Abstract
White wheat flour is a poor source of dietary fiber. Therefore a demand exists for enrichment of bread with non-digestible prebiotic ingredients that exert health-promoting effects. In this study, the effects of beta-glucan (BG) and resistant starch (RS) on the dough properties and bread-making characteristics were investigated. The water absorption of doughs increased with increasing BG and RS levels. Also, development time and farinograph quality number of BG-enriched doughs remained similar to that of the control while the doughs stability decreased, and all of these values decreased when the RS was added. BG was more effective in increasing the dough softening than RS. The resistance to deformation, energy, maximum resistance and ratio number values; increased with the addition of RS or BG, but their extensibility was decreased in comparison to the control. Formulation containing BG/RS combination showed the best farinograph (development time, stability) and extensograph (resistance and extensibility) parameters. The application of BG and RS had similar effect on specific volume, and moisture content while it caused a decrease in firmness after 5 days of storage.
Keywords: Prebiotic, Bread quality, Beta-glucan, Resistant starch, Dough rheology
Introduction
White bread is a staple food in the human diet in many countries and a popular and convenient cereal product. However, it is a poor source of dietary fiber, containing typically <2.5% fiber. In fact, white wheat bread is commonly used as a high glycemic index reference in glycemic response (Brouns et al. 2005). A demand therefore exists for the development of bread with substances that are non-digestible polysaccharides or partially resistant to the digestive process. Some components such as skim milk, fat, hydrocolloids, bran, acids, emulsifiers, carbohydrates, dietary fibers and gluten could be added to breads formulation to improve its organoleptic properties, nutritional value, and shelf life (Kaur and Singh 1999; Gujral and Singh 1999).
Functional foods either contain (or be added as) a component with a positive health effect or eliminate a component with a negative one, as they must be safe, healthy and tasty (Ejtahed et al. 2011). Addition of healthy components such as prebiotics to food products is a common approach to the development of these kinds of foods (Ferdousi et al. 2013). According to the definition by Roberfroid (2000), prebiotics are non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or more bacteria (probiotics) in the gastrointestinal tract, and thus improve host health (Homayouni 2009; Homayouni et al. 2012a).
Beta-glucan (BG) could be found in many natural sources such as barley, oat, yeast, bacteria, mushrooms and algae (Zhu et al. 2015) and they have different biological activities due to different molecular weights and sources (Du and Xu 2014). For example, BG from cereals decreases blood glucose and cholesterol levels (Zhu et al. 2015), but fungal BG improves the function of immune system (Du et al. 2015). Oat BG is a water soluble dietary fiber which mainly consists of the unbranched polysaccharides composed of (1 → 3), (1 → 4) linked β-d-glucopyranosyl units (Zhu et al. 2016). BGs are main components of starchy endosperm and aleurone cell walls of commercial cereals such as oat, barley and wheat grains. The cereals have BG from 1% in wheat grains, to 3–7% in oats, and 5–11% in barley (Lazaridou and Biliaderis 2007; Tessari and Lante 2017). Barley is the richest source for BG but has not been used in bakery products because it lacks gluten proteins and the end-products have poor sensory characteristics. Recent studies have demonstrated that BG-enriched wheat flour can produce dough with acceptable properties. BG has several physiological effects including the regulation of blood sugar and the lowering of the glycemic response because of viscosity development in the gut after a meal, therefore assisting in the control of diabetes, the reduction of serum cholesterol levels and the prevention of cancer (Daou and Zhang 2012; Tosh 2013; Ekström et al. 2017).
Starch is classified into three general types based on its rate of digestion: rapidly digestible (RDS), slowly digestible (SDS) and RS. RS, which commonly used as the dietary fiber in bread making, is resistant to digestion in the stomach, small intestine and colon, because RS is passing directly through them and is a natural component that is present in many foods, having some nutritional benefits such as positive effects on the digestive system, blood cholesterol levels, and glycemic response (Altuna et al. 2015; Hoffmann Sardá et al. 2016; Wang et al. 2016). When it reaches the large intestine, RS is fermented by large bowel microbiota. This increases the production of short-chain fatty acids (SCFAs) and fecal volume, but reduces the pH of feces. As a result, RS lowers blood glucose levels and therefore assists in the control of diabetes and plasma cholesterol levels, and therefore positively influences the function of the digestive tract and microbiota (Jenkins et al. 1998). RS is a linear polysaccharide of (1 → 4) α-d-glucan, essentially derived from the retrograded amylose fraction, and has a relatively low molecular weight (1.2 × 105 Da) (Tharanathan 2002). Therefore, RS levels are slightly increased by certain food processing methods, such as retorting, baking or drying at high temperatures (Homayouni et al. 2014).
Prebiotic dosage of BG and RS
In fact, the US Food and Drug Administration has claimed that the consumption of at least 3 g of BG per day, or 4 servings of a food product that supplies at least 0.75 g of BG per serving, has cholesterol lowering properties, with an effect on reducing the risk of heart disease (FDA 2008). Although the minimum healthy dose of RS is about 20 g/day (Cassidy et al. 1994), a low dosage in the range of 2.5–5 g/day has showed prebiotic effects as well (Bouhnik et al. 2004; Homayouni et al. 2012b; Homayouni et al. 2014).
The amount of RS and BG used in flour fortification depends on the particular starch being used, extraction procedures, the application, and other factors such as dosage and molecular weight. To our knowledge, no systematic studies have yet been performed on the effect of the supplementation of wheat flour with purified BG and RS in prebiotic dosage on dough rheology and bread characteristics. Therefore the present investigation was done to study the dough rheological and bread-making properties when supplemented with BG and RS in prebiotic dosages, in order to improve the nutritional quality of bread.
Materials and methods
Materials
White wheat flour, salt and yeast were purchased from local super markets. RS (high amylose corn starch) was obtained from Hi-maize 260, National Starch, USA. BG was supplied by promOat™, Biovelop international AB Company, Sweden.
Methods
Flour quality assessment
All of the quality tests for white wheat flour were performed in accordance with established official procedures used to characterize wheat flour properties: protein, moisture, ash, wet gluten, gluten index, sedimentation value and falling number were determined by using standard AACC methods (AACC 2000).
Farinograph tests
Separate doughs were fortified with at levels of 0.8, 1, 1.2% w/w BGs, 5.5, 8, 10.5% w/w RS, and one sample with the combination of 0.5% BG and 4% RS (w/w). All samples were calculated on a flour dry weight basis and were tested according to the AACC Method 2000 (method 54-21). The BG and RS in a dry powder form were first blended well with the wheat flour into the mixing bowl (300 g) of the farinograph (Brabender, Duisburg, Germany) that was connected with a circulating water pump and a thermostat which operated at 30 ± 0.2 °C. Farinograph water absorption, dough development time, dough stability, softening degree and farinograph quality number were then determined.
Extensograph tests
The control (unfortified), BG, and RS-enriched dough, as well as BG and RS enriched dough, at first were each prepared in the 300 g mixing bowl of the farinograph (Brabender, Duisburg, Germany). Salt and water were then added to produce the dough samples with a consistency of 500 BU (Brabender Units), followed by 5 min of mixing. A test piece (150 g) was shaped into a ball, shaped into a cylinder and clamped into the fermenting cabinet. After 45, 90, and 135 min reaction times in the fermenting cabinet at 30–32 °C, each dough piece was stretched in the Brabender extensograph by a hook until rupture, as described in the AACC Method 2000 (method 54-10). The stretching force was recorded as a function of time, and the resistance to constant deformation (resistance to extension), the extensibility and energy in 45, 90, 135 min. Only the maximum resistance at 135 min and ratio number values were discussed.
Bread-making process
A uniform dough bread-making process was employed. The basic (control dough) formula on 100 g flour (at 14% moisture basis) consisted of salt (2 g), compressed yeast (2 g) and the amount of water required reaching 500 BU of consistency by the farinograph. Wheat flour was blended well with each of BGs at levels of 0.8, 1, 1.2% w/w (wheat flour basis), RS at levels of 5.5, 8, 10.5% w/w, and one sample with the combination of 0.5% BG and 4% RS (w/w). Bread doughs were prepared by mixing all ingredients and fermenting in two-steps. After the first step, the fermented doughs were divided into four 500 g pieces, hand-moulded and put into tin pans for 45 min of proofing at 30 °C and then baked at 230 °C for 25 min. Following baking, the bread loaves were cooled at ambient conditions (for 2 h). Subsequently, for the aging effect on some quality parameters, breads were packed in sealed plastic bags at room temperature for 1, 3 or 5 days.
Specific volume
Weight and volume were measured 2 h after removal of bread loaves from the oven. The loaf volume was determined by the rapeseed displacement method and specific volume was calculated by dividing the volume by loaf weight (cm3/g).
Moisture content determination
Bread samples were stored for 1, 3 and 5 days (at 25 °C) in plastic bags, moisture content was determined according to the AACC method 44-16 (2000).
Bread firmness
Bread samples were stored at room temperature in plastic bags in order to determine the bread staling at 1, 3, and 5 days after preparation. This was performed with the Instron M350-10CT (500 N load cell, Rochdale, England) texture analyzer, using a probe of 36 mm diameter, according to the AACC method 74-09 (2000). Compression tests were recorded on two slices from the center of each loaf, with an average thickness of 25 mm, cut with a knife. Samples were compressed to 40% at 100 mm/min speed. Measurements from 3 bread loaves were taken for each formulation and sampling time.
Sensory analysis
Bread samples were subjected to sensory evaluation by a panel of eight trained individuals (ranging in age from 25 to 35, non-smokers) at room temperature. The parameters were evaluated using the scoring system recommended by Pyler (1973). The maximum scores for each parameter were: chewiness 15, crust 15, texture 15, color of crumb 10, appearance 10, aroma 15 and taste/flavor 20.
Statistical analysis
All experiments were carried out in triplicate and data were collected as mean ± SD or Median (Minimum to Maximum). Differences among means were identified by ANOVA (Analysis of Variance), followed by the Duncan’s multiple range test and considering significant P < 0.05. Kruskal–Wallis and post hoc tests were used to analyze the sensory data. These calculations were performed by the SPSS version 13 (SPSS INC, IL, Chicago, USA).
Results and discussion
Physicochemical properties of wheat flour were: 10.43% protein content, 0.629% ash content, 13.15% moisture content, 24.90% wet gluten, 72 gluten index, 22 (ml) sedimentation value and 557 (s) falling number value.
Farinograph measurements
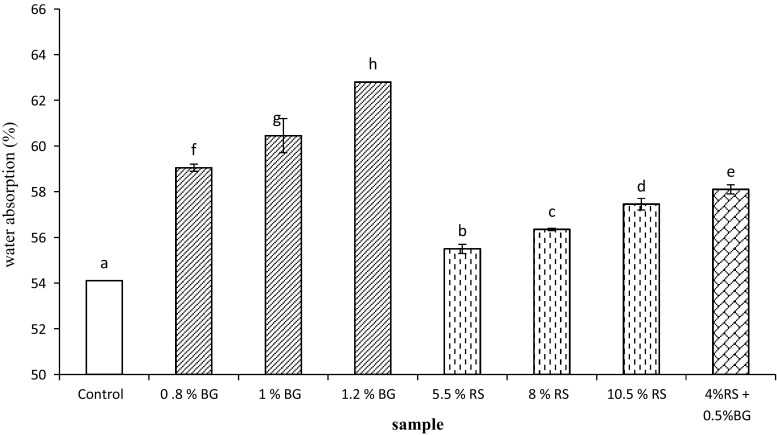
Figure 1 compares the water absorption results of prebiotics dough and control dough (without prebiotics addition). Both BG and RS increased the water absorption capacity however BG caused greater increase. RS increased moisture content of dough because of higher amylose content which has higher binding capacity than native starch (Zhiqiang et al. 1999). Also, increased water absorption has been reported in other studies where BG from different sources was used to fortify wheat flours (Skendi et al. 2009).
Fig. 1.
Comparison of water absorption values in dough supplemented with prebiotic components
According to the findings of Rosell et al. (2001) and Lazaridou et al. (2007), different hydrocolloids and dietary fiber increased water absorption due to the great number of hydroxyl groups existing in the fiber structure, which allow more water interactions through hydrogen bonding. Therefore, in this study, increasing dough water absorption was expected due to the high water absorption capacity of BG and RS. Such effects have been related to the high water absorbing capacity of these polysaccharides and their ability to compete for water with other components in dough system.
The amount of water added to the flour is usually adjusted to reach 500 BU at optimum development time; at this hydration level, high quality bread was produced. Therefore, the addition of these substances to the flour showed beneficial impact on bread-making properties.
Table 1 shows effect of supplemented BG and RS on farinographic character of flour. Addition of RS to the dough formula decreased the development time significantly (except for 8% RS) but the doughs with combination of BG and RS or only BG were similar to the control dough (except for 1% BG). Overall, the magnitude of the changes in development time of dough fortified with RS was greater than control dough.
Table 1.
Farinograph characteristics of dough supplemented with beta glucan (BG) and resistant starch (RS)
| Sample | Water absorption (%) | Development time (min) | Stability (min) | Degree of softening (BU) | Farinograph quality number |
|---|---|---|---|---|---|
| Control | 54.10 ± 0.00a | 1.95 ± 0.25c | 4.65 ± 0.15e | 97.50 ± 0.50a | 49.00 ± 1.00c |
| 0.8% BG | 59.05 ± 0.15f | 1.60 ± 0.20a,b,c | 2.95 ± 0.15c | 122.00 ± 2.00c | 38.50 ± 1.50b,c |
| 1% BG | 60.45 ± 0.75g | 1.55 ± 0.35a,b | 2.20 ± 0.90a,b | 133.00 ± 15.00d | 31.00 ± 16.00a,b |
| 1.2% BG | 62.80 ± 0.00h | 1.75 ± 0.05b,c | 2.70 ± 0.20b,c | 125.00 ± 1.00 c,d | 43.50 ± 2.50c |
| 5.5% RS | 55.50 ± 0.20b | 1.50 ± 0.00a,b | 3.25 ± 0.15c,d | 105.00 ± 4.00a,b | 27.50 ± 0.50a |
| 8% RS | 56.35 ± 0.05c | 1.60 ± 0.10a,b,c | 3.10 ± 0.40c | 103.50 ± 2.50a,b | 28.00 ± 2.00a |
| 10.5% RS | 57.45 ± 0.25d | 1.30 ± 0.10a | 2.00 ± 0.20a | 111.00 ± 0.00b | 23.50 ± 1.50a |
| 4% RS + 0.5% BG | 58.10 ± 0.20e | 1.70 ± 0.20b,c | 3.80 ± 0.10d | 102.50 ± 3.50a,b | 46.50 ± 3.50c |
Presented data are mean value of three replication ± SD. Means in columns followed by a different letter are significantly different (P < 0.05)
The farinograph properties of wheat flours are strongly dependent on protein content and quality. There was a direct relationship between the protein content and development time of different kinds of flour. Thus this reduction can be resulted from decreasing protein content that led to rapid development and hydration. There was a progressive (P < 0.05) decrease in dough’s stability value when BG, RS and their combination were added.
The combination of BG and RS in dough resulted to the same stability as the control dough. In contrast Mohamed et al. (2005) found that, with increased level of BG, there was no significant reduction in dough stability. It was noticed that the degree of softening increased with increasing the amount of BG, but RS had less effect than BG on dough softening and only the dough containing 10.5% RS increased the softening significantly (Table 1). The soften of doughs containing both of BG/RS was similar to the control. Dilution of gluten and interaction between fiber and gluten may result in an increase to the degree of dough softening. This conclusion confirmed the findings of MIŚ et al. (2012) which investigated the impact of adding fiber and oat whole meal to bread. In general, in comparison with the control dough, RS has affected softening less than BG. Although there was no significant difference between control and all samples, the addition of RS and 1% BG to the doughs formula decreased the farinograph quality number (FQN) which seems to be the result of BG and starch wheat flour complex.
According to Miyazaki et al. (2006), gluten makes the original dough network structure and plays an important role in dough and bread-making properties. Therefore, the dilution of gluten by bran leads to dough deterioration, breaking of the starch–gluten network structure and a decrease of consistency (Miś et al. 2012). Thus the substitution of some part of wheat flour with RS and BG resulted in protein content reduction, which was more significant in greater substitution levels. In contrast, some studies showed that, a few kinds of hydrocolloids and dietary fibers improved rheological properties of wheat flour such as water absorption, dough development time and dough stability and resistance (Lazaridou et al. 2007; Skendi et al. 2009). On the other hand, the dilution of gluten with fiber solely cannot explain all of the observed changes in the addition of fiber to wheat flour. The different results from the impact of various kinds of fibers on dough properties can be explained by reaction between fibers and wheat flour gluten protein. Also, discrepancies related to the influence of BG and RS on the dough and the bread quality properties may arise from differences in the molecular size, solubility and the concentration range of the polysaccharides, as well as the flour types used for supplementation among the various studies (Skendi et al. 2010).
Effect of BG and RS on extensograph parameters
The effects of BG, RS and combination of RS and BG addition on the extensograph parameters throughout 135 min of proofing time are shown in Table 2 and Fig. 2. At all resting times, all enriched samples exhibited higher resistance to extension values than did the control (except for 45 min). On the other hand, the lowest resistance values were obtained in the control dough. Overall, the resistance to extension values at all resting times for RS-enriched doughs were higher than BG-enriched doughs (except for BG 1.2% dough). The dough supplemented with both BG and RS seemed to result in resistance to extension values similar to the doughs with RS. This confirms the findings of Skendi et al. (2010) that showed that the addition of BG to poor bread-making quality flour increased extensibility and resistance to extension values up to the good bread-making quality flour. Thus, the addition of BG and RS resulted in an enhancement of the gas retention properties of dough and possible improvement of the gluten network structure during proofing.
Table 2.
Extensograph characteristics of dough supplemented with beta glucan (BG), resistant starch (RS) (at different level concentration) and the dough with both BG 0.5% and RS 4%
| Sample | Resistance to extension (BU) | Extensibility (mm) | Energy (cm2) | Maximum resistance (BU) | Ratio number | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 45 min | 90 min | 135 min | 45 min | 90 min | 135 min | 45 min | 90 min | 135 min | 135 min | 135 min | |
| Control | 211.50 ± 0.50c,d | 180.50 ± 4.50a | 155.50 ± 6.50a | 133.50 ± 2.50b,c | 135.00 ± 7.00d | 130.00 ± 1.00c | 45.00 ± 1.00a | 37.00 ± 1.00a | 30.00 ± 2.00a | 156.00 ± 6.00a | 1.20 ± 0.00a |
| 0.8% BG | 192.00 ± 2.00a | 192.00 ± 3.00a | 179.50 ± 0.50b | 140.50 ± 4.50c,d | 137.50 ± 1.50d | 120.00 ± 4.00b | 42.50 ± 2.50a | 39.50 ± 0.50a,b | 33.00 ± 1.00b | 192.00 ± 5.00b | 1.45 ± 0.05b |
| 1% BG | 199.00 ± 5.00a,b | 212.00 ± 0.00b | 180.00 ± 18.00b | 150.50 ± 1.50c,d | 121.50 ± 0.50b,c | 109.50 ± 8.50a | 43.00 ± 3.00a | 39.50 ± 0.50a,b | 29.50 ± 0.50a | 194.00 ± 20.00b | 1.70 ± 0.30c |
| 1.2% BG | 205.50 ± 1.50b,c | 275.50 ± 1.50d | 252.50 ± 7.50e | 139.00 ± 3.00c | 124.50 ± 1.50c | 107.00 ± 1.00a | 46.00 ± 1.00a | 51.50 ± 1.50e | 40.00 ± 1.00d,e | 253.00 ± 8.00f | 2.35 ± 0.05e |
| 5.5% RS | 218.50 ± 1.50d,e | 240.50 ± 0.50c | 225.00 ± 5.00d | 133.50 ± 10.50b,c | 118.50 ± 0.50a,b | 112.50 ± 2.50a | 46.00 ± 4.00a | 43.50 ± 0.50d,c | 38.00 ± 0.00c,d | 228.50 ± 3.50d,e | 2.00 ± 0.10d |
| 8% RS | 232.00 ± 16.00f | 238.50 ± 5.50c | 240.50 ± 14.50d,e | 121.00 ± 10.00a | 114.00 ± 0.00a | 123.00 ± 1.00b | 42.50 ± 0.50a | 41.00 ± 1.00b,c | 43.50 ± 2.50f | 244.00 ± 14.00e,f | 1.95 ± 0.15c,d |
| 10.5% RS | 218.50 ± 0.50d,e | 221.50 ± 7.50b | 203.50 ± 15.50c | 126.00 ± 5.00a,b | 118.00 ± 5.00a,b | 122.00 ± 1.00b | 44.00 ± 2.00a | 40.00 ± 1.00b | 36.50 ± 2.50c | 205.50 ± 15.50b,c | 1.70 ± 0.10c |
| 4% RS + 0.5% BG | 227.00 ± 3.00e,f | 242.50 ± 19.50c | 221.00 ± 11.00c,d | 131.50 ± 6.50a,b,c | 122.00 ± 1.00b,c | 125.50 ± 1.50b,c | 48.50 ± 3.50a | 45.50 ± 3.50d | 41.50 ± 1.50e,f | 222.00 ± 11.00c,d | 1.80 ± 0.10c,d |
Presented data are mean value of triplicates ± SD
Mean values signed with the same letters in particular columns are non-significant at 0.05 level of confidence
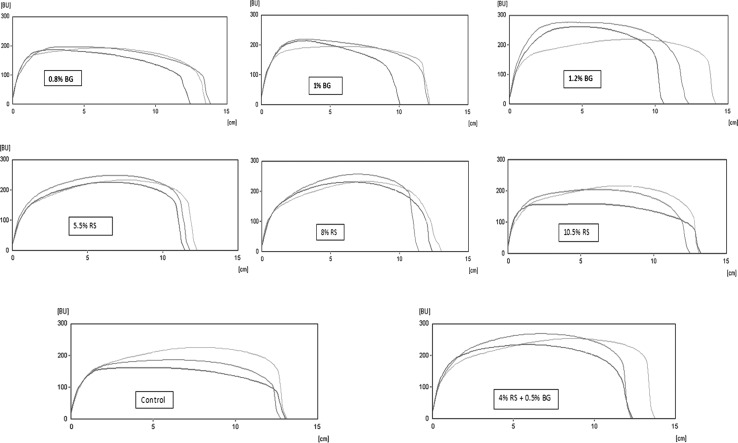
Fig. 2.
The impact of prebiotic beta glucan (BG), resistant starch (RS) on extensogram of doughs. The blue curves indicate proofing time during 45 min, red and green curves indicate it during 90 and 135 min respectively (colour figure online)
The extensibility of BG-enriched dough at 45 min proofing time remained similar but decreased after 90 and 135 of min proofing times compared to the control (Table 2). However, then were lower for doughs supplemented with RS at 135 min proofing time. RS produced a greater decreasing effect on the extensibility than BG (except for at 135 min) and the dough sample containing both BG and RS was similar to the control dough in extensibility values (except for 90 min). Desirable dough properties are usually associated with good dough resistance and extensibility values (Skendi et al. 2010). Therefore, desirable extensograph parameters (resistance and extensibility) were observed in the dough containing both BG and RS (Fig. 2).
At all resting times, the lowest energy values were obtained for control dough, whereas the dough supplemented with RS and BG had increased energy values. Generally, the results showed that increasing supplementation level increased the energy value of dough, with the exception of the sample containing 10.5% RS. Besides, energy values of dough with both BG and RS were generally higher than those of doughs with either BG or RS. The energy values of all doughs after 135 min of proofing time had decreased. The maximum resistance and ratio number values of doughs with BG, RS (at all substitution levels) and those with both of them were significantly higher than the control dough (only 135th minute values were discussed). It has been reported that maximum resistance and energy under extensograph can be used as indicators of dough strength (Preston and Hoseney 1991), thus the results of maximum resistance and energy at extensograph indicated that BG addition increases dough strength.
Specific volume
Specific volume values for loaves of bread are presented in Table 3. The volume of bread with addition of BGs was similar to the control whereas the addition of RS to flour reduced the specific volume of the bread and was similar to the control at 8% RS supplementation. Besides, the lowest specific volume was observed when combination of BG and RS was used. Similar behavior was observed by Korus et al. (2009) for gluten-free flour substituted with RS. Izydorczyk and Dexter (2008) reported a reduction in volume of wheat bread fortified with BG. This may depended on the molecular size, concentration of BGs and quality of wheat flour used (Skendi et al. 2010). It is generally agreed that addition of some hydrocolloids to wheat flour reduces loaf volume. This result can be explained by several phenomena:
Dilution of gluten.
Disruption of the gluten network structure by interactions between gluten and hydrocolloid material.
The strong water absorbing properties of hydrocolloids may suppress the amount of steam generated, resulting in reduced loaf volume (Gill et al. 2002).
Table 3.
Moisture content and crumb firmness of bread loaves stored for 1, 3 and 5 days at room temperature and specific volumes of breads
| Sample | Moisture content (%) | Crumb firmness (N) | Specific volume (cm3/g) | ||||
|---|---|---|---|---|---|---|---|
| 1 day | 3 days | 5 days | 1 day | 3 days | 5 days | ||
| Control | 35.08 ± 0.261,a | 34.19 ± 1.481,a | 26.18 ± 0.301,a | 1.03 ± 0.001,d | 1.10 ± 0.131,b,c | 6.95 ± 2.222,e | 3.68 ± 0.12c |
| 0.8% BG | 35.32 ± 0.481,a,b | 33.90 ± 0.192,a | 32.67 ± 0.903,d | 0.72 ± 0.121,b | 0.73 ± 0.111,2,a | 1.33 ± 0.322,a | 3.61 ± 0.01b,c |
| 1% BG | 37.01 ± 0.441,b,c | 32.11 ± 1.501,a | 28.11 ± 0.201,a,b | 0.92 ± 0.041,c,d | 0.91 ± 0.091,a,b | 4.05 ± 0.862,c,d | 3.62 ± 0.08b,c |
| 1.2% BG | 37.77 ± 1.171,c | 34.86 ± 1.881,a | 28.77 ± 0.791,a,b | 0.71 ± 0.071,b | 0.89 ± 0.062,a,b | 2.40 ± 0.233,a,b,c | 3.44 ± 0.20b,c |
| 5.5% RS | 36.26 ± 0.951,a,b,c | 35.33 ± 1.261,a | 29.24 ± 0.771,b,c | 0.51 ± 0.001,a | 0.69 ± 0.012,a | 1.76 ± 0.223,a,b | 2.95 ± 0.32a |
| 8% RS | 34.99 ± 1.561,a | 34.52 ± 1.641,a | 29.27 ± 3.361,b,c | 0.62 ± 0.081,a,b | 0.79 ± 0.011,a | 3.65 ± 0.132c,d | 3.62 ± 0.05b,c |
| 10.5% RS | 34.89 ± 1.281,a | 33.32 ± 1.151,a | 32.04 ± 2.361,c,d | 0.84 ± 0.091,c | 1.23 ± 0.071,c | 3.03 ± 0.562,b,c | 3.36 ± 0.06b |
| 4% RS + 0.5% BG | 34.68 ± 1.061,a | 31.79 ± 3.641,a | 30.27 ± 1.481,b,c,d | 1.26 ± 0.031,e | 2.94 ± 0.301,d | 5.27 ± 0.401,d | 2.97 ± 0.06a |
Presented data are mean values ± SD of triplicates
Values followed by the same small letter within the same column or same numbers in the same line are not significantly different (P > 0.05)
Another possible explanation for the decrease in bread volume with addition of RS to wheat flour is that during baking RS undergoes gelatinization (Miyazaki et al. 2006), while starch was not hydrolyzed by amylolytic enzymes produced by yeast. Yeast is therefore not able to utilize RS during fermentation, slowing down the overall fermentation process. The presence of undamaged starch granules could induce instability in cell walls (Liu and Scanlon 2003). These factors can negatively impact bread volume and cause uneven distribution of gas cells. The results of this study demonstrated that BG-enriched breads had greater specific volumes than those supplemented with RS. Therefore, the type and level of prebiotic supplementation seems to affect the rheological properties of the dough and consequently influence the specific loaf volume of the fortified bread loaves.
Influence of the BG and RS addition on moisture content of stored breads
By increasing level of BG addition, there was a continuous increase in moisture content of bread on the first day of storage (Table 3). Wang et al. (2017) enriched wheat flour with oat β-glucan, in a way that substituted wheat flour with oat β-glucan at levels varying from 1 to 5% (g/g), and showed similar behavior (Wang et al. 2017).
After 5 days of storage, all samples showed higher moisture content than did the respective control breads. Whereas, at the first day storage, only those breads made from BG had higher moisture content than their control. This demonstrated that the water retention capacity of the fortified bread with RS was higher than those containing BG. On the other hand, the rate of moisture content decreased in bread fortified with RS was lower than those with BG.
The moisture content of each of the bread did not decrease significantly during storage, except for those with 0.8% BG (Table 3). The results showed no significant difference among moisture contents of breads on the third day of storage.
Bread firmness
Generally crumb firmness of the breads decreased with addition of BG and RS. In previous studies, crumb softening was reported with the addition of hydrocolloids (Korus et al. 2009; Skendi et al. 2010).
Hardness increased by increasing BG and RS levels (on the first and fifth day of storage), but the values were generally smaller than the control (Table 3). The texture results showed that the firmness values of all BG and RS-supplemented bread crumbs, after 3 days of storage, were similar to or significantly lower than the control (except for breads with 10.5% RS and both BG and RS) (Table 3).
The increase in hardness of the bread crumb fortified with RS, at the high level of addition (10.5%), may be a consequence of the thickening of the wall surrounding gas cells, as proposed by Rosell et al. (2001). The bread fortified with both BG and RS exhibited higher firmness than the control after 1 and 3 days of storage. Crumb firmness of all breads was smaller than the control, after 5 days of storage. However, the hardening of each sample increased significantly during storage (expect for breads with the combination of BG and RS).
The decrease in the crumb firmness observed when BG and RS was added to the bread formula could be attributed to their higher water retention capacity, and a possible inhibition of the amylopectin retrogradation (Biliaderis et al. 1995), or it may be a consequence of an increase in the total area of gas cells (Skendi et al. 2010).
The effects of the hydrocolloids on starch gel structure and mechanical properties results from two opposite phenomena. First was due to an increase in the hardening as a consequence of the decrease in the swelling of the starch granules. Hydrocolloid added to wheat flour would compete for water with native wheat starch granules in the dough. This, in turn, might limit swelling and solubilisation of the starch during baking (Gill et al. 2002) and reduces the amylose leaking from the granules. The second was due to weakening effect on the composite starch network structure may be attributed to inhibition of the formation of cross-links among swollen granules. When there are no changes in the moisture content, the formation of a cross-linked network rather than the development of amylopectin crystallites in the ageing gluten–starch composite matrix causes bread firming (Schiraldi et al. 1996). In the present study, no significant losses of moisture in breads after 5 days of storage were noted, whereas the firmness of the bread crumbs increased significantly.
Hung et al. (2007) noticed that the rate of crumb hardening is influenced by water content and it is considered as one of the most important factors for bread staling. In this study the water content of BG and RS enriched breads was significantly higher than the control bread, so all breads exhibited softer breadcrumbs than the control measured after 5 days of storage.
Bread firmness is usually used as a tool to measure bread staling. However, staling is a very complicated process that cannot be explained by a single variant. It is perhaps a combination of these factors that determine the overall effect on the mechanical properties of the bread structure, an effect that is dependent on each specific hydrocolloid used for fortification.
Sensory
The sensory evaluation of the fresh bread was performed by eight trained panelists. The median, minimum and maximum ranks of breads characteristics supplemented with BG and RS, such as appearance, crust, texture, aroma, and taste/flavor are presented in Table 4. Only color and chewiness scores of breads were not significantly influenced by the addition of BG, RS and combination of RS/BG compared to the control.
Table 4.
Sensory scores of breads supplemented with beta glucan (BG), resistant starch (RS) (at different level concentration) and the dough with both BG 0.5% and RS
| Sample | Appearance | Crust | Aroma | Texture | Taste | Colour | Chewiness | Total score |
|---|---|---|---|---|---|---|---|---|
| Med | Med | Med | Med | Med | Med | Med | Med | |
| Control | 7.5a,b,c | 13.0a,b | 13.5a,b,c | 13.0a,b | 17.0a,b | 9.0a | 12.0a | 84.5a,b |
| 0.8% BG | 6.0a,b | 12.5a,b | 14.0b,c | 10.5a,b | 17.5b | 10.0a | 14.0a | 82.5a,b |
| 1% BG | 7.5a,b,c | 13.0a,b | 13.0a,b,c | 13.5a,b | 15.0a,b | 9.5a | 13.5a | 83.0a,b |
| 1.2% BG | 8.0a,b,c | 12.0a,b | 12.5a,b,c | 13.0a,b | 16.5a,b | 9.5a | 14.0a | 82.0a,b |
| 5.5% RS | 8.0b,c | 12.5a,b | 11.5a,b,c | 12.0a,b | 17.5a,b | 10.0a | 14.0a | 82.5a,b |
| 8% RS | 10.0d | 15.0b | 15.0c | 12.5b | 16.5a,b | 10.0a | 14.5a | 94.5b |
| 10.5% RS | 9.0c | 12.5a,b | 11.0a,b | 12.0a,b | 17.5a,b | 10.0a | 11.5a | 82.0a,b |
| 4% RS + 0.5% BG | 5.5a | 8.0a | 10.0a | 9.5a | 11.5a | 9.0a | 10.5a | 68.0a |
| P value | 0.000 | 0.011 | 0.020 | 0.039 | 0.045 | 0.365 | 0.310 | 0.019 |
Values are medians (minimums to maximums) of sensory evaluation; Different letters in the same column are significantly different (P < 0.05)
Results from sensory evaluation indicated that neither adding BG nor RS had any significant effects on appearance, crust, texture and total scores. Breads containing both of BG/RS were less appealing than breads containing 8% RS. RS has less effect on sensory properties than the other dietary fibers.
Panelists preferred the appearance properties of breads containing RS more than the bread enriched with both of BG/RS. Bread containing 8% RS was significantly better than all the other samples. Breads containing 0/8% BG had higher taste/flavor scores than breads containing both of BG/RS, whereas all sample breads were similar to the control. Increasing RS level from 8 to 10.5% caused a decrease in aroma scores. Results from sensory evaluation indicated that the use of these prebiotic compounds has no adverse effects on bread quality.
Conclusion
Β-glucan and resistant starch affected dough rheological properties significantly while not having an effect on the sensory properties of bread. Both of them were found to increase the shelf life of bread and decrease its staleness.
Acknowledgements
The authors would like to thank the Research Vice-Chancellor of Tabriz University of Medical Sciences for their financial support of this study.
References
- Altuna L, Ribotta PD, Tadini CC. Effect of a combination of enzymes on dough rheology and physical and sensory properties of bread enriched with resistant starch. LWT Food Sci Technol. 2015;64(2):867–873. doi: 10.1016/j.lwt.2015.06.024. [DOI] [Google Scholar]
- American Association of Cereal Chemists (AACC) Approved methods of American Association of Cereal Chemists. 10. St. Paul: American Association of Cereal Chemists (AACC); 2000. [Google Scholar]
- Biliaderis CG, Izydorczyk MS, Rattan O. Effect of arabinoxylans on bread-making quality of wheat flours. Food Chem. 1995;53:165–171. doi: 10.1016/0308-8146(95)90783-4. [DOI] [Google Scholar]
- Bouhnik Y, Raskine L, Simoneau G, Vicaut E, Neut C, Flourié B, Brouns F, Bornet FR. The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: a double-blind, randomized, placebo-controlled, parallel-group, dose-response relation study. Am J Clin Nutr. 2004;80:1658–1664. doi: 10.1093/ajcn/80.6.1658. [DOI] [PubMed] [Google Scholar]
- Brouns F, Bjorck I, Frayn K, Gibbs A, Lang V, Slama G, Wolever T. Glycaemic index methodology. Nutr Res Rev. 2005;18:145. doi: 10.1079/NRR2005100. [DOI] [PubMed] [Google Scholar]
- Cassidy A, Bingham S, Cummings J. Starch intake and colorectal cancer risk: an international comparison. Br J Cancer. 1994;69:937. doi: 10.1038/bjc.1994.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daou C, Zhang H. Oat beta-glucan: its role in health promotion and prevention of diseases. Compr Rev Food Sci Food Saf. 2012;11(4):355–365. doi: 10.1111/j.1541-4337.2012.00189.x. [DOI] [Google Scholar]
- Du B, Xu BJ. Oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) of β-glucans from different sources with various molecular weight. Bioact Carbohydr Diet Fibre. 2014;3:11–16. doi: 10.1016/j.bcdf.2013.12.001. [DOI] [Google Scholar]
- Du B, Lin CY, Bian ZX, Xu BJ. An insight into anti-inflammatory effects of fungal beta-glucan. Trends Food Sci Technol. 2015;41:49–59. doi: 10.1016/j.tifs.2014.09.002. [DOI] [Google Scholar]
- Ejtahed HS, Mohtadi Nia J, Homayouni Rad A, Niafar M, Asghari Jafarabadi M, Mofid V (2011) The effects of probiotic and conventional yoghurt on diabetes markers and insulin resistance in type 2 diabetic patients: a randomized controlled clinical trial. Iranian J Endocrinol Metab 13(1):1–8
- Ekström LMNK, Henningsson Bok EAE, Sjöö ME, Östman EM. Oat β-glucan containing bread increases the glycaemic profile. J Funct Foods. 2017;32:106–111. doi: 10.1016/j.jff.2017.02.027. [DOI] [Google Scholar]
- Ferdousi R, Rouhi M, Mohammadi R, Mortazavian AM, Khosravi-Darani K, Rad AH. Evaluation of probiotic survivability in yogurt exposed to cold chain interruption. Iran J Pharm Res. 2013;12(Suppl):139–144. [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Adminstration (FDA) (2008) Food labelling: specific requirements for health claims; health claims: soluble fiber from certain foods and risk of coronary heart disease (CHD). 21 Code of Federal Regulations, vol 2, pp 142–146
- Gill S, Vasanthan T, Ooraikul B, Rossnagel B. Wheat bread quality as influenced by the substitution of waxy and regular barley flours in their native and extruded forms. J Cereal Sci. 2002;36:219–237. doi: 10.1006/jcrs.2001.0458. [DOI] [Google Scholar]
- Gujral HS, Singh N. Effect of additives on dough development, gaseous release and bread making properties. Food Res Int. 1999;32:691–697. doi: 10.1016/S0963-9969(99)00148-9. [DOI] [Google Scholar]
- Hoffmann Sardá FA, Giuntini EB, Gomez MLPA, Lui MCY, Negrini JAE, Tadini CC, Lajolo FM, Menezes EW. Impact of resistant starch from unripe banana flour on hunger, satiety, and glucose homeostasis in healthy volunteers. J Funct Foods. 2016;24:63–74. doi: 10.1016/j.jff.2016.04.001. [DOI] [Google Scholar]
- Homayouni A (2009) Letter to the editor. Food Chem. 114(3):1073
- Homayouni A, Azizi A, Javadi M, Mahdipour S, Ejtahed H (2012a) Factors influencing probiotic survival in ice cream: a review. Int J Dairy Sci. 7(1):1–10
- Homayouni Rad A, Akbarzadeh F, Mehrabany EV (2012b) Which are more important: prebiotics or probiotics? Nutrition 28(11-12):1196–1197 [DOI] [PubMed]
- Homayouni A, Amini A, Keshtiban AK, Mortazavian AM, Esazadeh K, Pourmoradian S. Resistant starch in food industry: a changing outlook for consumer and producer. Starch-Stärke. 2014;66(1–2):102–111. doi: 10.1002/star.201300110. [DOI] [Google Scholar]
- Hung PV, Maeda T, Morita N. Dough and bread qualities of flours with whole waxy wheat flour substitution. Food Res Int. 2007;40:273–279. doi: 10.1016/j.foodres.2006.10.007. [DOI] [Google Scholar]
- Izydorczyk MS, Dexter JE. Barley β-glucans and arabinoxylans: molecular structure, physicochemical properties, and uses in food products. Food Res Int. 2008;41(9):850–868. doi: 10.1016/j.foodres.2008.04.001. [DOI] [Google Scholar]
- Jenkins DJA, Vuksan V, Kendall CWC, Würsch P, Jeffcoat R, Waring S, Mehling CC, Vidgen E, Augustin LSA, Wong E. Physiological effects of resistant starches on fecal bulk, short chain fatty acids, blood lipids and glycemic index. J Am Coll Nutr. 1998;17:609–616. doi: 10.1080/07315724.1998.10718810. [DOI] [PubMed] [Google Scholar]
- Kaur K, Singh N. Effect of acetic acid and CMC on rheological and baking properties of flour. J Food Qual. 1999;22:317–327. doi: 10.1111/j.1745-4557.1999.tb00560.x. [DOI] [Google Scholar]
- Korus J, Witczak M, Ziobro R, Juszczak L. The impact of resistant starch on characteristics of gluten-free dough and bread. Food Hydrocoll. 2009;23:988–995. doi: 10.1016/j.foodhyd.2008.07.010. [DOI] [Google Scholar]
- Lazaridou A, Biliaderis CG. Molecular aspects of cereal β-glucan functionality: physical properties, technological applications and physiological effects. J Cereal Sci. 2007;46:101–118. doi: 10.1016/j.jcs.2007.05.003. [DOI] [Google Scholar]
- Lazaridou A, Duta D, Papageorgiou M, Belc N, Biliaderis C. Effects of hydrocolloids on dough rheology and bread quality parameters in gluten-free formulations. J Food Eng. 2007;79:1033–1047. doi: 10.1016/j.jfoodeng.2006.03.032. [DOI] [Google Scholar]
- Liu Z, Scanlon MG. Predicting mechanical properties of bread crumb. Food Bioprod Process. 2003;81(3):224–238. doi: 10.1205/096030803322437992. [DOI] [Google Scholar]
- Miś A, Grundas S, Dziki D, Laskowski J. Use of farinograph measurements for predicting extensograph traits of bread dough enriched with carob fiber and oat wholemeal. J Food Eng. 2012;108:1–12. doi: 10.1016/j.jfoodeng.2011.08.007. [DOI] [Google Scholar]
- Miyazaki M, Van Hung P, Maeda T, Morita N. Recent advances in application of modified starches for breadmaking. Trends Food Sci Technol. 2006;17:591–599. doi: 10.1016/j.tifs.2006.05.002. [DOI] [Google Scholar]
- Mohamed AA, Rayas-Duarte P, Xu J, Palmquist DE, Inglett G. Hard red winter wheat/nutrim-OB alkaline fresh noodles: processing and texture analysis. J Food Sci. 2005;70:S1–S7. doi: 10.1111/j.1365-2621.2005.tb09056.x. [DOI] [Google Scholar]
- Preston KR, Hoseney RC (1991) Applications of the Extensigraph Handbook. In: Rasper F, Preston KR (eds) AACC International: St. Paul, MN
- Pyler EJ. Baking science and technology. Chicago: Siebel Publishing Company; 1973. [Google Scholar]
- Roberfroid MB. Prebiotics and probiotics: are they functional foods? Am J Clin Nutr. 2000;71(6):1682s–1687s. doi: 10.1093/ajcn/71.6.1682S. [DOI] [PubMed] [Google Scholar]
- Rosell C, Rojas J, De Barber CB. Influence of hydrocolloids on dough rheology and bread quality. Food Hydrocoll. 2001;15:75–81. doi: 10.1016/S0268-005X(00)00054-0. [DOI] [Google Scholar]
- Schiraldi A, Piazza L, Riva M. Bread staling: a calorimetric approach. Cereal Chem. 1996;73:32. [Google Scholar]
- Skendi A, Papageorgiou M, Biliaderis C. Effect of barley β-glucan molecular size and level on wheat dough rheological properties. J Food Eng. 2009;91:594–601. doi: 10.1016/j.jfoodeng.2008.10.009. [DOI] [Google Scholar]
- Skendi A, Biliaderis C, Papageorgiou M, Izydorczyk M. Effects of two barley β-glucan isolates on wheat flour dough and bread properties. Food Chem. 2010;119:1159–1167. doi: 10.1016/j.foodchem.2009.08.030. [DOI] [Google Scholar]
- Tessari P, Lante A. A multifunctional bread rich in beta glucans and low in starch improves metabolic control in type 2 diabetes: a controlled trial. Nutrients. 2017;9(3):297. doi: 10.3390/nu9030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharanathan RN. Food-derived carbohydrates—structural complexity and functional diversity. Crit Rev Biotechnol. 2002;22:65–84. doi: 10.1080/07388550290789469. [DOI] [PubMed] [Google Scholar]
- Tosh S. Review of human studies investigating the post-prandial blood-glucose lowering ability of oat and barley food products. Eur J Clin Nutr. 2013;67(4):310–317. doi: 10.1038/ejcn.2013.25. [DOI] [PubMed] [Google Scholar]
- Wang T, Soyama Sh, Luo Y. Development of a novel functional drink from all natural ingredients using nanotechnology. LWT Food Sci Technol. 2016;73:458–466. doi: 10.1016/j.lwt.2016.06.050. [DOI] [Google Scholar]
- Wang L, Ye F, Li S, Wei F, Chen J, Zhao G. Wheat flour enriched with oat β-glucan: a study of hydration, rheological and fermentation properties of dough. J Cereal Sci. 2017;75:143–150. doi: 10.1016/j.jcs.2017.03.004. [DOI] [Google Scholar]
- Zhiqiang L, Xiao-Su Y, Yi F. Effect of bound water on thermal behaviors of native starch, amylose and amylopectin. Starch/Stärke. 1999;11:406–410. doi: 10.1002/(SICI)1521-379X(199912)51:11/12<406::AID-STAR406>3.0.CO;2-K. [DOI] [Google Scholar]
- Zhu F, Du B, Bian Z, Xu B. Beta-glucans from edible and medicinal mushrooms: characteristics, physicochemical and biological activities. J Food Compos Anal. 2015;41:165–173. doi: 10.1016/j.jfca.2015.01.019. [DOI] [Google Scholar]
- Zhu F, Du B, Xu B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016;52:275–288. doi: 10.1016/j.foodhyd.2015.07.003. [DOI] [Google Scholar]