Abstract
Low-field nuclear magnetic resonance (LF NMR) was used to investigate the water mobility of salmon during cold storage and the correlation between texture, freshness, sensory quality and transversal relaxation times (T2) of salmon were studied. With the increasing of cold storage time, trapped water (T22), sensory, water holding capacity and cooking loss were descended while free water (T23), TVB-N and TBA were increased steadily, that reflected the quality of salmon quality visually. There was a significant correlation between sensory, hardness, TBA, cooking loss, K value and LF NMR parameters. The study showed that LF NMR was sensitive to different storage conditions which may be applied to monitor the quality of fish muscle, when the spoilage mechanism was affected by water properties and muscle structure.
Keywords: LF NMR, Water mobility, Quality, Cold storage
Introduction
Fresh Atlantic salmon (Salmo salar), belonging to the family Salmonidae, is one of the most traded and consumed fish products worldwide (Carlson and Hites 2005). Salmon is prone to deterioration with limited shelf life during cold storage because of physiochemical, enzymatic and microbial activity, which may lower the market value significantly (Zhou and Ackman 2003). Deterioration of the muscle can changes the protein structure and affect the mobility of water eventually (Sone 2012). Therefore, monitoring the mobility of water might be a prospective way to detect the quality of fish during storage (Gudjónsdóttir et al. 2011a, b).
Water is a significant component in aquatic product, which is essential for chemical and enzymatic reactions and microbial growth. Therefore, it has a direct effect on the stability of quality attributes of food during storage (Carneiro et al. 2015). Water is also the degradation product of muscle proteins. According to the mobility and the link between the molecules and the muscle structure, water can be separated into different groups (Anderson and Rinnan 2002). The low-field nuclear magnetic resonance (LF NMR) technique has been widely applied to gain further insight into the behavior of water molecules in muscle tissues (Pearce et al. 2011). As a non-destructive and non-invasive spectroscopic techniques, LF NMR requires only minimal or no sample preparation, which can investigate changes in the mobility of water in fish muscle during storage by detecting the relaxation of hydrogen protons (Gudjónsdóttir et al. 2011a, b; Sánchez-alonso et al. 2012). LF NMR provides constant relaxation times T1 (Longitudinal) and T2 (Transverse) which can reflect the mobility of water molecular (Aursand et al. 2008). T2 transversal relaxation times measured by LF NMR are reported to represent three different populations of water in muscle: strong bound water; bound or hydration water and free or weakly bound water (Bertram et al. 2001). Weakly boundwater, hydration water and free water, accounting for 90% of the water found in meat, are the most important variables during the cold storage and have a great significance in the study of food storage (Carneiro et al. 2015). Carneiro et al. (2015) studied the water mobility in muscle of salted sardine on different days of storage, by the use of low-field nuclear magnetic resonance. Physical and chemical analyses revealed a close correlation (p < 0.05) between quality parameters and the relaxation data. Previous studies shows that water is closely related to chemical reaction in food, including protein denaturation, fatty acid hydrolysis, enzyme activity change and starch gel, as well as rheological property such as viscoelastic (Horigane et al. 2013). Sánchez-alonso et al. (2014) obtained T2 transversal relaxation times of hake muscle measured by NMR after different freezing and storage conditions, found that T2 accurately reflect the quality changes of fish muscle. Shumilina et al. (2015) used high resolution NMR technique to monitor post-mortem changes in salmon fillets during storage at 4 and 0 °C. The data from this study shows that NMR spectroscopy provides the amount of all metabolites necessary for the calculation of the K-index used to express fish freshness, and a good correlation was found between the K-index increase and the formation of the undesired biogenic amines.
Although the quality changes of cold storage fish and water distribution of fish during different storage conditions using LF NMR were studied (Sánchez-alonso et al. 2012, 2014), quality changes is not yet compared with water distribution of salmon. Therefore, there is a great need for a correlation analysis between water distribution and texture, freshness and sensory quality of salmon during cold storage. The study was aimed to investigate the effects of storage temperatures on the quality of salmon examined with regard to physicochemical and spoilage indicators, demonstrate the possibility of NMR spectroscopy to monitor distribution and mobility of water that take place in salmon during cold storage, and find a rapid method to analyze the quality change of salmon.
Materials and methods
Sample preparation
About 6 kg of Salmon (S. salar) were obtained from aquatic products wholesale market of Tongchuan Road. The gutted fish were transported to laboratory in perforated polystyrene boxes with ice in 2 h. After washing in distilled water, the fish were cut and the steaks were divided in pieces of around 200 g and placed in separate plastic bags. The samples were divided into two groups randomly and stored at 4 and 0 °C, respectively. Sampling was carried out every 2 days.
Sensory evaluation
The sensory evaluation of samples was conducted according to the freshness grade guide (Meilgaard et al. 1999). A ten-member panel, comprised of the graduate students was asked to evaluate the organoleptic quality of the samples based on 5-point hedonic scales in descending order. The panelists were asked to be familiar with the rating scales beforehand. The total scores of four attributes including colour, odour, fleshy and tissue elasticity were added to represent the overall sensory quality. The acceptability was defined as having a sensory score of over 8.
Texture profile analysis
Hardness was performed by the use of a TA.XT Plus Texture Analyser (Stable Micro Systems, Ltd., Godalming, Surrey, UK). The fish sample was cut into small squares (10 mm × 10 mm × 10 mm). Each sample was compressed once using a cylindrical probe of 6 mm in diameter (P/6) on the platform under the following conditions: constant test speed, 1.0 mm/s; sample deformation, 50%. Six measurements were made for each sample in the same batch. The hardness of the fish samples was obtained and expressed as N/g.
Water holding capacity (WHC)
The water holding capacity of fish meat was determined according to the method of Gudjónsdóttir et al. (2013) with some modifications. In brief, the cutted fish meat were weighed precisely (about 2 g, recorded as M1) and after centrifugation at 5000 rpm at 4 °C for 10 min, the sample was weighed precisely again (recorded as M2). WHC (%) was calculated by the following formula:
| 1 |
Aw
The Aw of each 1 g sample was measured using a water activity meter (Aqualab Series 4TE, Decagon Devices Inc., Pullman, WA, USA) at 23 ± 2 °C according to the method described by Fundo et al. (2015). Each bacth was conducted in triplicate.
Cook loss
The raw fish samples were weighed precisely (recorded as C1) and weighed again after cooking in hot water (80 °C) for 10 min (recorded as C2) (Zell et al. 2010). The cook loss was calculated as the following formula:
| 2 |
Thiobarbituric acid-reactive substances (TBARS)
Thiobarbituric acid-reactive substances (TBARS) was determined by the method of Zhu et al. (2016) with some modifications. Briefly, a 5 g muscle sample was dispersed in 25 ml 20% trichloroacetic acid then homogenized for 60 s. After incubation at 0 °C for 1 h, the mixture was centrifuged at 8000 rpm at 4 °C for 10 min. Five ml filtrate was mixed with 0.02 M 2-thiobarbituric acid and heated at 100 °C for 20 min. The TBARS content, expressed as mg malonaldehyde per kg muscle sample, was calculated with the following equation:
| 3 |
where A532 is the absorbance at 532 nm.
Total volatile base-nitrogen (TVB-N)
The contents of TVB-N were determined using the FOSS method (2002). Samples were at exact dose of 5 g and massed, and then mixed with 0.5 g of magnesium oxide. The samples were distilled in a Kjeltec8400 apparatus, the distillate was transferred into a 25 g/L H3BO4 solution containing methyl red-bromocresol green mixed indicator and titrated with 0.01 M HCl. The results were expressed as mg of TVB-N per 100 g of salmon muscle.
K value
Nucleotide degradation was determined using high performance liquid chromatography (HPLC, SHIMADZU, LC-2010C HT, Kyoto, Japan) according to the method described by Kamalakanth and Ginson (2011) with slight modifications. Five kilograms of salmon meat was homogenized with 10 ml of 10% perchloric acid at 0 °C for 1 min and then centrifuged at 10, 000 g for 15 min at 4 °C. The supernatant obtained from two-times centrifugation by adding 5% perchloric acid were poured into a flask, and the pH of the supernatant was adjusted to 6.5 using 10 M and 1.0 M KOH. After the final volume adjusted to 50 ml with distilled water, the solution was filtrated through a 0.45 μm membrane before use. Compounds were identified and quantified based on the commercially obtained standards (ATP, ADP, AMP, IMP, Hx, and HxR). The K value was calculated as follows:
| 4 |
NMR measurements
The T2 relaxation times measurement
A LF NMR analyser minispec PQ 001 (Niumag, Ltd., Shanghai, China) with a proton resonance frequency of 21 MHz was used for the measurement. It was performed according to Sánchez-Alonso et al. (2012) as follows. Samples (about 10 g) cut from salmon fillets were placed in NMR tubes (60 mm in diameter). The measurements of the transverse relaxation time (T2) were carried out using the Carr-Purcell-Meiboom-Gill pulse sequence (CPMG). For each measurement 4 scans were acquired at an interval of 2 s with a total of 8000 echoes.
Proton magnetic resonance imaging (1H-MRI)
Nuclear magnetic resonance spectroscopy was performed by the method of Heijden et al. (2009) with slight modifications, using a LF NMR spectroscopy analyser MR 60 (Niumag, Ltd., Shanghai, China). The fish samples (about 10 g) were cut from salmon fillets and then placed in NMR tubes (60 mm in diameter). Proton density images were measured using the Multiple-Spin-Echo (MSE). The spectroscopy was performed with a repetition time of 500 ms and a echo time of 18.2 ms. Imaging planes were chosen through Larmor’s relation as follows:
| 5 |
where γ is the ratio between magnetic moment and moment of momentum, B0 is the number of the intensity of external magnetic fields.
By regulation of noise-signal ratio and the image sharpness, spectroscopy images were obtained.
Data analysis
At least three measurements for per fish samples were performed. The mean and standard deviation of data was performed in Microsoft Excel 2007 (Microsoft Cor., Redmond, USA). Line charts, scatter plots and bar graphs were drew by origin8.5 (Origin Lab, Northampton, USA). The relationship between each sensory, freshness and texture analysis and the transverse relaxation parameter was examined using SPSS 10.0 (SPSS Inc., Chicago., USA.) by Pearson correlations. Statistical significance was set as p < 0.05.
Results and discussion
Changes of sensory quality
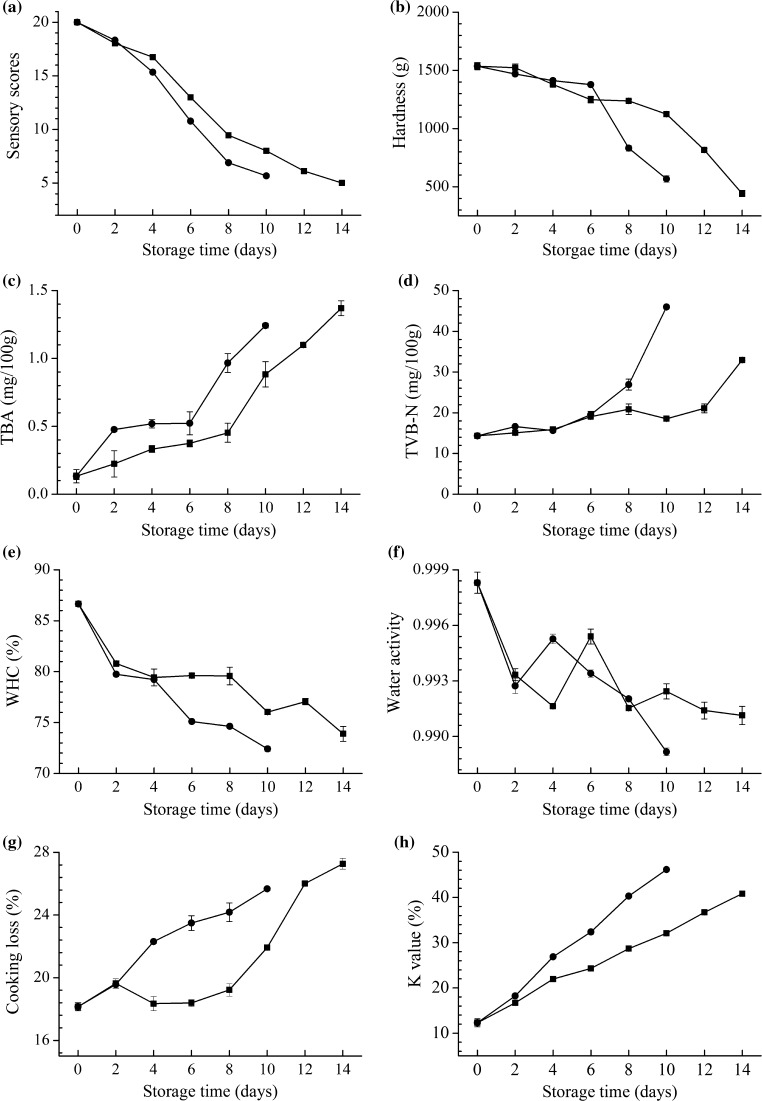
Figure 1a shows the sensory scores of samples stored at 0 and 4 °C. The changes of fish properties, such as proteins and lipids that contribute to microbiological and chemical variations (Ventanas et al. 2006), had a great effect on colour, odour, fleshy and tissue elasticity of the samples, which led to a decline in sensory evaluation during storage. Sensory scores of 9.46 for 0 °C and below 8 for 4 °C was observed for the samples on 8th day of storage, while the scores of 0 °C below 8 until day 12. Significant differences (p < 0.01) were detected in sensory scores between the fish stored at 0 °C and the fish stored at 4 °C.
Fig. 1.
Changes in the values of a sensory, b hardness, c TBA, d TVB-N, e WHC, f water activity, g cooking loss, h K value of salmon fillets storage at 0 °C (filled square) and 4 °C (filled circle). mean ± standard error
Physicochemical analysis
Hardness is the peak force (kg) sensed on the first curve cycle. As shown in Fig. 1b, the hardness value declined progressively during the process, this was in accordance with earlier studies (Li et al. 2011). The hardness of salmon stored at 4 °C was lower than those stored at 0 °C (p < 0.05).
Figure 1c shows the steady increase in TBA in storage period. Thiobarbituric acid index was used to evaluate the degree of lipid oxidation, which mainly comes from the degradation of polyunsaturated fatty acid in fish (Fuentes et al. 2013). The degradation products from lipid oxidation contribute to the off-flavor development in fresh salmon during refrigerated storage. The values of salmon increased from 0.1326 for 0 °C to 0.88335 and from 0.1326 to 1.24215 for 4 °C after 10 days. Comparisons between the different storage conditions showed that 0 °C was more effective than 4 °C to inhibit lipid oxidation, in agreement with Zhu et al. (2016) on catfish fillets.
TVB-N is widely used as an index to assess the quality and shelflife of fish and fish products (Fernández-Segovia et al. 2012; Rizo et al. 2015). Figure 1d displays the changes in TVB-N values of salmon during different storage conditions. At the very beginning of the storage, the TVB-N values of fresh fillet were 14.32 mg/100 g. The values of TVB-N increased steadily in 0 and 4 °C samples. The TVB-N content of the 0 °C samples increased by an average of 3.49 mg per day and that of the 4 °C samples by 6.53 mg per day. In contract, the TVB-N values of 4 °C fillets reached 45.99 mg/100 g by day 10, which exceeded the maximum acceptable value (30 mg/100 g) (Gökoglu and Cengiz 2004). Similar findings were also found in sardine whose TVB-N value changed from 13.2 mg/100 g to 64.8 mg/100 g during 10 days of refrigerated storage at 4 °C (Gökoğlu et al. 1998). Its increase is related to the activity of spoilage bacteria and endogenous enzymes (Kykkidou et al. 2009; Fuentes et al. 2011). The action of such enzymes results in the formation of compounds including ammonia, monoethylamine, dimethylamine and trimethylamine, which give fish a characteristic off-flavour (Liu et al. 2013). Therefore, the low values observed for fillets stored at 0 °C indicated a good quality.
It shows from Fig. 1e that the WHC decreased with storage time at 0 and 4 °C. It dropped from initial value of 86.64% to about 73.89% on 14th day of storage for salmon stored at 0 °C, and that of the 4 °C by 72.41% on 10th day of storage. The WHC and sensory quality decreased, reflecting a decrease in the water-protein interactions during refrigerated storage (Offer and Knight 1988; Jensen and Jørgensen 1997).
Water activity is the ability to bound water to muscle, may be defined as the ratio of steam partial pressure of solutions to vapor pressure of pure water (Wu 1995). The effect of storage temperature on water activity is revealed in Fig. 1f. The water activity of samples continues to decrease. Although water content of samples varied little during the storage (31.29–36.37%), compared to the samples stored at 0 °C, water activity of the fillets stored at 4 °C reduced more significantly.
Cook loss is an important index to reflect the water holding and oil holding capacity of fish muscle (Sheard et al. 1999). Storage temperature has a significant impact on cook loss of samples (Fig. 1g), the decline of cook loss of salmon fillets might be contributed to the oxidation of protein and lipid. Overall, the cooking loss increased with the storage time, and samples stored at 4 °C were higher than those stored at 0 °C. The statistical analysis showed differences between samples stored at two temperatures with a significance (p < 0.05).
K value is a value that reflects the degree of nucleotide degradation, and can directly reflect the freshness of fish, and its increase is mainly due to the sharp degradation of IMP into HxR and Hx (Zhu et al. 2016). The changes in the K value of samples under different storage conditions are shown in Fig. 1h. Correlation analysis showed that there are significant differences (p < 0.01) between two batches. There has been consistent growth in K value of salmon fillets during storage, the K value of samples stored at 4 °C increased faster than samples at 0 °C, which demonstrate the effectiveness of storage temperature to maintain the freshness of fish, in accordance with earlier studies (Zhang et al. 2016).
Study of the water distribution by LF NMR analysis
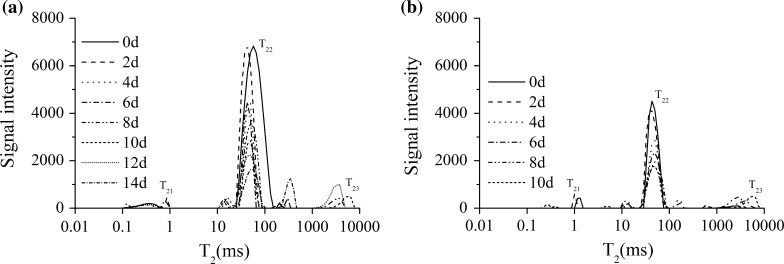
Figure 2a and b showed the T2 transverse relaxation time distribution of fillets from salmon stored at 0 and 4 °C. Three water populations were observed in Fig. 2, in accordance with previous research (Aursand et al. 2008). The population named as T21 with the shortest relaxation time (0.1–1 ms), was the immobile water tightly associated with macromolecules, the water represented by T22 component with a relaxation time centered at 28–86 ms was considered to be trapped within the protein-dense myofibrillar network, and the T23 component with the relaxation time about 114–7564 ms was due to the loose water in the extramyofibrillar space. The variations in water mobility may be due to variations in free energy of hydrogen bond between water and macromolecules in food (Andersen and Rinnan 2002). With the increasing storage time, the value of T22 decreased while T23 increased, the variations are obvious and in agreement with previous study (Ren et al. 2015).
Fig. 2.
Variation in T2 of salmon stored at 0 °C (a) and 4 °C (b)
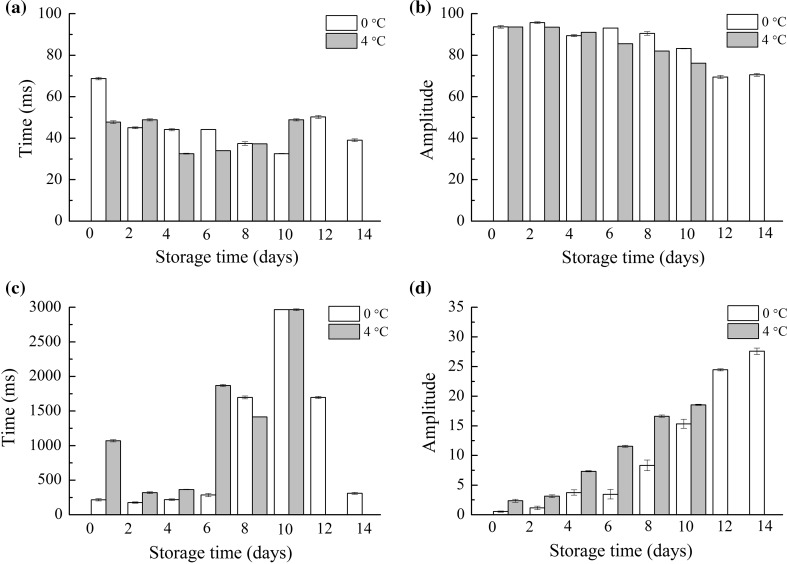
The changes of T22, A22, T23, and A23 of salmon stored at different temperatures are shown in Fig. 3a, b, c and d, respectively. No differences could be found in T22 time constants between salmon fillets stored at 0 and 4 °C whereas the A22 population tended to be lower in the samples during cold storage, accounting for 70–95% of the signal. The A22 of samples during storage at 4 °C reduced faster than samples at 0 °C. The T23 time of samples stored at 4 °C fluctuated during storage, while samples stored at 0 °C first increased and then declined constantly and its relative abundance increased, accounting for 2–27% of the signal, indicating the alteration of strong bound water to free water in salmon muscle. Changes in the values of T2 components may suggest the spoilage of the chilled meat, this may be evidenced by a slight increase in the value of A23, which was related to an amount of free water.
Fig. 3.
Mean relaxation time T22 (a) and T23 (c) and the corresponding amplitude A22 (b) and A23 (d) of LF NMR T2 relaxation data. Samples were stored at 0 °C (white) and 4 °C (grey)
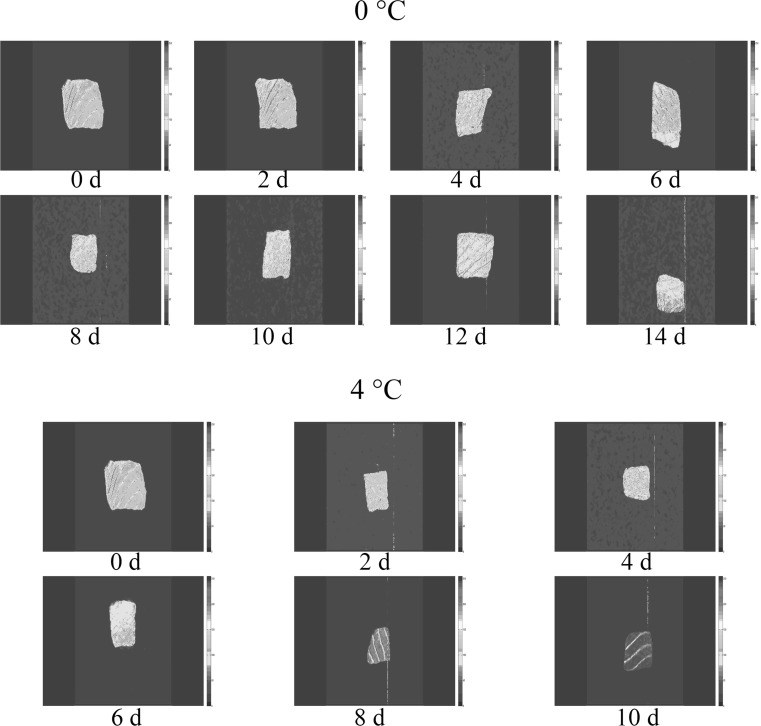
Magnetic resonance imaging (MRI) permits visual observations of the spatial, internal morphological organization and molecular distribution of water in high water content its food matrix. The intensity of the signal in the various zones of the sample may be directly proportional to water molecule content. Specifically, darker areas in an image may mean that there are less water/protons. A high content of ions and or contrast agent, etc. enhances relaxation, causing the area to become darker (Butz et al. 2005). As can be seen from MRI of salmon (Fig. 4), the brightness of samples changed from bright yellow to dark blue during storage, which indicated that a decline in water content in fish muscle decreased. Refrigerated storage induced changes in images in all samples, For the samples stored at 0 °C, a slight variation with images was found in the first 4 days, then fish was not until the 14th day that the fish deteriorated seriously and spoilage area radiated out to surface. From day 2, the fillets stored at 4 °C began to degrade and the color of meat in MRI image became bluer and darker than samples stored at 0 °C, indicating the loss of trapped water and loose water. This decrease of brightness may be contributed to the degradation and denaturalization of muscle protein. The results highlighted that the water distribution of salmon fillets was correlated with the quality changes, and greater water motobility was observed when the salmon stored at 4 °C than samples stored at 0 °C.
Fig. 4.
Magnetic resonance imaging (MRI) of salmon for storing at 0 and 4 °C
Relationship between LF NMR analysis, texture, freshness and sensory quality
Renou et al. (1985) firstly revealed that there was a relationship between T1 and T2 and traditional quality characteristics of pork meat, and later studies have confirmed a relationship between WHC, cook loss and LF NMR measurements (Gudjónsdóttir et al. 2011a, b). However, Tables 1 and 2 indicates that T21 did not correlate with quality parameters well. T22 and T23 of salmon stored at 0 °C were not only significantly correlated (p < 0.05) with cook loss and TBA, but also correlated well (p < 0.01) with WHC, K value. For samples stored at 4 °C, strong correlations between the NMR parameters (T22 and T23) and quality indicators including WHC, TVB-N, sensory scores, hardness, cook loss, TBA and K value were also found, No correlations were found between T2 and water activity.
Table 1.
Linear regression analyses of samples stored at 0 °C between the relaxation times and amplitudes of the populations of water and texture, freshness and sensory quality
| T2 | R | R2 | p |
|---|---|---|---|
| Correlation with TVB-N | |||
| T21 | − 0.45171 | 0.204042 | ns |
| T22 | − 0.74985 | 0.562275 | p < 0.01 |
| T23 | 0.82429 | 0.679454 | p < 0.01 |
| Correlation with water activity | |||
| T21 | 0.27313 | 0.0746 | ns |
| T22 | 0.58421 | 0.341301 | ns |
| T23 | − 0.62569 | 0.391488 | ns |
| Correlation with cooking loss | |||
| T21 | − 0.17866 | 0.031919 | ns |
| T22 | − 0.95825 | 0.918243 | p < 0.05 |
| T23 | 0.82429 | 0.679454 | p < 0.05 |
| Correlation with TBA | |||
| T21 | − 0.28964 | 0.083891 | ns |
| T22 | − 0.95547 | 0.912923 | p < 0.05 |
| T23 | 0.99037 | 0.980833 | p < 0.05 |
| Correlation with WHC | |||
| T21 | 0.44305 | 0.196293 | ns |
| T22 | 0.73829 | 0.545072 | p < 0.01 |
| T23 | − 0.81123 | 0.658094 | p < 0.01 |
| Correlation with hardness | |||
| T21 | 0.28053 | 0.078697 | ns |
| T22 | 0.92516 | 0.855921 | p < 0.01 |
| T23 | − 0.95898 | 0.919643 | p < 0.01 |
| Correlation with sensory | |||
| T21 | 0.47063 | 0.221493 | ns |
| T22 | 0.83676 | 0.700167 | p < 0.01 |
| T23 | − 0.91282 | 0.83324 | p < 0.01 |
| Correlation with K value | |||
| T21 | − 0.3798 | 0.144248 | ns |
| T22 | − 0.88812 | 0.788757 | p < 0.01 |
| T23 | 0.94375 | 0.890664 | p < 0.01 |
R regression coefficient, p significance level, ns no significant
Table 2.
Linear regression analyses of samples stored at 4 °C between the relaxation times and amplitudes of the populations of water and texture, freshness and sensory quality
| T2 | R | R2 | p |
|---|---|---|---|
| Correlation with TVB-N | |||
| T21 | 0.50068 | 0.25068 | ns |
| T22 | − 0.92687 | 0.859088 | p < 0.01 |
| T23 | 0.84553 | 0.714921 | p < 0.05 |
| Correlation with water activity | |||
| T21 | − 0.21962 | 0.048233 | ns |
| T22 | 0.81246 | 0.660091 | p < 0.05 |
| T23 | − 0.78921 | 0.622852 | ns |
| Correlation with cooking loss | |||
| T21 | 0.26647 | 0.071006 | ns |
| T22 | − 0.92229 | 0.850619 | p < 0.01 |
| T23 | 0.9597 | 0.921024 | p < 0.01 |
| Correlation with TBA | |||
| T21 | 0.14018 | 0.01965 | ns |
| T22 | − 0.92704 | 0.859403 | p < 0.01 |
| T23 | 0.92471 | 0.855089 | p < 0.01 |
| Correlation with WHC | |||
| T21 | 0.03376 | 0.00114 | ns |
| T22 | 0.86274 | 0.74432 | p < 0.05 |
| T23 | − 0.89655 | 0.803802 | p < 0.05 |
| Correlation with hardness | |||
| T21 | − 0.23405 | 0.054779 | ns |
| T22 | 0.94477 | 0.89259 | p < 0.01 |
| T23 | − 0.92238 | 0.850785 | p < 0.01 |
| Correlation with sensory | |||
| T21 | − 0.00615 | 0.000038 | ns |
| T22 | 0.96857 | 0.938128 | p < 0.01 |
| T23 | − 0.99686 | 0.99373 | p < 0.01 |
| Correlation with K value | |||
| T21 | 0.01289 | 0.000166 | ns |
| T22 | − 0.9616 | 0.924675 | p < 0.01 |
| T23 | 0.98822 | 0.976579 | p < 0.01 |
R regression coefficient, p significance level, ns no significant
Conclusion
The present study revealed the relationship between LF-NMR parameters and traditional quality indicators including sensory scores, hardness, TBA, TVB-N, WHC, water activity, cook loss, and K-value. The results showed that LF NMR T2 transverse relaxation time was significantly affected by storage conditions and period of salmon fillet during storage, and showed a strong correlation with the parameters related with water, such as cook loss and WHC, as well as quality indicators including sensory quality, hardness, TBA and TVB-N. The study highlighted the feasibility of application of proton spectroscopy (1H) by low-field NMR to monitor salmon quality deterioration during storage, which is associated with water mobility.
Acknowledgements
This work was financially supported by the National “13th Five-Year” Key Research and Development Program for Science and Technology Support [Grant No: 2016YFD0400106], Shanghai Science and Technology Key Project on Agriculture from Shanghai Municipal Agricultural Commission [Grant No: (2016) 1-1], and Shanghai Engineering Research Center Construction Special Fund from Shanghai Municipal Science and Technology Commission [Grant No: 16DZ2280300].
Contributor Information
Jing Xie, Phone: +86-21-61900353, Email: jxie@shou.edu.cn.
Yun-Fang Qian, Phone: +86 21 619 00 385, Email: yfqian@shou.edu.cn.
References
- Anderson CM, Rinnan A. Distribution of water in fresh cod. Environ Sci Technol. 2002;35(8):687–696. [Google Scholar]
- Aursand IG, Gallartjornet L, Erikson U, Axelson DE, Rustad T. Water distribution in brine salted cod (Gadusmorhua) and salmon (Salmo salar): a low-field 1H NMR study. J Agric Food Chem. 2008;56(15):6252–6260. doi: 10.1021/jf800369n. [DOI] [PubMed] [Google Scholar]
- Bertram HC, Andersen HJ, Karlsson AH. Comparative study of low-field NMR relaxation measurements and two traditional methods in the determination of water holding capacity of pork. Meat Sci. 2001;57(2):125–132. doi: 10.1016/S0309-1740(00)00080-2. [DOI] [PubMed] [Google Scholar]
- Butz P, Hofmann C, Tauscher B. Recent developments in noninvasive techniques for fresh fruit and vegetable internal quality analysis. J Food Sci. 2005;70(9):R131–R141. doi: 10.1111/j.1365-2621.2005.tb08328.x. [DOI] [Google Scholar]
- Carlson DL, Hites RA. Polychlorinated biphenyls in salmon and salmon feed: global differences and bioaccumulation. Environ Sci Technol. 2005;39(19):7389–7395. doi: 10.1021/es048023r. [DOI] [PubMed] [Google Scholar]
- Carneiro CDS, Mársico ET, Ribeiro RDOR, Conte-Júnior CA, Mano SB, Augusto CJC, Jesus EFOD. Low-field nuclear magnetic resonance (LF NMR 1H) to assess the mobility of water during storage of salted fish (Sardinellabrasiliensis) J Food Eng. 2015;169:321–325. doi: 10.1016/j.jfoodeng.2015.09.010. [DOI] [Google Scholar]
- Fernández-Segovia I, Fuentes A, Aliño M, Masot R, Alcañiz M, Barat JM. Detection of frozen-thawed salmon (Salmo salar) by a rapid low-cost method. J Food Eng. 2012;113(2):210–216. doi: 10.1016/j.jfoodeng.2012.06.003. [DOI] [Google Scholar]
- FOSS (2002) Determination of total volatile basic nitrogen of fresh fish and frozen fish. Application sub note, vol 8. Hillerød, Denmark, pp 16
- Fuentes A, Fernández-segovia I, Barat JM, Serra JA. Influence of sodium replacement and packaging on quality and shelf life of smoked sea bass (Dicentrarchuslabrax L.) Food Sci Technol. 2011;44(4):917–923. [Google Scholar]
- Fuentes A, Masot R, Fernández-Segovia I, Ruiz-Rico M, Alcañiz M, Barat JM. Differentiation between fresh and frozen-thawed sea bream (Sparusaurata) using impedance spectroscopy techniques. Innov Food Sci Emerg Technol. 2013;19(4):210–217. doi: 10.1016/j.ifset.2013.05.001. [DOI] [Google Scholar]
- Fundo JF, Amaro AL, Madureira AR, Carvalho A, Feio G, Silva CLM, Quintas MAC Fresh-cut melon quality during storage: an NMR study of water transverse relaxation time. J Food Eng. 2015;167:71–76. doi: 10.1016/j.jfoodeng.2015.03.028. [DOI] [Google Scholar]
- Gökoğlu N, Ozden O, Erkan N. Physical, chemical and sensory analyses of freshly harvested sardines (Sardina pilchardus) stored at 4 °C. J Aquat Food Prod Tech. 1998;7(2):5–15. doi: 10.1300/J030v07n02_02. [DOI] [Google Scholar]
- Gökoğlu N, Cengız E, Yerlıkaya P. Determination of the shelf life of marinated sardine (Sardina pilchardus) stored at 4 °CC. Food Control. 2004;15(1):1–4. doi: 10.1016/S0956-7135(02)00149-4. [DOI] [Google Scholar]
- Gudjónsdóttir M, Lauzon HL, Magnússon H, Sveinsdóttir K, Arason S, Martinsdóttir E, Rustad T. Low field nuclear magnetic resonance on the effect of salt and modified atmosphere packaging on cod (Gadusmorhua) during superchilled storage. Food Res Int. 2011;44(44):241–249. doi: 10.1016/j.foodres.2010.10.029. [DOI] [Google Scholar]
- Gudjónsdóttir M, Arason S, Rustad T. The effects of pre-salting methods on water distribution and protein denaturation of dry salted and rehydrated cod—a low-field NMR study. J Food Eng. 2011;104(1):23–29. doi: 10.1016/j.jfoodeng.2010.11.022. [DOI] [Google Scholar]
- Gudjónsdóttir M, Karlsdóttir MG, Arason S, Rustad T. Injection of fish protein solutions of fresh saithe (Pollachius virens) fillets studied by low field nuclear magnetic resonance and physicochemical measurements. J Food Sci Tech. 2013;50(2):228–238. doi: 10.1007/s13197-011-0348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijden GHAVD, Huinink HP, Pel L, Kopinga K. Non-isothermal drying of fired-clay brick, an NMR study. Chem Eng Sci. 2009;64(12):3010–3018. doi: 10.1016/j.ces.2009.03.012. [DOI] [Google Scholar]
- Horigane AK, Suzuki K, Yoshida M. Moisture distribution of soaked rice grains observed by magnetic resonance imaging and physicochemical properties of cooked rice grains. J Food Eng. 2013;57(1):47–55. [Google Scholar]
- Jensen HS, Jørgensen BM. A sensometric approach to cod-quality measurement. Food Qual Prefer. 1997;8(5–6):403–407. doi: 10.1016/S0950-3293(97)00029-3. [DOI] [Google Scholar]
- Kamalakanth CK, Ginson J. Effect of high pressure on K-value, microbial and sensory characteristics of yellowfin tuna (Thunnus albacares) chunks in EVOH films during chill storage. Innov Food Sci Emerg Technol. 2011;12(4):451–455. doi: 10.1016/j.ifset.2011.06.001. [DOI] [Google Scholar]
- Kykkidou S, Giatrakou V, Papavergou A, Kontominas MG, Savvaidis IN. Effect of thyme essential oil and packaging treatments on fresh Mediterranean swordfish fillets during storage at 4 °CC. Food Chem. 2009;115(1):169–175. doi: 10.1016/j.foodchem.2008.11.083. [DOI] [Google Scholar]
- Li XP, Li JR, Zhu JL, Wang YB, Fu LL, Xuan W. Postmortem changes in yellow grouper (Epinephelus awoara) fillets stored under vacuum packaging at 0 °CC. Food Chem. 2011;126(3):896–901. doi: 10.1016/j.foodchem.2010.11.071. [DOI] [Google Scholar]
- Liu D, Li L, Xia W, Regenstein JM, Zhou P. Biochemical and physical changes of grass carp (Ctenopharyngodon idella) fillets stored at −3 and 0 °C. Food Chem. 2013;140(1–2):105–114. doi: 10.1016/j.foodchem.2013.02.034. [DOI] [PubMed] [Google Scholar]
- Meilgaard MC, Carr BT, Civille GV. Sensory evaluation techniques. 3. Boca Raton: CRC Press; 1999. [Google Scholar]
- Offer G, Knight P (1988) The structural basis of water-holding in meat. Part 2: drip losses. In: Lawrie R (ed) Developments in meat science, vol 4. Elsevier Applied Science
- Pearce KL, Rosenvold K, Andersen HJ, Hopkins DL. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—a review. Meat Sci. 2011;89(2):111–124. doi: 10.1016/j.meatsci.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Ren XQ, Yu HH, Ma LZ. Quality changes of ground pork during cold storage determined by LF NMR. Food Res Dev. 2015;15:120–123. [Google Scholar]
- Renou JP, Monin G, Sellier P. Nuclear magnetic resonance measurements on pork of various qualities. Meat Sci. 1985;15(4):225–233. doi: 10.1016/0309-1740(85)90078-6. [DOI] [PubMed] [Google Scholar]
- Rizo A, Mañes V, Fuentes A, Fernández-Segovia I, Barat JM. Physicochemical and microbial changes during storage of smoke-flavoured salmon obtained by a new method. Food Control. 2015;56:195–201. doi: 10.1016/j.foodcont.2015.03.030. [DOI] [Google Scholar]
- Sánchez-alonso I, Martinez I, Sánchez-valencia J, Careche M. Estimation of freezing storage time and quality changes in hake (Merlucciusmerluccius, L.) by low field NMR. Food Chem. 2012;135(3):1626–1634. doi: 10.1016/j.foodchem.2012.06.038. [DOI] [PubMed] [Google Scholar]
- Sánchez-alonso I, Moreno P, Careche M. Low field nuclear magnetic resonance (LF NMR) relaxometry in hake (Merlucciusmerluccius, L.) muscle after different freezing and storage conditions. Food Chem. 2014;153(12):250–257. doi: 10.1016/j.foodchem.2013.12.060. [DOI] [PubMed] [Google Scholar]
- Sheard PR, Nute GR, Richardson RI, Perry A, Taylor AA. Injection of water and polyphosphate into pork to improve juiciness and tenderness after cooking. Meat Sci. 1999;51(4):371–376. doi: 10.1016/S0309-1740(98)00136-3. [DOI] [PubMed] [Google Scholar]
- Shumilina E, Ciampa A, Capozzi F, Rustad T, Dikiy A. NMR approach for monitoring post-mortem changes in Atlantic salmon fillets stored at 0 and 4 °C. Food Chem. 2015;184:12–22. doi: 10.1016/j.foodchem.2015.03.037. [DOI] [PubMed] [Google Scholar]
- Sone I. Spectral changes in fillet of Atlantic salmon as affected by freshness loss and spoilage during cold storage. Tromsø: University of Tromsø; 2012. [Google Scholar]
- Ventanas S, Estevez M, Tejeda JF, Ruiz J. Protein and lipid oxidation in Longissimus dorsi and dry cured loin from Iberian pigs as affected by crossbreeding and diet. Meat Sci. 2006;72(4):647. doi: 10.1016/j.meatsci.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Wu F. Water activity and determination of meat products. Meat Hyg. 1995;4:19–21. [Google Scholar]
- Zell M, Lyng JG, Cronin DA, Morgan DJ. Ohmic cooking of whole beef muscle—evaluation of the impact of a novel rapid ohmic cooking method on product quality. Meat Sci. 2010;86(2):258–263. doi: 10.1016/j.meatsci.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Xie J, Hao K, Zhao HQ. Effects of different cold storage conditions on quality of salmon. Sci Tech Food Ind. 2016;37:316–320. [Google Scholar]
- Zhou S, Ackman RG. Storage of off-flavors in adipocytes of salmon muscle. In: Rimando AM, Schrader KK, editors. Off-flavors in aquaculture. ACS symposium series 848. Washington: American Chemical Society; 2003. pp. 95–106. [Google Scholar]
- Zhu Y, Ma L, Yang H, Xiao Y, Xiong YL. Super-chilling (−0.7°C) with high-CO2 packaging inhibits biochemical changes of microbial origin in catfish (Clariasgariepinus) muscle during storage. Food Chem. 2016;206:182–190. doi: 10.1016/j.foodchem.2016.03.053. [DOI] [PubMed] [Google Scholar]