Abstract
The bacterial kinetics and quality indexes [sensory quality, total volatile basic nitrogen (TVB-N), thiobarbituric acid value, biogenic amine, and amino acids] were analyzed on salmon inoculated with Pseudomonas fluorescens during storage under different temperatures (30, 10, and 4 °C). The bacterial kinetics revealed that P. fluorescens showed a steady growth at low temperatures (10 and 4 °C). The TVB-N yield factors of the sample stored at 4 °C indicated that each bacterial cell of P. fluorescens displayed greater spoilage activity at low temperatures. A remarkable correlation was found between the production of biogenic amines and bacterial counts. The results also highlighted that P. fluorescens cultured at 4 °C had higher demand for amino acids.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2916-x) contains supplementary material, which is available to authorized users.
Keywords: Salmo salar, Pseudomonas fluorescens, Bacterial kinetic, Spoilage potential, Amino acid degradation
Introduction
Salmon (Salmo salar) is one of the most important commercial fish species in the world, which is favored by consumers due to the good flavor and nutritional properties. In particular, salmon is rich in protein and low in cholesterol, but it may provide nutrients for microbial growth. In the process of cold chain logistics, the deterioration of salmon attribute to microbial growth and metabolism, (Gunsen et al. 2011). The dominant microorganisms contributing to the spoilage in raw aquatic products are called the specific spoilage organisms (SSOs) (Gram and Dalgaard 2002). Small molecules such as volatile basic nitrogen, biogenic amines and organic acids are produced by the metabolism of SSOs in raw aquatic products, which could function as indicators of spoilage (Erikson et al. 2011).
The most common spoilage bacteria reported in salmon under aerobic and refrigerated storage conditions are Pseudomonas spp., Carnobacterium spp., and Shewanella spp. (Hatje et al. 2014; Picart et al. 2004; Sabrina et al. 2012). It is reported that Pseudomonas spp. was the most important spoilage microorganisms at low storage temperatures (He and Sun 2015), which may lead to the decomposition of nitrogenous substances and the production of ammonia, trimethylamine and hydrogen sulfide in salmon under cold chain logistics (Hu et al. 2013). This may lead to increase of enzymatic activity, denaturation of the muscle proteins and structural damage of membranes, which can result in increased weight loss, reduction of water-holding capacity, textural changes and off-odor. However, the correlation analysis between spoilage characteristics of Pseudomonas fluorescens and temperatures in salmon has not been established clearly.
Therefore, the aim of this study is to investigate the spoilage potential of P. fluorescens in salmon fillets stored at different temperatures (30, 10 and 4 °C). To do this, the sterile salmon fillets were inoculated with the P. fluorescens strains, and the changes in bacterial counts, sensory quality, total volatile basic nitrogen, thiobarbituric acid value, biogenic amines and total amino acids were determined.
Materials and methods
Bacterial strains
The bacteria strain P. fluorescens (NCBI accession no: SBW25) isolated from spoiled salmon previously was stored in sterilized brain heart influsion broth (BHI; Qingdao Hope Bio-Technology Co., Ltd., Qingdao, PR China) and glycerine (1:3) at − 80 °C. Before use, this bacteria strain was precultured in BHI at 30 °C for 18 h and then cultured in Tryptose soya broth (TSB; Qingdao Hope Bio-Technology Co., Ltd., Qingdao, PR China) at 27 °C until the counts reached 108 CFU/ml (Churchill et al. 2016).
Sample preparation and inoculation
Salmon sample purchased from a local aquatic market in Shanghai was transported in a foam box contained ice to laboratory within 30 min. Then the salmon sample was gutted and cut into fish fillets (60–75 g). The fillets were sterilized by soaked in 75% ethanol solution for 60 s and washed in sterile water 2 times (Qian et al. 2014). The pre-cultured bacteria strain solution was applied for the inoculation process. Each sterile fillet was immersed in a bacterial suspension (105 CFU/g) for 30 s. Non-inoculated fillets washed by sterile water were used as the control. All samples were drained for 10 s, packed in clean tray in a sterile environment and stored at 30, 10, and 4 °C, respectively. The related indexes were measured periodically during storage.
Sensory evaluation
The salmon samples were displayed on the clean sterile tray. The sensory characteristics were according to the method described by Sallam (2007). Ten trained panelists from the laboratory staff were requested to assess the general likeness of salmon fillets through appearance, odour, colour, dehydration and firmness independently. The scores were given in the decreasing order scale: 10–9 for excellent, 8–7 for good, 6–5 for acceptable, 4–3 for poor and 2–1 for very poor. The panelists should be familiar with the rating scales beforehand.
Microbiological analysis
Quantitative microbiological analysis were determined based on the method of Song et al. (2011). A 25 g of salmon fillets was aseptically weighed and homogenized with 225 ml sterilized saline water (0.85%, w/v) for 1–2 min. Then the homogenized sample was serially diluted (1:10) in sterile saline water for bacteriological analysis. The samples of 1 ml suitable dilution were inoculated in the plate count agar (PCA, Qingdao Hope Biol-Technology Co., Ltd., Qingdao, PR China) and Pseudomonas CFC selective agar (Qingdao Hope Biol-Technology Co., Ltd., Qingdao, PR China) separately. The plates of CFC selective agar were incubated at 30 °C for 2 days for the enumeration of Pseudomonas spp., and the plates of PCA were inoculated at 4 °C for 10 days for psychrotrops.
Total volatile basic nitrogen (TVB-N)
Total volatile basic nitrogen (TVB-N) was analyzed according to the method of Uriarte-Montoya et al. (2010). Five grams of minced meat from each sample was accurately weighed and measured by an Automatic Kjeldahl Apparatus (KjeltecTM8400; FOSS Quality Assurance Co., Ltd., Denmark). TVB-N values were expressed as mg N/100 g.
Thiobarbituric acid value (TBA)
The determination of TBA value was performed according to Rode and Hovda (2016). Salmon samples (5 g) were homogenized with 25 ml of 20% (w/v) trichloroacetic acid (Sinopharm Chemical Reagent Co., Ltd., Shanghai, PR China) and incubated at room temperature for 1 h. Then the samples were centrifuged at 8000g and 4 °C for 10 min. The supernatant was filtered and diluted with deionized water to the volume of 50 ml. Five milliliters of the solution was mixed with 5 ml 0.02 M thiobarbituric acid (Sinopharm Chemical Reagent Co., Ltd., Shanghai, PR China). The tubes were incubated in a water bath at 100 °C for 20 min. After cooling to room temperature, the absorbance was determined at 532 nm, and the TBA values were calculated according to the formula.
The results were presented as mg malonaldehyde/kg of samples.
Biogenic amine (BA)
The extraction and derivatization of biogenic amines for samples were performed according to the measures of Shukla et al. (2010) with some modifications. The mixture was filtered through a 0.45 μm membrane and stored at − 25 °C before analysis. The quantification of BA was measured by HPLC system (LC-2010C series, Shimadzu Co., Ltd., Japan). BA was separated by a C18 column (C18-WP, 4.6 mm × 150 mm, 5 μm, Shimadzu Co., Ltd., Japan). The solvents used as the mobile phase were 0.1 M ammonium acetate (A) and 0.1 M acetonitrile (B). The gradient program was described as following: 0–7 min, 45%A–50%A, 55%B–50%B; 7–25 min, 50%A–10%A, 50%B–90%B; 25–35 min, 10%A–45%A, 90%B–55%B; 35–45 min, 45%A–10%A, 55%B–90%B. The flow rate was 1 ml/min and the column apparatus were at 40 °C. A sample volume of 10 μl was injected and detected at 254 nm.
Total amino acid (TAA)
The TAAs of salmon samples were determined using an automatic amino acid analyzer (Hitachi Global Co., Ltd., Japan) (Deng et al. 2015). Salmon meat samples (100 mg) were homogenized with 6 ml hydrochloric acid (6 M) and hydrolyzed at 120 °C for 24 h. The hydrolysate was diluted to 50 ml by citrate buffer (pH 2.2) and 2 ml of the sample was filtered through 0.45 μm membrane before injecting to the analyzer.
Spoilage potential evaluation
The microbial colonies and the contents of, TVB-N were recorded. The yield factors were calculated as the following formula (Xu et al. 2011):
where (TVB-N)0 and (TVB-N)i represent the initial contents and the contents of TVB-N at sampling; (CFU)0 and (CFU)i represent the initial and bacterial counts of spoilage products at sampling.
Statistical analysis
All measurements were carried out in triplicate. Significance of differences defined at P ≤ 0.05 was used for all means data. Analysis of variance (ANOVA) and mean comparison between the data was evaluated statistically by using SPSS Version 15.0 for Windows (Inc., Chicago, IL, USA). Origin Pro V8.6 (Origin Lab, USA) was applied to render the data graphs.
Results
Changes in sensory quality
Changes in sensory quality of salmon at three different temperatures are shown in Table 1. The initial scores in sensory were 10. The sensory scores of samples at 30 °C decreased rapidly than samples stored at 10 and 4 °C, including the control group. The sensory rejection time in the inoculated group was described as flows: 30 °C for 18 h, 10 °C for 72 h, 4 °C for 144 h. The sensory scores of samples inoculated with P. fluorescens were lower than the control.
Table 1.
Sensory score of P. fluorescens inoculated on salmon fillets under different temperatures
| Storage time (hours) | Batches | |||||
|---|---|---|---|---|---|---|
| CK30 | P30 | CK10 | P10 | CK4 | P4 | |
| 0 | 10.00 ± 0.00a | 10.00 ± 0.00a | 10.00 ± 0.00a | 10.00 ± 0.00a | 10.00 ± 0.00a | 10.00 ± 0.00a |
| 6 | 8.11 ± 0.55b | 7.89 ± 0.27b | – | – | – | – |
| 12 | 6.56 ± 0.93c | 6.05 ± 0.71c | 8.97 ± 0.90b | 8.10 ± 0.47b | – | – |
| 18 | 5.20 ± 0.62d | 4.02 ± 0.82d | – | – | – | – |
| 24 | 2.06 ± 0.19e | 1.17 ± 0.31e | 7.52 ± 0.29b | 7.55 ± 0.49bc | 8.95 ± 0.61b | 8.64 ± 0.75b |
| 36 | – | – | 6.67 ± 0.27c | 6.42 ± 0.58c | – | – |
| 48 | – | – | 5.89 ± 0.32d | 5.61 ± 0.92cd | 7.49 ± 0.54c | 7.12 ± 0.42c |
| 60 | – | – | 5.49 ± 0.82de | 5.03 ± 0.39d | – | – |
| 72 | – | – | 4.09 ± 0.94e | 3.01 ± 0.35e | 6.42 ± 0.49d | 6.03 ± 0.29d |
| 84 | – | – | 3.51 ± 0.21e | 3.08 ± 0.52e | – | – |
| 96 | – | – | – | – | 5.51 ± 0.52e | 5.19 ± 0.68e |
| 108 | – | – | – | – | – | – |
| 120 | – | – | – | – | 5.30 ± 0.24e | 5.12 ± 0.44e |
| 132 | – | – | – | – | – | – |
| 144 | – | – | – | – | 5.07 ± 0.39e | 4.61 ± 0.37ef |
| 168 | – | – | – | – | 4.55 ± 0.24ef | 3.41 ± 0.33f |
| 192 | – | – | – | – | 3.92 ± 0.39f | 3.36 ± 0.50f |
CK30, CK10, and CK4 represent the non-inoculated samples stored at 30, 10 and 4 °C, respectively, while P30, P10, and P4 represent the inoculated samples stored at 30, 10 and 4 °C, respectively
Different letters in the same column indicated the significant differences between each batches (P < 0.05)
“—”: Not determined
Bacterial kinetic
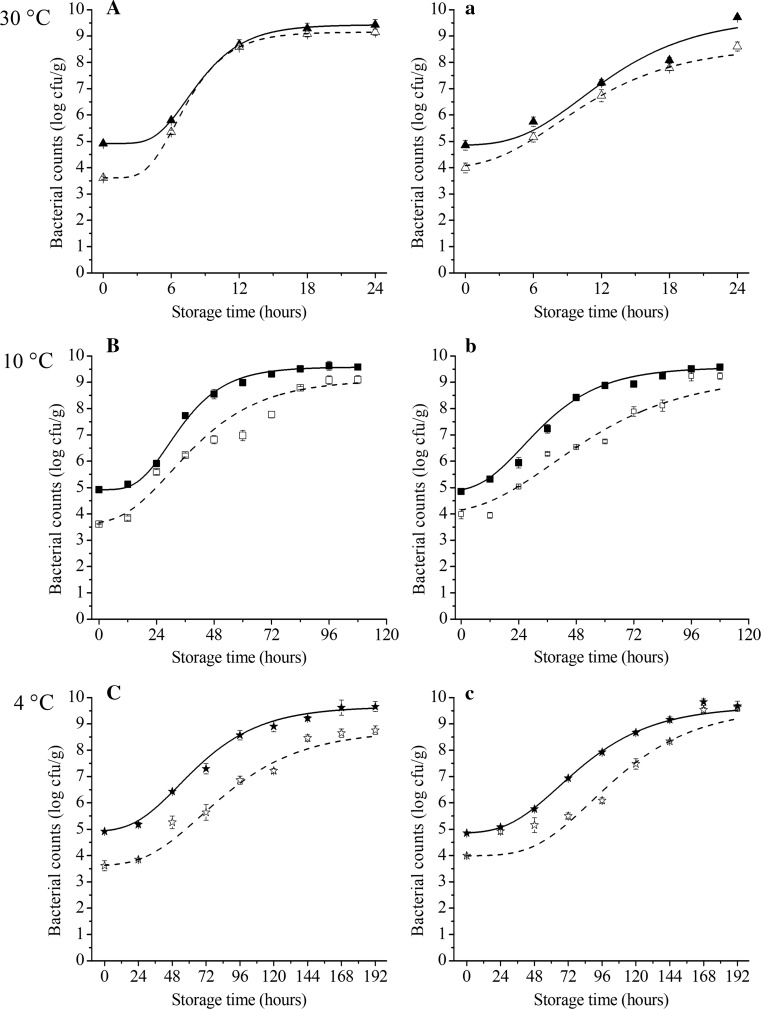
The evolution of P. fluorescens and psychrotrops at 30, 10 and 4 °C during the salmon storage is shown in the Fig. 1. During storage, the inoculated fillets showed higher bacterial counts than the control. The initial concentrations of inoculated samples and control samples were 4.9 and 3.6 log CFU/g. At 30 °C, P. fluorescens counts increased slowly in the first 6 h. Afterward, the concentration rapidly reached to 8.7 log CFU/g and there were no considerable differences between the inoculated and control until the end of storage. At 10 °C, inoculated group increased to 8.6 log CFU/g while control reached 6.8 log CFU/g. At 4 °C, P. fluorescens showed a lag phase of 20 h, then it increased rapidly from 20 to 72 h. The growth behavior of psychrotrops was similar with Pseudomonas spp.
Fig. 1.
The fitting growth curve of P. fluorescens (A, B, C) and psychrotrops (a, b, c) of salmon stored at 30 °C (open triangle: control, filled triangle: inoculated sample), 10 °C (open square: control, filled square: inoculated sample) and 4 °C (open star: control, filled star: inoculated sample)
Changes of total volatile basic nitrogen (TVB-N)
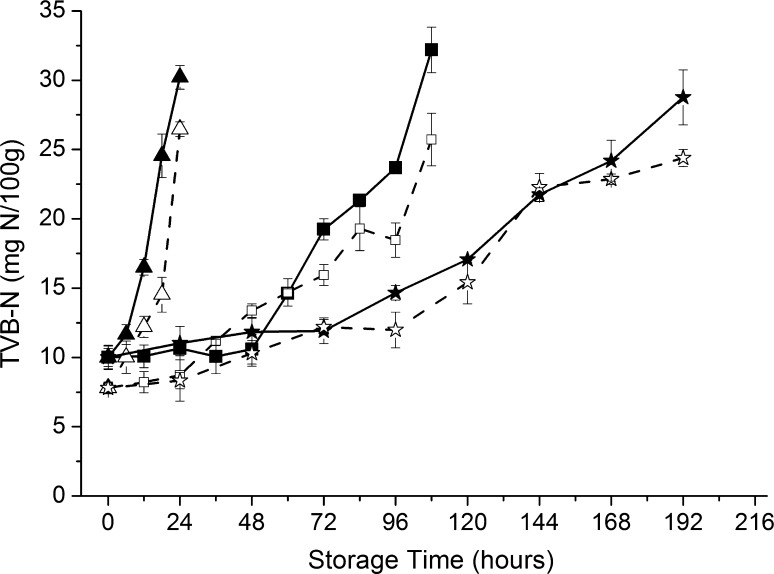
Variable production of TVB-N in salmon at three temperatures was observed in Fig. 2. The TVB-N values of inoculated samples at 30, 10, and 4 °C showed a significant increase after 3, 60 and 96 h. After 24 h, inoculated samples stored at 30 °C exceeded the limit of 30 mg N/100 g. A maximum TVB-N production was observed in inoculated samples at 10 °C for 120 h. However, the control samples stored at 4 °C remained low level of TVB-N (22.39 mg N/100 g) at the end of storage.
Fig. 2.
Changes of total volatile basic nitrogen of salmon stored at 30 °C (open triangle: control, filled triangle: inoculated sample), 10 °C (open square: control, filled square: inoculated sample) and 4 °C (open star: control, filled star: inoculated sample)
Changes of thiobarbituric acid value (TBA)
The changes in TBA under three temperatures (30, 10 and 4 °C) are listed in Table 2. The initial value of control and inoculated salmon fillets were very low (0.046 and 0.093 mg/kg, respectively). During the storage, TBA increased with the time at different temperatures. The TBA of fillets that inoculated with P. fluorescens increased rapidly than the control. At the end of storage, the TBA values of the control samples reached 1.221, 0.592 and 0.235 mg/kg, respectively, and the inoculated samples were 1.358, 0.752 and 0.302 mg/kg, respectively.
Table 2.
Thiobarbituric acid value of P. fluorescens inoculated on salmon fillets under different temperatures
| Storage time (hours) | Batches | |||||
|---|---|---|---|---|---|---|
| CK30 | P30 | CK10 | P10 | CK4 | P4 | |
| 0 | 0.046 ± 0.000d | 0.093 ± 0.000e | 0.046 ± 0.000g | 0.093 ± 0.000f | 0.046 ± 0.000h | 0.093 ± 0.000g |
| 6 | 0.218 ± 0.025c | 0.327 ± 0.017d | – | – | – | – |
| 12 | 0.531 ± 0.093b | 0.823 ± 0.141c | 0.062 ± 0.090f | 0.127 ± 0.087f | – | – |
| 18 | 1.039 ± 0.112a | 1.117 ± 0.102b | – | – | – | – |
| 24 | 1.221 ± 0.129a | 1.358 ± 0.091a | 0.081 ± 0.039b | 0.156 ± 0.043ef | 0.048 ± 0.002h | 0.080 ± 0.005f |
| 36 | – | – | 0.191 ± 0.003d | 0.225 ± 0.058e | – | – |
| 48 | – | – | 0.159 ± 0.022e | 0.314 ± 0.102d | 0.066 ± 0.005g | 0.095 ± 0.003e |
| 60 | – | – | 0.248 ± 0.042d | 0.353 ± 0.059d | – | – |
| 72 | – | – | 0.319 ± 0.074c | 0.482 ± 0.085c | 0.078 ± 0.004f | 0.117 ± 0.012d |
| 84 | – | – | 0.531 ± 0.031b | 0.648 ± 0.022b | – | – |
| 96 | – | – | 0.592 ± 0.091a | 0.752 ± 0.202a | 0.094 ± 0.010e | 0.158 ± 0.028bc |
| 108 | – | – | – | – | – | – |
| 120 | – | – | – | – | 0.120 ± 0.034d | 0.195 ± 0.064b |
| 132 | – | – | – | – | – | – |
| 144 | – | – | – | – | 0.151 ± 0.009c | 0.215 ± 0.097ab |
| 168 | – | – | – | – | 0.181 ± 0.041b | 0.267 ± 0.024a |
| 192 | – | – | – | – | 0.235 ± 0.027a | 0.302 ± 0.054a |
CK30, CK10, and CK4 represent the non-inoculated samples stored at 30, 10 and 4 °C, respectively, while P30, P10, and P4 represent the inoculated samples stored at 30, 10 and 4 °C, respectively
Different letters in the same column indicated the significant differences between each batches (P < 0.05)
“—”: Not determined
Variation of putrescine and cadaverine
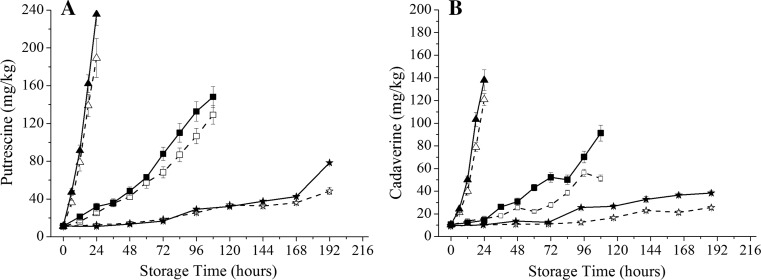
A rapid increase of cadaverine and putrescine was observed in the most samples during the storage at higher temperatures (Fig. 3). The initial values of putrescine and cadaverine were 11.31 and 10.51 mg/kg, respectively. The differences of cadaverine between the inoculated samples and the control became significant after 60 h at 10 °C and 96 h at 4 °C, respectively. At the end of storage, the values of putrescine from samples inoculated by P. fluorescens stored at 30 °C for 24 h, 10 °C for 108 h and 4 °C for 192 h increased to 235.77, 148.21, and 58.34 mg/kg respectively, while the control were only 189.25, 129.12, and 48.38 mg/kg, respectively. The values of cadaverine of inoculated samples also reached 137.98, 91.25 and 42.15 mg/kg after storage, while the control samples were only 120.89, 51.28, and 28.13 mg/kg respectively.
Fig. 3.
Variation of the putrescine (a) and cadaverine b of salmon stored at 30 °C (open triangle: control, filled triangle: inoculated sample), 10 °C (open square: control, filled square: inoculated sample) and 4 °C (open star: control, filled star: inoculated sample)
Changes of total amino acids (TAAs)
The contents of TAA in inoculated samples and non-inoculated samples that stored at 30 °C for 18 h, 10 °C for 60 h and 4 °C for 144 h are depicted in Table 2. The initial total contents of TAA were 212.03 mg/g. A significant decrease of TAA in the inoculated samples and the control was observed. Among them, the content of lysine decreased significantly from 19.60 to 13.19, 12.88 and 12.32 mg/g respectively in inoculated samples. The variation trend of arginine was similar with that of lysine, which decreased from 15.81 to 11.54, 13.18, and 9.43 mg/g.
Discussion
The spoilage potential of P. fluorescens on salmon at different temperatures was evaluated. In general, a significant decline was observed in the sensory properties during storage, especially for samples stored at 30 °C. The decreasing rate of the inoculated samples was higher at three temperatures in comparison with the control. At the initial period, no significant differences in sensory quality between the inoculated samples and the control were observed. After 144 h, the samples stored at 4 °C reached the sensory rejection point because of the production of exudates, off-odor, less firmness and more viscosity than the control. An intense dark orange colour of fillets was observed at 4 °C due to the oxidation of astaxanthin and carotenoids, similar to 30 °C. The efficient metabolic capacity of P. fluorescens was probably responsible for the modification because they produced TVB-N and BAs. It has been reported that the small molecular proteins in fillets were consumed by P. fluorescens (Cardinal et al. 2001). This result confirmed the previous study that P. fluorescens showed high protein hydrolytic ability at low temperatures.
The high temperature (30 °C) was considered to be the optimal temperature for the strain of P. fluorescens to grow (according to the pre-experiment, data not shown), while the low temperatures (10 and 4 °C) were regarded as the condition of cold storage. In general, the data suggested that P. fluorescens at 30 °C showed a shorter lag phase (3 h) and higher growth rate (0.81 CFU/h). Compared to the strains cultured at low temperatures (10 and 4 °C), variations of lag time (3–20.3 h) and maximum growth rate (0.81–0.05 CFU/h) were observed. The sterilizing treatment by 75% ethanol for 60 s could reduce the initial bacterial counts, so that the background bacteria (control group bacteria) only accounted for 4% of the inoculated P. fluorescens initially. During the whole culturing period, the counts of background bacteria were lower than that of inoculated P. fluorescens and only accounted for 12.5% at 4 °C at the end of storage.
The psychrotrophic growth dynamics were displayed in Fig. 1B. The proportion of psychrotrophic bacteria in salmon may be responsible for the spoilage of salmon during cold storage. In this work, the behavior of psychrotrops was similar to P. fluorescens, which indicated that P. fluorescens probably was the dominate group of spoilage bacteria at low temperature. This was in agreement with the results of Li et al. (2015). In addition, when bacteria, including P. fluorescens, were exposed to stress of low temperatures, a cold-adapted mechanism may occur in order to maintain microbiological activity (Serio et al. 2011).
TVB-N, a nitrogen volatile substance produced by protein decomposition, is widely used as an important indicator of the freshness of aquatic products. It can be found in Fig. 2 that TVB-N value gradually increased significantly with the time. The initial TVB-N value of fresh fillets (7.8 mg N/100 g) was in agreement with low initial bacterial counts. The results showed that TVB-N in the inoculated group was higher than the control group, especially at 10 °C, which indicated that P. fluorescens remained high TVB-N metabolic activity at low temperatures. At the end of storage, only inoculated groups achieved a high level of values at three temperatures, which can be attributed to the higher bacterial counts. Therefore, P. fluorescens might generally be regarded as an effective spoilage bacterium in this study. The changes in TVB-N values in the present work in salmon are similar to the work of Barraza et al. (2015).
A good correlation was observed between TVB-N value and bacterial counts during storage at different temperatures. In order to describe the correlation quantitatively, the yield factor was used to display the production of TVB-N per bacterial cell in inoculated samples. The spoilage potential was evaluated as log YTVB−N/CFU value (Fig. S1). After the same storage period, higher log YTVB−N/CFU were observed in samples stored at lower temperatures. After 24 h, the log YTVB−N/CFU value of samples stored at 4 °C was twice higher than the samples at 30 °C. Therefore, each cell of P. fluorescens displayed higher ability to produce TVB-N at low temperatures (10 and 4 °C) than at high temperature (30 °C).
As a widely used indicator of lipid oxidation, the value of TBA expressed as production of malondialdehyde (MDA) (Guillén-Sans and Guzmán-Chozas 1998). The initial TBA value of the inoculated samples was 0.093 mg/kg, which increased to 1.21, 0.752, and 0.302 mg/kg at the end of storage for 30, 10, and 4 °C. In this study, all samples showed an increase in TBA with storage time, including the control. Compared to the control group, P. fluorescens showed a slightly higher level of TBA value. Similar results were reported by Amanatidou et al. (2000). We assume that P. fluorescens might play a less important role in lipid oxidation.
Biogenic amines (BAs), a low molecular weight organic compounds with biological activity, are produced by the decarboxylation of amino acids (Kim et al. 2009). The most widely studied biogenic amines are histamine, cadaverine, and putrescine among aquatic products and a high level of microbial growth may easily lead to the immoderation of biological amine content (Silla Santos 1996; Sims et al. 1992). According to this study, cadaverine and putrescine were considered as the dominant BAs in salmon samples. P. fluorescens was active producer of BAs that were mentioned above. This work was in agreement with the literature (Rezaei et al. 2007), which reported that Pseudomonas spp. had a good linear correlation with the formation of cadaverine and putrescine. The accumulation of biogenic amines also can lead to food poisoning. Therefore, cadaverine and putrescine are recommended as the indicators of measuring safety and quality of white-muscle aquatic products such as salmon. (Prester 2011). BAs and other metabolites products could lead to changes in odor. In this study, putrescine and cadaverine showed a great correspondence with the sensory and microbial activity, in accordance with results obtained by Krizek et al. (2004). With the storage time, the production of cadaverine and putrescine was gradually increased. More significant differences between inoculated samples and non-inoculated samples were observed at 4 °C than at 30 °C, indicating that the P. fluorescens remained high decarboxylase activity at low temperature.
Cadaverine and putrescine can be produced by lysine, ornithine and arginine (Jørgensen et al. 2000). At the end of storage, the changes of most amino acids were observed from Table 3 since the formation of biogenic amines which attribute to decarboxylation of amino acids. Lysine could be transformed to cadaverine by P. fluorescens (De las Rivas et al. 2006). Compared to the control samples, a marked decline in lysine in the inoculated samples was observed. Arginine transformed into ornithine by action of enzymes (Coffino 2001). According to research (De las Rivas et al. 2006), the target gene of ornithine decarboxylase was found in P. fluorescens R2f. The rapid decarboxylation of putrescine in inoculated samples may have occurred.
Table 3.
Contents of total amino acid in salmon fillets at 30, 10 and 4 °C
| Amino acid | Total amino acid (mg/g) | ||||||
|---|---|---|---|---|---|---|---|
| 0 h | Control | Inoculated sample | |||||
| 30 °C | 10 °C | 4 °C | 30 °C | 10 °C | 4 °C | ||
| Asparaginic | 19.15 | 17.28 | 13.99 | 16.04 | 15.45 | 16.95 | 11.44 |
| Threonine | 13.64 | 12.77 | 9.22 | 9.80 | 13.24 | 10.30 | 11.62 |
| Serine | 9.47 | 8.25 | 8.49 | 7.73 | 9.34 | 8.91 | 9.00 |
| Glutamic | 31.65 | 31.34 | 28.92 | 26.25 | 23.42 | 20.92 | 25.25 |
| Glycine | 10.65 | 8.56 | 8.54 | 10.03 | 10.19 | 9.18 | 9.58 |
| Alanine | 14.23 | 13.44 | 11.95 | 11.74 | 13.24 | 12.24 | 10.94 |
| Cystine | 10.10 | 8.40 | 7.72 | 7.46 | 3.42 | 6.06 | 5.63 |
| Valine | 8.83 | 9.27 | 9.28 | 8.39 | 10.44 | 9.41 | 10.23 |
| Methionine | 8.86 | 7.98 | 7.34 | 8.08 | 7.18 | 7.34 | 7.98 |
| Isoleucine | 7.64 | 8.23 | 8.35 | 7.32 | 9.36 | 8.29 | 9.20 |
| Leucine | 16.45 | 13.95 | 15.46 | 15.72 | 12.63 | 15.95 | 16.42 |
| Tyrisine | 9.65 | 8.86 | 7.18 | 9.25 | 8.41 | 9.41 | 8.02 |
| Phenylalanine | 6.41 | 6.54 | 6.88 | 6.06 | 7.17 | 7.00 | 7.47 |
| Lysine | 19.60 | 16.45 | 14.39 | 15.32 | 13.19 | 12.88 | 12.32 |
| Histidine | 7.02 | 5.94 | 6.85 | 6.68 | 6.02. | 8.12 | 5.27 |
| Arginine | 15.81 | 12.73 | 11.58 | 13.43 | 11.54 | 13.18 | 9.43 |
| Proline | 4.87 | 4.02 | 4.53 | 4.64 | 2.45 | 3.83 | 3.52 |
| Total | 214.03 | 194.01 | 180.67 | 183.94 | 170.67 | 179.97 | 173.32 |
In conclusion, the growth of P. fluorescens may led to the accumulation of spoilage products including TVB-N, cadaverine and putrescine and the degradation of muscle protein and amino acids. Higher spoilage activity and more consumption of amino acids in salmon were observed when P. fluorescens cultured at low temperatures. The results highlighted that P. fluorescens may play an important role during cold storage of salmon. To understand the metabolize mechanism for P. fluorescens at low temperatures, more investigations are remained to be studied in future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Yield factor of TVB-N in the inoculated samples of salmon stored at 30 °C (△: control, ▲: inoculated sample), 10 °C (□: control, ■: inoculated sample) and 4 °C (☆: control, ★: inoculated sample). (TIFF 8220 kb)
Acknowledgements
This work was financially supported by National Natural Science Foundation of China [Grant Number: 31501551] and Shanghai Science and Technology Key Project on Agriculture from Shanghai Municipal Agricultural Commission [Grant Number: (2016) 1-1].
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2916-x) contains supplementary material, which is available to authorized users.
References
- Amanatidou A, Schlüter O, Lemkau K, Gorris LGM, Smid EJ, Knorr D. Effect of combined application of high pressure treatment and modified atmospheres on the shelf life of fresh Atlantic salmon. Innov Food Sci Emerg Technol. 2000;1:87–98. doi: 10.1016/S1466-8564(00)00007-2. [DOI] [Google Scholar]
- Barraza FAA, León RAQ, Álvarez PXL. Kinetics of protein and textural changes in Atlantic salmon under frozen storage. Food Chem. 2015;182:120–127. doi: 10.1016/j.foodchem.2015.02.055. [DOI] [PubMed] [Google Scholar]
- Cardinal M, Knockaert C, Torrissen O, Sigurgisladottir S, Mørkøre T, Thomassen M, Vallet JL. Relation of smoking parameters to the yield, colour and sensory quality of smoked Atlantic salmon (Salmo salar) Food Res Int. 2001;34:537–550. doi: 10.1016/S0963-9969(01)00069-2. [DOI] [Google Scholar]
- Churchill OJ, Fernandez-Piquer J, Powell SM, Tamplin ML. Microbial and sensorial models for head-on and gutted (HOG) Atlantic Salmon (Salmo salar) stored from 0 to 15 °C. Food Microbiol. 2016;57:144–150. doi: 10.1016/j.fm.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Coffino P. Regulation of cellular polyamines by antizyme. Nat Rev Mol Cell Biol. 2001;2:188–194. doi: 10.1038/35056508. [DOI] [PubMed] [Google Scholar]
- De las Rivas B, Marcobal Á, Carrascosa AV, Muñoz R. PCR detection of foodborne bacteria producing the biogenic amines histamine, tyramine, putrescine, and cadaverine. J Food Prot. 2006;69:2509–2514. doi: 10.4315/0362-028X-69.10.2509. [DOI] [PubMed] [Google Scholar]
- Deng Y, Luo Y, Wang Y, Zhao Y. Effect of different drying methods on the myosin structure, amino acid composition, protein digestibility and volatile profile of squid fillets. Food Chem. 2015;171:168–176. doi: 10.1016/j.foodchem.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Erikson U, Misimi E, Gallart-Jornet L. Superchilling of rested Atlantic salmon: Different chilling strategies and effects on fish and fillet quality. Food Chem. 2011;127:1427–1437. doi: 10.1016/j.foodchem.2011.01.036. [DOI] [Google Scholar]
- Gram L, Dalgaard P. Fish spoilage bacteria–problems and solutions. Curr Opin Biotechnol. 2002;13:262–266. doi: 10.1016/S0958-1669(02)00309-9. [DOI] [PubMed] [Google Scholar]
- Guillén-Sans R, Guzmán-Chozas M. The thiobarbituric acid (TBA) reaction in foods: a review. Crit Rev Food Sci Nutr. 1998;38:315–330. doi: 10.1080/10408699891274228. [DOI] [PubMed] [Google Scholar]
- Gunsen U, Ozcan A, Aydin A. Determination of some quality criteria of cold storaged marinated anchovy under vacuum and modified atmosphere conditions. Turk J Fish Aquat Sci. 2011;11:233–242. doi: 10.4194/trjfas.2011.0208. [DOI] [Google Scholar]
- Hatje E, Neuman C, Stevenson H, Bowman JP, Katouli M. Population dynamics of Vibrio and Pseudomonas species isolated from farmed Tasmanian Atlantic salmon (Salmo salar L.): a seasonal study. Microb Ecol. 2014;68:679–687. doi: 10.1007/s00248-014-0462-x. [DOI] [PubMed] [Google Scholar]
- He HJ, Sun DW. Toward enhancement in prediction of Pseudomonas counts distribution in salmon fillets using NIR hyperspectral imaging. LWT Food Sci Technol. 2015;62:11–18. doi: 10.1016/j.lwt.2015.01.036. [DOI] [Google Scholar]
- Hu Y, Huang Z, Li J, Yang H. Concentrations of biogenic amines in fish, squid and octopus and their changes during storage. Food Chem. 2013;135:2604–2611. doi: 10.1016/j.foodchem.2012.06.121. [DOI] [PubMed] [Google Scholar]
- Jørgensen LV, Dalgaard P, Huss HH. Multiple compound quality index for cold-smoked salmon (Salmo salar) developed by multivariate regression of biogenic amines and pH. J Agric Food Chem. 2000;48:2448–2453. doi: 10.1021/jf9909407. [DOI] [PubMed] [Google Scholar]
- Kim M-K, Mah J-H, Hwang H-J. Biogenic amine formation and bacterial contribution in fish, squid and shellfish. Food Chem. 2009;116:87–95. doi: 10.1016/j.foodchem.2009.02.010. [DOI] [Google Scholar]
- Krizek M, Vacha F, Vorlova L, Lukasova J, Cupakova S. Biogenic amines in vacuum-packed and non-vacuum-packed flesh of carp (Cyrinus carpio) stored at different temperatures. Food Chem. 2004;88:185–191. doi: 10.1016/j.foodchem.2003.12.040. [DOI] [Google Scholar]
- Li TT, Ding T, Zou ZY, Zhou K, Yi SM, Li JR. Differential prevalence of spoilage bacteria in salmon fillets during refrigerated storage and identification of predominant spoilage bacterial species. Mod Food Sci Technol. 2015;31:36–41. [Google Scholar]
- Picart L, Dumay E, Guiraud JP, Cheftel JC. Microbial inactivation by pressure-shift freezing: effects on smoked salmon mince inoculated with Pseudomonas fluorescens, Micrococcus luteus and Listeria innocua. LWT-Food Sci Technol. 2004;37:227–238. doi: 10.1016/j.lwt.2003.08.004. [DOI] [Google Scholar]
- Prester L. Biogenic amines in fish, fish products and shellfish: a review. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28:1547–1560. doi: 10.1080/19440049.2011.600728. [DOI] [PubMed] [Google Scholar]
- Qian YF, Xie J, Yang SP, Wu WH, Xiong Q, Gao ZL. In vivo study of spoilage bacteria on polyphenoloxidase activity and melanosis of modified atmosphere packaged Pacific white shrimp. Food Chem. 2014;155:126–131. doi: 10.1016/j.foodchem.2014.01.031. [DOI] [PubMed] [Google Scholar]
- Rezaei M, Montazeri N, Langrudi HE, Mokhayer B, Parviz M, Nazarinia A. The biogenic amines and bacterial changes of farmed rainbow trout (Oncorhynchus mykiss) stored in ice. Food Chem. 2007;103:150–154. doi: 10.1016/j.foodchem.2006.05.066. [DOI] [Google Scholar]
- Rode TM, Hovda MB. High pressure processing extend the shelf life of fresh salmon, cod and mackerel. Food Control. 2016;70:242–248. doi: 10.1016/j.foodcont.2016.05.045. [DOI] [Google Scholar]
- Sabrina M, Josiane C, Frédérique C, Mireille C, Marie-France P, Xavier D, Jean-Jacques J. Characterisation of the spoilage microbiota in raw salmon (Salmo salar) steaks stored under vacuum or modified atmosphere packaging combining conventional methods and PCR-TTGE. Food Microbiol. 2012;30:164–172. doi: 10.1016/j.fm.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Sallam KI. Chemical, sensory and shelf life evaluation of sliced salmon treated with salts of organic acids. Food Chem. 2007;101:592–600. doi: 10.1016/j.foodchem.2006.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serio A, Chaves-López C, Paparella A. Listeria monocytogenes isolated from the smoked salmon industry: growth potential under different environmental conditions. Food Control. 2011;22:2071–2075. doi: 10.1016/j.foodcont.2011.05.010. [DOI] [Google Scholar]
- Shukla S, Park HK, Kim JK, Kim M. Determination of biogenic amines in Korean traditional fermented soybean paste (Doenjang) Food Chem Toxicol. 2010;48:1191–1195. doi: 10.1016/j.fct.2010.01.034. [DOI] [PubMed] [Google Scholar]
- Silla Santos MH. Biogenic amines: their importance in foods. Int J Food Microbiol. 1996;29:213–231. doi: 10.1016/0168-1605(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Sims GG, Farn G, York RK. Quality indices for canned skipjack tuna: correlation of sensory attributes with chemical indices. J Food Sci. 1992;57:1112–1115. doi: 10.1111/j.1365-2621.1992.tb11275.x. [DOI] [Google Scholar]
- Song Y, Liu L, Shen H, You J, Luo Y. Effect of sodium alginate-based edible coating containing different anti-oxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala) Food Control. 2011;22:608–615. doi: 10.1016/j.foodcont.2010.10.012. [DOI] [Google Scholar]
- Uriarte-Montoya MH, Villalba-Villalba AG, Pacheco-Aguilar R, Ramirez-Suarez JC, Lugo-Sánchez ME, García-Sánchez G, Carvallo-Ruíz MG. Changes in quality parameters of Monterey sardine (Sardinops sagax caerulea) muscle during the canning process. Food Chem. 2010;122:482–487. doi: 10.1016/j.foodchem.2009.05.071. [DOI] [Google Scholar]
- Xu ZW, Li XY, Yang XS, Guo QY, Jiang CJ. Analysis on spoilage ability of dominant spoilage bacteria from marine fish. Food Mach. 2011;27:71–74. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Yield factor of TVB-N in the inoculated samples of salmon stored at 30 °C (△: control, ▲: inoculated sample), 10 °C (□: control, ■: inoculated sample) and 4 °C (☆: control, ★: inoculated sample). (TIFF 8220 kb)