Abstract
This study assessed the effect of whey protein substitution with isolated soy protein in protein bar (PB) formulations at 25% (PB2), 50% (PB3), or 75% (PB1) weight/weight on the proximate and mineral composition, sensory, and antidiabetic properties. Sensory evaluation was conducted within diabetic (DB) and non-diabetic (NDB) consumers by preference ranking and acceptance test. The formulations were analysed in terms of moisture, ash, protein, lipid, carbohydrates, fibers and mineral content. The consumers did not distinguish the formulations by preference ranking test. However, the acceptability test showed a rating of 9 most frequent for PB1 (36.30%), followed by PB2 and PB3 (both 34.09%), among DB consumers. The PB1 and PB3 showed higher content of total, soluble and insoluble fibers and, PB 2 presented higher carbohydrate content. Potassium, sodium and calcium showed the highest mineral content in the formulations. PB3 was assessed for glycaemic and lipidemic control in diabetics and non-diabetics female Wistar rats, for this 20% of PB was added in the ration consumed ad libitum, besides, the rats received 100 mg/kg b. w. by gavage daily. The treatment did not reduce significantly fasting glucose, lipid profile, or peripheral glucose disposal in DB or NDB rats. However, it significantly improved insulin tolerance test values in diabetic rats. The results suggest that the formulations showed good acceptance and potentially ameliorate insulin resistance both in control group and in animal model of type II diabetes.
Keywords: Sensory evaluation, Animal models of diabetes, Insulin sensitivity, Isolated soy protein, Type-2 diabetes
Introduction
According to the International Diabetes Federation (IDF) (2013), the prevalence of diabetes mellitus (DM) in South and Central American countries is estimated at 29.6 million people (9.4%) and is projected to increase to 38.5 million people by 2035. Brazil has the fourth highest prevalence of DM in the world, totalling 11.9 million diagnosed cases.
Poor nutrition is predominantly responsible for health conditions and critically affects the prevention and control of numerous chronic diseases such as obesity, DM, hypertension, and heart disease. In this sense, a balanced diet is recommended for their prevention or management, causing the demand for nutritious food increases worldwide (da Silva et al. 2014; Guevara-Cruz et al. 2012; Simmons et al. 2011).
An increasing demand for healthy and ready-to-eat foods have driven the protein bar (PB) market, particularly because these products are frequently associated with a healthy diet (Lobato et al. 2012). Due to the versatility of their formulation, bars can be supplemented with vitamins, minerals, or bioactive compounds without altering the acceptability (da Silva et al. 2014). Consequently, adding nutritional ingredients to PBs offers consumers the option to enhance the quality of their diet, especially those with type II DM.
Many phytocompounds obtained from plant or microbial metabolism has been incorporated in diabetic diet per a category of products called nutraceuticals. Nutraceuticals are defined by DeFelice (1989) as a food or part of a food that provides benefits health in addition to its nutritional content. Different studies have focused in natural products related to their alpha-glucosidase and alpha-amylase inhibition, effect on glucose uptake and glucose transporter, improvement in the insulin sensitivity, modulation of oxidative stress and others diabetic complications as well as others mechanism of action involved in glucose homeostasis (Prathapan et al. 2012; Rios et al. 2015; Sankar et al. 2015; Santini et al. 2017).
The soybeans (Glycine max(L.) Merr.), leguminous widely cultivated in Brazil, has been reported to be a functional food. In several studies involving humans and animals, soy prevented or controlled hyperglycaemia (Jayagopal et al. 2002; Zimmermann et al. 2012). In addition, some studies have provided evidence that incorporating soy into alternative foods helps reduce energy consumption and decreases glycaemic index. Besides, it provides macro and micronutrients such as high-quality protein, fibres, and various bioactive compounds that can reduce postprandial glycaemia and improves the lipid profile of individuals with dyslipidaemia (Simmons et al. 2011; Lobato et al. 2012). The antidiabetic effects of soy are related to the presence of isoflavones, particularly genistein and daidzein (Jonas et al. 1995; Fu et al. 2010; Kim and Lim 2013). However, other studies attribute this effect to proteins or to the combined consumption of proteins and isoflavones (Zimmermann et al. 2012).
In this context, the research proposal was to develop news PBs formulations with different concentrations of isolated soy protein (ISP), without sugar addition and supplemented with soy isoflavones, to conducted sensory tests with the PBs, in order to assess the most acceptable PB for effectiveness on glycaemic and lipidemic control in diabetic and non-diabetic model rats.
Materials and methods
Ingredients and reagents
The following ingredients were used to produce the PBs: ISP 90% (w/w) (free of genetically modified organisms; Bremil Food Industries, Passo Fundo, Brazil), WPC 30% (w/w) (Sooro Ingredientes, Paraná, Brazil), hydrolysed collagen (Peptiplus® SB, Gelita do Brasil, Maringá, Brazil), sucralose sweetener, citric acid anhydrous, soy isoflavones, malic acid (all PharmaNostra Insumos, Rio de Janeiro, Brazil), soy lecithin emulsion (Grings Alimentos Saudáveis, São João da Boa Vista, Brazil), 70% sorbitol solution (v/v) (PharmaNostra Insumos), vitamin E acetate oil (Via Farma, São Paulo, Brazil), PalmFat-370B (Agropalma, Belém, Brazil), and toasted soy (Só Soja do Brasil Ltda., Caldas Novas, Brazil). Other ingredients, such as NaCl, orange flavouring, glycerine, oat bran, and diet milk chocolate, were obtained from local markets.
The reagents used for proximate composition and minerals analyses were supplied by LabSynth®, Specsol® and assay kit to analyse fibre content from Sigma-Aldrich®. In addition, the following reagents were used in pharmacological test: Accu-Chek® Performa indicator strips to measure glucose (Roche, Brazil); and regular human insulin (Humulin®; Lilly, Brazil). For biochemical analyses weiner tests were supplied by M.S. Diagnóstica LTDA (Cuiabá, Brazil).
Formulations and processing
We developed three formulations (30-g each) substituting whey protein by isolated soy protein (ISP) at 25% (PB2), 50% (PB3), or 75% (PB1) weight/weight (Table 1), supplemented with soy isoflavones (44.48% w/w) as follow: different amounts of ISP and WPC were homogenised with others dry ingredients in a powder mixer, batter was made by adding dry ingredients to the wet ingredients (liquid and semi-solid), then the batter was shaped into a coated PVC container and placed overnight in freezer at −7 ± 1 °C, each PB was removed from PVC mould, wrapped and stored under refrigeration (10 ± 2 °C). 100 mg of isoflavones were homogeneously mixed in the batter, because concentration has been related with improvement in blood glucose levels between individual diabetics (Jayagopal et al. 2002; Curtis et al. 2012). To correct the isoflavones concentration to 100% we used 2.25 as correction factor.
Table 1.
Ingredients and proportions used in the formulation of 3 protein bars (PB1, PB2, and PB3)
| Formula ingredients | Function(s) | Formulas (g/100 g) | ||
|---|---|---|---|---|
| PB1 | PB2 | PB3 | ||
| Dry ingredients | ||||
| Whey protein concentrate | Source of protein | 6.66 | 20 | 13.33 |
| Isolated soy protein | Source of protein, fibre, and isoflavones | 20 | 6.66 | 13.33 |
| Oat bran | Source of fibre | 11.67 | 11.67 | 11.67 |
| Hydrolysed collagen | Source of protein and binding agent | 6.67 | 6.67 | 6.67 |
| Grains of toasted soy | Source of fibre, protein, isoflavones, and crunchiness | 3.33 | 3.33 | 3.33 |
| Sucralosea | Sweetener | 1.33 | 1.33 | 1.33 |
| Citric acid | Acidulant, preservative | 0.33 | 0.33 | 0.33 |
| Sodium chloride | Protein binding agent | 0.31 | 0.31 | 0.31 |
| Soy isoflavonesb | Bioactive substance | 0.24 | 0.24 | 0.24 |
| Malic acid | Acidulant | 0.13 | 0.13 | 0.13 |
| Liquid and semisolid ingredients | ||||
| Soy lecithin | Emulsifier | 7 | 7 | 7 |
| Sorbitol | Sweetener and humectant | 6.67 | 6.67 | 6.67 |
| Orange flavouring | Flavour and smell | 1.8 | 1.8 | 1.8 |
| Palm fat | Softness | 3.33 | 3.33 | 3.33 |
| Vitamin E | Antioxidant | 0.33 | 0.33 | 0.33 |
| Glycerine | Humectant | 0.2 | 0.2 | 0.2 |
| Diet milk chocolate | Coating | 30 | 30 | 30 |
| Total | 100 | 100 | 100 | |
Bold indicates proportion of substitution of whey protein by isolated soy protein in protein bar formulations at 25% (PB2), 50% (PB3), or 75% (PB1)
aDiluted in 1:50 parts of whey protein concentrate
b44.48% isoflavones containing 1.23% genistin, 0.14% genistein, 9.55% daidzein, 32.5% daidzein, 0.54% glycitein, and 0.52% glycitein, corrected by a factor of 2.24
Nutritional characterisation
Each formulation sample was prepared ten times, and each presentation was assessed in triplicate for moisture, ash, lipid, fibre, and protein content using a conversion factor of 6.25, according with the methods 925.09, 923.03, 920.39, 985.29 and 991.20 described in the AOAC Official Methods of Analysis, respectively (Hilrich 1990).
The efficiency of protein digestion was tested using the amino acid lysine and ammonium sulphate (NH4)2SO4 for the assessment of nitrogen loss. The carbohydrate content was estimated by subtracting the sum of moisture, ash, fibre, lipid, and protein content from 100 g. The calorie value (kcal 100 g−1) was determined by applying the Atwater conversion factors of 4 kcal g−1 for proteins and total sugars and 9 kcal g−1 for lipids (Lobato et al. 2012).
Mineral analysis
Absorption spectrometry with the Varian® Spectra AA20 spectrometer was used to analyse mineral contents under the follow experimental conditions: Fuel/oxidizing gases Acetylene/Air and Acetylene/nitrous oxide for calcium determination, spectral resolution and wave-length (0.5; 330.3 nm) for Na (1.0; 769.9 nm) K (1.0; 202.6 nm) Mg (0.2; 279.5 nm) Mn, (0.2; 248.3 nm) Fe, (1.0; 213.9 nm) Zn and (0.5;108 422.7 nm) Ca, respectively.” The quantifications were performed using external six-point analytical curve prepared in triplicate for Na (0.0–50.0 mg L−1), K (0.0–6.0 mg L−1), Ca (0.0–10.0 mg L−1), Mg (0.0–20.0 mg L−1), Fe (0.0–10.0 mg L−1), Mn (0.0–0.5 mg L−1) and Zn (0.0–3.0 mg L−1). All minerals were within the detection limit and quantification provided for the instrumental method used, except for Zn, which had a concentration below these limits. The determination coefficient (R2) for each mineral was >0.99, indicating an excellent correlation between absorbance and concentration and complying with the recommendations of the Brazilian Institute of Metrology, which suggests that R2 should be >0.90 (Ribani et al. 2004).
Acceptability
This project was submitted and approved by the Human Research Ethics Committee under opinion number 159-137, according to the Resolution 196/96 of the Brazilian National Health Council. Individuals aged 18–60 years was selected after answering a questionnaire about participants’ interest in diet and light products; habits and preferences regarding protein or cereal bars; and allergy to soy, lactose, or chocolate. Individuals with any of these allergies were excluded from the study.
The study was conducted with diabetics (DB) and non-diabetics (NDB) group of people. A total of 174 people participated in the tests (55.2% women and 44.8% men).
Preference ranking
The three different samples were presented to the participants (40 DB and 50 NDB) in containers coded with three numbers. To calculate the sum of the numbers, the participants were asked to order the samples in ascending order (1 = least preferred and 3 = most preferred) in order of preference with regard to flavour, according to the criteria established by the Brazilian National Standards Organization (1994).
Acceptance test and coefficient of concordance
At the second stage of the sensory analysis, the acceptability test was applied using a 9-point hedonic scale ranging from “extremely disliked” to “extremely liked.” For this test, 40 NDB and 44 DB were randomly selected to participate. The consistency of the mean values obtained from the ratings was assessed using the acceptability test, and the degree of dispersion of the opinions was assessed using the concordance coefficient (CC), according to Silva et al. (2010).
Pharmacological assessment
The antidiabetic effect of the PB chosen from the sensory analyses was assessed in Wistar rats (Rattus norvegicus). This study was previously approved by the Animal Ethics Committee of the Federal Institute of Mato Grosso, Campo Novo do Parecis Campus, according to Law 11.794 of 2008 (Presidency of the Republic of Brazil 2008) that regulates scientific research on animals. Forty-eight female rats (90 days old) weighing 150–200 g (Central Bioterium of UNIVAG, University Center of Várzea Grande) were housed in the animal facility at the pharmacology laboratory of this same institution at a controlled temperature of 25 ± 2 °C and relative humidity of 50 ± 5% with a 12-h light–dark cycle and ad libitum access to food and water.
Induction of type II diabetes
The rats (24) were induced with diabetes using a hyperlipidaemic diet and intraperitoneal streptozotocin using the methodology of Correia-Santos et al. (2012). The animals were provided a hyperlipidaemic diet for four weeks, on the fourth week, streptozotocin was administered intraperitoneally. Animals were considered diabetic if blood glucose levels were >200 mg/dL at 120 min in an oral glucose tolerance test (OGTT) (Correia-Santos et al. 2012).
Treatment
To test the antidiabetic potential of the PB supplemented with isoflavones, rations containing 20% of the PB were prepared together with 80% of Nuvilab® CR1 (Sogorb Indústria e Comércio Ltda, São Paulo, Brazil) ration recommended by the National Research Council and National Institutes of Health, USA. The animals received isoflavones diluted in water via gavage to ensure a daily ingestion of 100 mg/kg. The animals were divided into six groups of eight animals each that received one of the following treatments:
Group I: non-diabetic rats fed with Nuvilab® CR1 standard ration.
Group II: non-diabetic rats fed with a 20% ration of PB.
Group III: non-diabetic rats fed with a 20% ration of PB + isoflavones via gavage.
Group IV: diabetic rats fed with Nuvilab® CR1 standard ration.
Group V: diabetic rats fed with a 20% ration of PB,
Group VI: diabetic rats fed with a 20% ration of PB + isoflavones via gavage.
The animals undergone 30 days of treatment, with food and water ad libitum and isoflavones via gavage for groups III and VI. The non-diabetics were referred to as controls and the experimental groups were coded as untreated controls (C; Group I), controls treated with a modified ration (CTR; Group II), controls treated with a modified ration and isoflavones (CTRI; Group III), untreated diabetic controls (DC; Group IV), diabetics treated with a modified ration (DTR; Group V) and diabetics treated with a modified ration and isoflavones (DTRI; Group VI).
Oral glucose tolerance
After 30 days of treatment, the rats underwent an OGTT. Following a 15-h fast, the rats were anesthetized with intramuscular administration of xylazine (10 mg/kg) and ketamine (80 mg/kg). With analgesia confirmed, the first blood sample was taken from a single cut at the extremity of the animal’s tail (T0). The animals were then administered 20% dextrose (2 g/kg body weight) via gavage. Blood samples were collected 15, 30, 60, 90, and 120 min after the glucose overload. The blood glucose levels were determined using test strips (Accu-Check® Performa, Roche, São Paulo, Brazil), and the results were analysed by determining the areas under the curves (AUC) of serum glucose using the trapezoidal method in an Excel® spreadsheet.
Insulin tolerance
Forty-eight hours after the OGTT, an insulin tolerance test (ITT) was conducted. Before the test, the animals were fasted for 6 h, and baseline blood glucose levels were determined. Subsequently, standard insulin was administered intraperitoneally (1.5 U/kg p.c.). The clearance rate of glucose in plasma (t½) was calculated from the slope of the least squares analysis of the plasma glucose concentrations (as measured using test strips) 5–20 min after the insulin was injected (Accu-Check® Performa, Roche). ITT was calculated using the following formula: (0.693/t½) × 100.
Lipid profile
Upon completion of the experiments, the animals were anesthetized with xylazine (10 mg/kg) and ketamine (80 mg/kg) to collect blood from the vena cava into coagulant-free Vacutainer® tubes to determine TC, HDL, LDL, VLDL, and TG in an automated Wiener lab biochemical analyser (Model BT 3000/CB 350i; Wiener Lab Group, Rosario, Argentina). Animals were then euthanized with an overdose of xylazine and ketamine.
Experimental design and data analysis
The three PB formulations were analysed using a completely randomised design with 10 repetitions for each treatment for the proximate composition analyses. For the pharmacological assay, a completely randomised design of six treatments was used, each with eight repetitions and each animal representing an experimental unit.
All results of the proximate composition analysis of the PBs and of the pharmacological assays (OGTT, ITT, fasting glucose, and lipid profile) were statistically analysed using analysis of variance (ANOVA), with significance of the F test set at 5% probability; the Tukey–Kramer post hoc test was used to identify differences across the means. The results for the sensory analysis from the preference ranking test were assessed using the Friedman test, using the table by Newell and McFarlane, at a significance level of 5% (Brazilian National Standards Organization 1994). The results obtained from the 9-point hedonic scale were assessed using the frequency distribution of the points. The CCs of these ratings were assessed using the Consensor® program. All statistical analyses were conducted using GraphPadPrism® 5.0 software.
Results and discussion
Nutritional characterisation of protein bars
Proximate composition
PBs had a high protein content, as established in Resolution 54 by the National Health Surveillance Agency (ANVISA) (2012), because both formulations had 15–18 g protein/50 g PB, representing 20–24% of the daily recommended intake (DRI) for protein. Moreover, all formulations were defined as having a high fibre content, because they contained 11–13.5 g fibre/50 g PB (National Health Surveillance Agency, 2003). All formulations had a low calorific value, which was defined as 101–149 kcal/PB per Mahanna et al. (2009); a 30-g portion of PB1, PB2, or PB3 had a calorific value of 117.63, 123.26, and 121.07 kcal/PB, respectively.
There were no significant differences in lipid content among the formulations. There was no difference in soluble, insoluble, or total fibre between PB1 and PB3, but PB2 was significantly different from other. All formulations were different from one another with regard to carbohydrate content, PB1 showed the lowest value (9.11%). There was no significant difference in protein content between PB2 (31.38%) and PB3 (33.33%), but PB1 (37.42%) showed significantly greater than other two (Table 2).
Table 2.
Means of proximate composition (% wet weight) and mineral content of formulations of 3 protein bars (PB1, PB2, and PB3)
| Constituents (%) | Formula | ||
|---|---|---|---|
| PB1 | PB2 | PB3 | |
| Moisture | 1.2480 ± 0.10a | 0.9122 ± 0.16b | 0.9689 ± 0.16b |
| Ash | 2.4679 ± 0.25a | 2.8787 ± 0.18b | 2.7303 ± 0.15b |
| Protein (n × 6.25) | 37.42 ± 3.75a | 31.38 ± 3.08b | 33.11 ± 4.31b |
| Lipid | 22.88 ± 2.01a | 22.98 ± 1.84a | 23.94 ± 2.42a |
| Total fibre | 26.86 ± 0.70a | 22.21 ± 0.44b | 25.40 ± 0.37a |
| Soluble fibre | 23.24 ± 0.70a | 20.13 ± 0.44b | 21.96 ± 0.37a |
| Insoluble fibre | 3.62 ± 0.11a | 2.07 ± 0.11b | 3.43 ± 0.04a |
| Carbohydratea | 9.11 ± 1.07a | 19.53 ± 0.78b | 13.79 ± 0.71c |
| Macroelements | |||
| Sodium | 168.53 ± 1.64a | 160.02 ± 3.53a | 160.56 ± 1.22a |
| Potassium | 171.72 ± 0.9a | 190.54 ± 6.25b | 156.63 ± 3.91a |
| Magnesium | 21.68 ± 0.44a | 22.64 ± 1.15a | 20.86 ± 1.8a |
| Calcium | 86.76 ± 3.89a | 124.04 ± 4.34b | 99.34 ± 2.31a |
| Microelements | |||
| Manganese | 0.034 ± 0.008a | 0.030 ± 0.003a | 0.035 ± 0.007a |
| Iron | 1.44 ± 0.11a | 1.06 ± 0.09a | 1.47 ± 0.14a |
| Zinc | ≥QLb | ≥QL | ≥QL |
The results are expressed as mean ± standard deviation. Means with the same letters on the same line did not significantly differ between one another, as determined using Tukey’s test with a 5% significance level
aThe carbohydrate content was estimated by subtracting the sum of moisture, ash, fibre, lipid, and protein content from 100 g
bLess than the quantification limit (QL) ± standard error
PB1 had the highest content of protein and fibre, and these was directly associated with higher ISP content in this formulation. ISP consisted of approximately 20% fibre, of which 40–50% was soluble fibre; soluble fibre lowered LDL levels (Moraes et al. 2009). The higher concentration of ISP compared to WPC, which was composed of 30% protein and 70% other micronutrients and macronutrients, explained the higher carbohydrates content in PB1. PB2 had higher carbohydrates because of the presence of lactose and disaccharides, which were present at lower concentration in PB1.
The formulations in this study differed from existing bars available in market because they contained oatmeal as one of the fibre sources and were supplemented with soy isoflavones. Moreover, to meet the needs of those with a sugar-related metabolic disorder, such as DM, the bars did not contain added simple sugars, as recommended by the National Health Surveillance Agency (ANVISA) in Ordinance 29 (1998) for foods intended for controlled sugar intake diets.
Mineral analysis
The mineral content in the developed formulations originated from the raw materials used in their production, primarily WPC, ISP, and oatmeal (Avena sativa L.), i.e., there was no mineral or vitamin supplementation in the PBs, except vitamin E (tocopherol), which acted as an antioxidant in the formulations. Other ingredients in the bars did not significantly contribute to the mineral content (Table 2).
The three PB formulations were statistically equal with regard to Fe, Mg, Mn, and Na content (Tukey’s test, p < 0.005). However, PB2 contained higher Ca (124.30 mg/30 g), approximately 25% more than that of the other formulations, and more K (190.54 mg/30 g). This may be due to the higher WPC content, which was the ingredient with the highest Ca content, at 140.42 mg/100 g and K content of 1132 mg/100 g (Soral-Smietana et al. 2013). Ca and K are essential to maintain the health and homeostasis of organic functions, such as muscular contractions, heartbeat, insulin secretion, and nerve synapses.
The DRIs in the formulations were approximately 10% for Ca and 8% for Mg. The DRI for Mn was only 1.3%, as designated in Resolution 269 of 2005, which regulates the DRI for proteins, minerals, and vitamins (National Health Surveillance Agency 2005). The DRI for Na was only 6.7%, as designated in Resolution 360 of 2003 (National Health Surveillance Agency 2003).
Sensory analysis
Preference ranking
The Brazilian Association of Technical Standards states that a difference in preference among samples at a significance of 5% requires a difference between the sums of each sample pair of ≥21 for DB and ≥24 for NDB participants (Brazilian National Standards Organization 1994). When the two groups are combined, the difference must be ≥32. As shown in Table 3, there were no significant differences in flavour among the samples or among the different participant profiles, even when the two groups were combined. Thus, a structured acceptability test needed to be conducted as a follow-up test. The participants did not distinguish the sample with a high concentration of ISP from that with a low concentration, the formulations were capable of masking the rather unpleasant taste of soy, even with a high concentration of ISP. Paul et al. (2008) taste-tested a food product based on soy and powdered milk targeted at infants and concluded that the presence of milk did not influence the acceptance of soy, indicating that there was no significant difference between foods containing both soy and milk and those containing only soy. In addition, Bedani et al. (2013) verified that okara, a soybean-derived compound, did not affect the acceptance of different formulas of yogurt containing 0 or 50 g/L of okara. Based on these results, different soy-based foods with a reduced sugar content have been developed to increase consumption of healthy foods (Lobato et al. 2012; Pandey and Singh 2011; Romanchik-Cerpovicz et al. 2011) or even to provide alternatives for lactose intolerant individuals (Pandey and Singh 2011), given that such food items have been well accepted by these individuals.
Table 3.
Differences among the ranking preference for the flavour of the 3 protein bars (PB1, PB2, and PB3)
| Formulations | |||||
|---|---|---|---|---|---|
| Differences | PB2–PB1 | PB3–PB2 | PB3–PB1 | PB2–PB1 | PB1–PB3 |
| Diabetic | 5 | – | – | 8 | 3 |
| Non-diabetic | 10 | 11 | 21 | – | – |
| Combined data | 15 | 3 | 18 | – | – |
Differences were determined by subtracting the respective sums (p ≤ 0.05)
Acceptability
There were no significant difference in the preference ranking test, an acceptability test was conducted to assess the overall quality of the PBs.
The frequency of points with the NDB participants for each of the three formulations revealed that a rating of 7 was the most frequent (30%) for PB2; ratings of 6, 7, and 8 were the most frequent (20%) for PB1; and ratings of 8 (25%) and 9 (17.5%) were the most frequent for PB3. Among the DB participants, a rating of 9 was the most frequent for PB1 (36.3%), followed by PB2 and PB3 (both 34.09%). When the data were combined for both groups, a rating of 9 was more frequent (26.2%) for PB3 than for PB1 (25%).
To assess the consistency of the ratings for each sample, it was necessary to assess the degree of concordance between the participants; according to Silva et al. (2010), a higher CC indicates less dispersion of opinions. PB3 had the highest CC between the DB and NDB participants. Pessoa et al. (2011) verified that the highest CCs were found for the highest ratings, confirming that the best sample has the highest CC. Accordingly, PB3 was the most accepted formulation by the participants, as it had both the highest frequency of ratings of 9 and the highest CC. In this way, the PB3 was chosen to be assessed on the pharmacological tests.
A prospective study by the Institute of Food Technology in partnership with the Federation of Industries of the State of São Paulo (2010) revealed that the Brazilian population sought products based on taste and pleasure rather than health and well-being. This highlights the importance of taste-testing food before, during, and after developing a formulation, because the population is increasingly requiring improved overall food quality as well as a better nutritional and functional profile offered by the food product.
Therefore, the product taste-tested in the present study possessed the two main attributes currently sought by consumers, healthiness and taste, given that soy-based foods are becoming more accepted by consumers. Romanchik-Cerpovicz et al. (2011) assessed the acceptance of formulations of peanut butter cookies containing different amounts of soy, and a good level of acceptance was obtained for formulations with soy flour substitutions of 25, 50, and 75%. In contrast, Shogren et al. (2006) showed that the flavour of spaghetti fortified with soy was negatively affected when fortification exceeded 35% w/w.
The present study assessed formulations containing 20, 13.33, and 6.66% ISP, and all formulations had good acceptance; the 13.33% ISP formulation had the highest average acceptance and highest CC. However, the increase in acceptance of these formulations could not be attributed to the increase or decrease in soy concentration, because the PB1 formulation, with 20% ISP, had the second highest average in the acceptability test.
Pharmacological tests
Oral glucose and insulin tolerance tests
A number of human studies have demonstrated the benefit of consuming soy proteins or isoflavones for the control of type II DM and its complications, such as atherosclerosis, nephritis, and cardiovascular diseases (Guevara-Cruz et al. 2012). Animals with induced type II DM has also been used to primarily test the effect of soy and isolated aglycones on the glycaemic profile (Fu et al. 2010; Zimmermann et al. 2012). In this study, animals provide with diet containing 20% of PB3 incorporated in the standard ration treated or not treated with isoflavone via gavage and control group provided with only standard ration did not show significance difference in OGTT (p ≥ 0.05) among the experimental groups. However, with a 20% intake of PB and isoflavones, the AUC, which indicates the glycaemic response, was 32.9 and 11.6% lower in the DTRI group compared with the DTR and DC groups, respectively, and was 24.1% lower in the DTR group compared with the DC group (Fig. 1). In the control groups (non-diabetic), the AUC was 0.30 and 0.48% lower for the CTRI group than the C and CTR groups, respectively. In addition, the AUC in the CTR group was 0.19% greater than the C group. None of these results was statistically significant (p ≥ 0.05; Fig. 1).
Fig. 1.
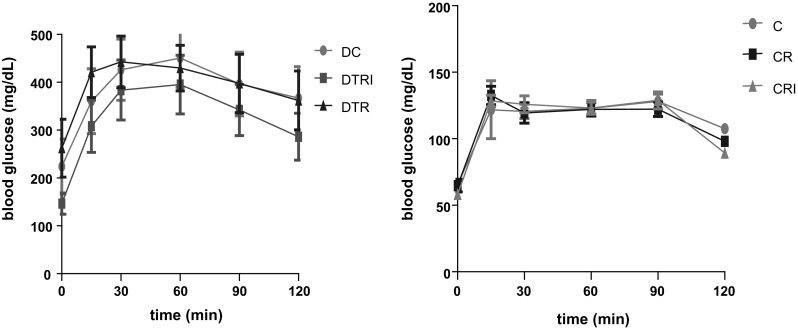
Oral glucose tolerance test and area under the curve of serum glucose 30 days after treatment in rats in the diabetic and control groups. Diabetic control (DC, n = 8), diabetic ration + isoflavone (DTRI, n = 8), and diabetic group with a modified ration group (DTR, n = 8), control group (C, n = 8), control group with a modified ration (CR, n = 8), and control group with a modified ration + isoflavones (CRI, n = 8). The differences between the means were determined using ANOVA and Tukey’s test with significance set at p ≤ 0.05. There were no significant differences among the diabetic groups
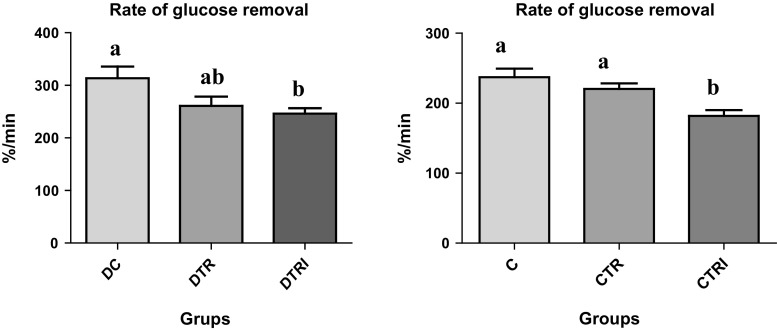
The DTRI group showed the highest rate of serum glucose removal (p ≤ 0.05) in the ITT compared with the DC and DTR groups (Fig. 2). Serum glucose removal also differed between the C and CTRI groups (p ≤ 0.01) and between the CTR and CTRI groups (p ≤ 0.05), indicating that consumption of isoflavones and ISP is associated with improved insulin sensitivity in tissues.
Fig. 2.
Insulin tolerance test 32 days after treatment in rats in the diabetic control group (DC, n = 8), diabetic group with a modified ration + isoflavones (DTRI, n = 8), diabetic group with a modified ration group (DTR, n = 8), control group (C, n = 8), control group with a modified ration (CR, n = 8), and control group with a modified ration + isoflavones (CRI, n = 8). The difference among the means was determined using ANOVA and Tukey’s test, with significance set at p ≤ 0.05. Different letters indicate a significant difference among groups
The AUC for glucose indicates that the effect of ISP on blood glucose levels depends on the isoflavone content of soy. However, Roblet et al. (2014) assessed the in vitro hypoglycaemic effect of ISP peptides obtained by electrodialysis without isoflavones and showed increased glucose uptake by muscle cells. This indicates that soy peptides and isoflavones might have synergistic effects.
Lavigne et al. (2000) showed that the consumption of soy protein (13.9 ± 0.6 mg/kg) for 28 days by Wistar rats improved glucose tolerance and insulin sensitivity. In other hand Sankar et al. (2015) found that soy isoflavones extract (150 mg/kg body weight/day) ameliorate insulin resistance in ovariectomized and high fat fed Wistar rats by a significant increase of expression of glucose transporter (GLUT4) and insulin receptor β (IRβ) proteins in hepatic, renal and adipose tissues.
Jayagopal et al. (2002) and Villa et al. (2009) showed that blood glucose levels decreased following dietary supplementation with isoflavones, suggesting a correlation between isoflavone consumption and decreased blood glucose levels in ovariectomized and control rats with high-fat diet-induced insulin resistance. Consumption of a raw extract of soy isoflavones for nine weeks decreased the AUC after intraperitoneal injection of glucose (Zhang et al. 2014). Moreover, supplementing the diet of diabetic mice with genistein had a protective effect on the kidneys, preventing diabetic nephropathy by regulating hyperglycaemia-induced oxidative stress and inflammation (Kim and Lim 2013). Studies with human volunteers also assessed the antidiabetic effectiveness of soy proteins and soy isoflavones. For example, Liu et al. (2010) showed no effect on glycaemic control or insulin resistance in Chinese menopausal pre-diabetic women treated for three or six months with soy protein (15 g) alone or supplemented with 100 mg of isoflavones. However, another study showed that postmenopausal diabetic women who consumed isoflavone aglycones (100 mg/day) for one year had improved peripheral insulin sensitivity and lipid profile, thus reducing the risk of cardiovascular disease (Curtis et al. 2012).
Jayagopal et al. (2002) reported similar results over a shorter period of time with postmenopausal diabetic women; plasma insulin, insulin resistance, glycated haemoglobin, and LDL decreased following consumption of 30 g soy protein containing 132 mg conjugated isoflavones for 12 weeks.
The mechanisms underlying the effects of isoflavones, particularly genistein, are still not fully understood. Studies have verified that genistein is a strong inhibitor of tyrosine kinases, including insulin receptors. Jonas et al. (1995) and Fu et al. (2010) showed that binding of genistein to the insulin receptor results in the intracellular accumulation of the signalling compounds cyclic adenosine monophosphate and Ca2 and to the activation of protein kinases A and C, both of which are intimately linked to pancreatic insulin synthesis and secretion.
However, Villa et al. (2009) suggested that the isoflavone-induced decrease in baseline glucose levels might be partially explained by the increase in peripheral glucose uptake, but that other mechanisms may also be involved, such as inhibition of glucose absorption by the brush-border intestinal cells through competition with glycones by β-glycosidases. Paradoxically, Zimmermann et al. (2012) showed that genetically diabetic mice (db/db) that were given soy-based diets with different concentrations of isoflavones had reduced hyperglycaemia and symptoms of type II DM, regardless of the isoflavone concentration. They also demonstrated that aglycones per se were not effective in the treatment of DM. Another study also showed that the daily consumption of isoflavones (132 mg) for three months had no effect on plasma glycated haemoglobin, insulin, or lipids in postmenopausal women with type II DM, suggesting that other soy compounds might be responsible for the health benefits observed in previous studies (González et al. 2007). This supports the theory that other soy compounds such as ISP are required to maximize the effect for decreased blood glucose levels.
Lipid profile and fasting glucose
The hypolipidaemic effect of ISP has been recognized in several countries; in Brazil, ANVISA approves this claim on food packaging labels if the portion size has ≥25 g ISP. The present study showed no significant improvements (p ≥ 0.05) in the lipid profile of diabetic or control rats. Nonetheless, in the DTRI group, TC was 23.65 and 12.75% lower and TG was 18.51 and 15.84% lower than in the DC and DTR groups, respectively.
There was also no significant difference in fasting glucose (Table 4); however, in the DTRI, group, fasting glucose was 30.1 and 14.6% lower than in the DC and DTR groups, respectively.
Table 4.
Assessment of biochemical parameters in control and diabetic rats, treated or untreated with 20% protein bar and isoflavones (100 mg/kg)
| Parameters (mg/dL) | Controls | Diabetics | Reference valuesa (mg/dL) | ||||
|---|---|---|---|---|---|---|---|
| C | CTR | CTRI | DC | DTR | DTRI | ||
| TC | 39.8 ± 0.67 | 34.87 ± 1.15 | 32 ± 1.85 | 42.28 ± 2.35 | 37 ± 2.87 | 32.28 ± 1.75 | 37–85 |
| HDL | 23.9 ± 1.23 | 22 ± 1.0 | 20.71 ± 1.58 | 23.57 ± 1.19 | 22.14 ± 1.86 | 24.14 ± 1.77 | – |
| LDL | 8.3 ± 0.84 | 4.87 ± 0.61 | 5.57 ± 0.60 | 6.14 ± 1.28 | 3.42 ± 1.06 | 2.85 ± 1.00 | – |
| VLDL | 7.6 ± 1.15 | 8 ± 0.88 | 5.71 ± 1.04 | 12.57 ± 1.39 | 13.57 ± 2.68 | 8.57 ± 1.42 | – |
| TG | 41.1 ± 2.04 | 36.25 ± 0.99 | 33.14 ± 1.01 | 54 ± 5.22 | 52.28 ± 3.52 | 44 ± 4.65 | 24–114 |
| FG | 65.5 ± 1.4 | 65.6 ± 3.1 | 63.5 ± 4.0 | 155.1 ± 47.7 | 132.4 ± 31.9 | 108.4 ± 13.9 | 92–138 |
The results are expressed as mean ± standard error. The difference among the means was determined using Tukey’s test, with significance set at p ≤ 0.05. There were no significant differences among the control and diabetic groups (ANOVA)
C control group, CTR control group with a modified ration, CTRI control group with a modified ration + isoflavones, DC diabetic control group, DTR diabetic group with a modified ration, DTRI diabetic group with a modified ration + isoflavones, TC total cholesterol, TG triacylglycerols, FG fasting glucose, HDL high-density lipoprotein, LDL low-density lipoprotein, VLDL very low-density lipoprotein
a Giknis and Clifford (2008)
In a study by Lobato et al. (2012), bars with a high concentration of soy protein and isoflavones were developed and assessed for use in the control of dyslipidaemia. Patients with dyslipidaemia who consumed the bars for 45 days experienced a moderate beneficial effect, including increased HDL and decreased TG; these results are consistent with those of the present study.
Although not significant, the present study showed an improvement in the lipid profile. The use of this PB by individuals with diabetes is validated based on the fact that diabetic complications include hyperlipidaemia associated with hyperglycaemia-induced inflammation.
Incorporating ISP into PB formulations improved the nutritional profile of PBs, providing the bars with a high protein and fibre content and low simple sugar content; these PBs can be incorporated into the diet of individuals with diabetes. In addition, higher concentrations of ISP did not affect the preference of PBs, as demonstrated by the lack of significant differences across formulations.
All formulations had higher frequencies of scores of 7, 8, and 9 in the acceptability test, indicating that it is possible to develop a formulation based on soy derivatives without the need to add simple sugars and supplemented with well-accepted isoflavones that will be accepted for taste by both individuals with diabetes as well as healthy individuals.
Although the results of OGTT were not statistically significant in the diabetic rats, supplementing the diet with PBs based on ISP and isoflavones tended to ameliorate tolerance to glucose in tissue cells. In addition, the ITT results in both the diabetic and non-diabetic control rats indicate that PBs supplemented with soy isoflavones improved insulin sensitivity in obese, diabetic rats.
In summary, our findings support that formulations comprising ISP and soy isoflavones can attenuate or prevent glucose metabolism disorder in animal models. Information gained from the present study provides valuable insights for future direction of research exploring the effects of soy compounds for the prevention and management of type II diabetes.
Acknowledgements
We thank the National Council for Scientific and Technological Development (CNPq) and the Research Support Foundation of Mato Grosso (FAPEMAT) for financial support. We also thank the Federal Institute of Mato Grosso, Bela Vista Campus, and the Laboratory of Instrumental Analysis at the Federal University of Mato Grosso and University Centre of Várzea Grande for providing the infrastructure required to conduct this study.
Abbreviations
- ANOVA
Analysis of variance
- ANVISA
Brazilian Health Surveillance Agency
- AUC
Area under the curve
- C
Control group
- Ca
Calcium
- CC
Concordance coefficient
- CTR
Control group with a modified ration
- CTRI
Control group with a modified ration + isoflavones
- D/V
Daily value
- DB
Diabetic individuals
- DC
Diabetic control group
- DL
Detection limit
- DM
Diabetes mellitus
- DRI
Daily recommended intake
- DTR
Diabetic group with a modified ration
- DTRI
Diabetic group with a modified ration + isoflavones
- Fe
Iron
- GLUT4
Glucose transporter
- HDL
High-density lipoprotein
- IRβ
Insulin receptor β
- ISP
Isolated soy protein
- ITT
Insulin tolerance test
- K
Potassium
- LDL
Low-density lipoprotein
- Mg
Magnesium
- Mn
Manganese
- Na
Sodium
- NDB
Non-diabetic individuals
- OGTT
Oral glucose tolerance test
- PB
Protein bar
- QL
Quantitation limit
- R2
Linear determination coefficient
- STZ
Streptozotocin
- TC
Total cholesterol
- TG
Triacylglycerol
- VLDL
Very low-density lipoprotein
- WPC
Whey protein concentrate
- Zn
Zinc
References
- Bedani R, Campos MM, Castro IA, Rossi EA, Saad SM. Incorporation of soybean by-product okara and inulin in a probiotic soy yoghurt: texture profile and sensory acceptance. J Sci Food Agric. 2013;94:119–125. doi: 10.1002/jsfa.6212. [DOI] [PubMed] [Google Scholar]
- Brazilian National Standards Organization (ABNT) (1994) Nbr 13170: Sensory analysis—methodology ranking test—procedure: Rio de Janeiro, Brasil
- Correia-Santos AM, Suzuki A, Anjos JS, Rêgo TS, Almeida KCL, Boaventura GT. Indução de diabetes tipo II por dieta hiperlipídica e baixa dose de streptozotocina em ratos Wistar. Medicina. 2012;45(4):436–444. [Google Scholar]
- Curtis PJ, Sampson M, Potter J, Dhatariya K, Kroon PA, Cassidy A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: a 1-year, double-blind, randomized, controlled trial. Diabetes Care. 2012;35:226–232. doi: 10.2337/dc11-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva EP, Siqueira HH, do Lago RC, Rosell CM, Vilas Boas EV. Developing fruit-based nutritious snack bars. J Sci Food Agric. 2014;94:52–56. doi: 10.1002/jsfa.6282. [DOI] [PubMed] [Google Scholar]
- DeFelice SL (1989) The nutraceutical revolution: fuelling a powerful, new international market. Harvard University Advanced Management Program in Biomedical Research and Development, Como, Italy
- Federation of Industries of the State of São Paulo (2010) Brasil Food Trends 2020. São Paulo: FIESP. http://www.brasilfoodtrends.com.br/Brasil_Food_Trends/index.html. Accessed 5 Aug 2016
- Fu Z, Zhang W, Zhen W, Lum H, Nadler J, Bassaganya-Riera J, Jia Z, Wang Y, Misra H, Liu D. Genistein induces pancreatic beta-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology. 2010;151:3026–3037. doi: 10.1210/en.2009-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giknis MLA, Clifford CB (2008) Clinical laboratory parameters for crl: WI (Han) rats. http://www.criver.com. Accessed 19 Oct 2016
- González S, Jayagopal V, Kilpatrick ES, Chapman T, Atkin SL. Effects of Isoflavone dietary supplementation on cardiovascular risk factors in type 2 diabetes. Diabetes Care. 2007;30(7):1871–1873. doi: 10.2337/dc06-1814. [DOI] [PubMed] [Google Scholar]
- Guevara-Cruz M, Tovar AR, Aguilar-Salinas CA, Medina-Vera I, Gil-Zenteno L, Hernández-Viveros I, López-Romero P, Ordaz-Nava G, Canizales-Quinteros S, Guillen Pineda LE, Torres N. A dietary pattern including nopal, chia seed, soy protein, and oat reduces serum triglycerides and glucose intolerance in patients with metabolic syndrome. J Nutr. 2012;142:64–69. doi: 10.3945/jn.111.147447. [DOI] [PubMed] [Google Scholar]
- Hilrich K. AOAC: official methods of analysis. 15. Virginia: Association of Official Analytical Chemists Inc; 1990. [Google Scholar]
- International Diabetes Federation (2013) IDF diabetes. (6th edition). http://www.idf.org/sites/default/files/Atlas-poster-2014_EN.pdf. Accessed 5 Aug 2016
- Jayagopal V, Albertazzi P, Kilpatrick ES, Howarth EM, Jennings PE, Hepburn DA, Atkin SL. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care. 2002;25:1709–1714. doi: 10.2337/diacare.25.10.1709. [DOI] [PubMed] [Google Scholar]
- Jonas JC, Plant TD, Gilon P, Detimary P, Nenquin M, Henquin JC. Multiple effects and stimulation of insulin secretion by the tyrosine kinase inhibitor genistein in normal mouse islets. Br J Pharmacol. 1995;114:872–880. doi: 10.1111/j.1476-5381.1995.tb13285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Lim Y. Protective effect of short-term genistein supplementation on the early stage in diabetes-induced renal damage. Mediat Inflamm. 2013;2013:1–14. doi: 10.1155/2013/510212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne C, Marette A, Jacques H. Cod and soy proteins compared with casein improve glucose tolerance and insulin sensitivity in rats. Am J Physiol Endocrinol Metabol. 2000;278:E491–E500. doi: 10.1152/ajpendo.2000.278.3.E491. [DOI] [PubMed] [Google Scholar]
- Liu ZM, Chen YM, Ho SC, Ho YP, Woo J. Effects of soy protein and isoflavones on glycemic control and insulin sensitivity: a 6-mo double-blind, randomized, placebo-controlled trial in postmenopausal Chinese women with prediabetes or untreated early diabetes. Am J Clin Nutr. 2010;91:1394–1401. doi: 10.3945/ajcn.2009.28813. [DOI] [PubMed] [Google Scholar]
- Lobato LP, Iakmiu Camargo Pereira AE, Lazaretti MM, Barbosa DS, Carreira CM, Mandarino JM, Grossmann MV. Snack bars with high soy protein and isoflavone content for use in diets to control dyslipidaemia. Int J Food Sci Nutr. 2012;63:49–58. doi: 10.3109/09637486.2011.596148. [DOI] [PubMed] [Google Scholar]
- Mahanna K, Moskowitz HR, Lee SY. Assessing consumer expectations for food bars by conjoint analysis. J Sens Stud. 2009;24:851–870. doi: 10.1111/j.1745-459X.2009.00241.x. [DOI] [Google Scholar]
- Moraes CL, Pastore GM, Sato HH, Park YK. Soy isoflavones and biological activities. São Paulo: Livraria Varela; 2009. [Google Scholar]
- National Health Surveillance Agency (ANVISA) (1998) Ordinance no 29, Food for special purposes. Brazil: Federative Republic of Brazil
- National Health Surveillance Agency (ANVISA) (2003) Resolution—RDC No 360 of December 23, 2003, Approval of the technical regulation on nutritional labeling of packaged foods, making nutrition labeling mandatory. Brazil: Federative Republic of Brazil http://portal.anvisa.gov.br/wps/content/Anvisa+Portal/Anvisa/Inicio/Alimentos/Publicacao+Alimentos/Rotulagem+de+Alimentos+2. Accessed 5 Aug 2016
- National Health Surveillance Agency (ANVISA) (2005) RDC No 269 of September 22, 2005: Technical regulation on the recommended daily intake (RDI) of protein, vitamins and minerals. Brazil: Federative Republic of Brazil
- Pandey A, Singh G. Development and storage study of reduced sugar soy containing compound chocolate. J Food Sci Technol. 2011;48:76–82. doi: 10.1007/s13197-010-0136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul KH, Dickin KL, Ali NS, Monterrosa EC, Stoltzfus RJ. Soy- and rice-based processed complementary food increases nutrient intakes in infants and is equally acceptable with or without added milk powder. J Nutr. 2008;138:1963–1968. doi: 10.1093/jn/138.10.1963. [DOI] [PubMed] [Google Scholar]
- Pessoa T, do Amaral DS, Duarte MEM, Cavalcanti MERM, Gurjão FF. Avaliação sensorial de goiabas passas obtida por técnicas combinadas de desidratação osmótica e secagem. Holos. 2011;4:127–147. doi: 10.15628/holos.2011.638. [DOI] [Google Scholar]
- Prathapan A, Krishna MS, Nisha VM, Sundaresan A, Raghu KG. Polyphenol rich fruit pulp of Aegle marmelos (L.) Correa exhibits nutraceutical properties to down regulate diabetic complications—An in vitro study. Food Res Int. 2012;48(2):690–695. doi: 10.1016/j.foodres.2012.06.008. [DOI] [Google Scholar]
- Presidency of the Republic of Brazil (2008) Law No. 11.794, of October 8, 2008. http://www.planalto.gov.br/ccivil_03/_Ato2007-2010/2008/Lei/L11794.htm. Accessed 5 Aug 2016
- Ribani M, Grespan Bottoli CB, Collins CH, Fontes Jardim ICS, Costa Melo LF. Validation for chromatographic and electrophoretic methods. Quim Nova. 2004;27:771–780. doi: 10.1590/S0100-40422004000500017. [DOI] [Google Scholar]
- Rios JL, Francini F, Schinella GR. Natural products for the treatment of type 2 diabetes mellitus. Planta Med. 2015;81:975–994. doi: 10.1055/s-0035-1546131. [DOI] [PubMed] [Google Scholar]
- Roblet C, Doyen A, Amiot J, Pilon G, Marette A, Bazinet L. Enhancement of glucose uptake in muscular cell by soybean charged peptides isolated by electrodialysis with ultrafiltration membranes (EDUF): activation of the AMPK pathway. Food Chem. 2014;147:124–130. doi: 10.1016/j.foodchem.2013.09.108. [DOI] [PubMed] [Google Scholar]
- Romanchik-Cerpovicz JE, Abbott AE, Dent LA. Sensory evaluation ratings and moisture contents show that soy is acceptable as a partial replacement for all-purpose wheat flour in peanut butter graham crackers. J Am Diet Assoc. 2011;111:1912–1916. doi: 10.1016/j.jada.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Sankar P, Zachariah B, Vickneshwaran V, Jacob SE, Sridhar MG. Amelioration of oxidative stress and insulin resistance by soy isoflavones (from Glycine max) in ovariectomized Wistar rats fed with high fat diet: The molecular mechanisms. Exp Geront. 2015;63:67–75. doi: 10.1016/j.exger.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Santini A, Tenore GC, Novellino E. Nutraceuticals: a paradigm of protective medicine. Eur J Pharm Sci. 2017;96:53–61. doi: 10.1016/j.ejps.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Shogren RL, Hareland GA, Wu YV. Sensory evaluation and composition of spaghetti fortified with soy flour. J Food Sci. 2006;71:S428–S432. doi: 10.1111/j.1750-3841.2006.00061.x. [DOI] [Google Scholar]
- Silva FAS, Duarte MEM, Cavalcanti-Mata MERM. New methodology for data interpretation of food sensorial analysis. Eng Agríc. 2010;30(5):967–973. doi: 10.1590/S0100-69162010000500018. [DOI] [Google Scholar]
- Simmons AL, Miller CK, Clinton SK, Vodovotz Y. A comparison of satiety, glycemic index, and insulinemic index of wheat-derived soft pretzels with or without soy. Food Funct. 2011;2:678–683. doi: 10.1039/c1fo10125k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soral-Smietana M, Zenon Zduñczyk Z, Wronkowska M, Juœkiewicz J, Zander L. Mineral composition and bioavailability of calcium and phosphorus from acid whey concentrated by various membrane processes. J Elem. 2013;18:18115–18125. [Google Scholar]
- Villa P, Costantini B, Suriano R, Perri C, Macrì F, Ricciardi L, Panunzi S, Lanzone A. The differential effect of the phytoestrogen genistein on cardiovascular risk factors in postmenopausal women: relationship with the metabolic status. J Clin Endocrinol Metab. 2009;94:552–558. doi: 10.1210/jc.2008-0735. [DOI] [PubMed] [Google Scholar]
- Zhang YB, Li LN, Zhao XY, Chen WH, Guo JJ, Fu ZH, Yang Y, Na XL. Effect of soy isoflavone crude extract supplementation on high fat diet-induced insulin resistance in ovariectomized rats. Biomed Environ Sci. 2014;27:49–51. doi: 10.3967/bes2014.014. [DOI] [PubMed] [Google Scholar]
- Zimmermann C, Cederroth CR, Bourgoin L, Foti M, Nef S. Prevention of diabetes in db/db mice by dietary soy is independent of isoflavone levels. Endocrinol. 2012;153:5200–5211. doi: 10.1210/en.2012-1490. [DOI] [PubMed] [Google Scholar]