Abstract
The aim of this study was to characterize 43 genotypes from five yam species [Dioscorea rotundata (Poir), Dioscorea alata (Linn), Dioscorea bulbifera (Linn), Dioscorea cayenensis (Lam) and Dioscorea dumetorum (Kunith) Pax] which are major land races in Nigeria in terms of their chemical composition, nutritional, anti-nutritional and mineral bioavailability. Findings showed that there was genotypic variation in terms of chemical composition, mineral profile and bioavailability of the minerals among the germplasm. D. bulbifera had the highest cell wall carbohydrates, (cellulose: 3.2%, hemicelluloses, 2.1%, lignin, 1.1%, acid detergent fibre (ADF) 3.2%, neutral detergent fibre (NDF) 6.4%), D. rotundata had the highest oxalate (606 mg/kg). In conclusion, intra and inter-species variations exist among the yam germplasm in terms of their chemical composition, anti-nutritional and mineral bioavailability. Phytate content of the yam genotypes did not affect the bioavailability of Zn but Ca was affected significantly. The Ox:Ca ratio in most of the yam varieties were below one, thus bioavailability of Ca in yam by oxalate is variety dependent.
Keywords: Yam specie, Bioavailability, Minerals, Anti-nutritional, Chemical composition
Introduction
Yams produce edible starchy storage tubers which are of cultural, economic and nutritional importance. In Nigeria, yam is highly prized because it is an economic and a ceremonial crop. However, the crop is still underutilized commercially though it has potential for enhanced value addition and can generate foreign exchange (Orkwor 1998). West Africa accounts for 91% of yam production in the world, while Nigeria accounts for 68% of the world’s annual total production of yams (50 million tonnes) (FAO 2009). Despite this, Nigeria is not the largest exporter of yam or yam products, and there exist very few products from yam in both local and International markets. In other words, yam utilization is limited to subsistence mode and very little is processed commercially. This may be adduced to lack of suitable raw materials; due to dearth of information on the food quality of the tubers [substantial effort is being put in research work directed towards increasing yam production, improving yam marketing system, breeding of disease resistant varieties (Bergh et al. 2012)] which can identify their industrial potentials and post harvest technologies that can be employed to utilize the tubers to minimize post-harvest losses. Food quality in yam include physico-chemical composition, nutritional (proximate, micronutrients, vitamins) and anti-nutritional factors (phytic acid, tannin, oxalate) which are inherent in the tuber and are significant in determining its utilization (Otegbayo et al. 2010). Characterization of major landraces in terms of their food quality will provide information on chemical, nutritional composition, functional and physicochemical properties of various yam species and varieties that are widely cultivated in the country and stimulate production of value added products through identification of potential end uses of the yam landraces.
Yam as a multi-species crop shows variation in its properties across species and varieties. Genotypic diversity of yams is wide, but very few reports are available on this diversity and variability in Nigerian yams. There have been reports on variability in chemical and physicochemical composition of yams from other countries; Jamaica (Muzac-Tucker et al.1993), Cote d’ivoire (Amani et al. 2004), Ethiopia (Tamiru et al. 2008), and Cameroon (Egbe and Treche 1984), but there is dearth of these information on Nigerian yam landraces. This research was focussed on characterizing genetic diversity in terms of chemical, nutritional, anti-nutritional composition, mineral profile and bioavailability in some major Nigerian yam species (D. rotundata, D. alata, D. bulbifera, D. cayenensis, and D. dumetorum).
Materials and methods
Materials
Forty-three varieties of yam from D. rotundata (27), D. alata (9), D. bulbifera (5), D. cayenensis (2), and D. dumetorum (2), species were used for this study. The materials were collected from two major yam growing ecological zones in Nigeria; FCT, Abuja (Paikonkore Lat 8°59′N long 7°2′E and Dobi Lat 9°4′N long 6°53″E), Oyo North (Kisi; Budo Gawe, Budo Sanni, Iwo Lat 7°40′N long 4°11′E) and were planted at the research farm of Bowen University, Iwo and harvested at maturity (number of cultivars of each species is in parenthesis).
Methods
Yam flour was prepared from the yam tubers by cutting off the proximal and distal ends of the tubers; the middle portion was used to prepare the flour for chemical analyses as described by the method of Lape and Treche (1994). Proximate analysis (moisture, crude protein and ash) were determined by AOAC procedures (AOAC 2012). Starch and Sugar content of the yam samples were assayed by the method of Dubois et al. (1956) and Mcready (1970). Non-starchy polysaccharides: insoluble dietary fiber was quantified as hemicelluloses, cellulose and lignin by the AACC (1992) method. Phytic acid was extracted and precipitated by the method of Wheeler and Ferrel (1971). Tannin was determined spectrophotometrically by the acidified vanillin method as modified by Chang et al. (1994). Oxalate was determined by the titrimetric method of AOAC (2012). Mineral analysis: potassium, calcium, iron, aluminum, zinc, cadmium, copper, magnesium, manganese, phosphorus, lead and selenium were determined according atomic emission spectrometry (ICPAES) method (Zarcinas et al. 1987). All analyses were done in triplicates and results are presented on dry weight basis (DWB). All reagents were analytical grades.
Statistical analysis
Data was analyzed using the SAS package (Statistical Analysis Systems of SAS Institute, Inc) Analysis of variance and means separations was calculated by the general linear models procedure (GLM). Clustering analysis was performed using PROC CLUSTER of SAS with Ward’s minimum variance method (Ward 1963). The SAS PROC TREE was used to generate the dendrogram. Principal component analysis (PCA) biplot of yam chemical components was carried out with PAST version 2.17c software using correlation matrix for between group variation method (Hammer et al. 2001).
Results and discussion
Chemical composition
The chemical composition of the yam genotypes in each species are presented on dry weight basis (DWB) in Table 1. The moisture content, protein, ash, sugar and starch in the yam genotypes ranged between 10–13.29, 3.2–8.71, 2.29–6.69, 0.77–4.6 and 49.57–86.73% respectively. D. alata (TDa) had the highest moisture content (12.58%) while D. rotundata (TDr) had the lowest mean (11%), D. bulbifera (TDb) and D. cayenensis (TDc) had 11.91 and 11.24% respectively. The moisture contents of these yams indicate that they will have high dry matter (DM) which is an important indication of good eating and textural quality in food products from root and tuber crops (Izutsu and Wani 1985).
Table 1.
Chemical composition of yam germplasm (%)
| MC | Protein | Ash | Sugar | Starch | NDF | ADF | Lig | Cellu | H.Cell | |
|---|---|---|---|---|---|---|---|---|---|---|
| TDb** | ||||||||||
| TDb 3079 | 10.58e | 5.69c | 3.91c | 0.77d | 52.17c | 6.70c | 3.24c | 1.10b | 3.36c | 2.15c |
| TDb 3084 | 12.68a | 5.89b | 5.29a | 1.56c | 69.98a | 7.14a | 3.42a | 1.20a | 3.72a | 2.23a |
| TDb 3072 | 11.68d | 5.23d | 3.65d | 3.86a | 54.34bc | 5.66d | 2.97c | 1.05bc | 2.69d | 1.92e |
| TDb 3086 | 12.21c | 4.60e | 4.31b | 2.66b | 70.64a | 6.88d | 3.36b | 1.15a | 3.52b | 2.21b |
| TDb 3069 | 12.42b | 8.71a | 4.43b | 3.15b | 57.78b | 5.62d | 3.04d | 1.04c | 2.59e | 2.00d |
| Mean | 11.91 b | 6.02 b | 4.32 b | 2.40 a | 60.97 a | 6.40 b | 3.20 b | 1.11 b | 3.19 b | 2.10 b |
| TDr*** | ||||||||||
| Gbongi | 11.12ghi | 5.58a | 3.22bc | 1.18lm | 86.73a | 6.22a | 2.95b | 1.54a | 3.27a | 1.42c |
| Mumuyi | 10.42nop | 5.27bc | 3.38a | 1.66hijk | 65.06ijk | 3.28ef | 1.33ijk | 0.48ghij | 1.97de | 0.85lm |
| Suba | 10.62lmno | 4.64ij | 3.50a | 1.64hijkl | 62.60kl | 2.67ij | 1.34ij | 0.00o | 1.33jkl | 1.34d |
| Kangan | 10.88ijkl | 4.40k | 3.60a | 2.46efg | 78.20b | 3.72d | 1.69gh | 0.63e | 2.04de | 1.06j |
| DanachaAbbja | 10.89ijkl | 4.41k | 3.60a | 1.33jklm | 60.94lm | 3.73d | 1.78fg | 0.43ljk | 1.96de | 1.35d |
| Adaka | 11.70cd | 4.32kl | 2.37jk | 2.94bcd | 66.38ij | 2.06l | 1.03o | 0.00o | 1.23klm | 1.03j |
| Gwari | 10.66klmn | 4.85gh | 2.64ghi | 1.44ijklm | 64.98ljk | 2.52jkl | 1.39i | 0.26mn | 1.13m | 1.13hi |
| Godiya | 10.62klm | 4.85gh | 2.80efgh | 1.01m | 64.90ijk | 6.31a | 3.10a | 1.04bc | 3.21a | 2.06a |
| Meccakwsa | 11.88cd | 5.54a | 2.29k | 1.32jklm | 66.74hij | 3.23efg | 1.21klm | 0.58efg | 2.02de | 0.63o |
| Akwuki | 11.57de | 5.19cd | 3.20c | 2.01fgh | 77.56bc | 2.41jkl | 1.17lmn | 0.27m | 1.24klm | 0.90l |
| Gbinra | 11.33efg | 4.60a | 2.82defg | 3.17b | 63.94jkl | 4.17c | 1.88f | 1.05bc | 2.29c | 0.83m |
| Mailemu | 12.82a | 3.96m | 2.44ijk | 1.73hij | 63.68jkl | 5.27b | 2.52c | 0.96cd | 2.75b | 1.56b |
| Orin | 11.35efg | 5.20cd | 2.60hi | 2.59de | 72.89def | 4.15c | 2.48c | 0.88d | 1.68gh | 1.60b |
| Yangbede | 10.26pa | 4.22l | 2.94def | 0.97m | 72.42def | 3.61d | 1.64h | 0.60ef | 1.97de | 1.05j |
| Ameh Kisi | 11.01hij | 5.13de | 3.02cd | 1.99gh | 71.89def | 2.96gh | 1.37ij | 0.17n | 1.59hi | 1.20efg |
| Jibo | 11.10ghi | 4.55j | 2.50ijk | 3.07bc | 73.07de | 3.17fg | 1.25jkl | 0.50fgh | 1.92def | 0.75n |
| Amula | 11.19fgh | 4.07m | 3.21bc | 1.89hi | 78.12bc | 5.40b | 2.27d | 0.94d | 3.13a | 1.33d |
| Lagos | 10.36op | 4.07m | 3.17c | 2.48def | 67.78ghi | 3.46de | 1.57h | 0.33lm | 1.90ef | 1.24e |
| Omi efun | 9.96r | 5.03ef | 2.47ijk | 2.51def | 66.60hij | 3.70d | 1.69gh | 0.54efgh | 2.02de | 1.15gh |
| Pepa | 10.57mno | 5.38a | 2.50ijk | 1.37jklm | 69.73fgh | 4.17c | 2.09e | 0.88d | 2.08d | 1.21ef |
| Ehorbia | 10.39nop | 4.82h | 2.73fgh | 1.75hij | 70.70efg | 2.06e | 1.10b | 2.28c | 0.96k | 0.96k |
| Coach | 12.51b | 4.69i | 3.02cde | 1.3klm | 59.14mn | 1.08no | 0.00o | 1.21lm | 1.08w | 1.08w |
| Olotan | 10.00qr | 4.30kl | 2.49ijk | 2.89bcde | 56.67n | 1.10mno | 0.37kl | 1.77fg | 0.73n | 0.73n |
| Oginni | 10.04qr | 4.23l | 2.58hij | 3.31b | 70.72efg | 1.30ijk | 0.00o | 1.27jklm | 1.30d | 1.30d |
| Danacha kisi | 11.48de | 4.27l | 2.89def | 3.26b | 75.01cd | 3.10a | 1.08b | 3.18a | 2.02a | 2.02a |
| Boki | 11.39ef | 4.96fg | 3.43ab | 2.66cde | 61.69lmn | 1.57h | 0.40jkl | 1.39jk | 1.17gh | 1.17gh |
| Ameh abj | 10.76jklm | 5.00f | 3.01cde | 4.61a | 74.05d | 1.64h | 0.48hijk | 1.43ij | 1.18efgh | 1.18efgh |
| Mean | 11.00 a | 4.76 a | 2.91 a | 2.17 a | 68.97 b | 3.73 a | 1.76 a | 0.57 a | 1.97 a | 1.18 a |
| TDa | ||||||||||
| Kesofunfun | 12.81c | 4.01cd | 4.62b | 3.14bc | 65.11cd | 3.78c | 2.10c | 0.87c | 1.68d | 1.34c |
| Sharmabulu | 12.47c | 3.21f | 4.04b | 2.10d | 73.36a | 2.40h | 1.54f | 1.04a | 0.86 u | 0.72g |
| TDa 291 | 12.63bc | 3.82de | 4.37b | 3.54b | 49.58f | 2.67c | 2.30b | 0.70f | 1.37f | 1.43b |
| Boko | 12.72bc | 6.73a | 4.32b | 1.02f | 60.80e | 4.74a | 2.85a | 0.96b | 1.89c | 1.81a |
| Ogunawatan | 12.33c | 5.11b | 4.29b | 2.11d | 63.38de | 3.47f | 1.93d | 0.00h | 1.54e | 1.23d |
| Olesunle | 12.16c | 4.96b | 6.69a | 2.85c | 70.97ab | 3.66e | 2.14c | 0.64g | 1.53e | 1.18e |
| TDa 93-36 | 12.29a | 3.32f | 4.30b | 4.14a | 67.70bc | 2.46g | 1.33g | 0.85cd | 1.15g | 1.31c |
| SharmaGd | 13.14ab | 3.38ef | 4.26b | 1.52e | 66.87bcd | 3.74d | 1.73c | 1.44a | 2.01b | 1.09f |
| TDa 92-2 | 12.28c | 4.35c | 3.81b | 4.12a | 66.91bcd | 4.25b | 1.93d | 0.55b | 2.32g | 1.09f |
| Mean | 12.58 c | 4.32 a | 4.41 b | 2.72 a | 64.96 ab | 3.57 a | 1.98 a | 0.74 a | 1.59 a | 1.24 a |
| TDc | ||||||||||
| Igangan | 11.67a | 7.15a | 2.64a | 4.40a | 61.20a | 4.40 | 2.48 | 1.02 | 1.92 | 1.46 |
| TDC 25-294 | 10.82b | 4.55b | 2.70a | 1.96b | 72.11a | 1.96 | 0.92 | 0.00 | 1.04 | 0.92 |
| Mean | 11.24 a | 5.85 b | 2.67 a | 2.15 a | 66.65 ab | 3.18 a | 1.70 a | 0.51 a | 1.48 a | 1.19 a |
Means of parameters are written in bold letters
Means with the same superscripts in the same column in each species are not significantly different at 5% level of significance
All analyses on dry weight basis (DWB)
MC moisture content, DF dietary fiber, NDF neutral detergent fiber, Lig lignin, Cellu cellulose, H.Cell hemicellulose
** TDb tropical Dioscorea bulbifera, TDa tropical Dioscorea alata, TDc tropical Dioscorea cayenensis
*** ND in TDc Iganga and TDc 25-294 means—not determined
Protein content decreased along the species as follows: TDb (6.02%) > TDc (5.85%) > TDr (4.76%) > TDa (4.32%) though there were significant variations among the cultivars in each species (Table 1). For example in TDb species, var TDb 3069 had 8.71% protein which is comparable to 8.3% protein content reported on maize (Grajales-García et al. 2012). Protein contents of these yams were lower than reported for Sri Lankan D. alata (10.16%) (Senanayake et al. 2012); Ethiopian yams (9.7%) (Tamiru et al. 2008) and some Indian varieties of TDb (15.75%) (Shanthakumari et al. 2008). The protein contents were however higher than 4.03–6.52% reported for Ghanaian yams (Polycarp et al. 2012), 5.3% for Indonesian yams (Aprianita et al. 2014); 1.2–1.8% for cassava (Charles et al. 2005) and 5.6% reported for sweet potato (Moongngarm 2013).
In terms of their sugar and starch content, TDb had a mean value of 2.44 and 63.7%, TDr; 2.16 and 69.02%, TDa 2.73 and 64.98% and TDc 2.15 and 66.65% respectively. TDr had the highest mean value for starch, while TDb had the lowest (60.97%). There was no significant difference (p < 0.05) in the sugar content of the yam species. Sugar and starch contents of yam can influence the eating quality (taste), textural quality and preference of the yam varieties by the consumers. The high starch content of these yam species may also indicate that their starches have potential of being exploited for industrial use. The low sugar content of these yams may be explained by the fact that they were freshly harvested tubers. Sugar contents of fresh tubers have been reported to be lower than those in stored tubers because during storage starch is metabolized to sugar leading to concomitant reduction in starch content of the stored tubersas sugar content increases (Otegbayo et al. 2012). Significant differences (p < 0.05) that existed in the sugar and starch contents of the yams both among the species and within the varieties of the same species may be due to genotypic differences and physiological maturity of the tuber. Starch content of different plants can vary because of differences in the activity of enzymes involved in starch biosynthesis (Krossmann and Lloyd 2000). However, the sugar and starch contents of these yams were lower than values reported by previous authors; (Baah et al. (2009) and Afoakwa et al. 2013).
The results of non-starchy carbohydrate contents are presented in Table 1. TDb had the highest mean value for cell wall carbohydrates compared to the other species. Neutral detergent fiber (NDF) which measures the insoluble dietary fiber (lignin, cellulose and hemicelluloses) is also an indicator of bulk and feed intake was higher than acid detergent fiber (ADF) in the entire yam species studied. ADF measures the soluble dietary fiber (pectin, cellulose and acid-insoluble hemicelluloses), it is an indicator of digestibility and energy intake. The implication of this is that the yam tubers will have higher digestible energy (the higher the NDF, the higher the digestible energy whereas the higher the ADF the lower the digestible energy). NDF ranged from 1.08 to 7.14% in the yam varieties with a mean of 6.40% in TDb, 3.73% in TDr, 3.57 and 3.17% in TDa and TDc respectively. Lignin content was between 0.17 and 1.77% in the yam species. TDa and TDc had significantly lower lignin content than TDb. Lignins are phenyl propanoid polymers of varying molecular weight which confers rigidity and toughness to cell walls of plants (Cho et al. 1997). Cellulose content ranged from 1.04 to 3.74% among the yam varieties with an average of 3.19% in TDb, 1.97, 1.59, and 1.48% in TDd, TDr, TDa, and TDc respectively. Hemicellulose content in the yam species ranged from 1.18% in TDr to 2.10% in TDd. Hemicelluloses consist of rigid highly branched rod-shaped polymers of neutral sugars xylan and xyloglucan, which is linked with cellulose, lignin and pectin by hydrogen bonding provides structural strength to cell plant cell walls. Otegbayo et al. (2011) reported that these non-starchy carbohydrates (lignin, cellulose, hemicelluloses) contributed significantly to the formation of firm and doughy texture of pounded yam made from both fresh and stored D. rotundata varieties. The high content of non-starchy carbohydrates in TDb may also indicate that it has high dietary fiber, it may be an indication that it will have higher resistant starch compared to the yam species, hence may have the potential to function as a functional food.
Generally, in terms of chemical composition amongst the yam species studied, TDb which is not the most preferred species had the highest protein, sugar, ash and non-starchy carbohydrates hence it seemed to be having a better nutritional profile amongst the species, while TDr (the most preferred species and highly prized) had the highest starch content (this may be responsible for the preference of the textural quality of its food product). There were both intra (between cultivars) and interspecies variations (among species) in the chemical composition of the yam germplasm; interspecies and intraspecies variation had also been reported in yams by Asiedu (1986) and Dansi et al. (2013).
Characterization of yam tubers based on their chemical composition
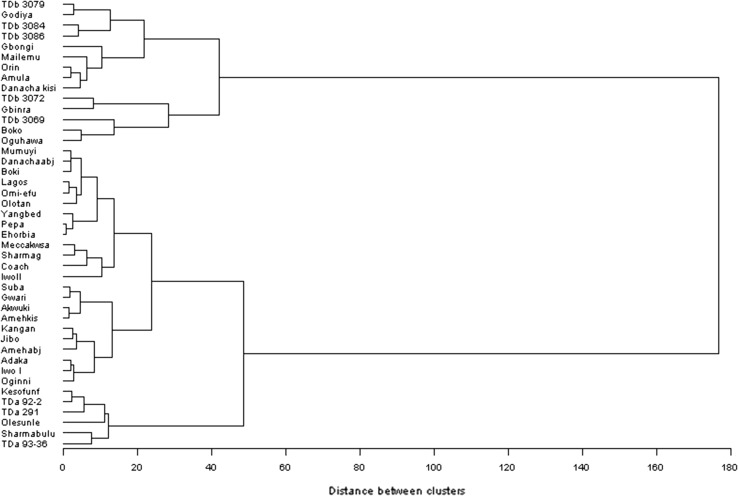
Principal component analysis was performed to characterize the yam germplasm based on their chemical composition; three homogenous clusters emerged (Fig. 1) and their dendrograms also revealed the three clusters clearly. Cluster I consist exclusively of D. alata species which are distinctly different from other yam species in terms of their sugar, ash and moisture contents. Cluster II consist distinctly of cultivars of D. rotundata and D. cayenensis the distinguishing feature of this cluster is their starch and dry matter content. The presence of D. rotundata and D. cayenensis exclusively in this group further reinstate earlier morpho-botanical and chemo-taxonomic studies which grouped these yam species into D. cayenensis-rotundata complex (Martin and Rhodes 1978; Tamiru et al. 2007). Cluster III consist of a mixture of cultivars from the three yam species: D. rotundata D. bulbifera and two cultivars of D. alata. The distinguishing chemical composition of this cluster is their non-starchy carbohydrate, protein and starch contents. From Table 2, among the yam species the first two components have Eigen values greater than 1 and accounted for 95.33% of the total variation; the first principal component accounted for 64.03% while the second accounted for 31.30% of variation in chemical composition among the yam species. Principal Component 1 recorded high positive loading for %ADL (0.3724) and %ADF (0.3591) while starch had a high negative loading (− 0.3617). However, for principal component 2, sugar (− 0.4544) and percentage moisture (− 0.4087) had high negative loadings while protein (0.4315) had a positive loading. This suggests that percentage moisture content and sugar content of yam species are closely related and highly correlated while yam species with reduced moisture content have higher protein content.
Fig. 1.
Dendrogram showing characterization of D. bulbifera, D. rotundata, and D. alata in terms of their chemical composition
Table 2.
Principal component analysis of yam varieties studied showing parameters contributing to variation in chemical composition and the percentage of total variation
| Chemical components | PC1 (64.03%) | PC2 (31.30%) |
|---|---|---|
| %MC | 0.2384 | − 0.4087 |
| Protein | 0.1165 | 0.4315 |
| Ash | 0.3240 | − 0.2703 |
| Sugar | 0.2026 | − 0.4544 |
| Starch | − 0.3617 | − 0.0275 |
| %NDF | 0.3375 | 0.2217 |
| %ADF | 0.3591 | 0.1613 |
| Cellulose | 0.3039 | 0.2702 |
| Hemicellulose | 0.3445 | 0.2181 |
Parameters with high negative loadings in both Principal components 1 and 2 (PC1 and PCI 2) are in italics
Anti-nutritional composition
The anti-nutritional compositions tannin, phytate and oxalate of the yam tubers are presented in Table 3. Tannin content of the tubers ranged from 56 to 1970 mg/kg on dry matter basis (DM) Significant differences (p < 0.05) existed in terms of tannin content among the species and among varieties within species. Tannins are water soluble phenolic compounds which precipitate protein by binding them irreversibly thus decreasing their digestibility and palatability. The reported lethal dose of tannin in plants is 7.6–9.0 g/kg (Alecto 1993). The tannin content reported in these yam tubers were lower than what was reported by Adeyeye et al. (2000) but was within the tolerable limits. Since yam is usually consumed in the cooked form, the tannin content would have been reduced during food processing before consumption, as a result of thermal degradation, denaturation and formation of insoluble complexes (Akin-Idowu et al. 2008).
Table 3.
Anti-nutritional composition, molar ratios (mol/kg) of calcium and zinc to phytate and molar ratios (mol/kg) calcium to oxalate
| Species/varieties | Oxalate (mg/kg) | Tannin (mg/kg) | Phytate (mg/kg) | {Ca}1 | {Zn}2 | {Phy}3 | {Ox}4 | Ca:Phy5 | Phy:Zn6 | {ca}{Phy}/{zn}7 | {Ox}:{ca}8 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| D. bulbifera | |||||||||||
| TDb 3079 | 632 | 884 | 458.03 | 10.28 | 0.17 | 0.79 | 7.03 | 14.81 | 4.20 | 0.43 | 0.68 |
| TDb 3084 | 484 | 1134 | 458.03 | 10.75 | 0.27 | 0.69 | 5.38 | 15.50 | 2.58 | 0.28 | 0.50 |
| TDb 3072 | 484 | 1573 | 307.21 | 10.96 | 0.22 | 0.47 | 5.38 | 23.55 | 2.09 | 0.23 | 0.49 |
| TDb 3086 | 633 | 1348 | 167.37 | 16.22 | 0.14 | 0.25 | 7.03 | 63.96 | 1.75 | 0.28 | 0.43 |
| TDb 3069 | 606 | 984 | 506.08 | 10.87 | 0.19 | 0.77 | 6.74 | 14.18 | 4.08 | 0.44 | 0.61 |
| Mean | 568.07 | 1187.6 | 379.35 | 11.82 | 0.20 | 0.57 | 6.31 | 26.40 | 2.94 | 0.33 | 0.55 |
| SD | 77.19 | 280.75 | 140.16 | 2.47 | 0.05 | 0.21 | 0.86 | 21.34 | 1.13 | 0.10 | 0.10 |
| D. rotundata | |||||||||||
| Gbongi | 631 | 104 | 261.79 | 1.89 | 0.15 | 0.40 | 7.02 | 4.76 | 2.65 | 0.05 | 3.72 |
| Mumuyi | 661 | 395 | 320.86 | 9.36 | 0.15 | 0.49 | 7.35 | 19.25 | 3.28 | 0.31 | 0.78 |
| Suba | 611 | 506 | 483.88 | 7.83 | 0.10 | 0.73 | 6.79 | 10.68 | 7.24 | 0.57 | 0.87 |
| Kangan | 625 | 143 | 350.84 | 4.14 | 0.22 | 0.53 | 6.95 | 7.78 | 2.38 | 0.10 | 1.67 |
| Danacha abuja | 571 | 144 | 350.84 | 7.86 | 0.19 | 0.53 | 6.35 | 14.78 | 2.8 | 0.22 | 0.80 |
| Adaka | 679 | 77 | 273 | 5.25 | 0.16 | 0.41 | 7.55 | 12.68 | 2.52 | 0.13 | 1.44 |
| Gwari | 612 | 427 | 674.58 | 7.49 | 0.12 | 1.02 | 6.81 | 7.33 | 8.21 | 0.61 | 0.91 |
| Godiya | 606 | 301 | 439.90 | 6.44 | 0.27 | 0.67 | 6.73 | 9.67 | 2.50 | 0.16 | 1.05 |
| Meccakwsa | 615 | 191 | 85.23 | 9.20 | 0.15 | 0.13 | 6.83 | 71.23 | 0.8 | 0.08 | 0.74 |
| Akwuki | 627 | 313 | 528.25 | 4.34 | 0.14 | 0.80 | 6.97 | 5.43 | 5.54 | 0.24 | 1.60 |
| Gbinra | 609 | 1971 | 106.53 | 12.99 | 0.15 | 0.16 | 6.77 | 80.47 | 1.06 | 0.14 | 0.53 |
| Mailemu | 599 | 840 | 185.6 | 3.36 | 0.14 | 0.28 | 6.66 | 11.93 | 2.03 | 0.07 | 1.98 |
| Orin | 671. | 606 | 237.84 | 6.55 | 0.20 | 0.36 | 7.46 | 18.17 | 1.79 | 0.12 | 1.14 |
| Yangbede | 487 | 155 | 242.22 | 7.46 | 0.17 | 0.37 | 5.41 | 20.33 | 2.16 | 0.16 | 0.73 |
| Ameh Kisi | 613 | 59 | 598.28 | 8.89 | 0.14 | 0.91 | 6.81 | 9.81 | 6.53 | 0.58 | 0.77 |
| Jibo | 647 | 389 | 223.63 | 4.52 | 0.21 | 0.34 | 7.19 | 13.32 | 1.61 | 0.07 | 1.59 |
| Amula | 554 | 473 | 215.11 | 3.12 | 0.18 | 0.33 | 6.16 | 9.57 | 1.83 | 0.06 | 1.97 |
| Lagos | 610 | 155 | 227.82 | 4.98 | 0.12 | 0.35 | 6.78 | 14.43 | 2.83 | 0.14 | 1.36 |
| Omi efun | 618 | 429 | 123.40 | 2.48 | 0.11 | 0.19 | 6.87 | 13.25 | 1.77 | 0.04 | 2.77 |
| Pepa | 640 | 303 | 249.01 | 3.33 | 0.16 | 0.38 | 7.12 | 8.82 | 2.33 | 0.08 | 2.13 |
| Ehorbia | 660 | 208 | 209.74 | 6.28 | 0.13 | 0.32 | 7.33 | 19.78 | 2.39 | 0.15 | 1.16 |
| Coach | 499 | 297 | 383.13 | 7.22 | 0.14 | 0.58 | 5.55 | 12.43 | 4.05 | 0.29 | 0.76 |
| Olotan | 642 | 56 | 246.38 | 5.70 | 0.18 | 0.37 | 7.13 | 15.26 | 2.11 | 0.12 | 1.25 |
| Oginni | 493 | 59 | 246.38 | 6.48 | 0.15 | 0.37 | 5.48 | 17.36 | 2.55 | 0.17 | 0.84 |
| Danacha kisi | 583 | 407 | 57.05 | 2.97 | 0.22 | 0.09 | 6.48 | 34.33 | 0.40 | 0.01 | 2.18 |
| Boki | 582 | 194 | 325.29 | 10.03 | 0.25 | 0.49 | 6.47 | 20.36 | 1.96 | 0.20 | 0.64 |
| Ameh abuja | 624 | 330 | 308.41 | 30.72 | 0.22 | 0.47 | 6.93 | 65.73 | 2.15 | 0.66 | 0.23 |
| Mean | 606.49 | 353.90 | 294.63 | 7.07 | 0.17 | 0.45 | 6.74 | 20.33 | 2.87 | 0.20 | 1.32 |
| SD | 50.12 | 374.22 | 149.13 | 5.41 | 0.04 | 0.23 | 0.56 | 18.54 | 1.89 | 0.18 | 0.77 |
| TDa | |||||||||||
| Kesofunfun | 502 | 660 | 52.88 | 8.67 | 0.095 | 0.08 | 5.59 | 108.16 | 0.84 | 0.07 | 0.64 |
| Sharmabulu | 641 | 238 | 146.12 | 5.03 | 0.14 | 0.22 | 7.13 | 22.67 | 1.58 | 0.08 | 1.41 |
| TDa 291 | 646 | 101 | 112.13 | 10.21 | 0.14 | 0.17 | 7.18 | 60.11 | 1.21 | 0.12 | 0.70 |
| Boko | 565 | 386 | 655.03 | 7.20 | 0.19 | 0.99 | 6.29 | 7.26 | 5.06 | 0.36 | 0.87 |
| Ogunawatan | 600 | 472 | 795.30 | 7.52 | 0.25 | 1.21 | 6.67 | 6.24 | 4.87 | 0.37 | 0.89 |
| Olesunle | 622 | 290 | 256.32 | 5.92 | 0.13 | 0.39 | 6.91 | 15.25 | 2.91 | 0.17 | 1.17 |
| TDa 93-36 | 649 | 362 | 115.00 | 8.39 | 0.12 | 0.17 | 7.21 | 48.15 | 1.49 | 0.13 | 0.86 |
| SharmaGd | 507 | 358 | 158.76 | 13.05 | 0.16 | 0.24 | 5.63 | 54.26 | 1.50 | 0.20 | 0.43 |
| TDa 92-2 | 604 | 258 | 144.54 | 9.99 | 0.09 | 0.22 | 6.71 | 45.60 | 2.49 | 0.25 | 0.67 |
| Mean | 593.26 | 328.40 | 270.68 | 8.94 | 0.15 | 0.41 | 6.59 | 40.86 | 2.44 | 0.19 | 0.85 |
| SD | 56.66 | 171.90 | 265.50 | 2.43 | 0.05 | 0.40 | 0.63 | 32.54 | 1.56 | 0.11 | 0.30 |
| TDd | |||||||||||
| Esuru pupa | 590 | ND | ND | 20.65 | 0.16 | ND | ND | ND | ND | ND | |
| 04-146 | ND | ND | ND | 17.95 | 0.12 | ND | ND | ND | ND | ND | 0.32 |
| TDc | |||||||||||
| Igangan | 494 | 103 | 322.47 | 10.00 | 0.13 | 0.37 | 5.49 | 7.38 | 3.20 | 0.12 | 2.03 |
| TDC 95-294 | 660.00 | 430 | 242.098 | 3.61 | 0.15 | 0.49 | 7.33 | 27.26 | 2.75 | 0.28 | 0.55 |
| Mean | 577.17 | 264.20 | 282.28 | 6.80 | 0.14 | 0.43 | 6.41 | 17.32 | 2.98 | 0.20 | 1.29 |
| SD | 117.14 | 227.78 | 56.83 | 5.06 | 0.08 | 0.25 | 1.30 | 14.06 | 0.32 | 0.11 | 1.05 |
| CVa | 6:1 | 15:1 | 0.5 | 1.1 | |||||||
Means of parameters are written in bold letters
Standard deviations (SD) of parameters are in italics
1mg of Ca/Mw (molecular weight),2 mg of Zn/MW of Zn, 3 mg of Phy/MW of phy, 4 mg of Ox/MW of Ox, 5 mg of Ca/MW/mg of Phy/MW, 6 mg of Phy/MW/Zn/MW, 7 (mol/kg Ca)(mol/kg phy)/(mol/kg Zn), 8 (mol/kg Ox)/(mol/kg Ca)
aCV is recommended critical value, ND not determined
The phytate in these yams ranged from 270.65 to 379.35 mg/kg DM. The phytate content of the yam tubers differed significantly (p < 0.05) among the species and between varieties in a species. Phytic acid (myoinositol (1, 2, 3, 4, 5, 6) hexakiphosphoric acid) is the storage form of phosphorus which is found in plant seeds and in many roots and tubers (Kumar et al. 2010). It has the ability to form insoluble complexes with positively charged food components such as protein, carbohydrate, minerals and trace elements. These complexes lead to reduced bioavailability of minerals such as calcium, zinc and iron, and formation of protein complexes which are resistant to protein proteolysis, alteration in their structure, decrease in protein solubility, digestibility and enzymatic activities (Shajeela et al. 2011). Food processing steps such as germination, fermentation, soaking, cooking or hydrothermal processing can reduce phytate content by dephosphorylation (removal of phosphate groups from the inositol ring which decreases the mineral binding strength of phytate).
Oxalate content of the yam tubers ranged between 487 and 671 mg/kg with an average of 568.07 mg/kg in TDb, 606 mg/kg in TDr, 593.26 mg/kg in TDa and 577 mg/kg in TDc. Oxalate is present in yam tubers in form of soluble oxalate, insoluble calcium oxalate (raphides) or a combination of the two forms. The intense irritation of the skin and mucous membrane when they come in contact with yam mucilage is due to the presence of calcium oxalate crystals (raphides). The reported lethal dose of oxalate in food is 2–5 g (Eka 1985). The oxalate content of these yam tubers does not pose a health hazard, because various processing methods that the yam tubers will be subjected to such as washing, boiling, blanching, roasting and other processing before consumption would have reduced the level of the oxalate significantly (Sakai 1979; Kumoro et al. 2014).
Mineral composition
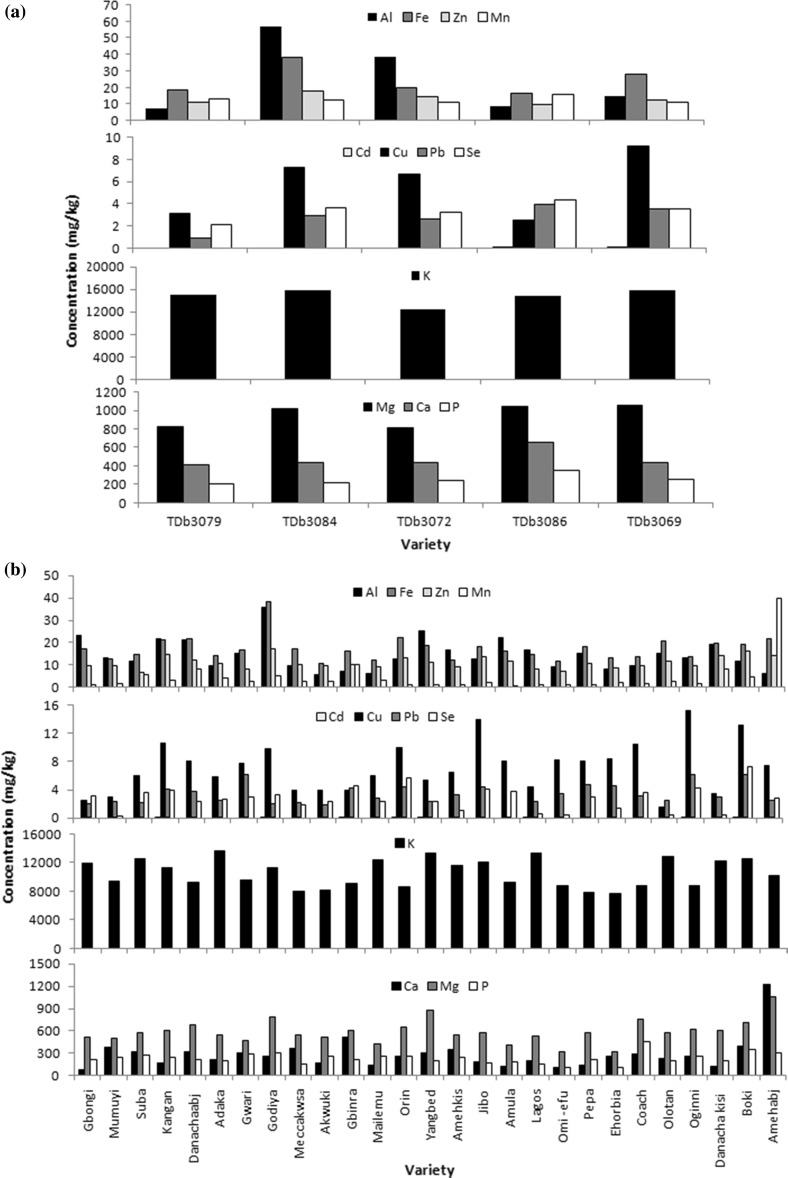
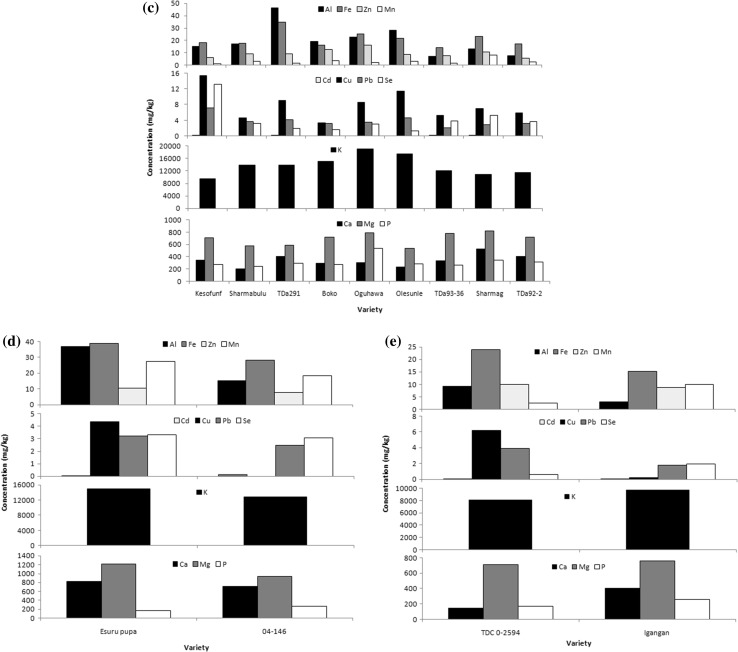
The mineral profile of the yam species are presented in Fig. 2a–e (supplementary file) twelve minerals; Al, Fe, Zn, Ca, Cd, Cu, K, Mg, Mn, P, Pb, and Se were determined. The result showed that these yam species are relatively good sources of both macro minerals (Ca, P, K and Mg) and micro minerals (Fe, Cu, Zn, Se and Mn) which are significant nutritionally. There were both intra species and interspecies variation in the mineral content of the yams. Generally, variations in mineral composition have been adduced to factors such as genetic component, environment effects, methods of estimation, cultural practices, time of planting and harvesting, chemical composition of the soil in which they were grown and amount of water available (Oluwatosin 1998; Eka 1985). Though the minerals were planted on the same soil, variation in the mineral contents of these yams may be due to genotypic differences and chemical composition of the soil.
Fig. 2.
Mineral composition of yam tubers. a Mineral composition of D. bulbifera. b Mineral composition of D. rotundata. c Mineral composition of D. alata. d Mineral composition of D. dumetorum. e Mineral composition of D. cayenensis
The most abundant mineral in all the yam species was Potassium. It ranged between 7.75 × 102 and 1.89 × 103 mg/kg among the varieties. This agreed with previous results (Polycarp et al. 2012; Baah et al. 2009). Potassium is a major intracellular cation which is involved in muscle contraction, transmission of nerve impulse and maintenance of fluid balance. TDb had the highest potassium content while TDr had the least. Iron content ranged between 10.71 and 38.87 mg/kg among all the yam varieties. The iron content of yam species reported in this study was in the order TDd > TDb > TDa > TDc > TDr. It ranged between 10.71 mg/kg (Akwuki; TDr) and 38.87 mg/kg (Esuru pupa-TDd) among all the yam varieties. The recommended daily allowance (RDA) of iron is between 11 and 18 mg/day (USDA 2015); most of these yam varieties have iron contents that can meet this RDA. Iron is an important oxygen carrier in haemoglobin in the blood and part of cytochromes a, b and c which are essential to the production of cellular energy by oxidative phosphorylation. Zinc (Zn) functions as a dietary and nutrient supplement in the body. The zinc content of the yam tubers in this study ranged between 7.97 and 17.56 mg/kg, with the highest zinc content (on the average) found in TDb (12.93 mg/kg). The order of the Zn content of these yam species was TDb > TDr > TDa > TDc > TDd). The RDA for Zinc according to USDA (2015) is between 8 and 11 mg/day hence consuming 500 g of most of these yam varieties is able to meet this RDA. Zn is necessary for biosynthesis of nucleic acid, cell metabolism (cell division) and growth. However, the Zn content of these yam tubers may be affected the presence of phytate in the tubers, which may reduce the bioavailability of dietary Zn by forming insoluble mineral chelates, that is Zn-phytic complex which is poorly absorbed from the gastrointestinal tract.
Calcium (Ca) content ranged between 75.74 and 1228.70 mg/kg in the yam tubers. In TDr the range was 75.74–1228.70 mg/kg, TDa: 200–522.06 mg/kg, TDc: 144–400 mg/kg, TDd: 717.88–825.17 mg/kg, TDb: 411.23–648.76 mg/kg. The calcium content of these yam tubers were however higher than reported values for each species and significant differences (p < 0.05) exist between varieties in each species. The RDA for calcium is 1300 mg/day consumption of 1 kg of TDb by a male will contribute about 36% of the RDA on the average while, other species; TDr, TDa, TDc and TDd will contribute 21, 25, 35 and 43% of the RDA respectively. The implication of these results is that these yam tubers should be a good source of calcium. Calcium as an important macro mineral is an important constituent of the bone [hydroxyapatite: Ca10(PO4)6(OH)2] with phosphorus and the extracellular fluid of the body. It has also been implicated in significantly affecting the textural quality of food products. Otegbayo et al. (2012) stated that the high calcium and pectin content and other soluble dietary fibre in TDr might have been responsible for the smooth texture of pounded yam made from its tubers.
The phosphorus content of the yam tubers ranged from 108.38 to 537.74 mg/kg. The lower value of phosphorus in these yam tubers may be due to phytic acid in yams which binds the phosphorus and render it unavailable for biochemical and nutritional utilization. It could also be due to the fact that phytic acid and starch are capable of combining via phosphate linkages. The magnesium content and manganese ranged from 320.89 to 1213.4 and 0.76 to 39.69 mg/kg respectively among the yam varieties. Among the five yam species D. bulbifera had the highest magnesium content. Cu ranged from 0.21 to 15.40 mg/kg with D.alata (kesofunfun) having the highest and D. dumentorum (04-146) had the least. RDA of Cu is 0.9 mg/day (USDA 2015), most of these yam varieties are able to meet this RDA. It is an important cell protective mineral. Selenium is part of the antioxidant defense system-glutathione peroxidase (GPx) which eliminates peroxide radicals. Low concentrations of Se are inhibitors of cancer (Tolonen 1989) and it is a modulator of variety of cellular functions. It ranged between 0 and 13.62 mg/kg with the highest on the average (3.39 mg/kg) found in D. bulbifera and the least (2.75 mg/kg) found in D. rotundata. The RDA of Selenium is 0.055 mg/day (USDA 2015) consumption of 1 kg of these yams is able to supply more than the RDA of this mineral per day. Lead and cadmium are regarded as toxic minerals because they compete with essential minerals for pathways and bind proteins. The cadmium content of the yam tubers ranged between 0.05 and 0.18 mg/kg, while for Pb the mean was of 2.81 mg/kg in TDb, 3.25 mg/kg in TDr, 3.80 mg/kg in TDa, 2.84 mg/kg in TDc and 3.15 mg/kg in TDd. These values were lower than the maximum allowable limit of 0.2 mg/day for cadmium and 6 mg/kg for lead (USDA 2015). Cadmium competes with zinc for binding sites and interferes with some of zinc’s essential functions. Presence of lead and cadmium in these yam tubers may be as a result of Pb in the soil in which they were planted as a result of waste deposition, soil erosion, sludge, air pollution car fumes, and smoke.
Interrelationship between calcium, zinc, phytate and oxalate
The molar ratios for phytate, oxalate, zinc and calcium were calculated to evaluate the effects of elevated levels of oxalate and phytate on the bioavailability of these minerals (Table 3). Phytate has been reported to decrease the absorption of Ca and Zn by forming insoluble Ca–phytate or Zn–phytate complexes which inhibit the absorption of Zn and iron (Bhandari and Kawabata 2004). The molar ratio for Phy:Zn ranged between 0.40 and 7.24 with a mean of 2.98 in TDc, 2.94 in TDb, 2.87 in TDr, 2.44 TDa and TDb (Table 3). These molar ratios were however lower than 21.5–22.7 and 10–12.4 previously reported (Adeyeye et al. 2000). According to Turnlund et al. (1984), Phy:Zn molar ratio of 15:1 has been implicated in low Zn bioavailability, our result was lower than this critical value indicating that the bioavailability of Zn in these yam tubers was high and it was not likely to be affected by phytate. According to Sandstrom (1997), when Phy:Zn molar ratio in a food is less than 5 then the food has good Zn bioavailability. Ellis et al. (1987) reported that the ratio of [Ca] × [Phy]/Zn is a better predictor of Zn bioavailability. They stated that, when the [Ca] × [Phy]/Zn ratio is greater than 0.50 mol/kg, there would be interferences with the availability of Zn. The [Ca] × [Phy]/Zn ratio of most of the yam varieties were lower than this critical value (0.50 mol/kg) indicating good zinc bioavailability (Table 3). The critical value of 6:1 must not be exceeded in order for Ca to be bioavailable (Oladimeji et al. 2000). The Ca:Phy molar ratios of the yam studied ranged from 4.76 to 65.74. Our result (Table 3) indicated that most of these yams [except Gbongi and akwuki (D. rotundata)] have molar ratios higher than the critical value of Ca:Phy 6:1 indicating that the bioavailability of calcium may be adversely affected by phytates in these yam tubers.
Oxalate content of plant product can affect or limit calcium bioavailability significantly when the ratio of Ox:Ca exceeds one (i.e. > 1), a high Ox:Ca ratio may cause chronic deficiency of calcium (Kelsay 1985). From our results (Table 3), the Ox:Ca ratio in most of the yams cultivars were below one. In all TDb varieties the ratio was less than 1, only two varieties in D. alata were above 1 (Sharma bulu and Olesunle) and many (15 out of 27) in D. rotundata were higher than 1. TDd (esuru pupa) was less than 1 (0.32) while in TDc (Igangan) the ratio was also less than 1 except var Igangan. This implies that varieties in which the Ox:Ca > 1 will need to be extensively treated; soaked, washed and well cooked before consumption. It also showed that limitation of bioavailability of calcium by oxalate in yam tubers is not specie dependent but cultivar/variety dependent.
Conclusion
From the result of this study, it can be concluded that these yam genotypes were good sources of bioavailable Zn but bioavailable calcium is considerably low due to presence of phytate and oxalate which limited the bioavailability of some cultivars. The levels of anti-nutritional factors (oxalate, phytate and tannin) were below the lethal dose. D. bulbifera which is a less preferred species had better nutritional profile than D. rotundata (the most preferred specie). There were both intra (between cultivars) and inter-specie variations (among species) in the chemical composition of the yam germplasm. This study presents the first characterization of large variety of yams from five yam species from Nigeria in terms of their biochemical, antinutritional and mineral bioavailability. This will serve as database for getting information on these properties and to select yam genotypes for various uses.
Acknowledgements
This study was funded by International Fund for Agricultural Development (IFAD) under the IFAD/IITA TAG 704 project for funding this research work, Activity 5.5.
References
- AACC . Approved methods of the American Association of Cereal chemists. 8. St Paul: AACC; 1992. [Google Scholar]
- Adeyeye EI, Arogundade LA, Akintayo ET, Aisida OA, Alao PA. Calcium, Zinc and phytate interrelationships in some foods of major consumption in Nigeria. Food Chem. 2000;71:435–441. doi: 10.1016/S0308-8146(00)00159-X. [DOI] [Google Scholar]
- Afoakwa EO, Polycarp D, Budu AS, Mensah-Brown H, Otoo E. Variability in biochemical composition and cell wall constituents among seven varieties in Ghanaian yam (Dioscorea sp.) germplasm. Afr J Food Agric Nutr Dev. 2013;13:8106–8127. [Google Scholar]
- Akin-Idowu PE, Odunola OA, Asiedu R, Maziya-Dixon B, Uwaifo AO. Variation in nutrient and anti-nutrient contents of tubers from yellow yam (Dioscorea cayenensis) genotypes grown at two locations. J Food Agric Environ. 2008;6:95–100. [Google Scholar]
- Alecto VA. Allelochemicals in plant food and feeding stuffs: nutritional, biochemical and physiopathological aspects in animal production. Vet Hum Toxicol. 1993;35:57–67. [PubMed] [Google Scholar]
- Amani NG, Buleon A, Kamenan A, Colonna P. Variability in starch and physico-chemical and functional properties of yam (Dioscorea spp.) cultivated in Ivory Coast. J Sci Food Agric. 2004;84:2085–2096. doi: 10.1002/jsfa.1834. [DOI] [Google Scholar]
- AOAC . Official methods of analysis of the Association of Official Analytical Chemists. 19. Gaithersburg: AOAC; 2012. [Google Scholar]
- Aprianita A, Vasiljevic T, Bannikova A, Kasapis S. Physicochemical properties of flours and starches derived from traditional Indonesian tubers and roots. J Food Sci Technol. 2014;51:3669–3679. doi: 10.1007/s13197-012-0915-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asiedu JJ. Processing and physical/chemical properties of tropical products. Pfaffenweiler: Centaurus; 1986. Yams; pp. 379–398. [Google Scholar]
- Baah FD, Maziya-Dixon B, Asiedu R, Oduro I, Ellis WO. Nutrition and biochemical composition of D. alata tubers. J Food Agric Environ. 2009;7:373–378. [Google Scholar]
- Bergh K, Orozco P, Gugerty MK, Anderson L (2012) Yam value chain: Nigeria. A report prepared for the Agricultural Policy Team of the Bill and Melinda Gates Foundation, Evans School of Policy Analysis and Research Brief, p 207
- Bhandari MR, Kawabata J. Assessment of anti-nutritional factors and bioavailability of calcium and zinc in wild yam (Dioscorea spp.) tubers of Nepal. Food Chem. 2004;85:281–287. doi: 10.1016/j.foodchem.2003.07.006. [DOI] [Google Scholar]
- Chang MJ, Collins JL, Baily JW, Coffey DL. Tannins related to cultivar, maturity, dehulling, and heating. J Food Sci. 1994;59:1034–1036. doi: 10.1111/j.1365-2621.1994.tb08183.x. [DOI] [Google Scholar]
- Charles AL, Sriroth K, Huang TC. Proximate composition, mineral contents, hydrogen cyanide and phytic acid of 5 cassava genotypes. Food Chem. 2005;92:615–620. doi: 10.1016/j.foodchem.2004.08.024. [DOI] [Google Scholar]
- Cho S, Devries JW, Prosky L (1997) The chemistry of non-saccharide fiber component. In: Dietary fiber analysis and applications. AOAC International, Gaithersburg, p 31
- Dansi AH, Dantsey-Barry I, Dossou-Aminon EK, N’kpenu AP, Agré YD, Sunu K, Kombaté YL, Loko M, Dansi P, Assogba Vodouhè R. Varietal diversity and genetic erosion of cultivated yams (Dioscorea cayenensis Poir—D. rotundata Lam complex and D. alata L.) in Togo. Int J Biodivers Conserv. 2013;5:223–239. [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Egbe TA, Treche S. Variability in the Chemical Composition of Yams grown in Cameroon. In: Terry ER, Doku EV, Arene OB, Mahungu NM, editors. Tropical root crops: Production and uses in Africa. Ottawa, Canada: IDRC; 1984. pp. 153–157. [Google Scholar]
- Eka OU. The chemical composition of yam tubers. In: Osuji G, editor. Advances in yam research: the biochemistry and technology of the yam tuber. Awka: University of Technology Publishers; 1985. pp. 110–141. [Google Scholar]
- Ellis R, Kelsay JL, Reynolds RD, Morris ER, Moser PB, Frazies CW. Phytate:zinc and phytate, calcium: zinc mill molar ratios in self-selected diets of American, Asian, Indians and Nepalese. J Am Diet Assoc. 1987;87:1043–1047. [PubMed] [Google Scholar]
- FAO . Food and Agriculture Organization Statistics database—agriculture. Rome: FAOSTAT; 2009. [Google Scholar]
- Grajales-García EM, Osorio-díaz P, Goñi I, Hervert-Hernández D, Guzmán-Maldonado SH, Bello-Pérez LA. Chemical composition, starch digestibility and antioxidant capacity of tortilla made with a blend of quality protein maize and black bean. Int J Mol Sci. 2012;13:286–301. doi: 10.3390/ijms13010286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer O, Harper DAT, Ryan PD. Paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4:9. [Google Scholar]
- Izutsu T, Wani K. Food texture and taste: a review. J Texture Stud. 1985;16:1–28. doi: 10.1111/j.1745-4603.1985.tb00677.x. [DOI] [Google Scholar]
- Kelsay JL. Effects of oxalic acid on calcium bioavailability. In: Kies C, editor. Nutritional bioavailability of calcium. Washington: American Chemical Society; 1985. pp. 105–116. [Google Scholar]
- Krossmann JJ, Lloyd J. Understanding and influencing starch biochemistry. Crit Rev Biochem Mol Biol. 2000;35:141–196. [PubMed] [Google Scholar]
- Kumar V, Sinha AK, Harinde RPSM, Becker K. Dietary roles of phytate and phytase in human nutrition. Rev Food Chem. 2010;120:945–959. doi: 10.1016/j.foodchem.2009.11.052. [DOI] [Google Scholar]
- Kumoro AC, Budiyati CS, Retnowati DS. Calcium oxalate reduction during soaking of giant taro (Alocasia macrorrhiza (L.) Schott) corm chips in sodium bicarbonate solution. Int Food Res J. 2014;21:1583–1588. [Google Scholar]
- Lape MI, Treche S. Nutritional quality of yam (Dioscorea dumentorum and D. rotundata) flours for growing rats. J Sci Food Agric. 1994;66:447–455. doi: 10.1002/jsfa.2740660405. [DOI] [Google Scholar]
- Martin FW, Rhodes AM. The relationship of Dioscorea cayenensis and Dioscorea rotundata. Trop Agric (Trinidad) 1978;55:193–206. [Google Scholar]
- Mcready RM. Determination of starch and dextrins. In: Joslyn AM, editor. Methods in food analysis, a series of monographs. 2. New York: Academic Press; 1970. pp. 522–557. [Google Scholar]
- Moongngarm A. Chemical compositions and resistant starch content in starchy foods. Am J Agric Biol Sci. 2013;8:107–113. doi: 10.3844/ajabssp.2013.107.113. [DOI] [Google Scholar]
- Muzac-Tucker I, Asemota HN, Ahmad MH. Biochemical composition and storage of Jamaican yams (Dioscorea sp.) J Sci Food Agric. 1993;62:219–224. doi: 10.1002/jsfa.2740620303. [DOI] [Google Scholar]
- Oladimeji MO, Okafor AA, Akindahunsi AF. Investigation of the bioavailability of zinc and calcium from some tropical tubers. Nahrung. 2000;44:2829–2834. doi: 10.1002/(SICI)1521-3803(20000301)44:2<136::AID-FOOD136>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Oluwatosin OB. Genetic and environmental variability in starch, fatty acids, and mineral nutrients composition in cowpea (Vigna unguiculata (L) walp) J Sci Food Agric. 1998;78:1–11. doi: 10.1002/(SICI)1097-0010(199809)78:1<1::AID-JSFA47>3.0.CO;2-H. [DOI] [Google Scholar]
- Orkwor GC. The importance of yams. In: Orkwor GC, Asiedu R, Ekanayake IJ, editors. Food yams: advances in research. Ibadan: IITA; 1998. pp. 1–11. [Google Scholar]
- Otegbayo BO, Bokanga M, Asiedu R. Physico-chemical composition of yam starch: Effect on textural quality of yam food product (Pounded yam) J Food Agric Environ. 2011;9:145–150. [Google Scholar]
- Otegbayo BO, Samuel FO, Kehinde AL, Sangoyomi TE, Okonkwo CC. Perception of food quality in yam by some Nigerian farmers. Afr J Food Sci. 2010;4:541–549. [Google Scholar]
- Otegbayo BO, Asiedu R, Bokanga M. Effect of storage on chemical composition and food quality of yam. J Food Process Preserv. 2012;36:438–445. doi: 10.1111/j.1745-4549.2011.00600.x. [DOI] [Google Scholar]
- Polycarp D, Afoakwa EO, Budu AS, Otoo E. Characterization of chemical composition and anti-nutritional factors in seven species within the Ghanaian yam (Dioscorea) germplasm. Int Food Res J. 2012;19:985–992. [Google Scholar]
- Sakai WS. Ariod root crops, acridity and raphides. In: Charallambous G, Inglett GE, editors. Tropical foods, chemistry and nutrition. New York: Academic Press; 1979. pp. 265–278. [Google Scholar]
- Sandstrom B. Bioavailability of zinc. Eur J Clin Nutr. 1997;51:S4–S8. [PubMed] [Google Scholar]
- Senanayake SA, Ranaweera KKDS, Bamunuarachchi A, Gunaratne A. Proximate analysis phytochemical and mineral constituents in four cultivars of yams and tuber crops in Srilanka. Trop Agric Res Ext. 2012;15:32–36. doi: 10.4038/tare.v15i1.5240. [DOI] [Google Scholar]
- Shajeela PS, Mohan VR, Jesudas LL, Soris PT. Nutritional and antinutritional evaluation of wild yam (Dioscorea spp.) Trop Subtrop Agroecosyst. 2011;14:723–730. [Google Scholar]
- Shanthakumari S, Mohan VR, Britto John DE. Nutritional evaluation and elimination of toxic principles in wild yam (Dioscorea species) Trop Subtrop Agroecosyst. 2008;8:319–325. [Google Scholar]
- Tamiru M, Becker CH, Brigitte LM. Genetic diversity in yam germplasm from Ethiopia and their relatedness to the main cultivated Dioscorea species assessed by AFLP markers. Crop Sci. 2007;47:1744–1753. doi: 10.2135/cropsci2006.11.0719. [DOI] [Google Scholar]
- Tamiru M, Mass BL, Pawelzik E. Characterizing diversity in composition and pasting properties of tuber flour in yam germplasm (Dioscorea spp.) from Southern Ethiopia. J Sci Food Agric. 2008;88:1675–1685. doi: 10.1002/jsfa.3263. [DOI] [Google Scholar]
- Tolonen M. Finnish studies on antioxidants with special reference to cancer, cardiovascular diseases and aging. Int Clin Nutr Rev. 1989;9:68–75. [Google Scholar]
- Turnlund JR, King JC, Keyes WR, Gong B, Michel MC. A stable isotope study of zinc absorption in young men: effects of phytate and alpha-cellulose. Am J Clin Nutr. 1984;40:1071–1077. doi: 10.1093/ajcn/40.5.1071. [DOI] [PubMed] [Google Scholar]
- USDA (2015). Dietary Guidelines Advisory Committee. Scientific report of the Guidelines Advisory Committee. Washington (DC): USDA and US Department of Health and Human Services. https://health.gov/dietaryguidelines/2015guidelines/appendix-7. Accessed 6 Jan 2017
- Ward JH. Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 1963;58:236–244. doi: 10.1080/01621459.1963.10500845. [DOI] [Google Scholar]
- Wheeler EL, Ferrel RE. A method for phytic acid determination in wheat and wheat fractions. Cereal Chem. 1971;48:312–316. [Google Scholar]
- Zarcinas BA, Cartwright B, Spouncer LR. Nitric acid digestion and multi-element analysis of plant material by inductively coupled plasma spectrometry. Commun Soil Sci Plant Anal. 1987;18:131–146. doi: 10.1080/00103628709367806. [DOI] [Google Scholar]