Abstract
With the objective to evaluate the modifications in the fruit quality, ‘Palmer’ mangoes were stored at 12.8 °C for 30 days in controlled atmosphere storage that contained a low level of oxygen (5 kPa) which was associated with increasing levels of carbon dioxide CO2 (0, 1, 5, 10, 15 and 20 kPa CO2). Controlled atmosphere storage did not effect mango respiration. However, transfer mangoes, that were previously stored at high levels of CO2 (5 kPa O2 + 15 kPa CO2 and 5 kPa O2 + 20 kPa CO2) to ambient temperature presented higher respiratory rates. No significant effects of increasing CO2 levels on color (L*, chromaticity, and hue angle), firmness, physical–chemical parameter and carbohydrate metabolism (total and reducing sugars, soluble pectin) were observed. After transfer to ambient temperature the mangoes ripened normally without any signs of CO2 injury. Therefore, the increment levels of CO2 neither improved the quality of the ‘Palmer’ mangoes nor presented a synergistic effect with low-oxygen when compared to 5 kPa O2-control.
Keywords: Mangifera indica L., Respiration, Softening, Shelf-life, Pectin, Carbohydrates
Introduction
Mango fruit (Mangifera indica L.) stands out as a produce of high commercial value in many tropical regions in the world (FAOSTAT 2014), however, as a climacteric and perishable fruit, mangoes ripen quickly at ambient temperature and their quality can only be maintained for 8 days under these conditions (Kader 2003a). Cold storage can be used to extend the shelf-life of mangoes for up to 16 days, but due to the development of chilling injury at temperatures below 13 °C (Teixeira and Durigan 2011), the use of controlled atmospheres (CA) with adequate levels of oxygen (O2) and carbon dioxide (CO2) can reduce the metabolism and delay mango ripening (Kader 2003a).
The use of CA for storage has proven to be a viable method to extend the shelf-life and maintain the quality of various tropical fruits (Kader 2003a, b). CA storage has been studied in many mango cultivars, e.g., ‘Kent’ (Trinidad et al. 1997; Bender and Brecht 2000), ‘Tommy Atkins’ (Lizada and Ochagavia 1997; Bender and Brecht 2000; Kim et al. 2007), ‘Manila’ (Ortega-Zaleta and Yahia 2000), ‘Haden’ (Bender et al. 2000), and ‘Palmer’ (Teixeira and Durigan 2011).
The general CA recommendation for storing mangoes is in an environment containing 5 kPa O2 and 5 kPa CO2 (Kader 1986), however some cultivars may have low tolerance to high levels of CO2 and problems with O2 levels lower than 2% (Bender and Brecht 2000). Mango CA storage (5 kPa O2 and 5 kPa CO2) at 12 °C has a shelf-life extended for up to 20 days, while the onset of off-flavors and skin discoloration only occurred in environments with 1 kPa O2 and/or CO2 levels higher than 15 kPa (Nakasone and Paull 1998). The wide variations in the results can be attributed to the differences in maturity stages, temperatures and cultivation practices.
When stored in CA, especially with CO2 levels higher than 25 kPa, mangoes generally present physiological disorders. Bender and Brecht (2000) reported that ‘Tommy Atkins’ mangoes produced more ethanol when stored in CA containing 50 kPa and 70 kPa CO2, and the respiratory rate was more intense when CO2 levels were higher than 45 kPa. The production of volatile compound responsible for the mango aroma was also affected in CO2 levels (> 6 kPa), and it was totally compromised in ‘Kensington Pride’ mangoes that were stored for up to 35 days in CA containing 6 kPa O2 and 2 kPa CO2 (Lalel et al. 2003). As for the antioxidant phytochemicals, hot water treatment (46 °C per 75 min) and CA storage (3 kPa O2 and 10 kPa CO2) did not adversely change the nutritional profile of ‘Tommy Atkins’ mangoes (Kim et al. 2007).
Although good results can be found, the response of mangoes to CA is not always positive, in some cultivars there is only a short increase in shelf-life (O’Hare and Prassad 1993) and in others the shelf-life can be extended for up to 1 month (Trinidad et al. 1997). There are various studies regarding CA storage for many mango varieties, yet there are no complete recommendations for ‘Palmer’ mangoes. Teixeira and Durigan (2011) reported a delay in the ripening process and better fruit quality when ‘Palmer’ mangoes were stored in CA containing 1–10 kPa O2 at 12.8 °C for up to 28 days, however there are no studies regarding the effects of CO2. Hence, the objective of this study was to evaluate the quality of ‘Palmer’ mangoes stored in CA containing low levels of oxygen in association with increasing levels of CO2.
Materials and methods
Plant material
Mango fruit (Mangifera indica L.) of the ‘Palmer’ cultivar were harvested on a commercial orchard located in Taiúva, São Paulo State, Brazil (21°7′ 26″S latitude; 48°27′1″W longitude; 627 m altitude). All fruits were considered physiologically matured, maturity stage 1 (Assis 2004). After harvesting, the mangoes were sorted, and did not receive any postharvest treatment (washing, hot water treatment, waxing, and fungicide spraying) in order to verify the effect of the increasing levels of CO2 in controlling postharvest decay.
Controlled atmosphere (CA) treatments
The fruits were placed in hermetically closed plastic buckets (20 L) which were ventilated with a humidified air flow of 100 mL min−1 and balanced with nitrogen (N2) in order to form the following gas concentrations: (1) 5 kPa O2-control; (2) 5 kPa O2 + 1 kPa CO2; (3) 5 kPa O2 + 5 kPa CO2; (4) 5 kPa O2 + 10 kPa CO2; (5) 5 kPa O2 + 15 kPa CO2; and (6) 5 kPa O2 + 20 kPa CO2. The sources of N2 and CO2 were commercial gas cylinders and compressed air for O2. To establish the different gas concentrations a flowmeter was used and set up according to Claypool and Keefer (1942), with slight modifications (Teixeira and Durigan 2011). The control atmosphere (5 kPa O2) was chosen based on our previous result of ‘Palmer’ mangoes stored in CA (Teixeira and Durigan 2011).
The experimental unit was considered a group of 4 fruits placed in the buckets with three repetitions per evaluation days (0, 15, and 30 days—36 fruits) of each gas mixture, totaling 216 fruits. The storage temperature was 12.8 ± 0.6 °C (RH ~ 95%) and fruit ripening was carried out in ambient conditions (25.2 ± 0.6 °C, 92.8 ± 2.4% RH). During CA storage, the fruits were sampled at 15 day intervals (0, 15, and 30 days) with the objective of verifying the effect of the gas mixtures on fruit quality. The evaluations were conducted immediately after the fruits were removed from the CA conditions (0, 15, and 30 days) and once more when the fruits were considered ripe (0 + 9, 15 + 7, and 30 + 5 days).
Atmospheric composition and respiration
The atmospheric composition (O2, CO2, and ethylene-C2H4) was determined daily using a gas analyzer (Dansensor Checkmate 9001, PBI Dansensor, Denmark). The respiration rate was determined using the Boyle–Charles’ Law according to Nakamura et al. (2003).
where Q, respiratory rate (mg CO2 kg−1 h−1); ΔC, difference in gas concentration (carbon dioxide) between input and output (%); F, gas flow from sampling chamber (mL min−1); ρ, gas density (g L−1); T0, storage temperature (K); T, constant (= 273.15); M, sample mass (kg).
The gas samples were taken from the inlet and outlet of the buckets with one hour time span (Bender and Brecht 2000) and then injected (0.3 mL gas samples) into a gas chromatograph (Finningan, model 9001, Finningan Corporation, San Jose, USA) equipped with stainless steel columns filled with Porapak-N and a molecular sieve (5A), thermal conductivity detectors (150 °C) and flame ionization, using nitrogen as the carrier gas (30 mL min−1) to determine the CO2 and ethylene concentrations, however it was not possible to determine ethylene due to the column sensibility. The chromatograms were acquired and analyzed with using Borwin software (Borwin version 1.20, JMBS Développements, Le Fontanil, France) and the area of the peaks compared with a gas standard (10 kPa O2, 11 kPa CO2, and 10 ppm C2H4).
Fruit quality evaluations
Fresh weight loss
Fresh fruit weight loss (FWL) was determined based on the difference in fruit mass from the different withdrawals using a semi-analytical scale with a precision of 0.01 g (Marte, model AS 2000, São Paulo, Brazil).
Firmness
The pulp firmness was determined using an Effegi Fruit Tester penetrometer (Bishop FT 327 Penetrometer, Alfonsine, Italy) with an 8.0 mm tip. Two analyses were performed on opposite sides of the equatorial region of each fruit after removing the peel. The results were expressed in Newton (N).
Color
The peel color was determined using a Minolta colorimeter (Model CR-400, Minolta Corp., Osaka, Japan) with an 8 mm aperture, which expresses the color parameter according to the “Commission Internacionale de L’Eclaraige” (CIE) as luminosity (L*), chromaticity and hue angle (McGuire 1992). Two readings were taken from each fruit on opposite sides of the equatorial region and the results were avereged.
Physical–chemical and chemical analysis
After withdraw from the CA conditions, the pulp of the fruit was homogenized (500 g) and 10 g was analyzed to soluble solids content (SSC) using a digital refratometer PR-101α (Atago, Tokyo, Japan), and 10 g to titatrable acidity (TA), according to the AOAC methods proc. 920.151, and 932-12, respectively, which allowed the calculus of the ratio SSC/TA. The pH was also determined (AOAC 1997-proc 945-27). The pulp was frozen at − 20 °C and this material was used to determine the contents of the total soluble sugars (TSS), according to the Anthrone method (Yemn and Willis 1954); and reduced sugars (RS) using the dinitrosalicylic acid (DNS) method (Miller 1959). Soluble pectin (PS) was extracted following the McCready and McComb (1952) method and the PS content was determined according to Bitter and Muir (1962).
Visual appearance evaluation
The fruit quality was determined based on appearance (1, very bad; 2, bad; 3, regular; 4, very good; and 5, excellent) as proposed by Teixeira and Durigan (2011). This analysis was carried out immediately after withdrawal from CA storage (0, 15, and 30 days), and also after 9, 7, and 5 days of transferring the fruit to ambient temperature (ripening). A total of 10 evaluations were taken per withdrawal of untrained panel.
Statistical analysis
Controlled atmosphere and visual appearance evaluation
The experiment was set up in a completely randomized design (CRD) in a factorial arrangement 6 × 3, as such: six gas mixtures (5 kPa O2-control, 5 kPa O2 + 1 kPa CO2, 5 kPa O2 + 5 kPa CO2, 5 kPa O2 + 10 kPa CO2, 5 kPa O2 + 15 kPa CO2, and 5 kPa O2 + 20 kPa CO2) with three withdrawals (0, 15, and 30 days), and three repetitions. The data was subjected to analysis of variance (ANOVA) using the PROC MIXED procedure of SAS (1999).
Fruit transfer to ambient temperature
The data was subjected to analysis of variance (ANOVA) following a completely randomized design (CRD) with 6 treatments (control and gas mixtures) and 3 repetitions using the GLM procedure of SAS (1999).
Results
Effect of increasing levels of CO2 on respiration
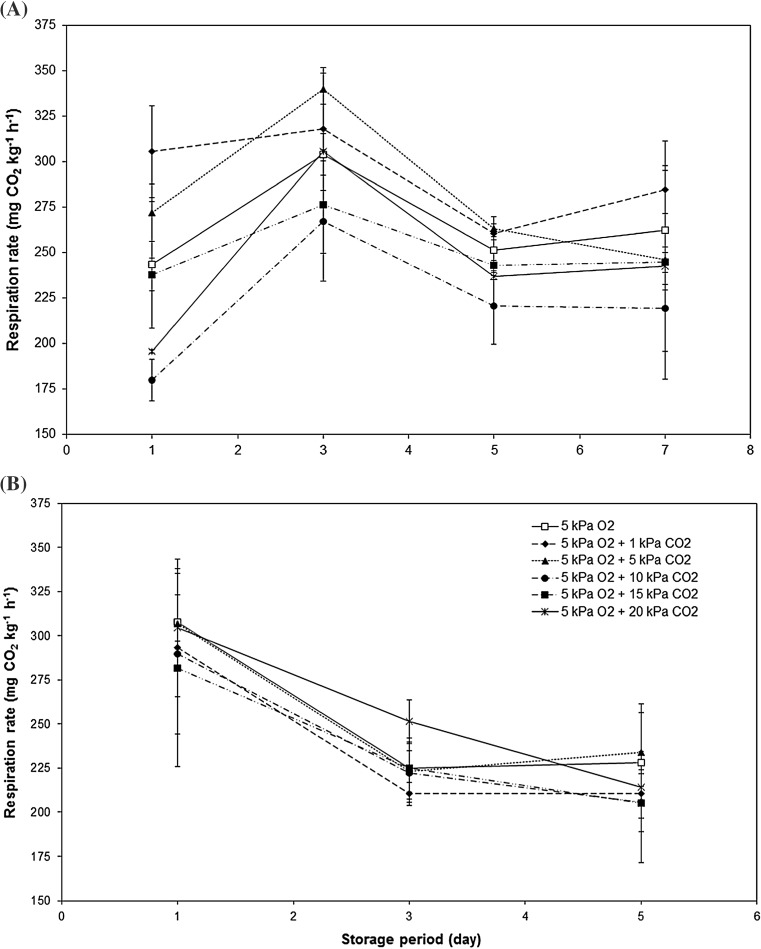
The increase in CO2 concentration did not influence mango respiratory activity (Fig. 1). Mangoes stored at 5 kPa O2 (control) presented the same respiration rate as those kept in the atmospheres that contained high levels of CO2 (Fig. 1). However, a significant effect of storage period on respiration rate was observed (p < 0.05). In the beginning of the storage period the production of CO2 was very high (42.84 mg kg−1 h−1), but stabilized after the third day onwards (Fig. 1). The respiration rates maintained unaltered for up to 25 days of storage until an increase in CO2 production was observed near the end of the storage period due to the onset of postharvest decay (Fig. 1). The respiration rates after 25 days were similar in all treatments, but were a little more accentuated in mangoes stored at 5 kPa O2, 5 kPa O2 + 1 kPa CO2, and 5 kPa O2 + 5 kPa CO2, (Fig. 1).
Fig. 1.
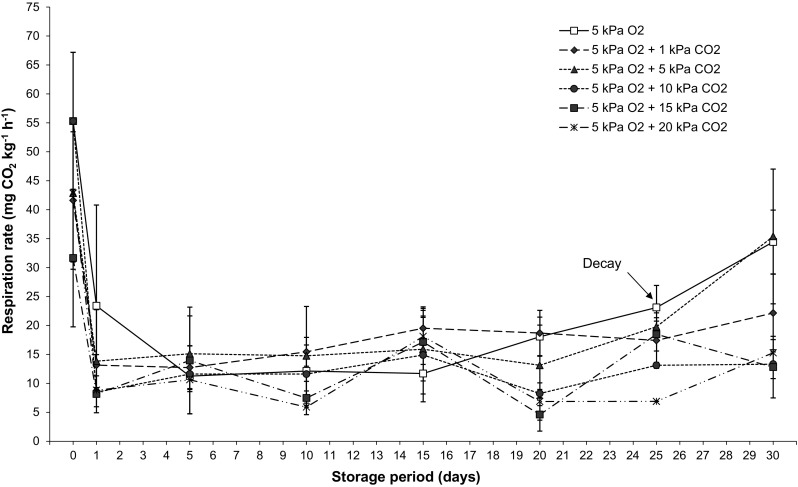
Effect of increasing levels of carbon dioxide (CO2) on ‘Palmer’ mango respiratory activity (mg CO2 kg−1 h−1) stored at 12.8 °C for up to 30 days. Vertical bars represent the standard deviation of three repetitions
After 15 days the mangoes were removed from CA storage to ambient conditions and the respiration rate was in fact affected by the different atmospheres (Fig. 2). Mangoes that had been stored at 5 kPa O2 + 10 kPa CO2 and 5 kPa O2 + 20 kPa CO2 presented the lowest rates (p < 0.05) while the other treatments did not present significant differences (Fig. 2a). After 3 days at ambient temperature there was an increase in respiration rate similar to climacteric peak, independent of the atmosphere in which the mangoes were previously stored, however CO2 production declined and remained unaltered for up to 7 days at ambient temperature (Fig. 2a). On the other hand, after 30 days of CA storage the respiration rates did not vary among the treatments, it was observed only a continuous reduction over time (Fig. 2b).
Fig. 2.
Effect of increasing levels of carbon dioxide (CO2) on ‘Palmer’ mango respiratory activity (mg CO2 kg−1 h−1) stored at 12.8 °C for 15 days and transfer to ambient (25.5 °C) for 7 (a) and 5 b more days. Vertical bars represent the standard deviation of three repetitions
Effect of increasing levels of CO2 on fruit quality
No significant statistical difference was observed in the storage atmospheres for all quality parameters and the few differences in chromaticity had little effect on fruit quality (Table 1). The storage period showed to have an effect on fresh weight loss (FWL), firmness and appearance (Table 1). The FWL was very low and even with the increments during storage and did not surpass 1.04% after 30 days of CA storage (Table 1). The fruit color did not change during CA storage with the fruit presenting dark (L* = 44.27 ± 0.36), few saturated (chromaticity = 11.13 ± 0.99), reddish (hue angle = 62.32 ± 8.29) peel, Table 1. Fruit firmness decreased during CA storage especially after the onset of postharvest decay (Table 1), which contributed to the deterioration in fruit quality. Although the appearance ranged from “excellent” to “very good” after 30 days of CA storage, all fruits received scores below the limit of commercialization independently of the storage environment (Table 1).
Table 1.
Effect of increasing levels of carbon dioxide (CO2) on ‘Palmer’ mango fresh weigh loss (FWL), color, firmness and appearance stored at 12.8 °C for up to 30 days
| Main effects | FWLa (%) | Color | Firmness (N) | Appearancee (5 to 1) | ||
|---|---|---|---|---|---|---|
| L*b | Chromaticityc | h°d | ||||
| Atmospheres (A) | ||||||
| 5.0 kPa O2-control | 0.62 ± 0.50 | 43.98 ± 1.74 | 12.61 ± 1.92 a | 77.93 ± 67.13 | 75.53 ± 50.26 | 3.74 ± 1.34 |
| 5.0 kPa O2 + 1.0 kPa CO2 | 0.61 ± 0.49 | 44.36 ± 1.25 | 11.47 ± 0.84 ab | 59.11 ± 17.14 | 76.23 ± 50.14 | 3.89 ± 1.15 |
| 5.0 kPa O2 + 5.0 kPa CO2 | 0.60 ± 0.50 | 44.04 ± 1.20 | 9.69 ± 1.68 b | 63.75 ± 14.51 | 64.79 ± 51.93 | 3.29 ± 1.46 |
| 5.0 kPa O2 + 10 kPa CO2 | 0.51 ± 0.41 | 44.68 ± 0.77 | 10.83 ± 1.15 ab | 55.90 ± 13.45 | 72.51 ± 48.67 | 3.93 ± 1.21 |
| 5.0 kPa O2 + 15 kPa CO2 | 0.49 ± 0.50 | 43.86 ± 0.65 | 11.56 ± 1.66 ab | 61.72 ± 34.81 | 74.99 ± 45.51 | 4.19 ± 1.41 |
| 5.0 kPa O2 + 20 kPa CO2 | 0.50 ± 0.42 | 44.69 ± 0.68 | 10.61 ± 1.40 b | 55.53 ± 10.32 | 79.61 ± 41.31 | 3.63 ± 1.82 |
| Storagef (B) | ||||||
| 0 | 0.00 ± 0.0 c | 44.16 ± 0.0 | 11.73 ± 0.0 | 56.80 ± 0.0 | 127.50 ± 0.0 a | 5.00 ± 0.0 a |
| 15 | 0.63 ± 0.17 b | 44.28 ± 1.16 | 10.89 ± 2.13 | 59.54 ± 17.09 | 73.48 ± 21.25 b | 4.06 ± 0.91 b |
| 30 | 1.04 ± 0.16 a | 44.36 ± 1.58 | 10.76 ± 1.93 | 75.62 ± 52.06 | 20.84 ± 11.25 c | 2.28 ± 1.01 c |
| Interaction | ||||||
| A × B | NS | NS | NS | NS | NS | NS |
Average values with the same letter in the columns are not statistically different by Tukey’s test (p < 0.05). Values in the column without letter are not statistically different by Tukey’s test (p < 0.05)
aFresh weight loss
bLuminosity
cChromaticity
dHue angle
eAppearance (5, excellent—1, very bad)
fStorage in days
NS non-significant interaction
The titratable acidity (TA) did not differ between treatments, but presented a significant reduction during CA storage, which was reflected in the increase of pH, especially at the end of the storage period (Table 2). The soluble solids content (SSC) increased during CA storage, independently from the storage atmosphere, and the fruit kept at 5 kPa O2 + 20 kPa CO2 presented the lowest SSC in relation to 5 kPa O2 + 5 kPa CO2 (Table 2). The ratio SSC/TA did not differ between treatments, but increased during CA storage mainly due to TA reduction and SSC increments (Table 2). The total soluble sugar (TSS), reduced sugar (RS) and soluble pectin (SP) content also increased during CA storage independently from the storage atmosphere, low SP was observed in the fruit maintained at 5 kPa O2 + 15 kPa CO2, and 5 kPa O2 + 20 kPa CO2 (Table 2).
Table 2.
Effect of increasing levels of carbon dioxide (CO2) on ‘Palmer’ mango titratable acidity (TA), soluble solids content (SSC), ratio SSC/TA, total soluble sugar (TSS), reducing sugar (RS), soluble pectin (SP) content and pH stored at 12.8 °C for up to 30 days
| Main effects | TA (g 100 g−1) | SSC (%) | SSC/TA | pH | TSS (g 100 g−1) | RS (g 100 g−1) | SP (mg 100 g−1) |
|---|---|---|---|---|---|---|---|
| Atmospheres (A) | |||||||
| 5.0 kPa O2-control | 0.53 ± 0.15 | 12.91 ± 3.49 ab | 28.84 ± 18.47 | 3.91 ± 0.22 ab | 9.02 ± 3.42 b | 3.29 ± 0.30 | 201.02 ± 136.88 ab |
| 5.0 kPa O2 + 1.0 kPa CO2 | 0.56 ± 0.14 | 13.20 ± 3.72 ab | 26.44 ± 14.05 | 3.85 ± 0.18 b | 10.79 ± 3.82 ab | 3.49 ± 0.34 | 184.96 ± 131.04 ab |
| 5.0 kPa O2 + 5.0 kPa CO2 | 0.48 ± 0.09 | 14.84 ± 4.13 a | 33.68 ± 16.96 | 4.07 ± 0.27 a | 11.88 ± 4.32 a | 3.52 ± 0.28 | 242.91 ± 134.56 a |
| 5.0 kPa O2 + 10 kPa CO2 | 0.49 ± 0.14 | 13.22 ± 3.76 b | 32.12 ± 20.68 | 4.00 ± 0.31 ab | 11.90 ± 3.90 a | 3.56 ± 0.49 | 205.51 ± 124.03 b |
| 5.0 kPa O2 + 15 kPa CO2 | 0.50 ± 0.12 | 13.50 ± 3.25 ab | 30.06 ± 14.92 | 3.98 ± 0.27 ab | 10.57 ± 3.71 ab | 3.61 ± 0.42 | 153.56 ± 112.18 b |
| 5.0 kPa O2 + 20 kPa CO2 | 0.49 ± 0.08 | 12.41 ± 2.66 b | 26.81 ± 9.75 | 3.92 ± 0.16 ab | 10.16 ± 2.79 ab | 3.57 ± 0.47 | 160.91 ± 110.78 b |
| Storagea (B) | |||||||
| 0 | 0.54 ± 0. a | 9.66 ± 0.0 c | 18.48 ± 0.0 b | 3.79 ± 0.0 b | 7.61 ± 0.0 c | 3.30 ± 0.0 b | 76.60 ± 0.0 c |
| 15 | 0.59 ± 0.11 a | 13.36 ± 2.45 b | 23.5 ± 7.74 b | 3.88 ± 0.17 b | 9.61 ± 2.59 b | 3.29 ± 0.27 b | 167.23 ± 94.96 b |
| 30 | 0.38 ± 0.10 b | 17.02 ± 1.54 a | 46.92 ± 14.64 a | 4.20 ± 0.22 a | 14.95 ± 2.11 a | 3.92 ± 0.34 a | 330.60 ± 55.50 a |
| Interaction | |||||||
| A × B | NS | NS | NS | NS | NS | NS | NS |
Average values with the same letter within the columns are not statistically different by Tukey’s test (p < 0.05). Values in the column without letter are not statistically different by Tukey’s test (p < 0.05)
NS non-significant interaction
aStorage in days
Effect of increasing levels of CO2 on fruit ripening
Mangoes were transferred to ambient temperature at the beginning of CA storage (day 0) and no significant differences were observed among treatments in respect to all quality parameters (Table 3). The fruit were considered completely ripe after 9 days at ambient temperature (25.5 ± 0.6 °C and 97.78 ± 4.1% RH). The initial and final color values for L* (44.16 and 45.99) and h° (56.80 and 52.77) were very similar though the chromaticity value increased from 11.72 to 24.53 (Table 3). The FWL reached values of 6.91 ± 0.75% in relation to the beginning of ambient temperature storage (Table 3). Considering the initial fruit firmness of 127.50 ± 1.75 N, sharp fruit softening process was observed as firmness declined to 7.85 ± 2.29 N after 9 days of shelf life at ambient temperature (Table 3). The appearance was severely affected due to the development of postharvest decay which led the fruit to receive low scores, 3.85 ± 0.59 (Table 3). The physical–chemical parameters did not differ among the treatments (Table 3), with fruit presenting average values of 0.19 ± 0.03 g; 100 g−1; 4.76 ± 0.19; 19.10 ± 0.87% and 101.81 ± 19.80 for TA, pH, SSC and ratio SSC/TA, respectively.
Table 3.
Effect of increasing levels of carbon dioxide (CO2) on ‘Palmer’ mango physical–chemical parameters after ripening in ambient temperature (25.2 °C) for 9 days
| Main effects | FWLa (%) | Initial colorb | Final colorc | Firmness (N) | Appearanced (5 to 1) | TAe (g 100 g−1) | SSCf (%) | SSC/TAg | pH | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | Chroma | Hue | L* | Chroma | Hue | ||||||||
| Atmospheres (A) | |||||||||||||
| 5.0 kPa O2-control | 6.32 | 44.55 | 11.81 | 53.23 | 46.49 | 27.80 | 48.05 | 6.23 | 3.89 | 0.19 | 18.80 | 108.00 | 4.73 |
| 5.0 kPa O2 + 1.0 kPa CO2 | 7.04 | 44.64 | 12.40 | 62.20 | 46.99 | 25.69 | 49.30 | 8.20 | 3.67 | 0.17 | 18.77 | 115.34 | 4.85 |
| 5.0 kPa O2 + 5.0 kPa CO2 | 6.79 | 44.40 | 12.63 | 60.11 | 47.04 | 23.84 | 57.68 | 8.15 | 4.22 | 0.20 | 19.67 | 99.29 | 4.68 |
| 5.0 kPa O2 + 10 kPa CO2 | 6.89 | 42.72 | 11.27 | 47.77 | 45.03 | 22.89 | 57.47 | 8.35 | 3.67 | 0.21 | 19.27 | 97.36 | 4.71 |
| 5.0 kPa O2 + 15 kPa CO2 | 6.95 | 43.77 | 12.11 | 52.86 | 44.36 | 23.09 | 46.44 | 8.45 | 4.00 | 0.20 | 18.93 | 95.39 | 4.71 |
| 5.0 kPa O2 + 20 kPa CO2 | 7.47 | 44.85 | 10.17 | 64.63 | 46.04 | 23.89 | 57.70 | 7.71 | 3.67 | 0.20 | 19.17 | 95.52 | 4.85 |
| SD (%) | 11.41 | 2.79 | 18.99 | 28.24 | 4.79 | 10.56 | 19.58 | 32.66 | 17.18 | 20.77 | 5.02 | 21.36 | 4.36 |
| F test | 0.6556 | 0.3763 | 0.7783 | 0.7760 | 0.6087 | 0.2377 | 0.5617 | 0.8932 | 0.8659 | 0.8299 | 0.8535 | 0.8287 | 0.8453 |
| MSD | 2.1614 | 3.3812 | 6.1079 | 43.97 | 6.0388 | 7.1050 | 28.32 | 7.0277 | 1.8142 | 0.1104 | 2.6308 | 59.64 | 0.5683 |
Average values with the same letter within the columns are not statistically different by Tukey’s test (p < 0.05). Values in the column without letter are not statistically different by Tukey’s test (p < 0.05)
aFresh weigh loss
bAfter withdraw
cAfter 9 days
d5 = excellent and 1 = very bad
eTitratable acidity
fSoluble solids content; gratio SSC/TA
gRatio SSC/TA
Similarly, mangoes transferred to ambient temperature after 15 days of CA storage were not affected by the increasing levels of CO2 (Table 4). Mangoes were considered completely ripe after 7 days at ambient temperature, 2 days less than the fruit transferred at the beginning of CA storage (Table 3) and there were no significant differences in fruit color (L* = 42.74 to L* = 45.48; h° = 54.83 to h° = 50.23). The chromaticity was affected by the atmospheres, yet without great influence in fruit quality (Table 4). The FWL was similar to the fruit transferred at day 0 (Table 3). Again, there was not a significant difference between the treatments and the FWL value was around 5.54 ± 0.86% (Table 4). A fast ripening process was observed with a sharp reduction in fruit firmness reaching final values of 5.75 ± 1.63 N. The deterioration in appearance was also due to the development of postharvest decay with fruits from all treatments scoring below the limit of commercialization (score = 3.0), being considered “regular” and/or “bad”, 2.7 ± 0.8 at the end of shelf life at ambient temperature (Table 4). The physical–chemical parameter also did not differ between the storage atmospheres with fruit presenting average values of 0.15 ± 0.02 g; 100 g−1; 4.99 ± 0.16; 20.05 ± 0.94% and 134.82 ± 18.34 for TA, pH, SSC, ratio SSC/TA, respectively (Table 4).
Table 4.
Effect of increasing levels of carbon dioxide (CO2) on ‘Palmer’ mango physical–chemical parameters after storage in CA for 15 days and ripening in ambient (25.2 °C) for 7 days
| Main effects | FWLa (%) | Initial colorb | Final colorc | Firmness (N) | Appearanced (5 to 1) | TAe (g 100 g−1) | SSCf (%) | SSC/TAg | pH | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | Chroma | Hue | L* | Chroma | Hue | ||||||||
| Atmospheres (A) | |||||||||||||
| 5.0 kPa O2-control | 5.41 | 43.93 | 13.57 | 44.43 | 47.93 | 22.49 a | 49.97 | 5.25 | 2.00 | 0.14 | 20.20 | 138.83 | 4.98 |
| 5.0 kPa O2 + 1.0 kPa CO2 | 6.01 | 43.86 | 10.23 | 81.93 | 47.00 | 17.56 ab | 64.19 | 6.30 | 3.11 | 0.16 | 19.80 | 124.47 | 4.91 |
| 5.0 kPa O2 + 5.0 kPa CO2 | 5.77 | 41.22 | 12.22 | 44.18 | 44.38 | 20.14 ab | 44.06 | 5.99 | 2.78 | 0.18 | 20.67 | 118.18 | 4.92 |
| 5.0 kPa O2 + 10 kPa CO2 | 5.70 | 42.17 | 11.55 | 52.16 | 45.10 | 18.03 ab | 50.38 | 5.25 | 2.67 | 0.15 | 19.80 | 137.29 | 5.03 |
| 5.0 kPa O2 + 15 kPa CO2 | 5.59 | 42.58 | 11.37 | 53.22 | 44.29 | 17.27 b | 49.71 | 6.36 | 2.89 | 0.14 | 19.47 | 137.24 | 5.07 |
| 5.0 kPa O2 + 20 kPa CO2 | 4.77 | 42.66 | 12.02 | 53.08 | 44.21 | 17.99 ab | 43.06 | 5.31 | 2.99 | 0.13 | 20.37 | 152.91 | 5.01 |
| SD (%) | 16.38 | 3.78 | 16.84 | 37.15 | 3.66 | 9.91 | 28.51 | 32.05 | 34.39 | 14.60 | 5.03 | 12.67 | 3.48 |
| F test | 0.6597 | 0.3550 | 0.5024 | 0.3093 | 0.0701 | 0.0354 | 0.5501 | 0.9303 | 0.7515 | 0.2529 | 0.7197 | 0.2555 | 0.8444 |
| MSD | 2.4903 | 4.4270 | 5.4616 | 56.89 | 4.5608 | 5.1398 | 39.25 | 5.0465 | 2.5838 | 0.0603 | 2.7593 | 46.83 | 0.4764 |
Average values with the same letter within the columns are not statistically different by Tukey’s test (p < 0.05). Values in the column without letter are not statistically different by Tukey’s test (p < 0.05)
aFresh weigh loss
bAfter withdraw
cAfter 7 days
d5 = excellent and 1 = very bad
eTitratable acidity
fSoluble solids content
gRatio SSC/TA
The mangoes that were transferred to ambient temperature after 30 days of CA storage were considered completely ripe after 5 days, 4 days less than the fruit that were initially transferred to ambient temperature, day 0 (Table 5). Upon removal from the buckets, the appearance of the fruit were completely compromised by postharvest decay (2.9 ± 1.3), and the decay development at ambient temperature was too severe that the fruit were considered unmarketable after 5 days (Table 5). The increase in CO2 concentration did not have a synergistic effect with low O2 to control softening after transfer to ambient temperatures, and fruit from all treatments presented low firmness values 3.46 ± 2.35 N after 5 days (Table 5). The color did not differ (L* = 42.90 to L* = 44.86; h° = 50.58 to h° = 54.83), but chromaticity increased (12.52–22.02), Table 5. As many repetitions were unable to be performed due to the presence of postharvest decay it was not possible to evaluate the data related to the physical–chemical parameters.
Table 5.
Effect of increasing levels of carbon dioxide (CO2) on ‘Palmer’ mango physical–chemical parameters after storage in CA for 30 days and ripening in ambient (25.2 °C) for 5 days
| Main effects | FWLa (%) | Initial colorb | Final colorc | Firmness (N) | Apperanced (5 to 1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| L* | Chroma | Hue | L* | Chroma | Hue | ||||
| Atmospheres (A) | |||||||||
| 5.0 kPa O2-control | 4.07 | 41.55 | 12.77 | 39.30 | 44.58 | 21.87 ab | 57.27 | 4.63 | 1.00 |
| 5.0 kPa O2 + 1.0 kPa CO2 | 4.80 | 42.48 | 11.54 | 52.98 | 46.78 | 22.37 ab | 58.42 | 2.53 | 1.00 |
| 5.0 kPa O2 + 5.0 kPa CO2 | 4.35 | 42.15 | 14.44 | 37.32 | 44.32 | 24.59 a | 43.68 | 4.14 | 1.00 |
| 5.0 kPa O2 + 10 kPa CO2 | 5.96 | 44.29 | 14.67 | 54.42 | 45.65 | 23.07 ab | 63.02 | 4.08 | 1.00 |
| 5.0 kPa O2 + 15 kPa CO2 | 4.85 | 43.03 | 11.43 | 55.67 | 44.34 | 20.69 ab | 49.44 | 1.85 | 1.00 |
| 5.0 kPa O2 + 20 kPa CO2 | 4.32 | 43.88 | 10.24 | 63.41 | 43.84 | 19.53 b | 57.16 | 3.52 | 1.00 |
| SD (%) | 26.24 | 3.64 | 13.19 | 26.15 | 4.08 | 8.21 | 17.30 | 73.33 | – |
| F test | 0.5171 | 0.3123 | 0.3650 | 0.2030 | 0.3556 | 0.0610 | 0.2257 | 0.7515 | 0.00 |
| MSD | 3.3987 | 4.2840 | 4.5276 | 36.25 | 5.0172 | 4.9586 | 26.00 | 6.9532 | 0.00 |
Average values with the same letter within the columns are not statistically different by Tukey’s test (p < 0.05)
aFresh weigh loss
bAfter withdraw
cAfter 5 days
d5 = excellent and 1 = very bad
Discussion
The lack of respiration rate responses of ‘Palmer’ mangoes to increasing levels of CO2 can be related to the tolerance of some mango cultivar to this gas. Bender and Brecht (2000) reported that atmospheres containing up to 25 kPa CO2 did not promote any alterations in the respiration rate of ‘Tommy Atkins’ mangoes, however, when these mangoes were stored in concentrations higher than 45 kPa the respiration rate was more intense and in much higher levels, 50 and 70 kPa, an irreversible injury occurred. Although atmospheric concentrations over 10 kPa CO2 inhibit the activity of key enzymes of glycolysis, such as ATP: phosphofructokinase and PPi: phosphofructokinase (Kader 1986), it also contributes to the decrease of the produce sensibility to ethylene, mainly at levels higher than 1 kPa CO2 (Kader 2003a), the increase in CO2 levels did not show any synergistic effects with low-oxygen (5 kPa O2). Therefore, the low O2 concentration was the determining factor to reduce the respiratory activity of the ‘Palmer’ mangoes and, consequently, leading to a delay in the ripening process, which did not differ between treatments. Teixeira and Durigan (2011) also reported a reduction in the respiration rate in ‘Palmer’ mangoes with atmospheres containing low O2.
Even though a suppression in the respiratory metabolism during CA storage was observed, ‘Palmer’ mangoes presented the ability to recover respiratory control after only a short exposition to ambient temperature (21 kPa O2 and 0.03 kPa CO2), which shows the inexistence of irreversible mitochondrial structure injury in consequence to a prolonged exposition to low O2 and/or high levels of CO2 (Bender and Brecht 2000). Therefore, ‘Palmer’ mangoes can be stored in CA containing 5 kPa O2 and up to 20 kPa CO2 without suffering negative effects to its respiratory metabolism.
The increasing levels of CO2 did not promote any modifications in the mangoes color when compared to low O2. As color changes depend on ethylene action, once present, this hormone triggers the ripening process and consequently the expression of many enzymes involved in chlorophyll breakdown and carotenoid synthesis (Kader 2003a). It is possible that the oxygen concentration of 5 kPa O2 was responsible for maintaining the mangoes color during CA storage, as in concentrations lower than 8 kPa O2 the ethylene production is reduced by the inhibition of the activity of the 1-amino-cyclopropane-1-carboxilic acid (ACC) synthase and of the ACC oxidase, both of which are key enzymes of the ethylene pathway (Kader 1995). In low O2 concentrations the ethylene biosynthesis is also inhibited by impeding the C2H4 to bind with the receptor that is responsible for triggering the autocatalytic response (Burg and Burg 1967). Among the different treatments, storage temperature can also be responsible for the absence of color modifications. When ‘Kensington Pride’ mangoes were stored at 13 °C, O’Hare (1995) reported that the fruit did not reach the typical color of this cultivar, similar to other mango varieties which presented reduced carotenoid synthesis (Medlicott et al. 1986).
The different atmospheres also did not significantly affect mango firmness, however a reduction was observed during CA storage, mainly at the end when postharvest decay was evident. Fungi presence can stimulate ethylene synthesis (Kader 2003a) and initiate the ripening process, mainly by expressing many enzymes involved in both starch and pectic molecule breakdown (Kader 2003a). Therefore, the development of decay was a determining factor for firmness reduction as the FWL was considered very low during CA storage.
The development of decay was also responsible for the quality degradation as after 30 days of CA storage all fruit received scores below the commercialization limit, independently of the atmospheric composition. In our previous study (Teixeira et al. 2009) we did not observe a high development of decay, however the elevate pluviometric indices in the summer (December and January) might have affected the onset of decay as humidity favors the development of Colletotrichum gloeosporioides (Penz.) Penz. & Sacc., the main organism involved in mango postharvest decay (Kefialew and Ayalew 2008). Even being an efficient technique to control postharvest decay, the increase of CO2 concentration up to 20 kPa did not control the development of decay.
The TA did not vary between treatments, but increased during CA storage, and affected the pH values. The TA content was maintained for up to 15 days of CA storage which shows the ripening control promoted by the cold temperature, as high TA values are commonly observed during mango cold storage (O’Hare 1995), which is related to organic acid retention by refrigeration. However, at the end of CA storage a slight decrease in TA was observed which normally occurs in mango ripening (Kader 2003a).
The SSC increased during CA storage independent of the atmospheric composition. In general, an increase in SSC is observed in ripening mangoes due to starch degradation (Khader 1992; Kader 2003a), which is also related to the increase in TSS and RS. Although the fixed low-oxygen (5 kPa O2) level has the capability to reduce the starch breakdown into glucose with the inhibition of amylase and maltase activities, and/or of glycose-1-phosphate by phosphorylase, the onset of decay might have led to the elevation of TSS and RS content, as this is normally observed during mango ripening (Nakasone and Paull 1998). Therefore, the starch degradation, based on the increase of TSS content, could also have affected the modification in fruit firmness, as well as the modification in pectic compounds.
The SP content also increased during CA independently of the atmospheric composition. Increase in soluble uronides have been reported during mango ripening in consequence of pronounced pulp softening (Mitcham and McDonald 1992). Teixeira and Durigan (2011) reported that ‘Palmer’ mangoes stored in low O2 (1, 5, and 10 kPa O2) practically did not present increase in SP, thus, again the presence of postharvest decay were the main factor which contributed to mango softening.
Even though the development of decay had promoted a slight ripening modification, upon withdrawal from CA conditions the fruit were not ready to be eaten, as the mangoes were still not completely ripe. For that reason and also to verify the presence of any injury caused by high CO2 concentrations, the fruit were transferred to ambient temperature. No effects were observed in the different atmospheres on fruit ripening nor injuries caused by high CO2. However, the ripening process was much faster after a period in CA storage. Other studies also reported an acceleration in the mango ripening process after a period in cold storage (Jenonimo and Kanesiro 2000). This phenomenon could be related to the elevation in ambient temperature (25.2 ± 0.6 °C) and also for the suppression of the gas control, especially low O2, in fruit ripening. Therefore, ‘Palmer’ mangoes can be stored in CA containing 5 kPa O2 and up to 20 kPa CO2 without negatively affecting its quality.
Conclusion
An increase in carbon dioxide concentration up to 20 kPa, associated with low-oxygen (5 kPa O2) did not present a synergistic effect in maintaining the fruit quality in ‘Palmer’ mangoes.
The development of postharvest decay was not controlled by the increase of the CO2 concentration (20 kPa), and it was the main cause of fruit quality deterioration during CA storage and ripening at ambient temperature.
No injuries were observed that were caused by high CO2 concentrations (20 kPa), even after being transferred to ambient temperature for up to 7 days.
‘Palmer’ mangoes can be stored in CA containing 5 kPa O2 and up to 20 kPa CO2 without negative effects to its quality, but low-oxygen (5 kPa O2) alone is just as effective as the association of both gases.
Acknowledgements
The authors would like to thank FAPESP for the financial support (proc. 2005/56159-1) and for the post-doctoral fellowship of the first author (proc. 2005/56160-0). We would also like to thank Dr. José Maria Monteiro Sigrist for his help in the construction of the flowboards.
References
- AOAC . Official methods of analysis of the Association of Official Analytical Chemists. 16. Arlington: Patricia Cuniff; 1997. [Google Scholar]
- Assis JS. Colheita e pós-colheita. In: Mouco MAC, editor. Cultivo da mangueira. Petrolina: EMBRAPA Semi-árido; 2004. [Google Scholar]
- Bender RJ, Brecht J. Respiração e produção de etanol e de etileno em mangas armazenadas sob diferentes concentrações de dióxido de carbono e oxigênio. Pesqui Agropecu Bra. 2000;35:865–871. doi: 10.1590/S0100-204X2000000500001. [DOI] [Google Scholar]
- Bender RJ, Brecht JK, Sargent SA. Mango tolerance to reduced oxygen levels in controlled atmosphere storage. J Am Soc Hortic Sci. 2000;125:707–713. [Google Scholar]
- Bitter T, Muir HM. A modified uronic acid carbazole reaction. Anal Biochem. 1962;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Burg SP, Burg EA. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967;42:144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claypool LL, Keefer RM. A colorimetric method for CO2 determination in respiration studies. Proc Am Soc Hortic Sci. 1942;40:177–186. [Google Scholar]
- FAOSTAT (2014) Food and agricultural commodities production. http://faostat.fao.org/site/339/default.aspx. Accessed 28 July 2015
- Jenonimo EM, Kanesiro MAB. Efeito da associação de armazenamento sob refrigeração e atmosfera modificada na qualidade de mangas ‘Palmer’. Rev Bras Fruticult. 2000;22:237–243. [Google Scholar]
- Kader AA. Biochemical and physiological basis for effects of controlled and modified atmospheres on fruits and vegetables. Food Technol. 1986;40:102–104. [Google Scholar]
- Kader AA. Regulation of fruit physiology by controlled/modified atmospheres. Acta Hortic. 1995;398:59–70. doi: 10.17660/ActaHortic.1995.398.6. [DOI] [Google Scholar]
- Kader AA. Postharvest biology and technology: an overview. In: Kader AA, editor. Postharvest technology of horticultural crops. Davis: University of California: Division of Agriculture and Natural Resources Publication; 2003. pp. 39–47. [Google Scholar]
- Kader AA. A summary of CA requirements and recommendations for fruits other than apples and pears. Acta Hortic. 2003;600:737–740. doi: 10.17660/ActaHortic.2003.600.112. [DOI] [Google Scholar]
- Kefialew Y, Ayalew A. Postharvest biological control of anthracnose (Colletotrichum gloesporioides) on mango. Postharvest Biol Technol. 2008;50:8–11. doi: 10.1016/j.postharvbio.2008.03.007. [DOI] [Google Scholar]
- Khader SESA. Effect of gibberellic acid and vapor gard on ripening, amylase and peroxidase activities and quality of mango fruits during storage. J Hortic Sci. 1992;67:855–860. doi: 10.1080/00221589.1992.11516318. [DOI] [Google Scholar]
- Kim Y, Brecht JK, Talcott ST. Antioxidant phytochemical and fruit quality changes in mango (Mangifera indica L.) following hot water immersion and controlled atmosphere storage. Food Chem. 2007;105:1327–1334. doi: 10.1016/j.foodchem.2007.03.050. [DOI] [Google Scholar]
- Lalel HJD, Singh Z, Tan SC. Elevated levels of CO2 in controlled atmosphere storage affects shelf live, fruit quality and aroma volatiles of mango. Acta Hortic. 2003;628:407–413. doi: 10.17660/ActaHortic.2003.628.51. [DOI] [Google Scholar]
- Lizada LA, Ochagavia A. Controlled atmosphere storage of mango fruits (Mangifera indica L) cvs Tommy Atkins and Kent. Acta Hortic. 1997;455:732–737. [Google Scholar]
- McCready PM, McComb EA. Extraction and determination of total pectin materials. Anal Chem. 1952;24:1586–1588. doi: 10.1021/ac60072a033. [DOI] [Google Scholar]
- McGuire RG. Reporting of objective color measurements. HorticScience. 1992;27:254–255. [Google Scholar]
- Medlicott AP, Reynolds SP, Thompson AK. Effects of temperature on the ripening of mango fruit (Mangifera indica L. var. Tommy Atkins) J Sci Food Agric. 1986;37:469–474. doi: 10.1002/jsfa.2740370506. [DOI] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Mitcham EJ, McDonald RE. Cell wall modification during ripening of ‘Keitt’ and ‘Tommy Atkins’ mango fruit. J Am Soc Hortic Sci. 1992;117:919–924. [Google Scholar]
- Nakamura N, Sudhakar RAO, Shiina T, Nawa Y. Effects of temperature and gas composition on respiratory behaviour of tree-ripe ‘Irwin’ mango. Acta Hortic. 2003;600:425–429. doi: 10.17660/ActaHortic.2003.600.63. [DOI] [Google Scholar]
- Nakasone HK, Paull RE. Tropical fruits. Wallingford: CAB International; 1998. [Google Scholar]
- O’Hare TJ. Effect of ripening temperature on quality and composition changes of mango (Mangifera indica L.) cv. Kensington. Aust J Exp Agric. 1995;35:259–263. doi: 10.1071/EA9950259. [DOI] [Google Scholar]
- O’Hare TJ, Prassad A. The effect of temperature and carbon dioxide on chilling symptoms in mango. Acta Hortic. 1993;343:244–250. doi: 10.17660/ActaHortic.1993.343.56. [DOI] [Google Scholar]
- Ortega-Zaleta D, Yahia EM. Tolerance and quality of mango fruit exposed to controlled atmosphere at high temperature. Postharvest Biol Technol. 2000;20:195–201. doi: 10.1016/S0925-5214(00)00137-X. [DOI] [Google Scholar]
- SAS . SAS User’s guide: statistics. 8. Cary: SAS Institute Inc.; 1999. [Google Scholar]
- Teixeira GHA, Durigan JF. Storage of ‘Palmer’ mangoes in low-oxygen atmospheres. Fruits. 2011;66:279–289. doi: 10.1051/fruits/2011037. [DOI] [Google Scholar]
- Teixeira GHA, Durigan JF, Santos LO, Ogassavara FO, Cunha Júnior LC (2009) Extending shelf-life of ‘Palmer’ cold stored mangoes using controlled atmosphere with different oxygen levels. In: 6th International postharvest symposium, Antalya (Turkey), May, 2009, p 119 (Abstract book)
- Trinidad M, Bósquez E, Escalona H, Días de León F, Pérez Flores L, Kerbel C, Ponce de León L, Muñoz C, Pérez L. Controlled atmosphere (5% CO2–5% O2 and 10% CO2–5% O2) do not significantly increase the shelf life of refrigerated Kent Mangoes. Acta Hortic. 1997;455:643–653. doi: 10.17660/ActaHortic.1997.455.83. [DOI] [Google Scholar]
- Yemn EW, Willis AJ. The estimation of carbohydrate in plant extracts by anthrone. Biochem J. 1954;57:508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]