Abstract
In this study, the effect of drying temperature on antioxidant activity, phenolic compounds, fatty acid composition and tocopherol content of citrus seeds and oils were studied. Kinnow mandarin seed, dried at 60 °C, exhibited the highest antioxidant activity. Orlendo orange seed had the maximum total phenolic content and α-tocopherol content, with a value of 63.349 mg/100 g and 28.085 mg/g (control samples), respectively. The antioxidant activity of Orlendo orange seed (63.349%) was higher than seeds of Eureka lemon (55.819%) and Kinnow mandarin (28.015%), while the highest total phenolic content was found in seeds of Kinnow mandarin, followed by Orlendo orange and Eureka lemon (113.132). 1.2-Dihydroxybenzene (13.171), kaempferol (10.780), (+)-catechin (9.341) and isorhamnetin (7.592) in mg/100 g were the major phenolic compounds found in Kinnow mandarin. Among the unsaturated fatty acids, linoleic acid was the most abundant acid in all oils, which varied from 44.4% (dried at 80 °C) to 46.1% (dried at 70 °C), from 39.0% (dried at 60 °C) to 40.0% (dried at 70 °C). The total phenolic content, antioxidant activity and phenolic compounds of citrus seeds and tocopherol content of seed oils were significantly affected by drying process and varied depending on the drying temperature.
Keywords: Citrus seed, Oil, Drying, Antioxidant activity, Phenolic compounds, Fatty acids, Tocopherol
Introduction
The genus Citrus (Rutaceae family) is an annual plant that is widely distributed in Mediterranean countries of Middle East and Southern Europe but also widely grows in other warm climates around the globe (Saidani et al. 2004). Citrus fruits are processed into different food products and substantial quantities of Citrus seeds are obtained as waste product which creates environmental and disposal problems (Matthaus and Özcan 2012). Plant seeds are important sources of oils for nutritional, industrial, and pharmaceutical applications (Aitzetmüller 1993). Some seed oils from other plants are already used for several purposes like blending with modified nutritional values, as ingredients in paint and varnish formulations, lubricants, pharmaceuticals, organic pesticides, plastics, dispersants, textiles, soaps, surface coating and oleo-chemicals, as well as oils for cosmetic purposes (Muuse et al. 1992; Hosamani and Sattigeri 2000). The high oil content makes the seed material interesting for the production of oil. The fatty acid composition of some Citrus seed oils has been identified by Saidani et al. (2004) and El-Adawy et al. (1999). The seeds of fruits such as oranges (Citrus sinensis) are shown to be promising sources of oils, rich in carotenoids, phenolic compounds, tocopherols, and phytosterols (Malacrida et al. 2012). In addition, the vegetable oils are considered as sources of carotenoids, phenolic compounds, tocopherols, and phytosterols (Jorge et al. 2016). The orange seed oils are rich in total carotenoids (19.01 mg/kg), total phenolic compounds (4.43 g/kg), α-tocopherol (135.65 mg/kg) and phytosterols (1304.2 mg/kg) (Jorge et al. 2016). The recovery of these phytochemicals and oil from seeds may involve use of elevated temperatures which may be is one of the most important factors affecting antioxidant activity. Generally, acceleration of the initiation reactions is being affected by heating. The α-tocopherol activity increased with increasing working temperature in the temperature range of 20–100 °C in all the stabilised substrates (Marinova and Yanishlieva 1998). The mechanism of action of some bioactive compounds may change due to variations in temperature. The objective of present study was to investigate the effects of heating on the on phenolic compound and tocopherol contents of citrus seeds, and to evaluate the effects of drying temperature on phenolic compounds, fatty acid compositions and tocopherol contents of citrus seed and oils.
Materials and methods
Materials
About 10 kg of fruit from each variety of Citrus species (Orlendo Orange, Kinnow mandarin and Eureka lemon) species were purchased from local markets in Riyadh in Saudi Arabia in December, 2015. The skin and pulp were removed from the seeds, and the seeds were washed and cleaned in an air screen cleaner to remove immature and broken seeds, and then stored in polypropylene bags at 4 °C temperature prior to further use. 100 seed samples from each species were analyzed.
Methods
Drying process
Conventional drying of Citrus seeds was currently carried out by commercial electrical ovens at (Nüve FN055 Ankara, Turkey, 55 L volume) at 60, 70 and 80 °C for 24 h. Citrus seed was placed on tray in a 5 cm thick. Seeds were mixed at regular intervals during drying and cooled in the desiccator after drying. The dried seeds were kept in refrigeration in a hermetically sealed glass jar until they were cooled.
Sample extraction
For phenolic compounds and antioxidants, seed samples were extracted according to Garcia-Salas et al. (2013) with some modifications. 2 g of ground samples were added to 10 ml of methanol. The mixture was shaken by vortex for 1 min and sonicated for 30 min, followed by centrifugation at 4500 rpm for 10 min. These steps were repeated twice and the supernatants were collected. The extract was concentrated at 37 °C in a rotary evaporator under the vacuum. The volume of the extracts was completed to 5 ml by methanol. Prior to injection, the extract was filtered through a 0.45 µm nylon filter. All analyses were made in triplicate.
Total phenolic content
Total phenol contents of obtained extracts were quantified by using the Folin–Ciaocalteu (FC) reagent as applied by Yoo et al. (2004) with some modifications. 1 ml of Folin–Ciacueltau was added and mixed for 5 min. Following the addition of 10 ml of 7.5% Na2CO3 solution tubes were mixed and the final volume was completed to 25 ml with deionised water. After 1 h, total phenolic contents were determined at 750 nm wave length in spectrophotometer. Gallic acid was used (0–200 mg/ml) as the standard for calibration curve. All determinations were performed in triplicate. The results were given as mg gallic acid equivalent (GAE)/100 g of fresh weight.
Antioxidant activity
The free radical scavenging activity of samples was determined using DPPH (1,1-diphenyl-2-picrylhydrazyl) according to Lee et al. (1998). The extract was mixed with 2 ml methanolic solution of DPPH. The mixture was shaken vigorously and allowed to stand at room temperature for 30 min. And the absorbance was recorded at 517 nm by using a spectrophotometer. All determinations were performed in triplicate.
Phenolic compounds
Phenolic compounds were determined using Shimadzu-HPLC equipped with PDA detector and Inertsil ODS-3 (5 µm; 4.6 × 250 mm) column. 0.05% acetic acid in water (A) and acetonitrile (B) mixture of mobile phase was used. The flow rate of the mobile phase was 1 ml/min at 30 °C and the injection volume was 20 µl. The peaks were recorded at 280 and 330 nm with PDA detector. The gradient program was as follows: 0–0.10 min 8% B; 0.10–2 min 10% B; 2–27 min 30% B; 27–37 min 56% B; 37–37.10 min 8% B; 37.10–45 min 8% B. The total running time per sample was 60 min.
Fatty acid composition
Citrus seed oils were esterificated according to ISO-5509 (1978) method with some modifications. Fatty acid methyl esters of samples were analyzed using gas chromatography (Shimadzu GC-2010) equipped with flame-ionization detector (FID) and capillary column (Tecnocroma TR-CN100, 60 m × 0.25 mm, film thickness: 0.20 µm). The temperature of injection block and detector was 260 °C. Mobile phase was nitrogen with 1.51 ml/min flow rate. Total flow rate was 80 ml/min and split rate was also 1/40. Column temperature was programmed 120 °C for 5 min and increased to 240 °C at 4 °C/min and held for 25 min at 240 °C. A standard fatty acid methyl ester mixture (Sigma Chemical Co.) was used to determine sample peaks. Commercial mixtures of fatty acid methyl esters were used as reference data for the relative retention times (AOAC 1990).
Tocopherol content
Tocopherol contents analysis was performed according to Spica et al. (2015). Oil sample (0.1 g) was dissolved in 10 ml of n-hexane and filtered through a 0.45 µm nylon fitler. HPLC analyses of tocopherols were determined using Shimadzu-HPLC equipped with PDA detector and LiChroCART Silica 60 (4.6 × 250 mm, 5µ; Merck, Darmstadt, Germany) column. Tocopherols were separated by isocratic chromatography using a mobile phase of 0.7% propan-2-ol in n-hexane. The flow rate of the mobile phase was 0.9 ml/min and the injection volume was 20 µl. The peaks were recorded at 295 and 330 nm with PDA detector. The total running time per sample was 30 min. Standard solutions of tocopherols (α, β, γ and δ-tocopherols) were constructed in the concentrations of 0–100 mg/l. All analyses were made in triplicate.
Statistical analysis
A complete randomized split plot block design was used, and all analyses were carried out three times and the results are mean ± standard deviation (MSTAT C) of independent citrus seed and oil samples (Püskülcü and İkiz 1989).
Results and discussion
Phenolic compounds and antioxidant activity of citrus seeds
Total phenolic contents and antioxidant activities, are presented in Table 1. The antioxidant activity of Orlendo orange seed (63.3%) was higher than seeds of Eureka lemon (55.8%) and Kinnow mandarin (28.0%), while the highest total phenolic content was found in the seed of Kinnow mandarin followed by Orlendo orange and Eureka lemon. Total phenolic content and antioxidant activity of all seeds were significantly affected by drying process. Additionally, the data showed differences according to drying temperature applied. The antioxidant activity of Kinnow mandarin seed showed an increase with drying at 60 °C (62.4%) and 70 °C (60.45%). Similarly, drying process at 60 and 70 °C provided an increase in antioxidant activity of Eureka lemon seed, while the drying at 80 °C caused a significant decrease in all seeds. Concerning total phenolic content, the increase was observed in Kinnow mandarin (170 mg/100 g) and Orlendo orange (129 mg/100 g) seeds when dried at 60 and 70 °C, respectively (p < 0.05). The increase and decrease in antioxidant activity were similar to total phenolic content of seeds. According to Sultana et al. (2015), total phenolic content of Citrus limon and Citrus pseudolimon were determined as 98.23 mg GAE/g extract and 106.06 mg GAE/g extract of dry matter, respectively. The antioxidant activity of Citrus limon was found as 39.98%. Total phenolic content of seeds was higher than our results, while antioxidant activity was also lower. The total polyphenols content of mandarin (Citrus reticulata) seed was found to vary from 0.68 to 2.11 mg GAE/g DW (Moulehi et al. 2012), while the total phenolic content of the orange (Citrus sinensis) seed extract ranged from 10.9 to 39.4 mg GAE/g (DW) (Molan et al. 2016).
Table 1.
Total phenolic contents and antioxidant activities of citrus seeds
| Sample | Temperature (°C) | Antioxidant activity (%) | Total phenolic content (mg/100 g) |
|---|---|---|---|
| Kinnow mandarin | Control | 28.015 ± 0.039*c | 158.160 ± 0.008c |
| 60 | 62.454 ± 0.006a** | 170.938 ± 0.021a | |
| 70 | 60.453 ± 0.014b | 163.993 ± 0.016b | |
| 80 | 12.533 ± 0.016d | 144.271 ± 0.007d | |
| Eureka lemon | Control | 55.819 ± 0.018c | 113.132 ± 0.021b |
| 60 | 58.346 ± 0.001a | 119.438 ± 0.012a | |
| 70 | 57.425 ± 0.022b | 111.771 ± 0.009c | |
| 80 | 43.286 ± 0.006d | 110.243 ± 0.005d | |
| Orlendo orange | Control | 63.349 ± 0.011b | 113.993 ± 0.010b |
| 60 | 60.927 ± 0.033c | 110.382 ± 0.004d | |
| 70 | 64.666 ± 0.012a | 129.688 ± 0.019a | |
| 80 | 51.132 ± 0.003d | 111.632 ± 0.009c |
* Mean ± SD; ** values within each column followed by different letters are significantly different (p < 0.05)
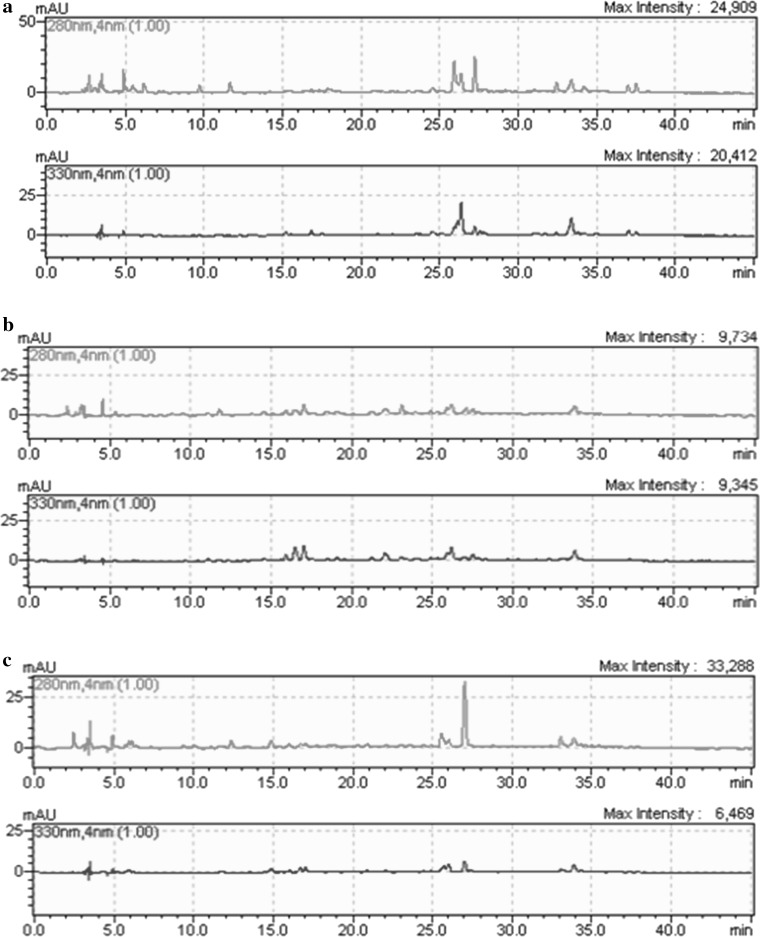
The phenolic compounds of citrus seeds are given in Table 2. 1.2-Dihydroxybenzene (13.171), kaempferol (10.780), (+)-catechin (9.341) and isorhamnetin (7.592) in mg/100 g were the major phenolic compounds found in Kinnow mandarin. 3.4-Dihydroxybenzoic acid (9.048), gallic acid (6.306) and rutin trihydrate (6.456) in mg/100 g were determined as the main phenolic compounds in Eureka lemon (p < 0.05). The predominant phenolic compounds of Orlendo orange in mg/100 g were apigenin 7 glucoside (21.936), (+)-catechin (8.906) and gallic acid (4.830). In addition to these phenolic compounds, all seeds had minor amounts of syringic acid, caffeic acid, p-coumaric acid, resveratrol, quercetin, trans-cinnamic acid and naringenin (Fig. 1). The results demonsrated that while drying at 60 °C caused a minor increase in 3.4-dihydroxybenzoic acid and apigenin 7 glucoside for Kinnow mandarin; 3.4-dihydroxybenzoic acid and (+)-catechin for Eureka lemon and Orlendo orange, a major decrease in 1.2-dihydroxybenzene, syringic acid and apigenin 7 glucoside for Kinnow mandarin, Eureka lemon and Orlendo orange, respectively. The amount of gallic acid content increased in all seeds dried at 70 °C. Although drying process at 80 °C resulted in minor reduction in phenolic compounds of all seeds, the contents of 3.4-dihydroxybenzoic acid, 1.2-dihydroxybenzene and quercetin showed a significant increase in Orlendo orange seed dried at both 70 and 80 °C.
Table 2.
Phenolic compounds of citrus seeds (mg/100 g)
| Control | 60 °C | 70 °C | 80 °C | |
|---|---|---|---|---|
| Kinnow mandarin | ||||
| Gallic acid | 5.113 ± 1.323*c | 6.185 ± 0.367b | 9.720 ± 0.797a | 4.496 ± 0.369d |
| 3.4-Dihydroxybenzoic acid | 4.412 ± 0.555b** | 12.113 ± 0.472a | 2.280 ± 0.718d | 3.641 ± 0.461c |
| (+)-Catechin | 9.341 ± 0.038a | 3.371 ± 0.142d | 6.105 ± 0.203b | 5.962 ± 0.270c |
| 1.2-Dihydroxybenzene | 13.171 ± 2.851a | 5.699 ± 0.258c | 2.517 ± 0.861d | 6.126 ± 0.236b |
| Syringic acid | 3.795 ± 0.248b | 3.330 ± 0.255b | 6.754 ± 0.990a | 1.651 ± 0.070c |
| Caffeic acid | 3.406 ± 0.700a | 0.667 ± 0.188c | 0.392 ± 0.008c | 1.537 ± 0.479b |
| Rutin trihydrate | 3.199 ± 0.578a | 0.635 ± 0.087b | 0.369 ± 0.054b | 0.405 ± 0.056b |
| p-Coumaric acid | 0.272 ± 0.009a | 0.155 ± 0.030c | 0.177 ± 0.035b | 0.152 ± 0.027c |
| Trans-ferulic acid | 6.150 ± 1.601a | 1.443 ± 0.067c | 1.418 ± 0.735c | 5.131 ± 0.851b |
| Apigenin 7 glucoside | 4.160 ± 0.493c | 12.854 ± 0.773a | 1.625 ± 0.790d | 8.230 ± 0.772b |
| Resveratrol | 0.128 ± 0.022c | 1.794 ± 0.112a | 0.524 ± 0.008c | 1.216 ± 0.098b |
| Quercetin | 3.816 ± 0.214b | 7.275 ± 0.048a | 2.681 ± 0.406c | 3.304 ± 3.610b |
| Trans-cinnamic acid | 0.296 ± 0.020b | 0.056 ± 0.007c | 0.410 ± 0.052a | 0.093 ± 0.112c |
| Naringenin | 3.398 ± 0.533a | 0.167 ± 0.056c | 1.954 ± 0.013b | 0.188 ± 0.010c |
| Kaempferol | 10.780 ± 0.279a | 5.642 ± 0.042c | 6.948 ± 0.452b | 5.601 ± 0.437c |
| Isorhamnetin | 7.592 ± 3.078b | 6.779 ± 0.230c | 9.258 ± 0.268a | 6.662 ± 0.250d |
| Eureka lemon | ||||
| Gallic acid | 6.306 ± 0.516b | 6.009 ± 0.196b | 11.221 ± 0.290a | 4.938 ± 0.201c |
| 3.4-Dihydroxybenzoic acid | 9.048 ± 1.019d | 12.666 ± 0.514a | 10.589 ± 0.661c | 11.649 ± 1.738b |
| (+)-Catechin | 4.459 ± 0.249c | 8.312 ± 0.328b | 12.577 ± 0.666a | 4.816 ± 0.394c |
| 1.2-Dihydroxybenzene | 4.821 ± 0.236b | 0.566 ± 0.025d | 3.687 ± 1.091c | 5.589 ± 0.448a |
| Syringic acid | 5.958 ± 0.858a | 1.242 ± 0.382c | 0.258 ± 0.079d | 1.810 ± 0.289b |
| Caffeic acid | 3.983 ± 0.294c | 4.763 ± 0.633b | 1.134 ± 0.143d | 5.323 ± 0.714a |
| Rutin trihydrate | 6.456 ± 0.591a | 2.377 ± 0.286b | 2.179 ± 0.559b | 0.195 ± 0.002c |
| p-Coumaric acid | 0.442 ± 0.215c | 0.524 ± 0.164b | 0.381 ± 0.194d | 1.202 ± 0.081a |
| Trans-ferulic acid | 2.673 ± 0.032a | 1.331 ± 0.154b | 0.682 ± 0.342c | 1.430 ± 0.343b |
| Apigenin 7 glucoside | 4.021 ± 1.158a | 1.466 ± 0.401c | 2.391 ± 0.624b | 1.930 ± 1.404c |
| Resveratrol | 0.422 ± 0.040a | 0.359 ± 0.058b | 0.187 ± 0.068d | 0.277 ± 0.005c |
| Quercetin | 4.865 ± 0.574b | 1.470 ± 0.162d | 5.199 ± 2.430a | 3.309 ± 0.356c |
| Trans-cinnamic acid | 0.066 ± 0.005c | 0.030 ± 0.006c | 0.157 ± 0.018b | 0.234 ± 0.017a |
| Naringenin | 0.378 ± 0.012b | 0.115 ± 0.001c | 0.547 ± 0.146a | 0.452 ± 0.063a |
| Kaempferol | 1.199 ± 0.238a | 1.237 ± 0.414a | 0.677 ± 0.211b | 0.614 ± 0.071b |
| Isorhamnetin | 0.901 ± 0.073c | 0.714 ± 0.026d | 1.102 ± 0.346b | 1.632 ± 0.372a |
| Orlendo orange | ||||
| Gallic acid | 4.830 ± 0.200b | 6.194 ± 0.118a | 6.356 ± 0.880a | 2.904 ± 0.867c |
| 3.4-Dihydroxybenzoic acid | 0.463 ± 0.037c | 3.224 ± 0.111b | 6.504 ± 0.529a | 3.439 ± 0.206b |
| (+)-Catechin | 8.906 ± 1.261c | 16.982 ± 0.541a | 5.268 ± 0.232d | 12.515 ± 0.411b |
| 1.2-Dihydroxybenzene | 2.745 ± 0.756d | 4.102 ± 1.180c | 24.984 ± 0.389a | 7.449 ± 0.752b |
| Syringic acid | 0.848 ± 0.040b | 0.848 ± 0.028b | 2.855 ± 0.048a | 0.634 ± 0.006c |
| Caffeic acid | 1.625 ± 0.151b | 2.839 ± 0.084a | 2.411 ± 0.046a | 0.983 ± 0.020c |
| Rutin trihydrate | 1.160 ± 0.025b | 1.139 ± 0.136d | 1.208 ± 0.041a | 1.146 ± 0.038c |
| p-Coumaric acid | 0.077 ± 0.025d | 0.173 ± 0.020c | 0.314 ± 0.015a | 0.223 ± 0.009b |
| Trans-ferulic acid | 0.261 ± 0.026d | 0.657 ± 0.023c | 1.255 ± 0.138a | 0.915 ± 0.156b |
| Apigenin 7 glucoside | 21.936 ± 0.850a | 2.488 ± 0.281c | 8.151 ± 0.092b | 1.138 ± 0.008d |
| Resveratrol | 0.145 ± 0.001b | 0.358 ± 0.060a | 0.345 ± 0.001a | 0.382 ± 0.021a |
| Quercetin | 0.531 ± 0.010d | 1.553 ± 0.190c | 10.375 ± 0.191a | 9.706 ± 0.635b |
| Trans-cinnamic acid | 0.115 ± 0.024c | 0.092 ± 0.013d | 0.622 ± 0.068a | 0.181 ± 0.007b |
| Naringenin | 0.256 ± 0.015c | 0.245 ± 0.031c | 0.708 ± 0.100a | 0.591 ± 0.081b |
| Kaempferol | 0.620 ± 0.028a | 0.575 ± 0.011b | –*** | – |
| Isorhamnetin | 0.932 ± 0.132c | 0.489 ± 0.034d | 1.210 ± 0.027b | 1.794 ± 0.005a |
* Mean ± SD; ** values within each column followed by different letters are significantly different (p < 0.05); *** nonidentified
Fig. 1.
Chromatograms of phenolic compounds of extract obtained from citrus seeds: a Kinnow mandarin; b Eureka lemon; c Orlendo orange
Fatty acid composition
Table 3 shows the fatty acid compositions of citrus seed oils. Among the unsaturated fatty acids, linoleic acid was the most abundant acid in all oils, which varied from 44.412% (dried at 80 °C) to 46.121% (dried at 70 °C), from 39.039% (dried at 60 °C) to 40.090% (dried at 70 °C), from 39.189% (dried at 80 °C) to 39.716% (dried at 60 °C), followed by oleic acid, ranged from 21.265% (dried at 70 °C) to 23.539% (control), from 23.220% (dried at 80 °C) to 23.897% (control), 23.097% (control) to 24.457% (dried at 80 °C) in Kinnow mandarin, Eureka lemon and Orlendo orange, respectively (p < 0.05). Concerning the saturated fatty acids, palmitic acid was observed to be dominant and the amount of palmitic acid higher than oleic acid content in seed oil of Orlendo orange. Besides the seed oil of Eureka lemon had maximum linolenic acid content, with a content varying from 7.841% (dried at 80 °C) to 8.061% (control). Results of fatty acid analysis showed that fatty acid profile of seed oils were not significantly affected by drying process compared to control conditions. Nevertheless the highest increase in content of palmitic acid (ranging from 15.770 to 23.423%) and the major decrease in content of oleic acid (ranging from 23.539 to 21.600%) were determined at Kinnow mandarin. According to Malacrida et al. (2012) report, the linoleic was the dominant fatty acid with percentages between 38.89 and 44.31% in the orange (Citrus sinensis), lemon (Citrus limon) and tangerine (Citrus reticulata) seed oils, followed by oleic acid, ranged from 20.80 (in lemon seed oil) to 27.78% (in tangerine seed oil) and palmitic acid, varied from 21.03 (in lemon seed oil) to 26.42% (in orange seed oil). Turkish citrus seed oil contained a significant amount of oleic acid (18.3–70.1%), linoleic acid (19.5–58.9%) and palmitic acid (5.1–28.3%) (Matthaus and Özcan 2012). Gültekin et al. (2016) reported that some citrus seed oils contained 23.29–28.625 palmitic, 21.72–32.16% oleic and 35.99–44.86% linolenic acids in a study conducted in 2008.
Table 3.
Fatty acid composition of citrus seed oils (%)
| Sample | Temperature (°C) | Myristic | Palmitic | Stearic | Oleic | Linoleic | Arachidic | Linolenic | Behenic | Arachidonic |
|---|---|---|---|---|---|---|---|---|---|---|
| Kinnow mandarin | Control | –*** | 15.770 ± 2.09*d | 2.620 ± 0.037b | 23.539 ± 2.275a | 45.927 ± 4.518b | 0.366 ± 0.126a | 4.924 ± 0.168a | 0.294 ± 0.004a | 0.108 ± 0.000d |
| 60 | – | 20.324 ± 0.046c** | 3.101 ± 0.003a | 23.486 ± 0.022a | 46.031 ± 0.019a | 0.308 ± 0.004d | 4.694 ± 0.015b | ND | 0.158 ± 0.003c | |
| 70 | – | 22.167 ± 0.055b | 3.591 ± 0.004a | 21.265 ± 0.020b | 46.121 ± 0.020a | 0.343 ± 0.000c | 4.530 ± 0.005c | 0.108 ± 0.003c | 0.189 ± 0.002b | |
| 80 | – | 23.423 ± 0.135a | 3.622 ± 0.021a | 21.600 ± 0.082b | 45.412 ± 0.170b | 0.360 ± 0.006b | 4.285 ± 0.014d | 0.124 ± 0.003b | 0.218 ± 0.010a | |
| Eureka lemon | Control | 0.155 ± 0.005a | 22.897 ± 0.142b | 3.508 ± 0.032b | 23.897 ± 0.168a | 39.462 ± 0.102a | 0.350 ± 0.012a | 8.061 ± 0.038a | 0.121 ± 0.008a | 0.226 ± 0.009b |
| 60 | 0.153 ± 0.009b | 22.961 ± 0.244b | 3.500 ± 0.054b | 23.680 ± 0.412b | 39.039 ± 0.531b | 0.325 ± 0.014b | 8.017 ± 0.032a | 0.089 ± 0.000b | 0.256 ± 0.008a | |
| 70 | 0.149 ± 0.003c | 23.181 ± 0.199a | 3.483 ± 0.005c | 23.310 ± 0.083c | 39.090 ± 0.054b | 0.329 ± 0.002b | 7.965 ± 0.025b | 0.088 ± 0.000b | 0.170 ± 0.006d | |
| 80 | 0.143 ± 0.006d | 23.242 ± 0.046a | 3.688 ± 0.015a | 23.220 ± 0.113c | 39.634 ± 0.153a | 0.351 ± 0.001a | 7.841 ± 0.027b | 0.099 ± 0.002b | 0.201 ± 0.000c | |
| Orlendo orange | Control | 0.108 ± 0.004a | 27.368 ± 0.647a | 4.198 ± 0.066d | 23.097 ± 0.274b | 39.649 ± 0.234b | 0.310 ± 0.016c | 3.374 ± 0.024a | 0.103 ± 0.000a | 0.213 ± 0.028a |
| 60 | 0.098 ± 0.000c | 26.756 ± 0.078b | 4.204 ± 0.010c | 23.682 ± 0.027b | 39.716 ± 0.028a | 0.298 ± 0.000d | 3.310 ± 0.005b | 0.073 ± 0.002c | 0.217 ± 0.003a | |
| 70 | 0.103 ± 0.006b | 26.861 ± 0.472b | 4.334 ± 0.074b | 23.570 ± 0.223b | 39.704 ± 0.133a | 0.321 ± 0.013b | 3.221 ± 0.029c | 0.084 ± 0.000b | 0.200 ± 0.018b | |
| 80 | 0.097 ± 0.004c | 26.217 ± 0.242b | 4.528 ± 0.055a | 24.457 ± 0.166a | 39.189 ± 0.022c | 0.331 ± 0.007a | 3.165 ± 0.018d | 0.081 ± 0.003c | 0.214 ± 0.017a |
* Mean ± SD; ** values within each column followed by different letters are significantly different (p < 0.05); *** nonidentified
Tocopherol contents
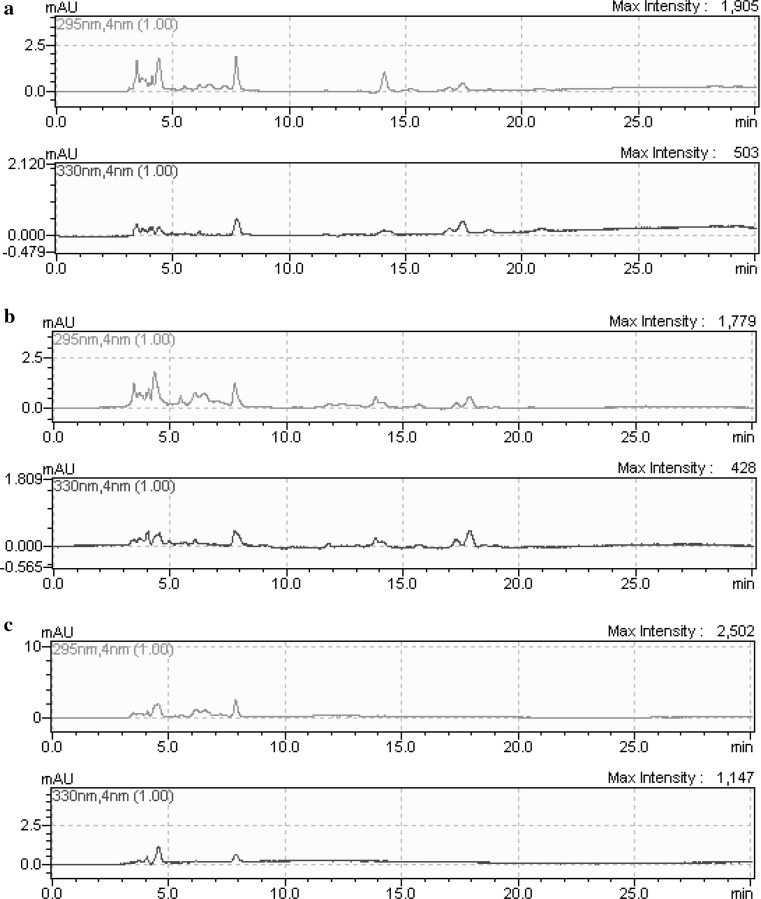
The tocopherol composition of seed oils is presented in Table 4. α-Tocopherol was the major isomer, followed by γ-tocopherol, while β-tocopherol and δ-tocopherol were not detected in any of the seed oils (Fig. 2). Orlendo orange seed oil showed the maximum α-tocopherol content, with a value of 28.08 mg/g (in control), whereas γ-tocopherol was never detected. Generally, it was found that tocopherol content of seed oils reduced comparing the control samples, after drying process was applied. Additionally, the decrease of α-tocopherol content was higher than γ-tocopherol content in Kinnow mandarin and Eureka lemon. α-tocopherol contents of seed oils of Kinnow mandarin, Eureka lemon and Orlendo orange decreased from 25.33 to 14.76 mg/g; from 21.96 to 13.808 mg/g; from 28.08 to 17.83 mg/g together with drying process at 80 °C, respectively. The highest reduction was also observed drying at 80 °C according to the control group in all seed oils. Malacrida et al. (2012) reported that α-tocopherol contents of orange (Citrus sinensis), lemon (Citrus limon) and tangerine (Citrus reticulata) seed oils in mg/kg were determined as 300, 102 and 116. According to Matthaus and Özcan (2012), Turkish citrus seed oil was characterized by higher amounts of α- and γ-tocopherols. C. paradisi, C. limon (Kütdiken) and C. limon (interdonato) had the highest α-tocopherol contents, with a range of 17.5, 13.0 and 10.9 mg/100 g, respectively. The increase or reduction of the analyzed compounds can be probably due to seed size, the maturity status, genetic structure and nutritional patterns of the plants, compounds of seeds. Also, it can be effective in applied heat treatment. Generally, heating causes an acceleration of the initiation reactions, and hence a decrease in the activity of the present or added antioxidants.
Table 4.
Tocopherol content of citrus seed oils (mg/g)
| Sample | Temperature | α-Tocopherol | γ-Tocopherol |
|---|---|---|---|
| Kinnow mandarin | Control | 25.322 ± 0.063*a | 14.875 ± 0.010b |
| 60 °C | 25.187 ± 0.006a** | 15.492 ± 0.007a | |
| 70 °C | 15.072 ± 0.040b | 12.225 ± 0.084c | |
| 80 °C | 14.762 ± 0.008c | 11.627 ± 0.232d | |
| Eureka lemon | Control | 21.967 ± 0.031a | 11.048 ± 0.093a |
| 60 °C | 19.213 ± 0.013b | 10.402 ± 0.009b | |
| 70 °C | 16.845 ± 0.311c | 10.840 ± 0.009b | |
| 80 °C | 13.808 ± 0.416d | 10.658 ± 0.011b | |
| Orlendo orange | Control | 28.085 ± 0.101a | –*** |
| 60 °C | 28.725 ± 0.021a | – | |
| 70 °C | 22.045 ± 0.161b | – | |
| 80 °C | 17.837 ± 0.010c | – |
* mean ± SD; ** values within each column followed by different letters are significantly different (p < 0.05); *** nonidentified
Fig. 2.
Chromatograms tocopherols of oils obtained from citrus seed oils: a Kinnow mandarin; b Eureka lemon; c Orlendo orange
Acknowledgements
The authors extend their appreciation to the International Scientific Partnership Program ISPP at King Saud University for funding this research work through ISPP# 0015.
References
- Aitzetmüller K. Capillary GLC fatty acid fingerprints of seed lipids—a tool in plant chemotaxonomy. J High Resolut Chrom. 1993;16:488–490. doi: 10.1002/jhrc.1240160809. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 15. Washington: Association of Official Analytical Chemists; 1990. [Google Scholar]
- El-Adawy TA, Rahman EH, El-Bedawy AA, Gafar AM. Properties of some citrus seeds. Part 3. Evaluation as a new source of protein and oil. Nahrung. 1999;43:385–391. doi: 10.1002/(SICI)1521-3803(19991201)43:6<385::AID-FOOD385>3.0.CO;2-V. [DOI] [Google Scholar]
- Garcia-Salas P, Gomez-Caravaca AM, Arraez-Roman D, Segura-Carretero A, Guerra-Hernandez E, Garcia-Villanova B, Fernandez-Gutierrez A. Influence of technological processes on phenolic compounds, organic acids, furanic derivatives, and antioxidant activity of whole-lemon powder. Food Chem. 2013;141:869–878. doi: 10.1016/j.foodchem.2013.02.124. [DOI] [PubMed] [Google Scholar]
- Gültekin Ö, Özcan MM, AlJuhaimi F. Some physicochemical properties, fatty acid composition, and tocopherol contents of Citrus seed oils. Riv Sostanze Grasse. 2016;93:47–51. [Google Scholar]
- Hosamani KM, Sattigeri RM. Industrial utilization of Rivea ornata seed oil: a moderate source of vernolic acid. Ind Crops Prod. 2000;12:93–96. doi: 10.1016/S0926-6690(00)00041-8. [DOI] [Google Scholar]
- ISO-International Organization for Standardization . Animal and vegetable fats and oils preperation of methyl esters of fatty acids. Geneve: ISO; 1978. pp. 1–6. [Google Scholar]
- Jorge N, da Silva AC, Aranha CPM. Antioxidant activity of oils extracted from orange (Citrus sinensis) seeds. An Acad Bras Ciênc. 2016;88(2):951–958. doi: 10.1590/0001-3765201620140562. [DOI] [PubMed] [Google Scholar]
- Lee SK, Mbwambo ZH, Chung HS, Luyengi L, Games EJC, Mehta RG. Evaluation of the antioxidant potential of natural products. Comb Chem High Throughput Screen. 1998;1:35–46. [PubMed] [Google Scholar]
- Malacrida CR, Kimura M, Jorge N. Phytochemicals and antioxidant activity of citrus seed oils. Food Sci Technol Res. 2012;18(3):399–404. doi: 10.3136/fstr.18.399. [DOI] [Google Scholar]
- Marinova EM, Yanishlieva NV. Antioxidative action of quercetin and morin in triacylglycerols of sunflower oil at ambient and high temperatures. Seifen Ole Fette Wachse. 1998;124:10–16. [Google Scholar]
- Matthaus B, Özcan MM. Chemical evaluation of citrus seeds, an agro-industrial waste, as a new potential source of vegetable oils. Grasas Aceites. 2012;63(3):313–320. doi: 10.3989/gya.118411. [DOI] [Google Scholar]
- Molan A, Ismail MH, Nsaif RH. Phenolic contents and antioxidant activity of peels and seeds of orange (Citrus sinensis) cultivated in Iraq. World J Pharm Pharm Sci. 2016;5(4):473–482. [Google Scholar]
- Moulehi I, Bourgou S, Ourghemmi I, Tounsi MS. Variety and ripening impact on phenolic composition and antioxidant activity of mandarin (Citrus reticulate Blanco) and bitter orange (Citrus aurantium L.) seeds extracts. Ind Crops Prod. 2012;39:74–80. doi: 10.1016/j.indcrop.2012.02.013. [DOI] [Google Scholar]
- Muuse BG, Cuperus FP, Derksen JTP. Composition and physical properties of oils from new oilseed crops. Ind Crops Prod. 1992;1:57–65. doi: 10.1016/0926-6690(92)90046-X. [DOI] [Google Scholar]
- Püskülcü H, İkiz F. Introduction to statistic. İzmir: Bilgehan Press; 1989. p. 333. [Google Scholar]
- Saidani M, Dhifi W, Marzouk B. Lipid evaluation of some Tunisian citrus seeds. J Food Lipids. 2004;11:242–250. doi: 10.1111/j.1745-4522.2004.01136.x. [DOI] [Google Scholar]
- Spica MJ, Kraljic K, Koprivnjak O, Skevin D, Zanetic M, Katalinic M. Effect of agronomical factors and storage conditions on the tocopherol content of Oblica and Leccino virgin olive oil. J Am Oil Chem Soc. 2015;92:1293–1301. doi: 10.1007/s11746-015-2688-2. [DOI] [Google Scholar]
- Sultana B, Anwar F, Mushtaq M, Alim M. Citrus residues: a potential source of phenolics with high antioxidant values. Int Food Res J. 2015;22(3):1163–1168. [Google Scholar]
- Yoo KM, Lee KW, Park JB, Lee HJ, Hwang IK. Variation in major antioxidants and total antioxidant activity of Yuzu (Citrus junos Sieb ex Tanaka) during maturation and between cultivars. J Agric Food Chem. 2004;52:5907–5913. doi: 10.1021/jf0498158. [DOI] [PubMed] [Google Scholar]