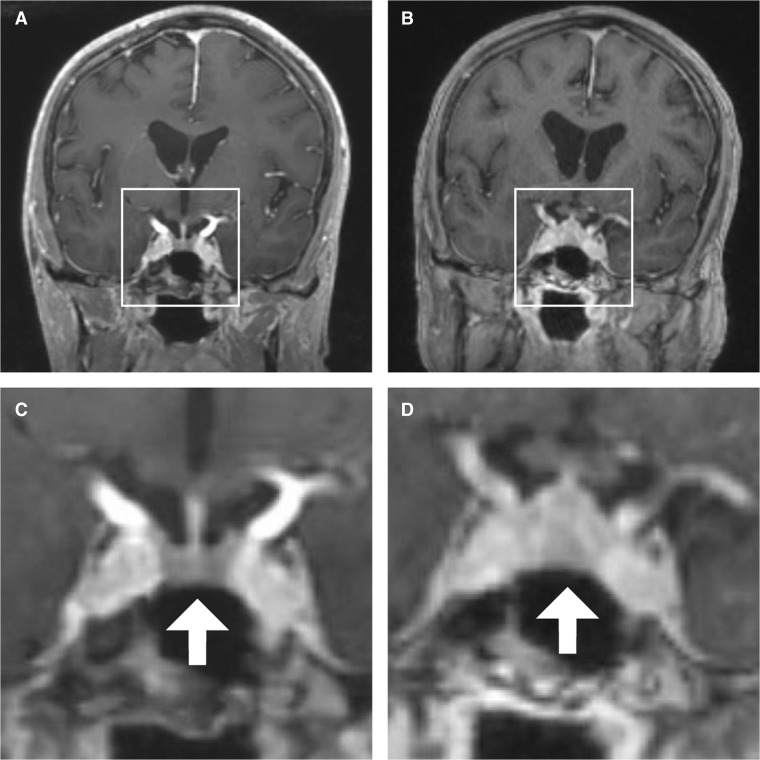
Figure 1.
Coronal views of the patient’s brain on MRI. A Before ipilimumab and nivolumab initiation. B Five months after ipilimumab and nivolumab initiation. C Inset of panel A, with a normal-sized pituitary gland (arrow) and pituitary stalk. D Inset of panel B, with diffuse enlargement of the pituitary gland (arrow) and thickening of the pituitary stalk.
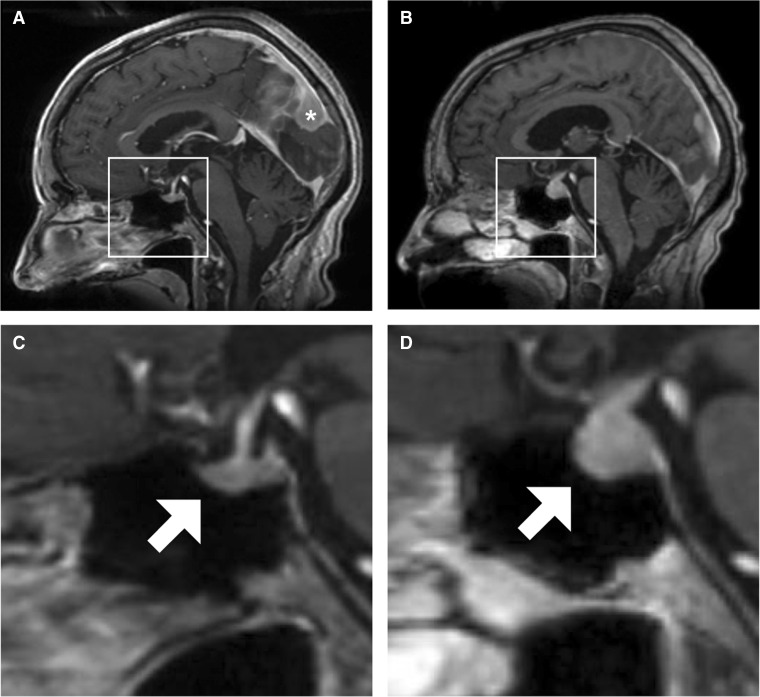
Figure 2.
Sagittal views of the patient’s brain on MRI. A Before ipilimumab and nivolumab initiation. The patient had a preexisting parafalcine meningioma (asterisk). B Five months after ipilimumab and nivolumab initiation. C Inset of panel A, with a normal-sized pituitary gland (arrow) and pituitary stalk. D Inset of panel B, with diffuse enlargement of the pituitary gland (arrow) and thickening of the pituitary stalk.
Acknowledgments
Contributors
There are no additional contributors.
Funding Sources
There are no funding sources, internal or external.
Prior Presentations
There are no prior presentations.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Joshi MN, Whitelaw BC, Palomar MT, Wu Y, Carroll PV. Immune checkpoint inhibitor-related hypophysitis and endocrine dysfunction: clinical review. Clin Endocrinol (Oxf) 2016;85:331–9. doi: 10.1111/cen.13063. [DOI] [PubMed] [Google Scholar]
- 2.Faje A. Immunotherapy and hypophysitis: clinical presentation, treatment, and biologic insights. Pituitary. 2016;19:82–92. doi: 10.1007/s11102-015-0671-4. [DOI] [PubMed] [Google Scholar]