Abstract
The present study aims survey of fungi causing deterioration of millets during storage, detection of aflatoxigenic fungal strains ans assessment of chemically characterized Gaultheria fragrantissiuma Wall essential oil (EO) and its major component methyl salicylate (MS) as plant based preservative. Essential oil (EO) and its major component methyl salicylate (MS) as plant based preservative was evaluated. In this study a total of 13 fungal species along with toxigenic strain of Aspergillus flavus were isolated from the millets. Chemical characterization of G. fragrantissima EO through GC–MS analysis revealed Methyl salicylate (98.04%) as major component. The EO significantly inhibited growth and aflatoxin B1 production by toxigenic strain of A. flavus LHP (B)-7 at 1.0 and 0.7 µl ml−1 respectively. In addition, EO exhibited remarkable antioxidant activity (IC50 7.5 µl ml−1). EO and MS showed non phytotoxic nature on germination of millets. The LD50 of the EO was 3833.33 µl kg−1 for mice showing favourable safety profile. In view of side effects of synthetic preservatives, the study recommends G. fragrantissima EO as a safe plant based preservative to enhance shelf-life of food commodities during storage.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2842-y) contains supplementary material, which is available to authorized users.
Keywords: Afltoxin B1, Antifungal, Antioxidant, Essential oil, Gaultheria fragrantissima
Introduction
Millets are one of the major sources of carbohydrates, proteins and minerals (calcium, iron and fibers) in the Indian dietaries. India occupies top position in annual production of millets (FAO 1995). Currently, millet grains are receiving significant attention because of their inherent nutraceutical compounds, which could be used as functional food for human being as well as for livestock (Li et al. 2008). Unfortunately, climatic conditions of tropical and sub tropical countries are highly congenial to growth of molds and secretion of associated mycotoxins causing qualitative and quantitative losses of food grains and thereby, reducing their shelf-life. Patulin, zearalenone, fumonsin, and aflatoxin are the major group of mycotoxins secreted by different prevalent storage molds such as Penicillium spp., Fusarium spp. and Aspergillus spp. respectively (Prakash et al. 2015). Among mycotoxins, aflatoxin B1 mainly produced by toxigenic strain of A. flavus and A. parasiticus has received global attention because of their hepatocarcinogenic, mutagenic, teratogenic and immunosuppressive nature (Leontopoulos et al. 2003; Williams et al. 2004; Prakash et al. 2015). AFB1 has been recognized as Class 1 human carcinogen by the International Agency for Research on Cancer (IARC) and World Health Organization (WHO) (WHO-IARC 1993). Apart from molds, food commodities are also prone to oxidative deterioration during storage, which could negatively affect its nutritional value. Moreover, there are reports on acceleration of oxidative stress resulting in an increase of toxic reactive oxygen species resulting into generation of enumerable free radicals in affected food commodities due to aflatoxin contamination. (Prakash et al. 2015). Hence, an ideal food preservative should be antimicrobial against food borne fungi, inhibitory to aflatoxin secretion as well as having antioxidant activity in order to enhance their shelf life.
Several synthetic preservatives are currently being used in the management of post harvest losses of food grains. However, due to the recent reports on their adverse effects to human health and environment, these products are currently not much more entertained by health conscious consumers. In this context, plant derived products could play a significant role in the development of eco-friendly food preservatives and may be used as a safer alternative to synthetic ones. Some of the plant based bioactive compounds such as azadirachtin, pyrethrin, carvone, and allyl isothiocynate have received global attention due to their pesticidal properties and potency to enhance shelf life of food commodities (Varma and Dubey 1999; Dwivedy et al. 2015).
Among plant-derived products, EOs of traditionally used aromatic plants drags considerable attention because of their volatile and ephemeral nature. A number of plant EOs such as thyme oil (Thymus capitatus), rosemary oil (Rosemarinus officinalis) and Ocimum gratissimum, Cinnamomum zeylanicum and their formulations are currently being used as preservatives and are kept in Generally Recognized As Safe (GRAS) by the Food and Drug Administration (FDA) and Environmental Protection Agencey (EPA) (Prakash et al. 2015). Essential oils based preservatives have proved their efficacy as antifungal agents against a wide range of molds (Prakash et al. 2012; Soliman and Badeaa 2002; Dwivedy et al. 2016), pathogenic microbes (Burt 2004) and as antioxidant (Tomaino et al. 2005; Kedia et al. 2015). Hence, in the present investigation it has been thought desirable to assess the bioactivity of EO of G. fragrantissima against food borne fungi, aflatoxin secretion and as antioxidant agent. Gaultheria fragrantissima is a member of genus Gaultheria family Ericaceae commonly known as winter green oil. Its application as an antimicrobial, antileishmanial, and antidiabetic agent has been well explore (Prajapati et al. 2003). However, reports are silent about the efficacy of Gaultheria EO against food born molds, aflatoxin production and its antioxidant activity.
The current study was performed to investigate inherent mycoflora associated with two prevalent used millet Pennisetum glaucum (Bajra) and Sorghum bicolor (Jowar). Thereafter, a comparative study was performed to evaluate the efficacy of chemically characterized G. fragrantissima EO and its major compound Methyl salicylate against isolated molds and aflatoxin secretion by the toxigenic strain A. flavus LHP(B)-7. In addition, antioxidant potential and safety limit profile was also investigated so as to recommends it as a safe plant based preservative to enhance the shelf-life of food commodities during storage.
Materials and methods
Chemicals and equipments
Acetone, chloroform, methanol, sodium sulfate anhydrous, tween-20, sodium hypochlorite, toluene, isoamyl alcohol, sodium chloride, potassium hydroxide, absolute ethanol, n-heptane, Potato Dextrose Agar (PDA) medium (Potato, 200 g; Dextrose, 20 g; Agar, 15 g and distilled water 1000 ml, pH 5.6 ± 0.2), Silica gel (13% CaSO41/2H2O as binder), SMKY medium;(Sucrose, 200 g; MgSO4·7H2O, 0.5 g; KNO3, 0.3 g and yeast extract, 7 g; 1000 ml distilled water), Potato Dextrose Broth (PDB) medium and 2,2-diphenyl-1-picrylhydrazil (DPPH) were procured from HiMedia Laboratories Pvt. Ltd., Mumbai, India. Hydro-distillation apparatus (Merck Specialities Pvt. Ltd., Mumbai, India), centrifuge, UV transilluminator (Zenith Engineers, Agra, India), gas chromatography and mass spectrometry (GC–MS) (PerkinElmer Auto XL GC, MA) and spectrophotometer (Systronics India Ltd., Mumbai, India) were the major equipment used in the present investigation.
Moisture content and pH of millet samples
Six months stored millet grains of four varieties of Bajra (P. glaucum) viz. Pusa 322, Pusa 23, ICMV 155 and Mainpuri and Jowar (S. bicolor) viz.CSH 16, were procured from the local market of Varanasi, Uttar Pradesh, India during the period (August–September). After procurement, the samples were kept in sterilized polybags for 1 month at laboratory condition (Temp = 25 ± 2 °C; Humidity = 45 ± 5%) to observe the further microbial contamination. For assessment of moisture content, weighed amount of individual samples were dried at 100 °C till their weight remained constant and the moisture content was calculated. For pH measurement, 1:10 sample: distilled water suspension of each sample was prepared, put in 200 ml beaker and subjected to magnetic stirrer for 24 h. The pH of the suspension was measured using pH meter (Mandeel 2005).
Mycoflora analysis
Mycoflora analysis of selected millet samples was carried out following Aziz et al. (1998) with slight modification. Millet samples were surface sterilized and ground and 10 g of each powdered sample was homogenized in 90 ml sterile distilled water in Erlenmeyer flask (250 ml). Then, it was mechanically homogenized at constant speed for 15 min on electronic shaker. The sample-water suspension was allowed to stand for 10 min. Fivefold (10−4) serial dilutions were prepared and 1 ml aliquot of each dilution was aseptically surface plated and evenly distributed with the help of sterilized L-shaped glass spreader on freshly prepared PDA medium (Fraedrich and Miller 1995). The plates were incubated at 27 ± 2 °C for 7 days and inspected daily. Counting and identification of fungal species was done by cultural and morphological characteristics following Raper and Fennel (1977) for the genus Aspergillus, Pitt (1979) for the genus Penicillium and Domsch et al. (1980) for other molds. The percent relative density of different fungi and their occurrence frequency in each sample was calculated following Singh et al. (2008). The cultures of fungal isolates were maintained on PDA media for further study.
Selection of most toxigenic strain of Aspergillus flavus
Different randomly selected colonies of A. flavus isolated from the individual sample were screened for the production of the aflatoxin B1 using SMKY liquid medium composed of sucrose 200 g, potassium nitrate 0.3 g, magnesium sulfate 0.5 g, and yeast extract 7 g; in 1 L of distilled water (Dienner and Davis 1966). The method of Kumar et al. (2007) was used for the estimation of aflatoxin B1. A fungal disc of 5 mm diameter from 7 days old culture of A. flavus grown on PDA medium was aseptically inoculated on 25 ml of the SMKY medium in each 100 ml flask. The flasks were put in incubator at 28 ± 2 °C for 10 days. After incubation, content of each flask was filtered through Whatmann filter paper No. 1. The fungal mats (mycelia) were dried in oven at 100 °C for 12 h (Singh et al. 2008) and mycelial weight was determined. The filtrate was extracted with 20 ml of chloroform in a separating funnel. The extract was evaporated to dryness on water bath at 70 °C and the residue was dissolved in 1 ml of chloroform. The detection of aflatoxin B1 (AFB1) produced was done by the thin layer chromatography (TLC). 50 µl of the chloroform extract spotted on TLC plate along with the standard of AFB1, was run in toluene:isoamyl alcohol:methanol (90:32:2 v/v/v). The developed plates were dried in air and aflatoxin B1 was observed in ultra violet fluorescence analysis cabinet at wavelength of 360 nm. Initially detection of the intensity of AFB1 was made on visual basis by comparing the colour and intensity of fluorescence of the sample and standard of AFB1 toxin spots. The quantitative assessment of AFB1 was performed by spectrophotometer. For quantitative estimation of AFB1 spots on TLC plate were scrapped out and dissolved in 5 ml of glacial methanol, shaken and centrifuged at 3074 g for 5 min. Optical density of supernatant was recorded at the wavelength of 360 nm and the amount of AFB1 present in the sample was calculated according to the following formula (Soares and Rodriguez-Amaya 1989):
D, absorbance; M, molecular weight (312); E, molar extinction coefficient of AFB1 (21,800) and L, path length (1 cm).
Isolation of essential oil
The fresh leaves of G. fragrantissima were collected from Upper Shillong, Meghalaya, India. The plant was identified using authentic flora and a voucher specimen (LHP-GAU- ERI- 06/14) was deposited in Laboratory of Herbal Pesticides, Center of Advanced Study in Botany, Banaras Hindu University, Varanasi, India. Prior to hydrodistillation leaves were thoroughly washed by doubled distilled water then subjected to hydrodistillation using Clevenger’s apparatus (Prasad et al. 2009) for 4 h. The volatile fraction of EO was separated and water traces was removed by adding anhydrous sodium sulfate. The isolated EO was collected in sterilized glass vial and kept at 4 °C for the experimental process.
Chemical characterization of essential oil
The G. fragranantissima EO was subjected to gas chromatography (PerkinElmer Auto XL GC, MA, USA) equipped with a flame ionization detector and the GC condition were: EQUITY-5 column (60 m × 0.32 mm × 0.25 µm); H2 was the carrier gas; column head pressure 10 psi; oven temperature program isotherm 2 min at 70 °C, 3 °C/min gradient to 250 °C, isotherm 10 min; injection temperature, 250 °C; detector temperature 280 °C. GC–MS analysis was performed using PerkinElmer Turbomass GC–MS. The GC column was EQUITY-5 (60 m × 0.32 mm × 0.25 µm) fused silica capillary column. The GC conditions were: Injection temperature, 250 °C; column temperature, isothermal at 70 °C for 2 min, then programmed to 250 °C at 37 °C/min and held at this temperature for 10 min; ion source temperature, 250 °C. Helium was the carrier gas. The effluent of the GC column was introduced directly into the source of MS and spectra obtained in the EI mode with 70 eV ionization energy. The sector mass analyzer was set to scan from 40 to 500 amu for 2 s. The identification of individual compounds was based on their retention times relative to those of authentic samples and matching spectral peaks available with Wiley, NIST and NBS mass spectral libraries or with the published data (Adams 2007).
Antifungal assays
Determination of minimum inhibitory concentration (MIC) and minimum fungitoxic concentration (MFC) of EO and its major component methyl salicylate (MS)
The fungitoxic potential of the EO and its compound Methyl salicylate (MS) against the toxigenic strain A. flavus [LHP(B)-7] was measured in terms of minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) by poisoned food technique following Mishra et al. (2013), with slight modifications. Requisite amounts the EO and Methyl salicylate (MS) (0.1–1.2 µl/ml) were dissolved separately in Petri plates containing 0.5 ml tween-20 (for homogenization), 9.5 ml PDA medium was poured in plates to obtain different concentrations. Plates were serving as control contained tween-20 (0.5 ml) and PDA (9.5 ml) medium only. A fungal disc (5.0 mm diameter) of A. flavus taken from 7 day old culture was aseptically inoculated to prepared Petri dishes and incubated at 27 ± 2 °C for 7 days. The minimum concentration of EO and its compound at which fungal growth of A. flavus was absent considered as the MIC.
The percent inhibition of fungal growth was calculated by the following formula:
where dc, average diameter of fungal colonies in control sets and dt, average diameter of fungal colonies in treatment sets.
For assessment of MFC, the inhibited fungal discs of oil treated sets were re-inoculated on fresh PDA medium, and the lowest concentration at which revival of the fungal growth is checked was considered as the MFC (Prakash et al. 2012).
Determination of minimum aflatoxin B1 inhibitory concentration (MAIC) of EO and its major component Methyl salicylate (MS)
The minimum aflatoxin B1 inhibitory concentrations (MAIC) of EO and its compound Methyl salicylate (MS) was recorded against the toxigenic strain A. flavus LHP(B)-7 following Shukla et al. (2008). Requisite amounts of EO and MS were dissolved separately in 0.5 ml tween-20 and 24.5 ml SMKY broth medium in 100 ml conical flasks so as to procure different concentrations of EO and compound respectively (0.1 µl ml−1–1.0 µl ml−1 and 0.1 µl ml−1–1.1 µl ml−1). Conical flasks containing only tween-20 (0.5 ml) and SMKY medium (24.5 ml) served as controls. Thereafter, flasks were inoculated with 50 µl of spore suspension (approx. 106 spores ml−1) of the toxigenic strain A. flavus [LHP(B)-7] and incubated at 28 ± 2 °C for 10 days. After incubation, the contents of each flask were filtered (Whatman No. 1) and the biomass of filtered mycelium was dried at 80 °C (for 12 h) and weighed. The filtrates were extracted with 20 ml of chloroform in a separating funnel. The extracts were evaporated to dryness on a water bath at 70 °C and the residues were re-dissolved in 1 ml of chloroform. The detection of AFB1 produced was done by the thin layer chromatography method. Fifty microliter (50 µl) of the chloroform extracts were spotted on TLC plates along with the standard of AFB1, and the TLC plates were run in toluene: isoamyl alcohol: methanol (90:32:2 v/v/v). The developed plates were air-dried and AFB1 was observed in an ultraviolet fluorescence analysis cabinet at the wavelength of 360 nm. Initial detection of the intensity of AFB1 in the control set was made on a visual basis by comparing the color and intensity of fluorescence of the samples and standard spot. The quantitative estimation of AFB1 was done by a spectrophotometer. For quantitative estimation, spots of AFB1 on TLC plate were scraped out and dissolved in 5 ml of cold methanol, shaken, and centrifuged at 3074 g for 5 min. The optical densities of supernatants were recorded at the wavelength of 360 nm and the amount of AFB1 present in the sample was calculated, following Soares and Rodriguez-Amaya (1989).
where D, absorbance; M, molecular weight (312); E, molar extinction coefficient of AFB1 (21,800); and l = path length (1 cm).
Fungitoxic spectrum of G. fragrantissima EO
The toxicity of G. fragrantissima EO against 13 dominant food borne fungal isolates from millet sample during mycoflora analysis, was evaluated by food poisoned technique. The oil at its MIC (viz.1.0 µl ml−1) was added separately to Petri plates containing 0.5 ml tween-20 and 9.5 ml PDA medium. A 5 mm disc from 7 days old colony from each fungus was separately placed in the center of the prepared Petri plates. Plates serving as control contained only fungal inoculum in tween-20 (0.5 ml) and 9.5 ml PDA. The plates of both treatment and control sets were incubated at 27 ± 2 °C for 7 days. The percent inhibition and nature of toxicity (fungistatic/fungicidal) was determined.
Antioxidant activity of G. fragrantissima (EO) and its major component Methyl salicylate (MS)
Free radical scavenging activity of the G. fragranatisisma EO and MS were measured by recording the extent of bleaching of a DPPH solution from purple to yellow following Prakash et al. (2012). Different concentrations (1.0–30 and 1.0–100 µl/ml) of the EO and MS were added to 0.004% DPPH solution in methanol (5 ml). After 30 min incubation at room temperature (27 ± 2 °C), the absorbance was measured against a blank at 517 nm using a spectrophotometer. The antioxidant activity was measured through scavenging of DPPH free radical with reduction in absorbance of the sample. The IC50 of the test compounds, which represented the concentration that caused 50% neutralization of DPPH radicals, was measured from the graph plotting percentage inhibition against concentration.
where, Ablank is the absorbance of the control (without test material) and Asample is the absorbance of the test material.
Safety profile of G. fragrantissima EO
The safety profile of the EO was determined on male mice (Mus musculus L., average weight 30 g, age 3 months) by recording their LD50 values which represent the lethal dose of EO per unit weight for killing of 50% population of test animals (Singh et al. 2009). A stock solution of tween-20 and distilled water (1:1) was prepared. Different doses of EO (from 0.05 to 0.5 ml were orally given to each group of animal (10 mice) separately with 0.5 ml stock solution. In control set, equal dose of tween-20 and distilled water was given. After 24 h, the mortality of the test animals was recorded and LD50 of EO was calculated by probit analysis (Finney 1971).
Phytotoxicity assay of G. fragrantissima for EO and MS
Phytotoxic assessment was performed in terms of germination of millet seeds fumigated for 3 months to EO at 1.0 µl ml−1 and MS at 1.1 µl ml−1 following Shukla et al. (2009). After 3 month exposure, 20 randomly selected seeds from each set were placed in Petri plates containing sterilized moistened blotting paper. Control set comprised untreated millet seeds. Thereafter, Petri dishes were sealed with paraffin film and placed in incubator for germination. The percent germination and viability of seeds of control and treated sets were recorded at regular interval of time.
Statistical analysis
Experiments were performed in triplicate, analyzed as mean ± standard error subjected to one way analysis of variance (ANOVA). Means are separated by Tukey’s multiple range tests when ANOVA was significant (p < 0.05). The analysis of data was performed with the SPSS program version 16.0 (IBM Corporation).
Results
The pH and moisture content of millet samples ranged from 6.23 to 6.87 and 13.56 to 14.86% respectively. During mycoflora analysis, a total of 916 fungal isolates belonging to 7 different genera and 13 species were identified (Table 1).
Table 1.
Mycoflora associated with selected millet samples
| Millet samples | Fungal isolates | Total colonies | Total species | Moisture content (%) | pH | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A.f. | A.n. | A.t. | A.s. | A.m. | A.a. | R.s. | C.l. | C.s. | P.i. | P.p. | M | M.s. | |||||
| P. glaucum var. Pusa 322 | 17 | 17 | 12 | 65 | 12 | − | − | − | − | − | − | − | 2 | 125 | 6 | 14.34 ± 0.09bc | 6.53 ± 0.10b |
| P. glaucum var. Pusa 23 | 12 | 2 | 3 | − | − | 1 | 2 | − | − | 1 | 1 | − | 1 | 23 | 5 | 13.56 ± 0.12c | 6.27 ± 0.01b |
| P. glaucum var. ICMV 155 | 16 | 17 | − | 667 | 12 | − | − | − | − | − | − | − | 2 | 714 | 5 | 14.81 ± 0.50ab | 6.23 ± 0.03b |
| P. glaucum var. Mainpuri | 8 | 7 | − | 19 | − | − | 2 | 1 | − | − | − | − | − | 37 | 5 | 15.39 ± 0.04a | 6.46 ± 0.11b |
| S. bicolour var. CSH 16 | 1 | − | − | − | − | − | 4 | − | 2 | 2 | − | 2 | 6 | 17 | 6 | 14.86 ± 0.03ab | 6.87 ± 0.04a |
| Total isolates | 54 | 43 | 15 | 751 | 24 | 1 | 8 | 1 | 2 | 3 | 1 | 2 | 11 | 916 | |||
| Relative density | 5.90 | 5.73 | 1.64 | 81.99 | 2.62 | 0.11 | 0.88 | 0.11 | 0.22 | 0.33 | 0.11 | 0.22 | 1.20 | ||||
RD relative density, A.f. Aspergillus flavus, A.n. Aspergillus niger, A.t. Aspergillus terreus, A.s. Aspergillus sydowi, A.m. Aspergillus minutus, , A.a. Alternaria alternata, R.s. Rhizopus stolonifer, C.l. Curvularia lunata, C.s. Cheatomium spirale, P.i. Penicillium italicum, P.p Penicillium purpurogenum, M. Mucor sp M.s. Mycelia sterlia
The means followed by same letter in the same column are not significantly different according to ANOVA and Tukey’s multiple comparison tests
During the assessment of aflatoxigenicity of A. flavus isolated from the millet samples, 17 isolates from Bajra and one isolate from Jowar were recorded to secrete aflatoxin. The aflatoxigenic isolate A. flavus strain LHP(B)-7 isolated from Bajra produced maximum aflatoxin B1 (2199.84 µg/l), hence, it was selected as test fungus for further detailed investigation.
EO obtained from G. fragrantissima fresh leaves was pale yellow in colour with pungent cool odor. The yield of EO was 4–6 ml/kg through Clevenger’s hydro distillation apparatus. Methyl salicylate (MS) was identified as the major component (98.04%) of EO through GC–MS analysis (supplementary Fig. 1 and Table 2).
Table 2.
Chemical composition of Gaultheria frgrantissima Wall. EO identified through GC–MS analysis
| S. no. | Compound | Retention time (min) | % |
|---|---|---|---|
| 1 | Methyl salicylate | 11.351 | 98.035 |
| 2 | Ethyl salicylate | 13.787 | 0.069 |
| Total | 98.104 |
The MIC of G. fragrantissima for EO and MS against A. flavus strain LHP(B)-7 was recorded as 1.0 and 1.1 µl ml−1 respectively (Table 3). At MIC dose, EO inhibited complete growth of all the food borne molds exhibiting a broad range of antifungal efficacy except for A. terreus (75%), A. sydowii (82%) and A. minutes (79%). At MIC, EO exhibited fungicidal nature for A. alternata, Curvularia lunata and Mycelia sterilia while it was fungistatic for A. flavus, A. niger, A. terreus, A. sydowii, A. minutes, P. italicum, P. purpurogenum, Chaetomium spirale, Rhizopus stolonifer and Mucor sp. (Table 4).
Table 3.
Effect of different concentrations of G. fragrantissima Wall. EO and Methyl salicylate on mycelial weight, aflatoxin B1 production in SMKY medium
| Conc (µl ml−1) | G. fragrantissima EO | MS | ||
|---|---|---|---|---|
| MDW | AFB1 content | MDW | AFB1 content | |
| CNT | 288.67 ± 1.20a | 2161.10 ± 74.37a | 321.00 ± 3.06a | 2819.44 ± 43.72a |
| 0.1 | 284.00 ± 4.862 0a | 1846.23 ± 16.53b | 286.00 ± 2.08b | 2318.53 ± 57.84b |
| 0.2 | 276.00 ± 1.73a | 1817.61 ± 49.58b | 279.00 ± 1.15b | 1975.05 ± 37.89c |
| 0.3 | 235.00 ± 3.46b | 1746.05 ± 8.26b | 263.00 ± 2.52c | 1803.30 ± 36.01d |
| 0.4 | 211.67 ± 4.91c | 1517.06 ± 74.36c | 232.33.00 ± 1.86d | 1502.75 ± 21.86e |
| 0.5 | 202.20 ± 6.66c | 1488.44 ± 41.32c | 218.00 ± 4.62d | 100.18 ± 57.84f |
| 0.6 | 176.67 ± 1.86d | 114.49 ± 38.16d | 205.00 ± 4.16e | 14.31 ± 08.26f |
| 0.7 | 144.00 ± 2.08e | 0.00d | 129.33 ± 3.84f | 0.00f |
| 0.8 | 75.67 ± 9.96f | 0.00d | 117.00 ± 8.39f | 0.00f |
| 0.9 | 17.00 ± 1.73g | 0.00d | 40.00 ± 4.36g | 0.00f |
| 1.0 | 0.00g | 0.00d | 22.67 ± 4.67h | 0.00f |
| 1.1 | 0.00g | 0.00d | 0.00i | 0.00f |
Values are mean (n=3) ±standard error
Conc. concentration (µl ml−1), MDW mycelial dry weight (mg)
The means followed by same letter in the same column are not significantly different according to ANOVA and Tukey’s multiple comparison tests
Table 4.
Fungitoxic spectrum and nature of toxicity of G. fragrantissima EO at 1.0 µl ml−1 concentration against some food borne molds
| S. no. | Name of fungus | % Inhibition | Nature of toxicity |
|---|---|---|---|
| 1 | Aspergillus flavus | 100 ± 0.00a | Static |
| 2 | Aspergillus niger | 100 ± 0.00a | Static |
| 3 | Aspergillus terreus | 75 ± 1.73c | Static |
| 4 | Aspergillus sydowii | 82 ± 2.65b | Static |
| 5 | Aspergillus minutus | 79 ± 1.53b | Static |
| 6 | Penicillium italicum | 100 ± 0.00a | Static |
| 7 | Penicillium purpurogenum | 100 ± 0.00a | Static |
| 8 | Alternaria alternata | 100 ± 0.00a | Cidal |
| 9 | Chaetomium spirale | 100 ± 0.00a | Static |
| 10 | Curvularia lunata | 100 ± 0.00a | Cidal |
| 11 | Rhizopus stolonifer | 100 ± 0.00a | Static |
| 12 | Mucor sp. | 100 ± 0.00a | Static |
| 13 | Mycelia sterilia | 100 ± 0.00a | Cidal |
Values are mean (n = 3) ± standard error. The means followed by same letters in the same column are not significantly different according to ANOVA and tukey’s multiple comparision tests
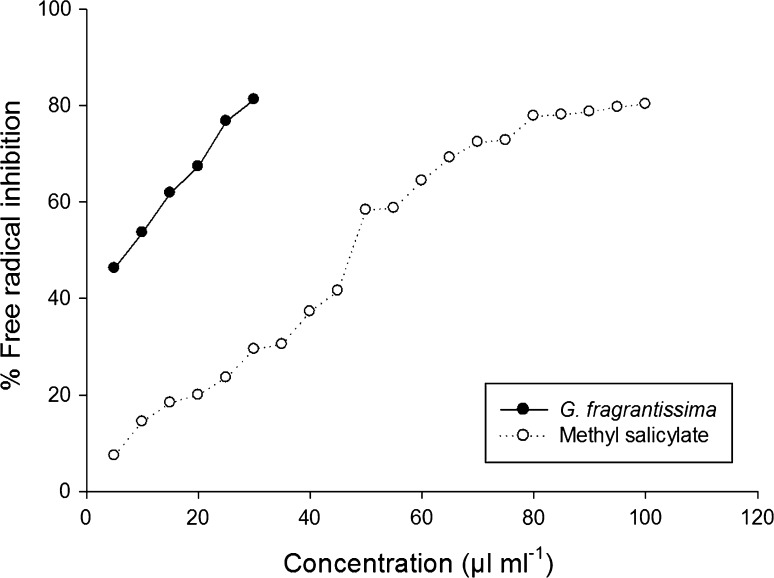
During aflatoxin inhibitory assay, a significant dose dependent activity has been observed. Both EO and MS caused complete inhibition of aflatoxin secretion by the aflatoxigenic strain of A. flavus LHP(B)-7 at 0.7 µl ml−1 (Table 3). Free radical scavenging activity of G. fragrantissima for EO and MS was recorded as 7.5 and 47.72 µl ml−1 respectively (Fig. 1). During seed germination assay, no significant difference in germination of millets seed was observed between control set and EO as well as MS treated sets. The safety limit profile of essential oil was recorded through oral toxicity assay on mice. LD50 value of G. fragrantissima for EO was recorded as 3833.33 µl/kg.
Fig. 1.
Free radical scavenging activity of G. fragrantissima EO and Methyl salicylate
Discussion
During mycoflora analysis, the millets samples were found to be heavily contaminated with different mold species. Prior to mycoflora analysis, moisture content and pH of the samples were determined as these abiotic factors play significant role in molds proliferation. In the present investigation, pH and moisture content in the millet samples ranged from 6.23 to 6.86 and 13.56 to 15.35% respectively, which were under highly favorable limit for mold growth and proliferation. Though, moisture content and pH value of both millet grains were found in same range, the degree of extent of mold infestation was more in Bajra compared to Jowar. Hence, the findings suggested that apart from moisture content and pH, storage practice and chemical profile of the samples also influenced mold infestations as has been earlier reported by Prakash et al. (2010). In addition, seeds were found to be contaminated with the toxigenic strain of A. flavus. Hence, it inferred that the samples were prone to qualitative as well as quantitative losses by fungi during prolong storage.
As it has been reported that minor compound can also significantly influence the bioactivity of intact EO (Burt 2004; Prakash et al. 2015), Moreover, different climatic conditions, soil types, part of plant and time of harvesting also significantly caused variation in chemical profile of essential oils (Burt 2004). Such apparent variations in the chemical profile of the EO would significantly influence its biological activity. Hence, prior to recommendation as food additive it is advisable that the chemical composition of EOs should be characterized. Therefore, in the present investigation EO was chemically characterized through GC–MS analysis to explore its chemical profile which would be helpful to agri-firms during its formulation for large scale application.
The GC–MS of the EO revealed MS (98.04%) as the major component. Thereafter, in the present investigation a comparative analysis was made between EO and MS for all the test parameters to explore the role of major compound in biological activity of EO.
A significant dose dependent activity was observed for growth and toxin inhibition against the aflatoxigenic strain of A. flavus for both EO and MS. EO and MS caused complete inhibition of aflatoxin secretion below their MIC concentration suggesting their two different modes for growth and toxin inhibition. Similar supportive observations have earlier been observed by Rasooli and Razzaghi-abyaneh (2004), Tian et al. (2012) during the study of aflatoxin inhibitory potential of EOs. In addition, EO and MS exhibited pronounced antifungal activity at 1.0 and 1.1 µl/ml respectively against all the isolated molds. The finding suggested that the antifungal and aflatoxin inhibitory activity of EO was significantly contributed by its major compound MS.
Oxidation is a multilateral process that brings about undesirable changes related to food quality, organoleptic characteristics, safety and nutritional quality of food items. Further, it has been reported that there is a positive correlation between the aflatoxin production and free radical generation (Prakash et al. 2015). Therefore, in present investigation, free radical scavenging activity of the EO and MS were recorded through DPPH analysis, one of the most commonly used assay for the measurement of free radical scavenging activity of plant products. The IC50 of EO was found quite lower than that of its major compound MS reflecting that the minor compound act synergistically, thereby, enhancing the radical scavenging activity of intact EO as has been earlier emphasized by Burt (2004). In addition, EO showed high antioxidant activity compared to earlier reported plant essential oils viz., Zataria multiflora and Thymus caramanicus (Safaei-Ghomi et al. 2009), Chrysopogon zizanioides (L.) Roberty, Lavandula angustifolia Mill. Pelargonium odorantissimum L’Her, Feronia limonia (L.) Swingle (Prakash et al. 2012) strengthening its application as a plant based antioxidant to prevent the oxidative deterioration of food items.
Seed samples exposed to EO and MS showed 100% germination, suggesting their non-phytotoxic nature. Hence, test EO and MS could be recommended for preservation of food commodities stored for consumption as well as sowing purposes.
The LD50 of the EO was recorded as 3833.33 µl kg−1 body weight on mice which was higher than the values for previously reported botanicals, viz., pyrethrum (350–500 mg kg_1), carvone (1640 mg kg_1), and some commercial fungicides, Bavistin (1500 mg kg_1), Wettasul −80 and Nystatin (Prakash et al. 2011). The high LD50 value of the EO indicates its favourable safety profile on mammals during its application as preservatives. The major component of the EO is methyl saliycylate and many naturally occurring salicylates are used at low doses in a wide variety of foods and beverages for fragrance. In addition, MS have been granted Generally Recognized as Safe (GRAS) status by the Flavor and Extract Manufacturers’ Association (Panel et al. 2007).
G. fragrantissima plants grow luxuriantly in Himalayan region of India and sufficient raw materials will be available for extraction of essential oil in bulk. Keeping in view the medicinal importance of the plant and based on the findings on antimicrobial and aflatoxin inhibitory nature of its essential oil and high LD50 value on mice, the EO has potentiality as plant based safe and economical preservative for enhancement of shelf life of millets during storage from biodeterioration caused due to food borne molds, aflatoxins as well as mycotoxin and lipid peroxidation.
Based on strong antifungal, antiaflatoxigenic and antioxidant efficacy, the G. fragrantissima EO could be recommended as plant-based preservative against the quantitative and qualitative fungal as well as oxidative losses of millet grains during storage. However, further investigations during storage conditions are required to evaluate its efficacy in food system in large scale.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
Authors are thankful to Department of Biotechnology (DBT), New Delhi, India for financial assistance as DBT sponsored project (Project No. P07-575) and also thankful to ethical committee, Banaras Hindu University for ethical approval as well as coordinator, Centre of Advanced Studies (CAS) in Botany, Banaras Hindu University, Varanasi for laboratory facility.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2842-y) contains supplementary material, which is available to authorized users.
References
- Adams RP. Identification of essential oil components by gas chromatograph/mass spectrometry. Carol Stream, IL: Allured Publishing Corporation; 2007. [Google Scholar]
- Aziz NH, Youssef YA, El-Fouly MZ, Moussa LA. Contamination of some common medicinal plant samples and spices by fungi and their mycotoxins. Bot Bull Acad Sinica. 1998;39:279–285. [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and their potential application in foods: a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Dienner UI, Davis N. Aflatoxins production by isolates of Aspergillus flavus. Phyopathology. 1966;56:1390–1393. [PubMed] [Google Scholar]
- Domsch KH, Gams W, Anderson TH. Compendium of soil fungi, vols. 1 and 2. London: Academic Press; 1980. p. 857. [Google Scholar]
- Dwivedy AK, Kumar M, Upadhyay N, Dubey NK. Green chemistry in agricultural pest management programmes. Med Chem. 2015;S2:005. [Google Scholar]
- Dwivedy AK, Kedia A, Kumar M, Dubey NK. Essential oils of traditionally used aromatic plants as green shelf life enhancers for herbal raw materials from microbial contamination and oxidative deterioration. Curr Sci. 2016;110(2):143–145. [Google Scholar]
- FAO (1995) Accessed on 20-01-2015. Web https://www.google.co.in/url?sa=t&rct=j&q=&esrc=s&source=web&cd=3&cad=rja&uact=8&ved=0ahUKEwjC_pe_yrjKAhVVCI4KHcTHA5QQFggnMAI&url=http%3A%2F%2Fwww.fao.org%2Fdocrep%2Fw1808e%2Fw1808e0c.htm&usg=AFQjCNFaV5KLrQGfI-ximc97mO_p0YVgnQ&sig2=JO84YqKc2JEGVIW6DGz6yg
- Finney JD. Probit analysis. London: Cambridge University Press; 1971. p. 333. [Google Scholar]
- Fraedrich SW, Miller T. Mycoflora associated with slash-pine seeds from cones collected at seed orchards and cone-processing facilities in the southeastern USA. Eur J Forest Pathol. 1995;25:73–82. doi: 10.1111/j.1439-0329.1995.tb00321.x. [DOI] [Google Scholar]
- Kedia A, Prakash B, Mishra PK, Singh P, Dubey NK. Botanicals as eco friendly biorational alternatives of synthetic pesticides against Callosobruchus spp. (Coleoptera: Bruchidae)—a review. J Food Sci Technol. 2015;52:1239–1257. doi: 10.1007/s13197-013-1167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Mishra AK, Dubey NK, Tripathi YB. Evaluation of Chenopodium ambrosioides oil as a potential source of antifungal, antiaflatoxigenic and antioxidant activity. Int J Food Microbiol. 2007;115:159–164. doi: 10.1016/j.ijfoodmicro.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Leontopoulos D, Siafaka A, Markaki P. Black olives as substrate for Aspergillus parasiticus growth and aflatoxin B1 production. Food Microbiol. 2003;20:119–126. doi: 10.1016/S0740-0020(02)00080-1. [DOI] [Google Scholar]
- Li J, Chen Z, Guan X, Liu J, Zhang M, Xu B (2008) Optimization of germination conditions to enhance hydroxyl radical inhibition by water-soluble protein from stress millet. J Cereal Sci 48(3):619–624
- Mandeel QA. Fungal contamination of some imported species. Mycopatholgia. 2005;159:291–298. doi: 10.1007/s11046-004-5496-z. [DOI] [PubMed] [Google Scholar]
- Mishra PK, Singh P, Prakash B, Kedia A, Dubey NK, Chanotiya CS. Assessing essential oil components as plant-based preservatives against fungi that deteriorate herbal raw materials. Int Biodeterior Biodegrad. 2013;80:16–21. doi: 10.1016/j.ibiod.2012.12.017. [DOI] [Google Scholar]
- Panel T, Belsito D, Bickers D, Bruze M, Calow P, Greim H, Tagami H. A toxicologic and dermatologic assessment of salicylates when used as fragrance ingredients. Food Chem Toxicol. 2007;45(1):318–361. doi: 10.1016/j.fct.2007.09.066. [DOI] [PubMed] [Google Scholar]
- Pitt JL. The genus Penicillum. Sidney: Academic Press; 1979. p. 423. [Google Scholar]
- Prajapati ND, Purohit SS, Sharma AK, Kumar T. A hand book of medicinal plants, a complete source book. Jodhpur: Shyam; 2003. [Google Scholar]
- Prakash B, Shukla R, Singh P, Kumar A, Mishra PK, Dubey NK (2010) Efficacy of chemically characterized Piper betle L. essential oil against fungal and aflatoxin contamination of some edible commodities and its antioxidant activity. Int J Food Microbiol 142(1):114–119 [DOI] [PubMed]
- Prakash B, Shukla R, Singh P, Mishra PK, Dubey NK, Kharwar RN. Efficacy of chemically characterized Ocimum gratissimum L. essential oil as an antioxidant and a safe plant based antimicrobial against fungal and aflatoxin B1 contamination of spices. Food Res Int. 2011;44:385–390. doi: 10.1016/j.foodres.2010.10.002. [DOI] [Google Scholar]
- Prakash B, Singh P, Kedia A, Dubey NK. Assessment of some essential oils as food preservatives based on antifungal, antiaflatoxin, antioxidant activities and in vivo efficacy in food system. Food Res Int. 2012;49:201–208. doi: 10.1016/j.foodres.2012.08.020. [DOI] [Google Scholar]
- Prakash B, Kedia A, Mishra PK, Dubey NK. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities - Potentials and challenges. Food Control. 2015;47:381–391. doi: 10.1016/j.foodcont.2014.07.023. [DOI] [Google Scholar]
- Prasad CS, Shukla R, Kumar A, Dubey NK. In vitro and in vivo antifungal activity of essential oils of Cymbopogon martini and Chenopodium ambrosioides and their synergism against dermatophytes. Mycoses. 2009;53:123–129. doi: 10.1111/j.1439-0507.2008.01676.x. [DOI] [PubMed] [Google Scholar]
- Raper KB, Fennel DL. The genus Aspergillus. Huntington, New York: Krieger Publishing Corporation; 1977. [Google Scholar]
- Rasooli I, Razzaghi-Abyaneh M. Inhibitory effects of thyme oils on growth and aflatoxin production by Aspergillus parasiticus. Food Control. 2004;15:479–483. doi: 10.1016/j.foodcont.2003.07.002. [DOI] [Google Scholar]
- Safaei-Ghomi J, Ebrahimabadi AH, Djafari-Bidgoli Z, Batooli H. GC/MS analysis and in vitro antioxidant activity of essential oil and methanol extracts of Thymus caramanicus Jalas and its main constituent carvacrol. Food Chem. 2009;115:1524–1528. doi: 10.1016/j.foodchem.2009.01.051. [DOI] [Google Scholar]
- Shukla R, Kumar A, Prasad CS, Srivastava B, Dubey NK. Antimycotic and antiaflatoxigenic potency of Adenocalymma alliaceum Miers. on fungi causing biodeterioration of food commodities and raw herbal drugs. Int Biodeterior Biodegrad. 2008;62:348–351. doi: 10.1016/j.ibiod.2007.11.006. [DOI] [Google Scholar]
- Shukla R, Kumar A, Singh P, NK Dubey. Efficacy of Lippia alba (Mill.) N.E. Brown essential oil and its monoterpene aldehyde constituents against fungi isolated from some edible legume seeds and aflatoxin B1 production. Int J Food Microbiol. 2009;135:165–170. doi: 10.1016/j.ijfoodmicro.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Singh P, Srivastava B, Kumar A, Dubey NK. Fungal contamination of raw materials of some herbal drugs and recommendation of Cinnamomum camphora oil as herbal fungitoxicant. Microb Ecol. 2008;56:555–560. doi: 10.1007/s00248-008-9375-x. [DOI] [PubMed] [Google Scholar]
- Singh P, Kumar A, Dubey NK, Gupta R. Essential oil of Aegle marmelos as a safe plant-based antimicrobial against postharvest microbial infestations and aflatoxin contamination of food commodities. J Food Sci. 2009;74:302–307. doi: 10.1111/j.1750-3841.2009.01223.x. [DOI] [PubMed] [Google Scholar]
- Soares LMV, Rodriguez-Amaya DB. Survey of aflatoxins, ochratoxin A, zearalenone and sterigmatocystin in some Brazilian foods by using multi-toxin thin layer chromatographic method. J Assoc Off Anal Chem. 1989;72:22–26. [PubMed] [Google Scholar]
- Soliman KM, Badeaa RI. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem Toxicol. 2002;40:1669–1675. doi: 10.1016/S0278-6915(02)00120-5. [DOI] [PubMed] [Google Scholar]
- Tian J, Huang B, Luo X, Zeng H, Ban X, He J, Wang Y. The control of Aspergillus flavus with Cinnamomum jensenianum Hand.-Mazz essential oil and its potential use as a food preservative. Food Chem. 2012;130:520–527. doi: 10.1016/j.foodchem.2011.07.061. [DOI] [Google Scholar]
- Tomaino A, Cimino F, Zimbalatti V, Venuti V, Sulfaro V, De Pasquale A, Saija A. Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem. 2005;89:549–554. doi: 10.1016/j.foodchem.2004.03.011. [DOI] [Google Scholar]
- Varma J, Dubey NK. Perspective of botanical and microbial products as pesticides of tomorrow. Curr Sci. 1999;76:172–179. [Google Scholar]
- WHO-IARC Working Group, International Agency for Research on Cancer (IARC) (1993) Some naturally occurring substances: Food items and constituents. In: IARC monographs on the evaluation of carcinogenic risk to humans, vol. 56. World Health Organization, Lyon
- Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr. 2004;80:1106–1122. doi: 10.1093/ajcn/80.5.1106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.