Abstract
This study focuses on the influence of operating conditions on Alcalase-catalyzed egg white protein hydrolysis performed in a continuously stirred tank reactor coupled with ultrafiltration module (10 kDa). The permeate flow rate did not significantly affect the degree of hydrolysis (DH), but a significant increase in process productivity was apparent above flow rate of 1.9 cm3 min−1. By contrast, an increase in enzyme/substrate (E/S) ratio provided an increase in DH, but a negative correlation was observed between E/S ratio and productivity. The relationship between operating conditions and antioxidant properties of the hydrolysates, measured by three methods, was studied using Box-Behnken experimental design and response surface methodology. The statistical analysis showed that each variable (impeller speed, E/S ratio, and permeate flow rate) had a significant effect on the antioxidant capacity of all tested systems. Nevertheless, obtained response functions revealed that antioxidative activity measured by DPPH, ABTS and FRAP methods were affected differently by the same operating conditions. High impeller speeds and low permeate flow rates favor ABTS while high impeller speeds and high permeate flow rates had a positive effect on the DPPH scavenging activity. On the other hand, the best results obtained with FRAP method were achieved under moderate operating conditions. The integration of the reaction and ultrafiltration membrane separation in a continuous manner appears to be a right approach to improve and intensify the enzymatic process, enabling the production of peptides with desired antioxidant activity.
Keywords: Egg-white protein hydrolysis, Alcalase, Continuous membrane reactor, Operating conditions, Antioxidant properties
Introduction
Recently, the production of novel antioxidant compounds has become imperative in biochemical, medical and food sciences, since free radicals and reactive oxygen species cause serious health issues (Fan et al. 2014). The emphasis is placed on finding the alternative natural antioxidants, which would efficiently substitute synthetic antioxidants because of the indications of their toxic nature (de Oliveira et al. 2015). Proteins from plant and animal sources were found to be a suitable source of natural antioxidants, since their hydrolysates obtained under controlled conditions showed an antioxidant capacity comparable to the ones of commonly used synthetic antioxidants (Bhat et al. 2015; Malaguti et al. 2014; Sarmadi and Ismail 2010). Among them, egg white proteins (EWPs) are an adequate source for the production of bioactive peptides intended for human consumption considering nutritional quality and functional properties (Van der Plancken et al. 2005; Yu et al. 2014).
Enzymatic hydrolysis of animal and plant proteins is a preferred tool for obtaining hydrolysates with specific and preserved peptides compared to the traditional chemical acid or alkali route, due to the high selectivity and mild conditions of enzymatic processes, notably with the application of the appropriate reaction engineering. Their release from related intact proteins has been shown to be affected by various factors such as protein source, pre-treatment, type and amount of enzyme, substrate concentration, DH, temperature, pH, and process operating conditions (Yu et al. 2014). Regarding EWP hydrolysates, amongst the three proteases, the hydrolysate prepared using Alcalase has shown to be the most effective in both, scavenging DPPH and ABTS radicals (Jakovetić et al. 2015; Stefanović et al. 2014) and reducing power (Lin et al. 2012). However, other enzymes such as protease from plant (e.g. papain) and animal sources (digestive enzymes, e.g., pepsin and trypsin) have also been reported in the literature, especially for the production of various EWP hydrolysates with antihypertensive (ACE) inhibitory properties (Chen and Chi 2012).
Traditionally, the enzymatic hydrolysis of raw natural proteins is conducted in batch bioreactors presenting certain difficulties such as limited productivity and yield, product inhibition, enzyme inactivation due to enzyme autolysis and/or hydrodynamic forces. A feature that also hampers the enzymatic process is the formation of higher oligopeptides beside di- and tripeptides. After completing the process in the classical batch bioreactor, these compounds must be separated from low-molecular products and biocatalyst, for example by passing through ultrafiltration membranes or by column chromatography techniques, in order to obtain a more uniform product with the desired range of molecular mass. For this application, the integration of reaction and one or more unit operations such as ultrafiltration membrane separation is a well-established method to improve and intensify biochemical processes. The enzymatic reaction can also be coupled with a membrane module in a continuous process, enabling higher reactor productivity since a biocatalyst is confined to the reaction zone while allowing the enzyme reuse and the removal of the products (Berends et al. 2014).
In spite of the industrial importance of the egg-white as a multifunctional food ingredient, systematic investigations of the effects of process operating conditions and reactor design on the EWP hydrolysis and the antioxidant capacity of the obtained hydrolysates have been reported only in a few cases (Chiang et al. 2008; Jakovetić et al. 2015).In addition, the batch bioreactor is mainly used and only little information about this reaction in the membrane reactor system is available.
The aim of this research was to design an efficient continuous and intensified enzymatic membrane process for the production of EWP hydrolysates with improved antioxidant capacity. For this purpose, the effects of operating factors on the efficiency of EWP hydrolysis in the continuous enzymatic membrane reactor (CEMR) was investigated in terms of reactor productivity and antioxidant properties of the obtained hydrolysates. RSM and Box-Behnken experimental design were used to evaluate effects of three key operational parameters: impeller speed, E/S ratio and permeate flow rate on the antioxidant activity measured by ABTS, DPPH, and FRAP methods since each assay reflects a different aspect of the antioxidant behavior of the hydrolysates. A comparison of the relationship between operating conditions and antioxidant capacity measured by different methods is also presented.
Materials and methods
Materials
All hydrolyses were performed using commercial pasteurized egg-white as a substrate, which was a kind gift from the Jata Emona d.o.o (Ljubljana, Slovenia). 2,2′-Azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,4,6-tris (2-pyridyl)-s-triazine (TPTZ) were all purchased from Sigma Aldrich (St. Louis, USA). Protease from Bacillus licheniformis, Alcalase®, (EC 3.4.21.14), with the claimed activity of 2.4 Anson unit (AU) g−1was also purchased from Sigma Aldrich. Membrane module, Vivaflow 50 10000MWCO PES consisted of the polyethersulfone (PES) membrane with an active area of 50 cm2was purchased from Sartorius Stedim Biotech (Goettingen, Germany). Two peristaltic pumps (Pump Drive 5201 Heidolph Instruments GmbH & Co, Schwabach, Germany) were used for the flow control.
Preparation of EWP hydrolysates
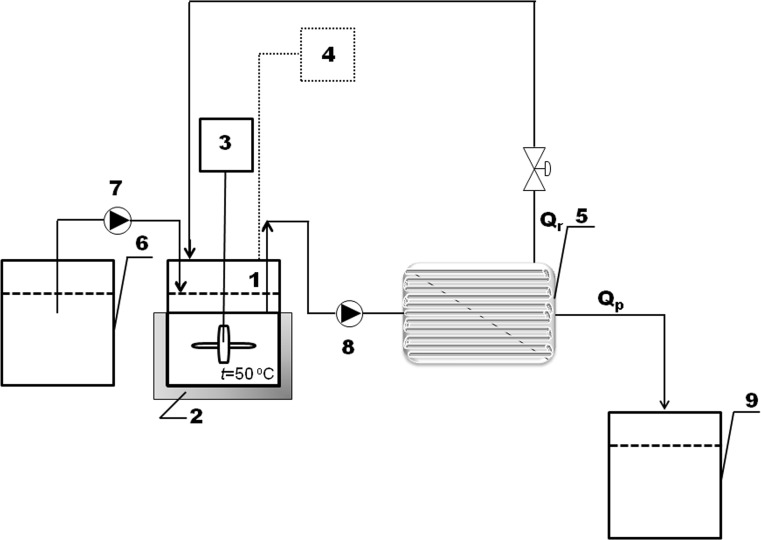
EWP water solution (100 g kg−1) was prepared under the constant stirring, pretreated by heating at 75 °C for 30 min and then adjusted to 50 °C and the pH to 8. Enzymatic hydrolyses were performed in the continuously stirred tank reactor (CEMR, 1) consisted of a 300 cm3glass vessel with a water jacket (2), a mechanical stirrer (3) equipped with the four-bladed propeller and a pH electrode (4) (Fig. 1). The reactor is coupled with PES membrane unit with the molecular cut-off of 10 kDa (5). The reaction mixture was continuously pumped from the reactor tank (1) into the membrane module using a peristaltic pump (8). The permeate was collected in the reservoir (9). The fresh substrate was added at the desired flow rate from the reservoir (6) using a peristaltic pump (7).
Fig. 1.
Schematic representation of enzymatic hydrolysis of EWPs in CEMR: 1- CEMR consisted of a 300 cm3 glass vessel with a water jacket (2), 3-mechanical stirrer equipped with four-bladed propeller; 4-pH electrode; 5- PES membrane unit; 6-the reactor tank; 7 and 8- peristaltic pumps; 9- reservoir
The progress of the reaction was followed using pH stat method. DH was determined with the following equation (Guadix et al. 2006):
| 1 |
M B represents the amount of base necessary to keep pH constant (mol), V P and V R are volumes of permeate and reaction mixture respectively (dm3), S F is the protein concentration in the feed (g dm−3), h T = 7.67·10−3 (mol g−1 of protein) presents number of peptide bonds in the substrate, K w = 5.8·10−14 mol2 dm−6 is the ionic product of water at 50 °C, α = 0.88 is the average degree of dissociation of the α-amino groups.
In order to obtain kinetic constants for EWP hydrolysis in the CEMR previously developed mathematical model, assuming the zero-order kinetics of hydrolysis with respect to substrate and second-order kinetics of enzyme deactivation, is employed (Prieto et al. 2010):
| 2 |
where n is the number of enzyme uses and t n (min) is the cumulated time of the consecutive hydrolysis, x is DH (%), k h (min−1) and k d (dm3 min−1 g−1) are the rate constants for hydrolysis and deactivation, respectively, (g dm−3) is the enzyme concentration and is the initial substrate concentration (g dm−3). The experimental data were fitted to the proposed kinetic model using Matlab software. Overall, the process productivity expressed as mg of obtained proteins divided by the added enzyme activity units in the system and reaction time.
Determination of protein concentration
Protein concentration in samples was determined using the standard Lowry method, which was previously described elsewhere (Lowry et al. 1951).
Statistical analysis
The experimental planning was done according to the three-factor Box-Behnken experimental design with 17 experimental points (5 central points) as shown in Table 1. The impeller speed (100–500 rpm), E/S ratio (15–30 g of enzyme kg−1 of egg-white), and permeate flow rate (1.6–2.5 cm3 min−1) were the variables investigated. These variables were chosen based on the results obtained in a preliminary study (data not shown) and literature data. (Demirhan et al. 2010; Nouri et al. 1997).
Table 1.
Box–Behnken design with actual/coded values for the three variables, experimentally obtained and model predicted responses
| Run | Variable levels | Experimental ABTS (%) | Predicted ABTS (%) | Experimental DPPH (%) | Predicted DPPH (%) | Experimental FRAP (µmol Fe2+ dm−3) | Predicted FRAP (µmol Fe2+ dm−3) | ||
|---|---|---|---|---|---|---|---|---|---|
| X 1 | X 2 | X 3 | |||||||
| 1 | 100 (−1) | 15 (−1) | 2.05 (0) | 85 | 84.94 | 42.67 | 41.54 | 19 | 13 |
| 2 | 500 (1) | 15 (−1) | 2.05 (0) | 87.78 | 87.14 | 39.75 | 39.76 | 37 | 34 |
| 3 | 100 (−1) | 30 (1) | 2.05 (0) | 85.71 | 86.87 | 43.11 | 45.46 | 17 | 20 |
| 4 | 500 (1) | 30 (1) | 2.05 (0) | 89.52 | 89.07 | 44.9 | 43.68 | 2.819 | 8.45 |
| 5 | 100 (−1) | 22.5 (0) | 1.60 (−1) | 85.4 | 84.36 | 42.44 | 44.23 | 27 | 30 |
| 6 | 500 (1) | 22.5 (0) | 1.60 (−1) | 89.37 | 89.41 | 25.5 | 28.51 | 42 | 35 |
| 7 | 100 (−1) | 22.5 (0) | 2.50 (1) | 82.06 | 82.01 | 43.8 | 40.79 | 23 | 22 |
| 8 | 500 (1) | 22.5 (0) | 2.50 (1) | 80.32 | 81.36 | 54.76 | 52.97 | 21 | 26 |
| 9 | 300 (0) | 15 (−1) | 1.60 (−1) | 82.54 | 83.38 | 47.82 | 45.02 | 27 | 36 |
| 10 | 300 (0) | 30 (1) | 1.60 (−1) | 90.79 | 90.94 | 50.95 | 48.94 | 32 | 27 |
| 11 | 300 (0) | 15 (−1) | 2.50 (1) | 83.97 | 83.82 | 51.62 | 55.53 | 28 | 27 |
| 12 | 300 (0) | 30 (1) | 2.50 (1) | 80.95 | 80.11 | 58.57 | 59.45 | 21 | 18 |
| 13 | 300 (0) | 22.5 (0) | 2.05 (0) | 86.03 | 85.91 | 43.11 | 43.1 | 56 | 55 |
| 14 | 300 (0) | 22.5 (0) | 2.05 (0) | 87.00 | 85.91 | 45 | 43.1 | 60 | 55 |
| 15 | 300 (0) | 22.5 (0) | 2.05 (0) | 85.01 | 85.91 | 43.2 | 43.1 | 52 | 55 |
| 16 | 300 (0) | 22.5 (0) | 2.05 (0) | 85.72 | 85.91 | 41 | 43.1 | 53 | 55 |
| 17 | 300 (0) | 22.5 (0) | 2.05 (0) | 85.82 | 85.91 | 43.2 | 43.1 | 55 | 55 |
The antioxidant capacity measured by ABTS, DPPH and FRAP methods was set as a response variable. Experimental data were fitted with an empirical second-order polynomial equation including effects of linear, quadratic and interaction terms of tested variables.
| 3 |
where, are independent variables (impeller speed, E/S ratio, and permeate flow rate) which affect the predicted response y (antioxidant capacity measured by y 1 ABTS, y 2 DPPH, and y 3 FRAP methods), β 0, β i, β ii, β ij are coefficients for intercept, linear, quadratic and interaction terms, respectively. The coefficients of the response function and their statistical significance were evaluated by the response surface regression analysis, using the statistical software Design Expert Statistical Software package 8.0.7.1 (Stat Ease Inc., Minneapolis, USA).
All experiments were carried out in triplicate and the results are expressed as mean ± SD. The significance of a difference of means between two distributions was evaluated using Student t test while mean values of multiple groups were analyzed by one-way analysis of variance (ANOVA). All analyses were done using Microsoft Office Excel 2007 software (Microsoft Corporation, WA, USA). Significance was determined at p < 0.05.
Antioxidant capacity measured by DPPH radical scavenging method
The DPPH radical scavenging activity of hydrolysates and peptide fractions was analyzed according to the method previously described (Jiang et al. 2014). Namely, 0.5 cm3 of hydrolysate and 0.5 cm3 of 0.1 mmol dm−3 methanolic DPPH solution were mixed and left in dark for 30 min and then absorbance was measured at 517 nm.
| 4 |
A s and A b represent the absorbance of the sample solution in the presence and in the absence of the DPPH, respectively, and A c is absorbance of the control solution with DPPH.
Antioxidant capacity measured by ABTS radical scavenging method
The method was based on the ABTS·+ radical cation decolorization (Re et al. 1999). ABTS·+ radical was produced in the reaction between 7 mmol dm−3 ABTS solution and 2.45 mmol dm−3 potassium persulfate (final concentration), left in dark for 12–16 h, and then diluted with 5 mmol dm−3 phosphate buffer, pH 7.4, until the absorbance of 0.700 (± 0.02) was reached. The 5 μl of the hydrolysates was mixed with 500 µl of prepared ABTS·+ solution and after 5 min absorbance was measured at 734 nm.
| 5 |
where, A s represents the absorbance of the sample solution in the presence of the ABTS·+and A c is the absorbance of the control solution with ABTS·+.
Antioxidant capacity measured by ferric reducing power
The FRAP assay is based on the hydrolysates ability to reduce ferric tripyridyl triazine (Fe III-TPTZ) to the blue-colored ferrous complex at low pH, as previously described (Milutinović et al. 2013). Samples (buffer for blank), 25 µl were vigorously mixed with 750 µl of FRAP solution and after 5 min absorbance was measured at 593 nm. A standard curve was prepared using FeSO4 7H2O (0.1–1 mmol dm−3). All results were expressed as µmol Fe2+ dm−3.
Results and discussions
Protein hydrolysis in the CEMR: the influence of the operating parameters on process efficiency
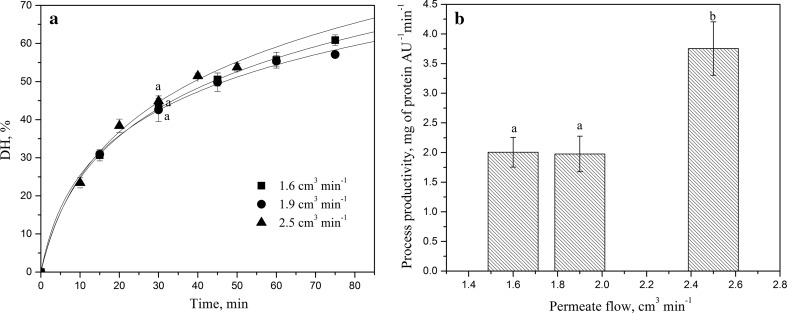
The effects of operating parameters on the process efficiency and antioxidant capacity were evaluated during hydrolysis of EWPs in the CEMR, coupled with the selected membrane module (10 kDa) as shown in Fig. 1. Process efficiency was first evaluated in terms of the achieved DH and process productivity. Results of DH versus time for different permeate flow rates, obtained in the continuous mode are represented in Fig. 2a. Solid lines present the kinetic fit of the experimental data for different permeate flow rates and Table 2 reports rate constants for hydrolysis and enzyme deactivation. Interestingly, no significant differences in the DH are observed between different permeate flow rates. This result was confirmed by ANOVA test (p > 0.05). For any tested permeate flow rate, the reaction progresses rapidly for the first 30 min, and then the rate of hydrolysis subsequently decreases. It seems to be useful to model the combined effects of an operating parameter on the intrinsic kinetic rate and deactivation of the biocatalyst and optimize the parameter with respect to both aspects. The value of the hydrolysis constant appeared to be slightly higher for the lowest applied permeate flow rate, revealing a slightly positive effect of residence time on the reaction progress. This can be explained by prolonged and better contact between enzyme and substrate. By contrast, the highest permeate flow rate provides the lowest k h, but the value of the deactivation rate kinetic constant is also the lowest, making that the achieved DH is apparently unchanged.
Fig. 2.
a Experimental (points) and fitted data (solid lines) of EWP hydrolysis in the continuous enzymatic membrane reactor system for different permeate flow rates. Values with different superscripts for experimental hydrolysis time (30 min) differ significantly (p < 0.05). b The influence of permeate flow rate on the process productivity. Reaction conditions: pH 8.0, 50 °C, E/S ratio 20 g of enzyme kg−1 of egg-white, and stirring with 4-bladed propeller. Values with different superscripts for tested permeate flow rates differ significantly (p < 0.05)
Table 2.
Values of the kinetic constants obtained in the Alcalase-catalyzed EWPs hydrolysis in the continuous stirred tank membrane reactor system at different permeate flow rates and with different E/S ratios
| Permeate flow rate (cm3 min−1) | E/S ratio (g of enzyme kg−1 of egg white) | |||||
|---|---|---|---|---|---|---|
| 1.6 | 1.9 | 2.5 | 15 | 20 | 30 | |
| k h (min−1) | 4.63 | 4.03 | 4.01 | 2.15 | 3.86 | 4.56 |
| k d (dm3 min g−1) | 0.073 | 0.078 | 0.063 | 0.024 | 0.067 | 0.109 |
| R2 | 0.9998 | 0.9961 | 0.9962 | 0.9961 | 0.9961 | 0.9997 |
When optimizing the operating parameters in the CEMR, both conversion and productivity should be evaluated.Fig. 2 b displays the process productivity for different permeate flow rates. No significant change in the productivity was found for permeate flow rate ranging from 1.6 to 1.9 cm3 min−1 (p > 0.05). However, a significant increase in process productivity was apparent for hydrolysis for flow rate above 1.9 cm3 min−1 (p < 0.05). The increase in permeate flow rate from 1.9 to 2.5 cm3 min−1 increased the process productivity by 80%. Similar results were reported in the continuous production of 5′-nucleotides using ultrafiltration membrane reactor, where the permeate flow rate did not have a significant effect on the concentration of produced nucleotides, but seemed to significantly affect process productivity (Shi et al. 2009). Higher permeate flow rates have not been tested because of the corresponding membrane pressures, which could damage membrane, as well as lower rates because they take an excessively long time to achieve steady state.
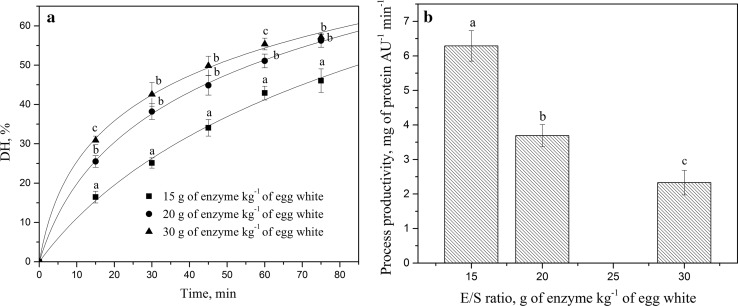
E/S ratio is another important parameter which has to be optimized in an enzymatic reaction since the enzyme cost often causes limitation of industrial enzymatic processes. Degrees of hydrolysis obtained in this series as a function of E/S ratio are shown in Fig. 3a. Solid lines represent the best fit of experimental data from the proposed kinetic model for different E/S ratios. It can be observed that experimental and predicted data apparently correlate well, confirming the validity of the applied model. The achieved degree of hydrolysis was higher for higher E/S ratio, as expected. The same trend was observed for kinetic constants` values. Specifically, k h was more than two folds higher for the E/S ratio of 15 g kg−1 than for 30 g kg−1(Table 2). This was simply because more active enzyme provided more enzyme–substrate complexes, hence more extensive hydrolysis. The positive influence of the E/S ratio on the DH has been previously reported in the hydrolysis of oil seed meal proteins (Das et al. 2012). Nevertheless, enzyme cost and auto-digestion phenomenon of proteases require finding the optimal E/S ratio, since an excess of substrate actually protects the enzyme from auto-cleavage (Prieto et al. 2008).
Fig. 3.
a Experimental (points) and fitted data (solid lines) of EWP hydrolysis in the CEMR for different E/S ratios. Values with different superscripts for each experimental hydrolysis time differ significantly (p < 0.05). b The influence of different E/S ratios on the process productivity. Reaction conditions: pH 8.0, 50 °C, 4-bladed propeller, permeate flow rate 2.5 cm3 min−1. Values with different superscripts for tested E/S ratios differ significantly (p < 0.05)
Figure 3b reveals that the highest process productivity is achieved with the lowest E/S ratio. Keeping that in mind, the necessity for finding a reasonable compromise between achieved DH and process productivity is an absolute imperative. Overall, E/S ratio of 20 g of enzyme kg−1 of egg-white represents the reasonable compromise between achieved values of DH and process productivity for this specific hydrolysis.
Optimization of operating parameters with respect to antioxidant activity by statistical design
The antioxidant activity of protein hydrolysates and peptides, as common components of food formulations, has been widely studied and well documented. When discussing their antioxidant activity, the application of at least two methods is recommended due to the differences between the test systems investigated (Schlesier et al. 2002). Therefore, three methods were used for evaluation of EWP hydrolysates antioxidant capacity in the current research, namely ABTS, DPPH and FRAP methods.
Generally, the hydrolysis favors the antioxidant capacity of obtained hydrolysates as compared to the control pasteurized non-hydrolyzed EWP solution. This beneficial effect of EWP hydrolysis seems to be the most apparent with ABTS method, as presented in Table 1, where the control sample expresses the ability to scavenge only 7% of the aforementioned radical. The maximum ABTS·+ scavenging activity of 90.86 ± 0.11% is achieved in the exp. No 10, when the process parameters are as follows: impeller speed 300 rpm, E/S ratio 30 g of enzyme kg−1; and permeate flow rate 1.6 cm3 min−1. The beneficial effect of protein hydrolysis on the ABTS·+ radical quenching ability has been previously reported for whey (Dryáková et al. 2010) and soy proteins (de Oliveira et al. 2015), EWPs (Lin et al. 2013; Stefanović et al. 2014), zein (Zhu et al. 2008), etc.
The results of the second-order response surface model were examined by analysis of variance (ANOVA) and Fischer’s F-test. The calculated F-value of 20.24 indicates that the model is significant while R 2-value of 0.9239 reveals that the model could explain 92.39% of the variability for the response. The following regression equation representing the empirical relation between ABTS·+ scavenging activity and impeller speed (x 1), E/S ratio (x 2), and permeate flow rate (x 3), is obtained:
| 6 |
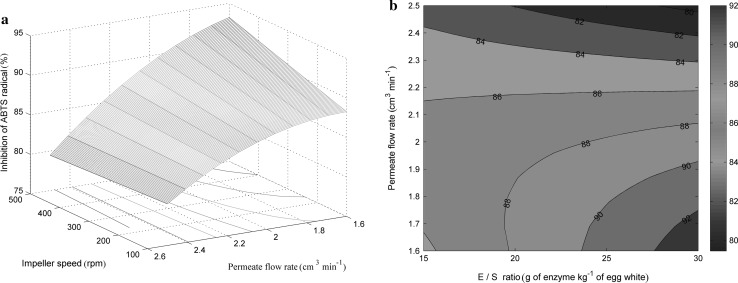
The analysis reveals that all tested variables have a significant effect on the ability of the obtained hydrolysates to scavenge ABTS·+ radical, within the experimentally tested ranges. The most relevant variable seems to be permeate flow rate, but the effects of E/S ratio, impeller speed, or E/S ratio-permeate flow rate and impeller speed-permeate flow rate interactions are also significant. The quadratic term of permeate flow rate is also significant, indicating that a response is a quadratic function with a local maximum.
The effect of permeate flow rate and impeller speed on the ABTS·+ scavenging activity of the hydrolysates is presented in Fig. 4a. The impeller speed showed an interactive negative effect with permeate flow rate on the scavenging activity. The highest antioxidant activity seems to be achieved with the highest impeller speed and the lowest value of the permeate flow rate. At lower values of permeate flow rate, an increase of impeller speed causes an increase in antioxidant activity. At higher or lower impeller speeds, however, an increase in permeate flow rate leads to the decrease in antioxidant activity. This was probably closely related to the DH since at the lowest permeate flow rate the hydrolysis constant had the highest value (Table 2). CEMR showed that the overall mixing process is the result of two independent operations: liquid mixing due to stirring and due to recycling of reaction mixture. At lower values of both impeller speed and permeate flow both of these processes are at insufficient level, therefore mass transfer limitations are apparent, resulting in low DH. However, both parameters influence the enzyme reaction in two different ways: through a change in the rate constants for hydrolysis and/or through a change in the enzyme deactivation rate constant. Thus, higher impeller speeds enable efficient mass transfer contributing to an efficient hydrolysis and formation of short-chained peptides, which are usually responsible for antioxidant activity. On the other hand, the deactivation seems to dominate with raising the flow rate. The lowest antioxidant activity is observed at the highest values of both permeate flow and impeller speed. These conditions probably lead to the inhibition of the enzyme due to the strong hydrodynamic forces (mixing and enforced flow), causing an incomplete reaction (low DH), and concurrently inhibiting the release of bioactive peptides.
Fig. 4.
a The effects of impeller speed and permeate flow rate on the ABTS·+ quenching activity of the obtained hydrolysates for E/S ratio of 30 g of enzyme kg−1. b The effects of E/S ratio and permeate flow rate on the ABTS·+ quenching activity of the obtained hydrolysates for impeller speed of 500 rpm
As shown in Fig. 4b the highest value of the ABTS·+ quenching ability is achieved with the highest E/S ratio and the lowest permeate flow rate. At any value of E/S ratio, the permeate flow rate does not seem to increase the ABTS·+ quenching activity. By contrast, at low permeate flow rates, an increase in E/S ratio significantly improves the activity. This was probably closely linked to the extent of hydrolysis since these conditions favor the protein hydrolysis. Lower flow rates enable longer contact between enzyme and substrate while high E/S ratios enable conditions for the formation of more enzyme–substrate complexes (see values of hydrolysis constants in Table 2). The positive influence of the E/S ratio on the quality of obtained hydrolysates assessed as the ABTS·+ quenching activity has been previously reported in the hydrolysis of several proteins (de Oliveira et al. 2015; Vaštag et al. 2010).
The antioxidant activity evaluated as the ability of the hydrolysate to scavenge DPPH· radical was also tested systematically. This method was based on the ability of the tested compound to reduce the odd electron of the nitrogen atom in DPPH by donating a hydrogen atom to form corresponding hydrazine (Kedare and Singh 2011). Similarly to the ABTS·+quenching ability, linear regression coefficients representing the effects of permeate flow rate, E/S ratio and impeller speed are found to be all significant, while the permeate flow rate is the most significant factor. The effects of impeller speed-permeate flow rate interaction as well as quadratic terms of all tested variables are also significant. Nevertheless, there is an apparent difference between the obtained response functions of different antioxidant test systems, revealing an opposite trend of these effects on antioxidant activity measured by DPPH and ABTS methods. The regression model describing the DPPH· scavenging activity is as follows:
| 7 |
The values for the Fisher test and R 2 are 13.99 and 0.9158 respectively, indicating that the model is significant. The highest DPPH· radical scavenging activity of 59.01 ± 0.62 is obtained in exp. No 12, conducted under the following conditions: impeller speed 300 rpm, E/S ratio 30 g of enzyme kg−1 and permeate flow rate 2.5 cm3 min−1, while the lowest value of the ABTS·+ radical inhibition is observed in the same experiment. The effect of permeate flow is somewhat different than in Eq. 6, since the positive quadratic coefficient is significant and the positive interaction with impeller speed is apparent. The differences between optimum conditions for achieving the highest ABTS·+ and DPPH· antioxidant activities are probably the consequence of different solubility and diffusivity of tested radicals (Zhu et al. 2008). ABTS·+ seems to be water soluble while DPPH· is oil-soluble radical and the DPPH radical assay is a model system for testing the capacity of lipophilic antioxidants. Similar antioxidant activity (56%) measured by DPPH method has been reported for EWP hydrolysates ultrafiltrated through the membrane of 10 kDa (Lin et al. 2013). The same authors obtained even higher antioxidant activities measured by the DPPH method with the fractions less than 1 kDa (69%) while ABTS scavenging activity remained unchanged. Such result suggests that more hydrophobic amino acid residues are exposed at smaller peptides, hence they are more accessible to the DPPH· radical. This is in agreement with previous studies where DPPH radical-scavenging activity of hydrolysates and/or peptides has also been shown to be associated with the higher content of hydrophobic amino acids (Li et al. 2008). The increase in DPPH· scavenging activity with protein hydrolysis has been previously reported for a variety of natural proteins (Lin et al. 2012).
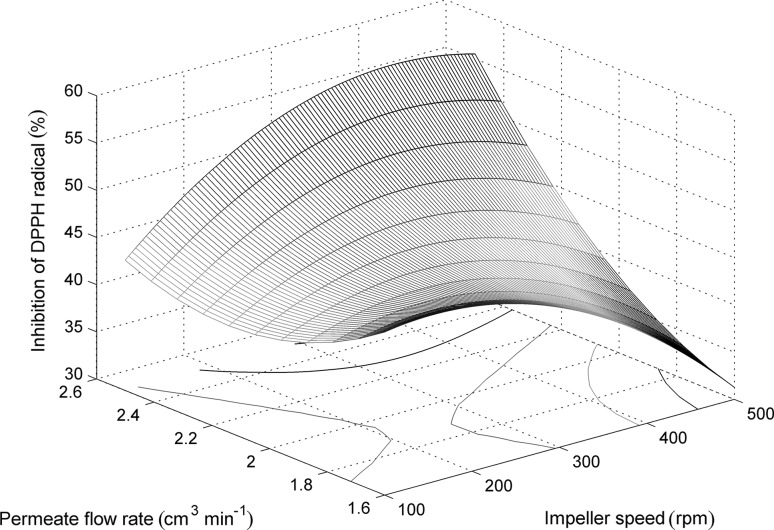
Unlike for the ABTS quenching activity, the optimum conditions for obtaining hydrolysates with improved DPPH scavenging activity are at high permeate flow rates and high impeller speeds (Fig. 5). It is interesting since these operating conditions do not favor hydrolysis revealing that the DH is not the only criterion that determines the antioxidant activity of the hydrolysates. This could be related to the relatively hydrophobic character of polyethersulfone membrane, causing accumulation and/or adsorption of more hydrophobic peptides at the membrane surface (Qu et al. 2010). The interactions between the membrane (or the layer of the hydrophobic peptides accumulated at the surface) and hydrophobic peptides would be favored at higher flow rates because of the greatest presence of peptide molecules near the membrane surface, resulting in a selective transmission of hydrophobic peptides over hydrophilic ones.
Fig. 5.
The effects of impeller speed and permeate flow rate on the DPPH quenching activity of the obtained hydrolysates for E/S ratio of 15 g of enzyme kg−1 of egg white
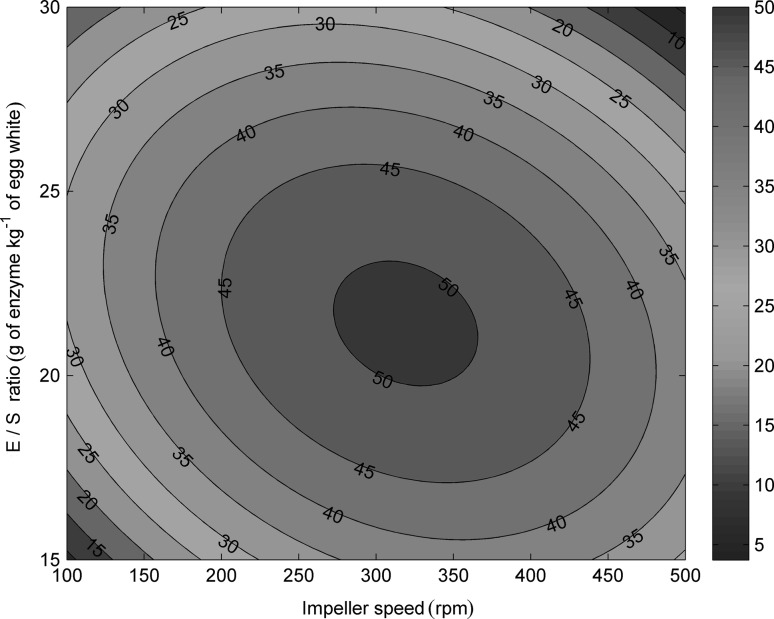
The antioxidant capacity of EWP hydrolysates was also evaluated using the FRAP assay, which is based on the ability of the hydrolysates to reduce a TPTZ–Fe(III) complex to a TPTZ–Fe(II) complex. Unlike, ABTS and DPPH method, results obtained with FRAP assay indicate that hydrolysis of the EWP does not potentiate increase in the antioxidant capacity of every obtained hydrolysate (Table 1), since untreated egg-white expressed activity equivalent to the 20 µmol dm−3 of Fe2+. Nevertheless, the hydrolysate obtained in the experiment No. 13-17 conducted at central points under the following conditions: E/S ratio 22.5 g of enzyme kg−1; impeller speed 300 rpm and permeate flow rate 2.05 cm3 min−1 provides 2.8 fold increase in the ferric ion reducing activity. Statistical analysis shows that the model is significant, with the F value of 15.92 and R 2 value of 0.9253. The obtained regression equation is as follows:
| 8 |
It seems clear that both impeller speed and E/S ratio have a maximum (310 rpm, 22.4 g of enzyme kg−1, Fig. 6). These results suggest that enzymatic hydrolysis should be performed under strictly controlled and optimized process conditions resulting in a hydrolysate that contains biologically active peptides with particular antioxidant capacity.
Fig. 6.
The effects of impeller speed and E/S ratio on the antioxidant activity measured by FRAP method. Permeate flow rate is 2.05 cm3 min−1
Conclusion
In conclusion, hydrolysis of EWPs in the CEMR produces beneficial effects on the antioxidant capacity. Notable diversity in the antioxidant capacity depending on the operating conditions and tested systems may be clearly observed. Response equations are obtained, making it possible to predict the operating conditions required to obtain hydrolysates with specific antioxidant activities. This finding confirms that the control of hydrolysis conditions enables the production of the desired product quality, which could satisfy strict needs of the food industry.
Acknowledgements
This work was supported by EUREKA Project E!6750 and III-46010 from the Ministry of Education, Science and Technological Development of the Republic of Serbia.
References
- Berends P, Appel D, Eisele T, Rabe S, Fischer L. Performance of enzymatic wheat gluten hydrolysis in batch and continuous processes using Flavourzyme. LWT Food Sci Technol. 2014;58:534–540. doi: 10.1016/j.lwt.2014.03.035. [DOI] [Google Scholar]
- Bhat ZF, Kumar S, Bhat HF. Bioactive peptides of animal origin: a review. J Food Sci Technol. 2015;52:5377–5392. doi: 10.1007/s13197-015-1731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chi YJ. Antioxidant, ACE inhibitory activities and functional properties of egg white protein hydrolysate. J Food Biochem. 2012;36:383–394. doi: 10.1111/j.1745-4514.2011.00555.x. [DOI] [Google Scholar]
- Chiang WD, Tsou MJ, Weng CH, Tsai TC. Production of angiotensin I-converting enzyme inhibitor derived from egg white protein hydrolysates using a membrane reactor. J Food Drug Anal. 2008;16:54–60. [Google Scholar]
- Das R, Ghosh S, Bhattacharjee C. Enzyme membrane reactor in isolation of antioxidative peptides from oil industry waste: a comparison with non-peptidic antioxidants. LWT Food Sci Technol. 2012;47:238–245. doi: 10.1016/j.lwt.2012.01.011. [DOI] [Google Scholar]
- de Oliveira CF, Correa AP, Coletto D, Daroit DJ, Cladera-Olivera F, Brandelli A. Soy protein hydrolysis with microbial protease to improve antioxidant and functional properties. J Food Sci Technol. 2015;52:2668–2678. doi: 10.1007/s13197-014-1317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirhan E, Apar DK, Özbek B. Sesame cake protein hydrolysis by alcalase: effects of process parameters on hydrolysis, solubilisation, and enzyme inactivation. Korean J Chem Eng. 2010;28:195–202. doi: 10.1007/s11814-010-0316-2. [DOI] [Google Scholar]
- Dryáková A, Pihlanto A, Marnila P, Čurda L, Korhonen HT. Antioxidant properties of whey protein hydrolysates as measured by three methods. Eur Food Res Technol. 2010;230:865–874. doi: 10.1007/s00217-010-1231-9. [DOI] [Google Scholar]
- Fan S, Hu Y, Li C, Liu Y. Optimization of preparation of antioxidative peptides from pumpkin seeds using response surface method. PLoS ONE. 2014;9:e92335. doi: 10.1371/journal.pone.0092335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadix A, Camacho F, Guadix EM. Production of whey protein hydrolysates with reduced allergenicity in a stable membrane reactor. J Food Eng. 2006;72:398–405. doi: 10.1016/j.jfoodeng.2004.12.022. [DOI] [Google Scholar]
- Jakovetić S, Luković N, Jugović B, Gvozdenović M, Grbavčić S, Jovanović J, Knežević-Jugović Z. Production of antioxidant egg white hydrolysates in a continuous stirred tank enzyme reactor coupled with membrane separation unit. Food Bioprocess Technol. 2015;8:287–300. doi: 10.1007/s11947-014-1402-y. [DOI] [Google Scholar]
- Jiang H, Tong T, Sun J, Xu Y, Zhao Z, Liao D. Purification and characterization of antioxidative peptides from round scad (Decapterus maruadsi) muscle protein hydrolysate. Food Chem. 2014;154:158–163. doi: 10.1016/j.foodchem.2013.12.074. [DOI] [PubMed] [Google Scholar]
- Kedare S, Singh RP. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol. 2011;48:412–422. doi: 10.1007/s13197-011-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Jiang B, Zhang T, Mu W, Liu J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH) Food Chem. 2008;106:444–450. doi: 10.1016/j.foodchem.2007.04.067. [DOI] [Google Scholar]
- Lin S, Guo Y, You Q, Yin Y, Liu J. Preparation of antioxidant peptide from egg white protein and improvement of its activities assisted by high-intensity pulsed electric field. J Sci Food Agric. 2012;92:1554–1561. doi: 10.1002/jsfa.4742. [DOI] [PubMed] [Google Scholar]
- Lin S, Jin Y, Liu M, Yang Y, Zhang M, Guo Y, Jones G, Liu J, Yin Y. Research on the preparation of antioxidant peptides derived from egg white with assisting of high-intensity pulsed electric field. Food Chem. 2013;139:300–306. doi: 10.1016/j.foodchem.2013.01.048. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Malaguti M, Dinelli G, Leoncini E, Bregola V, Bosi S, Cicero AFG, Hrelia S. Bioactive peptides in cereals and legumes: agronomical, biochemical and clinical aspects. Int J Mol Sci. 2014;15:21120–21135. doi: 10.3390/ijms151121120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milutinović M, Šiler-Marinković S, Antonović D, Mihajlovska K, Pavlović M, Dimitrijević-Branković S. The antioxidant properties of dried extracts from the spent espresso coffee. Hem Ind. 2013;67:261–267. doi: 10.2298/HEMIND120410074M. [DOI] [Google Scholar]
- Nouri L, Legrand J, Popineau Y, Belleville P. Enzymatic hydrolysis of wheat proteins Part I. Enzymatic kinetics and study of limited hydrolysis in a batch stirred reactor. Chem Eng J. 1997;65:187–194. doi: 10.1016/S1385-8947(97)00002-8. [DOI] [Google Scholar]
- Prieto CA, Guadix EM, Guadix A. Influence of temperature on protein hydrolysis in a cyclic batch enzyme membrane reactor. Biochem Eng J. 2008;42:217–223. doi: 10.1016/j.bej.2008.06.018. [DOI] [Google Scholar]
- Prieto CA, Guadix EM, Guadix A. Optimal operation of a protein hydrolysis reactor with enzyme recycle. J Food Eng. 2010;97:24–30. doi: 10.1016/j.jfoodeng.2009.08.041. [DOI] [Google Scholar]
- Qu P, Tang H, Gao Y, Zhang L. Polyethersulfone composite membrane blended with cellulose fibrils. BioResources. 2010;5:2323–2336. [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Sarmadi BH, Ismail A. Antioxidative peptides from food proteins: a review. Peptides. 2010;31:1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Schlesier K, Harwat M, Böhm V, Bitsch R. Assessment of antioxidant activity by using different in vitro methods. Free Radic Res. 2002;36:177–187. doi: 10.1080/10715760290006411. [DOI] [PubMed] [Google Scholar]
- Shi L-E, Ying G-Q, Tang Z-X, Chen J-S, Xiong W-Y, Wang H. Continuous enzymatic production of 5′-nucleotides using free nuclease P1 in ultrafiltration membrane reactor. J Membr Sci. 2009;345:217–222. doi: 10.1016/j.memsci.2009.09.001. [DOI] [Google Scholar]
- Stefanović A, Jovanović J, Grbavčić S, Šekuljica N, Manojlović V, Bugarski B, Knežević-Jugović Z. Impact of ultrasound on egg white proteins as a pretreatment for functional hydrolysates production. Eur Food Res Technol. 2014;239:979–993. doi: 10.1007/s00217-014-2295-8. [DOI] [Google Scholar]
- Van der Plancken I, Van Loey A, Hendrickx ME. Combined effect of high pressure and temperature on selected properties of egg white proteins. Innov Food Sci Emerg Technol. 2005;6:11–20. doi: 10.1016/j.ifset.2004.10.002. [DOI] [Google Scholar]
- Vaštag Ž, Popović L, Popović S, Krimer V, Peričin D. Hydrolysis of pumpkin oil cake protein isolate and free radical scavenging activity of hydrolysates: influence of temperature, enzyme/substrate ratio and time. Food Bioprod Process. 2010;88:277–282. doi: 10.1016/j.fbp.2009.12.003. [DOI] [Google Scholar]
- Yu Z, Yin Y, Zhao W, Chen F, Liu J. Application and bioactive properties of proteins and peptides derived from hen eggs: opportunities and challenges. J Sci Food Agric. 2014;94:2839–2845. doi: 10.1002/jsfa.6670. [DOI] [PubMed] [Google Scholar]
- Zhu L, Chen J, Tang X, Xiong YL. Reducing, radical scavenging, and chelation properties of in vitro digests of alcalase-treated zein hydrolysate. J Agric Food Chem. 2008;56:2714–2721. doi: 10.1021/jf703697e. [DOI] [PubMed] [Google Scholar]