Abstract
The objective of the study was to determine the antimicrobial activity of Swamp Cranberry (Vaccinium oxycoccos) fruit and pomace extracts (FSCE and PSCE) and their efficiency in minced pork meat. Ethanol (96 and 40%) and water were used for raw material extraction. Organic acids, flavonols, terpenes and stilbenes composition of the extracts was determined using HPLC. Minimal inhibitory concentration and minimum bactericidal/fungicidal concentration were determined for bacteria and fungi strains using the broth macrodilution method. The growth inhibition of Staphylococcus aureus, Listeria monocytogenes, Salmonella Enteritidis, and Escherichia coli in inoculated fresh minced pork meat containing 2.5% we-PSCE or we-FSCE (prepared by using 40% ethanol) were evaluated within 6 days of refrigeration storage. Swamp Cranberry pomace extracts contained stilbenes and more organics acids and flavonols than fruit extracts. Extracts inhibited Gram-positive bacteria strains stronger than Gram-negative, regardless of used raw material. The extracts did not show antifungal activity. Water–ethanol extracts (we-FSCE and we-PSCE) had stronger antibacterial properties than ethanolic extracts (e-FSCE and e-PSCE) and aqueous extracts (w-FSCE and w-PSCE). A 2.5% addition of we-PSCE or we-FSCE to minced pork meat resulted in a reduction of the number of pathogenic cells by 4 log cycles after 4 days of refrigeration storage. Baked burgers containing 2.5% of these extracts obtained high ratings for color, taste, odor, juiciness, and overall acceptability that did not differ statistically from control samples. Extracts from Swamp Cranberry constitute interesting candidates for natural preservatives of minced pork meat.
Keywords: Swamp Cranberry, Vaccinium oxycoccos, Pomace, Antimicrobial, Minced pork
Introduction
Fresh pork can be important dietary source of protein, selenium, and thiamin, and also can contribute to the consumption of microelements, vitamins and fat in the diet of consumers (Murphy et al. 2011). Among approximately 30 types of available pork meat and its products, minced pork meat is consumed on a regular basis by a large percentage of consumers (Verbeke et al. 2010). Minced meat constitutes a different ecological environment for microorganisms than intact meat products. Mincing destroys tissue structure of the muscles, and microorganisms that contaminate their surface become mixed in meat mass. The environment favoring microorganism development is created as a result of these actions (Honikel 2004). Therefore, minced meat is a potentially hazardous food product with short shelf-life (Andritsos et al. 2012).
Safety issues affect the shaping of consumers’ attitudes toward meat. Due to consumers’ expectations, a constant search for new methods that could be used in prolonging meat shelf-life has been conducted. Presently, the most modern meat preservation methods include preserving with high hydrostatic pressure (HHP), ionizing radiation, use of active packaging, modified atmosphere packaging, steam pasteurization, and use of organic preservatives and natural antimicrobial substances (Aymerich et al. 2008). A significant advantage of natural antimicrobial substances is their safety and low risk of overdosing. In recent years, antimicrobial and antioxidant activity of plant extracts and essential oils was tested in pork meat (Krisch et al. 2010), beef (Fernández-López et al. 2005; Wu et al. 2009; Gadallah and Fattah 2011) and turkey (Raghavan and Richards 2007).
Cranberry (Vaccinium oxycoccos L.) fruits are rich in natural antioxidants, i.e. polyphenolic compounds: anthocyanins, flavonols, phenolic acids, and proanthocyanidins, which is attributed to the strong antioxidant properties (Rauha et al. 2000). Therefore, cranberry extracts have been proposed to be used as an additive to meat products for inhibiting unfavorable storage changes of lipids and muscle pigments (Raghavan and Richards 2007; Kathirvel et al. 2009; Ganhão et al. 2010). Furthermore, researchers exhibit growing interest in antimicrobial activity of cranberry. Antibacterial action of American cranberry (Vaccinium macrocarpon) concentrate has been shown (Wu et al. 2008) along with a mechanism of specific action of different classes of cranberry compounds, including organic acids, phenolic compounds, and anthocyanins on microorganisms (Lacombe et al. 2010).
Little has been done to evaluate the antibacterial action of Vaccinium oxycoccos. The study of Ermis et al. (2015) indicates that these cranberry concentrates were able to inhibit the growth of xerophilic and non-xerophilic fungi. To date, no studies have been conducted on antimicrobial activity toward meat microbiota, including food-borne pathogens. The objective of this study was to obtain fruit and pomace extracts of Swamp Cranberry and determine bioactive compound content, antimicrobial activity of the extracts and efficiency of antibacterial action of selected extracts in minced pork meat. The results will provide knowledge on the functional suitability of Swamp Cranberry extracts as natural meat preservatives.
Materials and methods
Materials
Fresh, raw pork meat from ham (Semimembranosus musculus) and Swamp Cranberry (Vaccinium oxycoccos L.) fruits, which were originated from marshy-forest area (N 51 47.999′ E 22 15 52.0019′) were bought in a local shop (Warsaw, Poland). Ripe fruits of red color without visible damage were selected for the study. Swamp Cranberry fruit pomace was obtained after pressing using low-speed juicer (Omega 8006, Omega Products Inc., USA) at 70 rpm.
Preparation of Swamp Cranberry fruit and pomace extracts (SCEs)
The ethanolic and water extracts of Swamp Cranberry fruits or pomace were prepared according to the extraction method, which was described in our earlier work (Gniewosz et al. 2014) with modification. Raw material (1 kg) extracted with 5 l of ethanol (96 or 40%) or water. The single-stage extraction was carried out in a prototype raw material extraction apparatus 3EU01 (OBR Pleszew, Poland). Fruits were extracted at 70 °C for 2 h and pomace at 40 °C for 2 h. Extracts were filtered through a Whatman No. 2 (Whatman International Ltd., Maidstone, England) and vacuum evaporated on a rotary evaporator (Rotovaporator R-215, Büchi, Flawil, Switzerland). The concentrated extracts were lyophilized at −48 °C for 72 h (Alpha 1-4, Christ, Germany). Three Swamp Cranberry fruit extract lyophilizates such as: e-FSCE was prepared by 96% ethanol, we-FSCE was prepared by using 40% ethanol and w-FSCE was prepared by water, and analogously three Swamp Cranberry fruit pomace lyophilizates (e-PSCE, we-PSCE and w-PSCE) were obtained. Powdered extracts were stored at 4 °C in the dark.
HPLC analysis of SCE compounds (phenolic acids, flavonols, terpenes and stilbenes)
The HPLC analyses were carried out on an Agilent 1200 system (Palo Alto, CA, USA), composed of a quaternary pump, autosampler, diode array detector (DAD), and HP ChemStation software Version 10.01. The column used was a Zorbax Eclipse XDB C18 (150 mm × 4.6 mm i.d.; 5 μm particle size) from Agilent Technologies (Palo Alto, CA, USA), maintained at 25 °C. The mobile phase was a combination of solvent A (0.05% (v/v) trifluoroacetic acid [TFA] in ddH2O) and solvent B (acetonitrile). The obtained extracts were filtered through a 0.45 µm Millipore membrane filter before injection. The injection volume was 10 μl. A flow rate of 0.8 ml/min and detection wavelength were set at 280 nm and 325 nm (Harris et al. 2007). The standards were purchased from ChromaDex (Irvine, USA). The content of the determined compounds was calculated in mg/100 g dry weight of extracts.
Test microorganisms and preparation of inoculum
Reference strains originated from the American Type Culture Collection (ATCC, Manassas, VA, USA), clinical strains originated from the National Institute of Public Health-National Institute of Hygiene (NIPH, Warsaw, Poland), and the strain isolated from food originated from the Division of Milk Biotechnology (WULS, Warsaw University of Life Sciences-SGGW, Poland). The study used 9 strains of Gram-positive bacteria (Staphylococcus aureus ATCC 25923, Staphylococcus aureus NIPH A-529, Staphylococcus epidermidis ATCC 12228, Micrococcus luteus ATCC 9341, Enterococcus faecalis ATCC 29212, Listeria monocytogenes NIPH 17/11, Bacillus cereus ATCC 11778, Bacillus cereus WULS 15, Bacillus subtilis ATCC 6633) and 10 strains of Gram-negative bacteria (Salmonella Enteritidis ATCC 13076, Salmonella Enteritidis NIPH 322/11, Salmonella Typhimurium NIPH-NIH 300/11, Shigella sonnei NIPH “s”, Escherichia coli ATCC 25922, Escherichia coli O26 NIPH 152/11, Klebsiella pneumoniae ATCC 13883, Enterobacter aerogenes ATCC 13048, Proteus mirabilis ATCC 35659, Pseudomonas aeruginosa ATCC 27853) and three fungi strains (Aspergillus niger ATCC 9142, Rhizopus arrhizus ATCC 11145, and Saccharomyces cerevisiae ATCC 9763).
All the strains were stored at –78 °C and revived on agar plate. The bacterial strains were cultured on Nutrient Agar (NA, BTL, Łódź, Poland) and incubated at 37 °C for 18 h. Bacterial inocula were prepared in sterile 0.85% NaCl (w/v) solution to reach a population of approximately 108 CFU/ml. Molds were cultured on Sabouraud Agar (SA, Merck, Darmstadt, Germany) at 22 °C until spores were formed (ca. 14 days). After the culture, 1 ml of physiological salt was added onto the thallus’ surface and was mixed well to achieve a suspension of spores. Yeasts were cultured on SA at 28 °C for 24 h. Cells of yeasts or mold conidia were counted in a hemocytometer. The inoculum of fungal populations was prepared in the concentration of 106 CFU/ml.
MIC and MBC/MFC determination of Swamp Cranberry extracts (SCEs)
The minimum inhibitory concentration (MIC) and the minimum bactericidal (MBC) and fungicidal concentration (MFC) of extracts against the tested strains were measured using the broth macrodilution method (CLSI 2009). Freshly prepared tubes contained 10 serial twofold dilutions of extract in 2 ml of Müller Hinton Broth (MHB, Merck, Darmstadt, Germany) (for bacteria) or Sabouraud Broth (SB, Merck, Darmstadt, Germany) (for yeasts and mold) (range from 50 to 0.098 mg/ml). A tube without extract was chosen as the control. Initial pH level was measured in the prepared media. The concentration of bacterial inoculum was 5 × 105 CFU/ml, and that of yeast and mold was 5 × 104 CFU/ml. Tubes with bacteria were incubated at 37 °C for 24 h and those with fungi at 28 °C for 72 h. Afterward, the growth of strains in test tubes with different concentrations of extract was checked visually and compared with the control sample. MIC was recorded as the lowest concentration of extract that completely inhibited visible growth of the organisms. The evaluation of MIC was carried out in triplicate.
In order to determine the minimum bactericidal (MBC) and fungicidal (MFC) Concentration of extract, 100 µl of the culture medium from the tube showing neither bacterial nor fungal growth were re-inoculated onto Müller Hinton Agar (Merck, Darmstadt, Germany) (for bacteria) or SA (for fungi) plates, which were incubated at 37 °C for 24 h (for bacteria) or at 28 °C for 72 h (for fungi). The plates were checked for growth of colonies. MBC/MFC was determined as the lowest extract concentration, at which no growth occurred on the plates.
In addition, the minimal pH value of growth of each test bacteria in MHB medium was determined. Two series of MHB were prepared with pH values from 4.0 to 6.0 with accuracy of 0.1. The pH of medium was regulated using 0.5 M HCl. The initial pH of control medium MHB was 7.3. Bacterial inoculum (0.1 ml) prepared as above was transferred to each tube. The growth of bacteria in each tube was compared with the control sample. The tube with medium with the lowest pH value, in which growth was observed, indicated minimum pH value for tested bacteria.
On the basis of MIC value, an antibacterial percentage activity of extract was determined:
Percentage of activity indicates total antibacterial potency of individual extracts, i.e. it determined the number of bacterial strains susceptible to one specific extract.
Evaluation of the antibacterial activity of SCEs in minced pork meat
Antibacterial activity of selected SCEs was evaluated in minced pork meat. Cube-shaped pieces of meat with the edge of 5 cm were immersed in ethanol (96% v/v) and were heated over the flame of a burner. Then, a surface layer of meat with thickness of 3 mm was cut off from each side using a sterile scalpel. Remaining pieces of meat were crushed in a mincer with screen with 4 mm opening size, sterilized with ethanol (96% v/v). Test strains i.e. S. aureus NIPH-NIH A-529, L. monocytogenes NIPH-NIH 17/11, E. coli O26 NIPH-NIH 152/11, S. Enteritidis NIPH-NIH 322/11 were individually cultured in 10 ml Nutrient Broth (NB, BTL, Łódź, Poland) at 37 °C for 24 h. Then, the bacteria strains were cultured again in 20 ml NB under the same conditions as mentioned above. Broth was centrifuged at 4 °C for 5 min at 12,857×g (Centrifuge 5804R, Eppendorf, Germany). Bacterial sediment was initially washed with sterile 0.1% (w/v) peptone–water solution (BTL, Łódź, Poland) and then was suspended in 20 ml sterile 0.1% peptone–water solution (approximately 9 log CFU/ml). Four strains were mixed, again diluted (approximately 8 log CFU/ml), and were placed in minced pork meat samples. 600 g of minced pork meat was divided into six portions of 100 g each, which were placed in sterile stomacher bags. To three samples, 1 ml of culture was added in order to obtain approximately 6 log CFU/g. Instead of inoculum to two remaining samples, 1 ml sterile 0.1% peptone–water solution was added. Then, the samples were thoroughly mixed using sterile rod. To four samples, appropriate extract powder in concentration of 2.5% (w/w) was added and then thoroughly mixed. Following samples were obtained: (1) (meat + inoculum + we-FSCE), (2) (meat + inoculum + we-PSCE), positive control (3) (meat + inoculum), negative controls: (4) (meat + we-FSCE), (5) (meat + we-PSCE), (6) (meat without additives). Each sample was divided into four portions of 25 g each and placed in sterile filter stomacher bags and stored at 4 °C. Test was repeated three times for each treatment. The pH value was measured in all samples. Mean pH value of minced pork meat was 6.59 ± 0.06, and minced pork meat with addition of we-FSCE or we-PSCE was 6.24 ± 0.07 and 6.29 ± 0.06, respectively. During 6 days of storage, pH values of samples did not differ significantly.
Microbiological examination was conducted on day 0 (30 min after the addition of extract to the meat) and the 2nd, 4th and 6th day. 25 g samples mixed with 225 ml sterile 0.1% peptone–water and homogenized in Stomacher 400C Lab Blender (Seward, London, UK) at room temperature for 2 min. Then, decimal dilutions were prepared, after which 100 μl were transferred to surfaces of two plates from Baird Parker Agar and Palcam Listeria Agar from BTL (Łódź, Poland), Hektoen Agar and Chromogenic Coliform Agar from Merck (Dortmund, Germany). Plates were incubated at 37 °C for 24 h. After incubation, characteristic colonies were counted, and the result was expressed in CFU/g of meat. In order to confirm species identity of bacteria, colonies were randomly selected, which were stained with Gram method and inspected using commercial diagnostic equipment (API, bioMérieux, France).
Preparation and sensory evaluation of minced pork meat burgers containing we-PSCE or we-FSCE
The sensory analysis was conducted by a panel of 10 trained assessors, who were students or workers of the WULS-SGGW (Warsaw, Poland) and were trained according to international standards (ISO 1993). To 975 g of fresh minced pork meat, 25 g of we-PSCE or we-FSCE was added. The control sample comprised fresh minced pork meat without addition of extract. Then, burgers weighing 50 g were hand-formed, wrapped in aluminum foil and baked at 160 °C for 25 min in a commercial electric oven (Whirlpool, Poland). Baked hot burgers were sliced into four portions and served to assessors for sensory evaluation. Each assessor received all three coded samples at the same time, placed on a white porcelain plate. The following parameters were evaluated: color, taste, odor, texture and juiciness, and overall acceptability of tested samples. Assessors were required to score each descriptor on an unstructured scale anchored by the terms low intensity (0) and high intensity (10). Assessors were provided with a bottle of still water at room temperature in order to rinse their mouths.
Statistical analysis
All tests were conducted in three replicates. Numbers of cells were transformed to log10 CFU/g for statistical analysis. All data were expressed as mean ± standard deviation (SD). Statistical tests were performed using the Statistica version 10PL computer program (StatSoft Inc., Poland). One-way analysis of variance was carried out. The significance of differences between mean values was assessed using Tukey-test at a significance level of p < 0.05.
Results and discussion
Chemical composition of SCEs and their effect on inhibiting the growth of tested microorganisms
Table 1 presents bioactive components found in extracts obtained from Swamp Cranberry fruits and pomace. In the chemical composition of w-FSCE, a dominant presence of benzoic acid, followed by p-coumaric acid and chlorogenic acid was determined. Caffeic acid and gentisic acid contents were significantly lower. Among the present flavonols, the content of quercetin and myricetin was three times higher than epicatechin and isorhamnetin. This extract did not contain terpenes and stilbenes. In the composition of e-FSCE, the benzoic acid and the remaining acid content was two times higher (except for gentisic acid) than in the chemical composition of w-FSCE. In addition, e-FSCE was distinguished by higher flavonols content, especially quercetin, than the remaining Swamp Cranberry fruit extracts and also the presence of ursolic acid. In the composition of we-FSCE, the gentisic acid concentration was higher than in the remaining Swamp Cranberry fruit extracts.
Table 1.
HPLC quantification of soluble components identified in Swamp Cranberry extracts
| Component | Fruit extracts | Pomace extracts | ||||
|---|---|---|---|---|---|---|
| w-FSCE | e-FSCE | we-FSCE | w-PSCE | e-PSCE | we-PSCE | |
| [mg/100 g ± SD] | ||||||
| Benzoic acid | 99.6 ± 1.4a | 214.6 ± 1.7e | 143.7 ± 1.4c | 191.2 ± 1.3d | 115.0 ± 2.3b | 195.7 ± 1.3d |
| p-Coumaric acid | 66.3 ± 0.8a | 77.0 ± 1.2b | 78,0 ± 1.1b | 157.9 ± 1.2c | 175.0 ± 2.1d | 207.9 ± 3.9e |
| Chlorogenic acid | 61.0 ± 0.4a | 96.3 ± 0.4c | 89.5 ± 0.8b | 286.4 ± 2.4e | 408.7 ± 4.6f | 262.9 ± 3.1d |
| Caffeic acid | 0.7 ± 0.1a | 1.4 ± 0.2b | 0.9 ± 0.2a | 33.9 ± 3.1c | 36.5 ± 1.3c | 98.5 ± 2.6d |
| Gentisic acid | 0.3 ± 0.1a | 0.3 ± 0.1a | 4.9 ± 0.6b | 33.6 ± 0.9c | 41.9 ± 0.6d | 45.7 ± 1.2e |
| Sum of acids | 227.9 | 389.5 | 360.0 | 702.9 | 777.0 | 810.7 |
| Quercetin | 8.0 ± 0.5a | 15.4 ± 1.3c | 10.4 ± 0.5b | 10.7 ± 1.2b | 25.2 ± 2.2d | 27.3 ± 0.6d |
| Myricetin | 8.4 ± 0.2a | 11.2 ± 0.5c | 10.0 ± 0.1b | 31.8 ± 1.5d | 49.2 ± 3.6e | 65.3 ± 2.2f |
| Epicatechin | 3.1 ± 0.3c | 6.3 ± 0.9e | 4.1 ± 0.9d | 1.5 ± 0.4a | 5.7 ± 0.3e | 2.2 ± 0.4b |
| Isorhamnetin | 2.1 ± 0.1c | 3.5 ± 0.7d | 3.0 ± 0.3d | 0.2 ± 0.1a | 1.5 ± 0.5b | 1.3 ± 0.2b |
| Sum of flavonols | 21.5 | 36.3 | 27.6 | 44.3 | 81.5 | 96.0 |
| Terpenes (ursolic acid) | 0.0 ± 0.0 | 12.0 ± 1.1a | 0.0 ± 0.0 | 0.0 ± 0.0 | 15.0 ± 1.6b | 0.0 ± 0.0 |
| Stilbenes (resveratrol) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 12.2 ± 0.4b | 9.7 ± 0.8a | 16.7 ± 2.3c |
Values are mean ± SD of three separate experiments. Different superscript letters within the same row indicate significant (p ≤ 0.05) differences according to Tukey test
Swamp Cranberry pomace extracts were characterized by higher organic acid and flavonols content compared to Swamp Cranberry fruit extracts and the occurrence of reservatrol, not found in fruit extracts of the plant (Table 1). E-PSCE was characterized by the highest chlorogenic acid content, which was approximately 1.5 times higher than in w-PSCE and we-PSCE. On the contrary, the composition of we-PSCE revealed the highest content of the following acids: p-coumaric, caffeic and gentisic, myricetin and resveratrol compared to all the obtained extracts. we-PSCE was also distinguished by the highest caffeic acid content, which was approximately three times higher than in the remaining extracts.
On the basis of the obtained results, it can be said that extract composition was a composite result of the type of solvent, raw material, and temperature of extraction. The extraction of higher amount of compounds was possible by using 96% ethanol and 40% ethanol than water. In aqueous extracts (w-FSCE and w-PSCE), lower organic acid and flavonols content was determined, independent of raw material used. There were no big differences between the content of bioactive components in ethanol and water–ethanol extracts, and therefore considering the production of extracts in the future, it is preferred for economic reasons, to use as a solvent of 40% ethanol. The presence of terpenes and stilbenes was determined primarily in pomace extracts (w-PSCE, e-PSCE, we-PSCE) which stems from the fact, that these compounds are found mainly in fruit skins (Frighetto et al. 2008). According to Wang and Stretch (2001), reservatol content in fresh American cranberry juice is very low (0.024 mg/100 g). Concentration of this compound in the obtained pomace extracts was 25–40 times higher than in the studied juice. The extracts obtained from pomace at lower temperature were characterized by almost two times higher organic acid and flavonols content than obtained from fruits using hot extraction method. It should be noted that numerous bioactive compounds found in plants are in chemically bounded formulas, for instance, glycosides or esters (Singh et al. 2009) which were not determined in this study.
Antimicrobial action of Swamp Cranberry fruit and pomace extracts
Table 2 presents Swamp Cranberry extract’s antimicrobial activity, which depended on species. Inhibitory action of extracts toward Gram-positive bacteria (MIC 0.78–6.25 mg/ml) was stronger than toward Gram-negative bacteria (MIC 6.25–12.5 mg/ml). In most cases, MBC were higher than MIC values. The extracts exhibited the most significant activity toward two Gram-positive bacteria strains, i.e. M. luteus and S. epidermidis. Among Gram-negative bacteria, the most sensitive to tested extracts was P. mirabilis and the most resistant was the strain E. aerogenes.
Table 2.
MIC, MBC/MFC values of Swamp Cranberry fruit and pomace extracts
| Strain | Fruits extracts | Pomace extracts | Min pH** | ||||
|---|---|---|---|---|---|---|---|
| w-FSCE | e-FSCE | we-FSCE | w-PSCE | e-PSCE | we-PSCE | ||
| MIC/pH* (MBC; mg/ml) | |||||||
| Gram-positive bacteria | |||||||
| S. aureus ATCC 25923 | 1.56/6.02 (12.5) | 6.25/4.72 (12.5) | 1.56/6.46 (12.5) | 1.56/5.64 (12.5) | 1.56/5.86 (3.13) | 1.56/6.10 (6.25) | 5.2 |
| S. aureus NIPH A-529 | 1.56/6.02 (6.25) | 3.13/5.68 (12.5) | 3.13/5.45 (12.5) | 1.56/5.64 (12.5) | 3.13/4.94 (12.5) | 1.56/6.10 (6.25) | 5.0 |
| S. epidermidis ATCC 12228 | 3.13/5.00 (6.25) | 3.13/5.68 (6.25) | 1.56/6.46 (6.25) | 3.13/4.89 (6.25) | 1.56/5.86 (12.5) | 1.56/6.10 (3.13) | 5.3 |
| M. luteus ATCC 9341 | 1.56/6.02 (3.13) | 3.13/5.68 (6.25) | 1.56/6.46 (3.13) | 1.56/4.89 (3.13) | 1.56/5.86 (1.56) | 1.56/6.10 (1.56) | 5.4 |
| E. faecalis ATCC 29212 | 3.13/5.00 (12.5) | 6.25/4.72 (12.5) | 1.56/6.46 (6.25) | 3.13/4.89 (12.5) | 3.13/4.94 (12.5) | 1.56/6.10 (6.25) | 4.6 |
| L. monocytogenes 17/11 | 3.13/5.00 (25.0) | 6.25/4.72 (25.0) | 6.25/4.58 (25.0) | 6.25/4.37 (25.0) | 3.13/4.94 (25.0) | 3.13/5.25 (25.0) | 4.9 |
| B. cereus ATCC 11778 | 6.25/4.40 (12.5) | 6.25/4.72 (6.25) | 6.25/4.58 (6.25) | 3.13/4.89 (6.25) | 1.56/5.86 (6.25) | 1.56/6.10 (6.25) | 4.9 |
| B. cereus WULS 15 | 1.56/6.02 (>50) | 3.13/5.68 (>50) | 1.56/6.46 (>50) | 1.56/5.64 (>50) | 1.56/5.86 (>50) | 0.78/6.51 (>50) | 5.0 |
| B. subtilis ATCC 6633 | 1.56/6.02 (3.13) | 3.13/5.68 (3.13) | 1.56/6.46 (3.13) | 1.56/5.64 (3.13) | 3.13/4.94 (3.13) | 1.56/6.10 (1.56) | 5.2 |
| Gram-negative bacteria | |||||||
| S. enteritidis ATCC 13076 | 12.5/3.88 (25.0) | 6.25/4.72 (12.5) | 12.5/4.00 (25.0) | 12.5/3.88 (12.5) | 12.5/3.70 (12.5) | 12.5/4.01 (25.0) | 4.5 |
| S. enteritidis NIPH 322/11 | 12.5/3.88 (25.0) | 6.25/4.72 (12.5) | 12.5/4.00 (25.0) | 12.5/3.88 (12.5) | 12.5/3.70 (12.5) | 12.5/4.01 (25.0) | 4.5 |
| S. typhimurium NIPH300/11 | 6.25/4.40 (12.5) | 6.25/4.72 (12.5) | 12.5/4.00 (12.5) | 12.5/3.88 (12.5) | 12.5/3.70 (25.0) | 12.5/4.01 (12.5) | 4.5 |
| S. sonnei NIPH “s” | 6.25/4.40 (6.25) | 6.25/4.72 (6.25) | 6.25/4.58 (6.25) | 6.25/4.37 (12.5) | 12.5/4.35 (12.5) | 6.25/4.53 (6.25) | 4.6 |
| E. coli ATCC 25922 | 12.5/3.88 (12.5) | 6.25/4.72 (25.0) | 6.25/4.58 (12.5) | 6.25/4.37 (6.25) | 6.25/4.35 (12.5) | 6.25/4.53 (12.5) | 4.5 |
| E. coli NIPH 152/11 | 6.25/4.40 (12.5) | 6.25/4.72 (12.5) | 6.25/4.58 (12.5) | 6.25/4.37 (12.5) | 12.5/3.70 (25.0) | 6.25/4.53 (25.0) | 4.5 |
| K. pneumoniae ATCC 13883 | 12.5/3.88 (25.0) | 12.5/4.15 (25.0) | 6.25/4.58 (25.0) | 12.5/3.88 (25.0) | 12.5/3.70 (25.0) | 6.25/4.53 (25.0) | 5.2 |
| E. aerogenes ATCC 13048 | 12.5/3.88 (25.0) | 12.5/4.15 (25.0) | 12.5/4.00 (25.0) | 12.5/3.88 (25.0) | 12.5/3.70 (25.0) | 12.5/4.01 (25.0) | 4.5 |
| P. mirabilis ATCC 35659 | 6.25/4.40 (12.5) | 6.25/4.72 (12.5) | 6.25/4.58 (12.5) | 3.13/4.89 (6.25) | 6.25/4.35 (6.25) | 3.13/5.25 (6.25) | 5.0 |
| P. aerugionosa ATCC 27853 | 6.25/4.40 (25.0) | 6.25/4.72 (12.5) | 6.25/4.58 (12.5) | 6.25/4.37 (12.5) | 6.25/4.35 (6.25) | 6.25/4.53 (12.5) | 4.5 |
| Fungi | |||||||
| A. niger ATCC 9142 | >50/<3.07 (>50) | 50/3.25 (>50) | >50/<3.05 (> 50) | >50/<3.2 (>50) | 50/2.83 (>50) | 50/3.1 (>50) | nt |
| R. arrhizus ATCC 11145 | >50/<3.07 (>50) | 50/3.25 (>50) | >50/<3.05 (>50) | >50/<3.2 (>50) | 25/3.23 (>50) | >50/<3.1 (>50) | nt |
| S. cerevisiae ATCC 9763 | >50/<3.07 (>50) | 50/3.25 (>50) | >50/<3.05 (50) | >50/<3.2 (>50) | 50/2.83 (>50) | >50/<3.1 (>50) | nt |
MIC minimal inhibitory concentration, MBC/MFC minimum bactericidal/fungicidal concentration, nt no tested
* pH—initial pH value of MHB medium, corresponding to MIC
** min pH—critical pH values for the growth of strains tested in MHB medium with inorganic acid (HCl)
Simultaneously, no fungistatic and fungicidal effects of the majority of extracts were observed (MIC and MBC > 50 mg/ml). Very weak fungistatic activity was exhibited only by e-FSCE and e-PSCE, possibly due to small ursolic acid content (not present in the remaining extracts) (Table 1). Becker et al. (2005) found that strong antifungal properties of plant extracts obtained from Lythrum salicaria resulted from the presence of ursolic acid. Similar research results presented by other authors (Shai et al. 2008; Mahlo and Eloff 2014).However, Becker et al. (2005) make it clear that higher ursolic acid concentrations in extracts effectively inhibit fungal growth on one hand, but on the other, they exhibit acute toxicity, which limits their usage in fighting fungal infections in plants.
Organic acids found in extracts caused a decrease of pH level in culture medium. Simultaneously, it was noted that pH of medium with the same MIC (to the concentration of 6.25 mg/ml) differed depending on the type of extract. It was noted that after addition of pomace extracts, pH values of media were lower than after the addition of fruit extracts, independent of solvent.
Greater effect of acidic environment on inhibiting Gram-negative bacteria than Gram-positive was determined, especially in media containing w-FSCE, w-PSCE, or e-PSCE. The vast majority of pH values of media with MIC ranged from 3.88 to 4.72, and in most cases pH values were lower than minimum pH values tolerated by Gram-negative bacteria strains (4.5–5.2) (Table 2). In such environment, weak organic acids present in extracts are found in undissociated state (pH below pKa value in the range between pH 3 and 5 for the majority of weak organic acids) (Doores 1993) and efficiently inhibit microbiota growth. According to Adams and Moss (2000) undissociated acid molecules pass from an external environment of low pH where the equilibrium favours the undissociated molecule to the high pH of the cell cytoplasm. At this higher pH, the equilibrium shifts in favour of the dissociated molecules, so the acid ionizes producing protons which will tend to acidify the cytoplasm. The cell will try to maintain its internal pH by neutralizing or expelling the protons leaking in but this will slow growth as it diverts energy from growth-related functions. If the external pH is sufficiently low and the extracellular concentration of acid high, the burden on the cell becomes too great, the cytoplasmic pH drops to a level where growth is no longer possible and the cell eventually dies. Therefore, a part of antibacterial activity of cranberry extracts toward these bacteria was a result of low pH level (Puupponen-Pimiä et al. 2005). Nohynek et al. (2006) suggest that these acids are partially engaged in lipopolysaccharides (LPS) release from the outer membrane of Gram-negative bacteria, thus contributing to its permeability.
In the majority of Gram-positive strains, acid environment did not play a significant role in inhibiting their growth because a vast majority of pH levels in media with extract MIC was above the critical pH level required for the growth of test strains (pH 4.72–6.46). Lacombe et al. (2010) exhibited that phenolic compounds and anthocyanins present in cranberry extracts, contrary to organic acids, retain antibacterial effect at neutral pH, although anthocyanins at neutral pH exhibit lower antibacterial activity, possibly due to instability occurring under such conditions. The antimicrobial effect of cranberry, as in other berries, may be caused by numerous mechanisms, because they contain different compounds and combinations of their various chemical forms (Nohynek et al. 2006).
Comparison of antimicrobial activity of Swamp Cranberry extracts
In order to compare antibacterial activity of tested extracts, a percentage activity was calculated (A %) (Table 3). None of the tested extracts inhibited strains at lowest concentrations (0.098, 0.195, and 0.39 mg/ml). At 0.78 mg/ml concentration, the inhibiting effect was shown only by one extract (we-PSCE). It was determined that at 1.56–6.25 mg/ml concentrations, extracts prepared by using 40% ethanol were distinguished by a broader spectrum of activity than aqueous and ethanolic extracts (prepared by 96% ethanol). Only at the concentration of 12.5 mg/ml and higher, all tested Swamp Cranberry extracts inhibited 100% tested strains.
Table 3.
Antibacterial percentage activity of Swamp Cranberry extracts (A%)
| MIC (mg/ml) | Fruits extracts | Pomace extracts | ||||
|---|---|---|---|---|---|---|
| w-FSCE | e-FSCE | we-FSCE | w-PSCE | e-PSCE | we-PSCE | |
| 0.098 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.195 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.39 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.78 | 0 | 0 | 0 | 0 | 0 | 5 |
| 1.56 | 26 | 0 | 32 | 32 | 24 | 42 |
| 3.13 | 42 | 26 | 47 | 47 | 47 | 53 |
| 6.25 | 74 | 74 | 74 | 63 | 71 | 79 |
| 12.5 | 100 | 100 | 100 | 100 | 100 | 100 |
| 25.0 | 100 | 100 | 100 | 100 | 100 | 100 |
| 50.0 | 100 | 100 | 100 | 100 | 100 | 100 |
A (%) = (100 × number of strains inhibited by tested extract)/(total number of tested strains). Percentage of activity indicates total antibacterial potency of individual extracts
The differences in the content of determined bioactive compounds probably had an effect on broader spectrum and strength of action of tested extracts. we-PSCE, which had the broadest spectrum of activity at 1.56–66.25 mg/ml (Table 3), contained more flavanols and organic acids in chemical composition than the remaining extracts (Table 1). According to Cushnie and Lamb (2005), the antibacterial activity of flavonoids is based on several mechanisms of cell membrane damage by perforation or fluctuation, inhibition of nucleic acid synthesis, energy metabolism (by inhibition of NADH-cytochrome c reductase), inhibition of cell wall synthesis (by inhibition of d-alanine-d-alanine synthetase) and inhibition of cell membrane synthesis (due to FabG, Fabl, Fabz, Rv0636 or III KAS enzyme inhibition).
Evaluation of antibacterial efficiency of we-FSCE and we-PSCE in minced pork meat
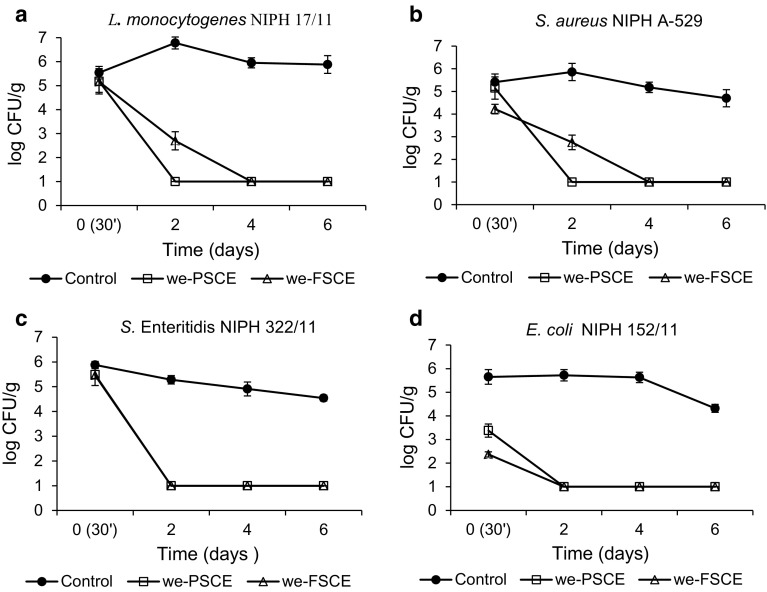
Efficiency of Swamp Cranberry extracts protection against obligatory pathogens in minced pork meat was tested. To do this, extracts exhibiting in vitro broad antibacterial spectrum (A%) at concentrations 1.56–6.25 mg/ml i.e. we-PSCE or we-FSCE were added to meat (Fig. 1).
Fig. 1.
Change of bacterial count in minced pork meat containing 2.5% we-PSCE or we-FSCE during refrigeration storage (4 °C)
After adding extracts to minced pork meat inoculated with pathogens, a decrease in the number of cells was observed. Only 30 min after addition of we-FSCE, a decrease in the number of S. aureus cells by 1 logarithmic cycle and by 3 logarithmic cycles of E. coli was observed which may stem from very rapid effect of the extract on bacterial cells in meat. In the tested raw material, we-PSCE exhibited more efficient inhibiting effect on the pathogens growth than we-FSCE. After 2 days, the effect of this extract caused a statistically significant decrease in the number of cells in meat sample by 4 logarithmic cycles (p < 0.05). In the meat samples with we-FSCE, after 2 days of storage, a statistically significant decrease (p < 0.05) in the number of Gram-positive bacteria by 3 logarithmic cycles (Fig. 1a, b) and Gram-negative by 4 logarithmic cycles (Fig. 1c, d) was observed.
The obtained results indicate that during storage, the tested extracts inhibited the growth of Gram-negative bacteria more efficiently than Gram-positive bacteria. Populations of L. monocytogenes and S. aureus were destroyed after 2 days only in meat with the addition of we-PSCE. Above dependencies may result from synergistic effect of extract and refrigeration conditions of sample storage. Onyango et al. (2012) demonstrate that S. aureus bacteria can adapt to low temperatures and retain good viability under such conditions thanks to morphological, ultra- structural, and biochemical changes in the composition of the cell wall. This factor may be of importance for effect of cranberry extract, the activity of which in such a situation may be impeded. Similarly, L. monocytogenes bacteria are thermotolerant and thanks to protective proteins of thermal shock can retain good viability under low temperatures (Novak and Juneja 2003) which could affect their better survivability in the tested sample. Raw pork it’s self is of importance because this food matrix as it provides a source of protection to pathogens that is attributed to its large surface area. A surface area that is increased upon the mincing of the pork. There is more of a chance for bacteria to settle in a niche protecting them coming into contact with intervention. Holzapfel (1998) suggests better Gram-positive bacteria survivability than Gram-negative in meat raw materials, especially under low temperature conditions.
There has not been conducted much research into the use of cranberry extracts as natural preservatives in meat. Apostolidis et al. (2008) investigated inhibition of growth of L. monocytogenes strain in cooked ground beef by synergistic action of water soluble cranberry and oregano extracts (1:1) with added sodium lactate. The authors have shown that the combination of the extract from both plants was characterized by a better antimicrobial effect than each extract alone, and the addition of sodium lactate, which enhanced the antimicrobial effect of the extracts, provided a preservative that could be used in food production. The effectiveness of synergistic action of bioactive compounds of cranberries and oregano against L. monocytogenes is also confirmed by other authors (Lin et al. 2004).
Sensory evaluation of burgers with addition of we-PSCE or we-FSCE
Table 4 presents average evaluation of color, taste, odor, texture and juiciness, and overall acceptability of pork burgers with and without 2.5% addition of we-FSCE or we-PSCE. Assessors awarded burgers with high marks for all evaluated attributes. Obtained results indicate that addition of we-FSCE or we-PSCE at concentration 2.5% to pork burgers did not significantly affect their perception by consumers. Wu et al. (2009) report that beef burgers with addition of American cranberry concentrate at concentrations 2.5, 5.0, and 7.5% also received good overall acceptability by consumers. Raghavan and Richards (2007) indicate the possibility of using ethanolic extracts from cranberry pomace as natural antioxidants used in mechanically separated turkey meat.
Table 4.
Mean panel scores for attributes of pork burgers with 2.5% we-FSCE or we-PSCE
| Attribute | Untreated (control) | we-FSCE 2.5% |
we-PSCE 2.5% |
|---|---|---|---|
| Mean panel score ± SD | |||
| Color | 6.85 ± 1.31a | 7.42 ± 1.37a | 7.62 ± 1.90a |
| Taste | 6.85 ± 1.58a | 7.76 ± 1.32a | 7.98 ± 1.60a |
| Odor | 7.55 ± 1.40a | 7.92 ± 1.51a | 8.04 ± 1.47a |
| Texture | 7.85 ± 0.85a | 7.72 ± 0.71a | 7.65 ± 0.85a |
| Juiciness | 7.99 ± 0.59a | 8.01 ± 0.88a | 8.10 ± 0.91a |
| Overall acceptability | 7.73 ± 0.53a | 8.17 ± 1.09a | 8.35 ± 1.23a |
Average score of 10 assessors and measuring attributes on defined 100 mm line scale line scales. Different letters within the same row mark significantly differences means (Tukey test, p ≤ 0.05)
Conclusion
Results of the study show that Swamp Cranberry fruit and pomace extracts have antibacterial activity, but do not have antifungal activity. Stronger antibacterial properties were exhibited by ethanolic than aqueous extracts, independently of used raw material. Extract of 2.5% content in minced pork meat was sufficient to reduce the number of pathogenic cells, without any negative effect on organoleptic characteristics of burgers indicated potential use Swamp Cranberry extracts as natural preservatives. Use of Swamp Cranberry pomace as a raw material for the production of extracts allows for management of food industry waste and reducing the cost of obtaining natural preservatives.
References
- Adams MR, Moss MO. Factors affecting the growth and survival of micro-organisms in food. In: Royal T, editor. Food microbiology. 2. Cambridge: Society of Chemistry; 2000. pp. 28–29. [Google Scholar]
- Andritsos ND, Mataragas M, Mavrou E, Stamatiou A, Drosinos EH. The microbiological condition of minced pork prepared at retail stores in Athens, Greece. Meat Sci. 2012;91:486–489. doi: 10.1016/j.meatsci.2012.02.036. [DOI] [PubMed] [Google Scholar]
- Apostolidis E, Kwon YI, Shetty K. Inhibition of Listeria monocytogenes by oregano, cranberry and sodium lactate combination in broth and cooked ground beef systems and likely mode of action through proline metabolism. Int J Food Microbiol. 2008;128(2):317–324. doi: 10.1016/j.ijfoodmicro.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Aymerich T, Picouet PA, Monfort JM. Decontamination technologies for meat products. Meat Sci. 2008;78(1–2):114–129. doi: 10.1016/j.meatsci.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Becker H, Scher JM, Speakman JB, Zapp J. Bioactivity guided isolation of antimicrobial compounds from Lythrum salicaria. Fitoterapia. 2005;76:580–584. doi: 10.1016/j.fitote.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard (M07-A8) 8. Wayne: Clinical and Laboratory Standards Institute; 2009. [Google Scholar]
- Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrobial Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doores S. Organic acids. In: Davidson PM, Branen AL, editors. Antimicrobials in food. 2. New York: Marcel Dekker; 1993. pp. 95–136. [Google Scholar]
- Ermis E, Hertel C, Schneider C, Carle R, Stintzing F, Schmidt H. Characterization of in vitro antifungal activities of small and American cranberry (Vaccinium oxycoccos L. and V. macrocarpon Aiton) and lingonberry (Vaccinium vitis-idaea L.) concentrates in sugar reduced fruit spreads. Int J Food Microbiol. 2015;204:111–117. doi: 10.1016/j.ijfoodmicro.2015.03.017. [DOI] [PubMed] [Google Scholar]
- Fernández-López J, Zhi N, Aleson-Carbonell L, Pérez-Alvarez JA, Kuri V. Antioxidant antibacterial activities of natural extracts: application in beef meat balls. Meat Sci. 2005;69:371–380. doi: 10.1016/j.meatsci.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Frighetto RTS, Welendorf RM, Nigro EN, Frighetto N, Siani AC. Isolation of ursolic acid from apple peels by high speed counter-current chromatography. Food Chem. 2008;106(2):767–771. doi: 10.1016/j.foodchem.2007.06.003. [DOI] [Google Scholar]
- Gadallah MGE, Fattah AAA. The antibacterial effect of mango seed kernel powder in minced beef during refrigerated storage. World J Dairy Food Sci. 2011;6(2):219–228. [Google Scholar]
- Ganhão R, Morcuende D, Estévez M. Protein oxidation in emulsified cooked burger patties with added fruit extracts: influence on colour and texture deterioration during chill storage. Meat Sci. 2010;85:402–409. doi: 10.1016/j.meatsci.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Gniewosz M, Synowiec A, Kraśniewska K, Przybył JL, Bączek K, Węglarz Z. The antimicrobial activity of pullulan film incorporated with meadowsweet flower extracts (Filipendulae ulmariae flos) on postharvest quality of apples. Food Control. 2014;37:351–361. doi: 10.1016/j.foodcont.2013.09.049. [DOI] [Google Scholar]
- Harris CS, Burt AJ, Saleem A, Le PM, Martineau LSC, Haddad PS, Bennett SAL, Arnason JT. A single HPLC-PAD-APCI/MS method for the quantitative comparison of phenolic compounds found in leaf, stem, root and fruit extracts of Vaccinium angustifolium. Phytochem Anal. 2007;18:161–169. doi: 10.1002/pca.970. [DOI] [PubMed] [Google Scholar]
- Holzapfel WH. The Gram-positive bacteria associated with meat and meat-products. In: Davies A, Board R, editors. Microbiology of meat and poultry. London: Blackie Academic & Professional; 1998. [Google Scholar]
- Honikel KO. Minced Meats. In: Jensen WK, editor. Encyclopedia of meat sciences. New York: Elsevier; 2004. pp. 854–856. [Google Scholar]
- ISO (1993) International Organization for Standardization (ISO), Sensory analysis: general guidance for the selection, training and monitoring of assessors—Part 1. Selected Assessors. ISO, Geneva, Switzerland ISO 8586–1:1993 (E)
- Kathirvel P, Gong Y, Richards MP. Identification of the compound in a potent cranberry juice extract that inhibits lipid oxidation in comminuted muscle. Food Chem. 2009;115:924–932. doi: 10.1016/j.foodchem.2009.01.007. [DOI] [Google Scholar]
- Krisch J, Pardi Z, Tserennadmid R, Papp T, Vágvölgyi C. Antimicrobial effects of commercial herbs, spices and essential oils in minced pork. Acta Biol Szeged. 2010;54(2):131–134. [Google Scholar]
- Lacombe A, Wu VCH, Tyler S, Edwards K. Antimicrobial action of the American cranberry constituents; phenolics, anthocyanins, and organic acids against Escherichia coli O157:H7. Int J Food Microbiol. 2010;139:102–107. doi: 10.1016/j.ijfoodmicro.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Lin YT, Labbe RG, Shetty K. Inhibition of Listeria monocytogenes in fish and meat systems using oregano and cranberry synergies. Appl Environ Microbiol. 2004;70:5672–5678. doi: 10.1128/AEM.70.9.5672-5678.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlo SM, Eloff JN. Acetone leaf extracts of Breonadia salicina (Rubiaceae) and ursolic acid protect oranges against infection by Penicillium species. South Afr J Botany. 2014;93:48–53. doi: 10.1016/j.sajb.2014.03.003. [DOI] [Google Scholar]
- Murphy MM, Spungen JH, Bi X, Barraj LM. Fresh and fresh lean pork are substantial sources of key nutrients when these products are consumed by adults in the United States. Nutrition Res. 2011;31(10):776–783. doi: 10.1016/j.nutres.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Nohynek LJ, Alakomi HL, Kähkönen MP, Heinonen M, Helander IM, Oksman-Caldentey KM, Riitta H, Puupponen-Pimiä RH. Berry phenolics: antimicrobial properties and mechanisms of action against severe human pathogens. Nutrition Cancer. 2006;54(1):18–32. doi: 10.1207/s15327914nc5401_4. [DOI] [PubMed] [Google Scholar]
- Novak JS, Juneja VK. Effects of refrigeration or freezing on survival of Listeria monocytogenes Scott A in under-cooked ground beef. Food Control. 2003;14:25–30. doi: 10.1016/S0956-7135(02)00048-8. [DOI] [Google Scholar]
- Onyango LA, Dunstan RH, Gottfries J, von Eiff C, Roberts TK. Effect of low temperature on growth and ultra-s of Staphylococcus spp. PLoS ONE. 2012;7(1):e29031. doi: 10.1371/journal.pone.0029031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puupponen-Pimiä R, Nohynek L, Hartmann-Schmidlin S, Kähkönen M, Heinonen M, Määttä-Riihinen K, Oksman-Caldentey KM. Berry phenolics selectively inhibit the growth of intestinal pathogens. J Appl Microbiol. 2005;98:991–1000. doi: 10.1111/j.1365-2672.2005.02547.x. [DOI] [PubMed] [Google Scholar]
- Raghavan S, Richards MP. Comparison of solvent and microwave extracts of cranberry press cake on the inhibition of lipid oxidation in mechanically separated Turkey. Food Chem. 2007;102:818–826. doi: 10.1016/j.foodchem.2006.04.049. [DOI] [PubMed] [Google Scholar]
- Rauha JP, Remes S, Heinonen M, Hopia A, Kähkönen M, Kujala T, Pihlaja K, Vuorela H, Vuorela P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int J Food Microbiol. 2000;56:3–12. doi: 10.1016/S0168-1605(00)00218-X. [DOI] [PubMed] [Google Scholar]
- Shai LJ, McGaw LJ, Aderogba MA, Mdee LK, Eloff JN. Four pentacyclic triterpenoids with antifungal and antibacterial activity from Curtisia dentata (Burm.f) C.A. Sm. leaves. J Ethnopharmacol. 2008;119:238–244. doi: 10.1016/j.jep.2008.06.036. [DOI] [PubMed] [Google Scholar]
- Singh AP, Wilson T, Kalk AJ, Cheong J, Vorsa N. Isolation of specific cranberry flavonoids for biological activity assessment. Food Chem. 2009;116:963–968. doi: 10.1016/j.foodchem.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke W, Pérez-Cueto FJA, de Barcellos MD, Krystallis A, Grunert KG. European citizen and consumer attitudes and preferences regarding beef and pork. Meat Sci. 2010;84:284–292. doi: 10.1016/j.meatsci.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Wang SY, Stretch AW. Antioxidant capacity in cranberry is influenced by cultivar and storage temperature. J Agric Food Chem. 2001;49(2):969–974. doi: 10.1021/jf001206m. [DOI] [PubMed] [Google Scholar]
- Wu VCH, Qiu XJ, Bushway A, Harper L. Antibacterial effects of American cranberry (Vaccinium macrocarpon) concentrate on foodborne pathogens. LWT Food Sci Tech. 2008;41:1834–1841. doi: 10.1016/j.lwt.2008.01.001. [DOI] [Google Scholar]
- Wu VCH, Qiu XJ, de los Reyes BG, Lin CS, Pan YP. Application of cranberry concentrate (Vaccinium macrocarpon) to control Escherichia coli O157:H7 in ground beef and its antimicrobial mechanism related to the down regulated slp, hdeA and cfa. Food Microbiol. 2009;26(1):32–38. doi: 10.1016/j.fm.2008.07.014. [DOI] [PubMed] [Google Scholar]