Abstract
In this study, the effect of barley malt process on antioxidant activity, carotenoid content, oil yield, phenolic compounds and fatty acid composition of barley, green malt and malt was investigated. The highest antioxidant activity (79.80%) and total phenolic content (122.43 mg/100 g) was observed in green malt. Carotenoid content of green malt (1.71 µg/g) was higher than those of barley and malt. Green malt had the maximum (+)-catechin (69.06 mg/100 g), 1,2-dihydroxybenzene (37.21 mg/100 g), quercetin (30.78 mg/100 g) and isorhamnetin (22.44 mg/100 g) content. Oil contents of samples ranged from 1.73 to 2.13% and showed increase with malting process. While barley lipids contained 18.53% palmitic, 19.94% oleic and 51.74% linoleic acids, malt oil contained 17.33% palmitic, 15.62% oleic and 56.56% linoleic acids. Linoleic acid content increased during malting process while oleic and palmitic acid content decreased.
Keywords: Barley, Green malt, Malt, Phenolic compounds, Oil yield, Fatty acid
Introduction
The barley (Hordeum vulgare) belongs to Poaceae family and is used for animal feed, production of malt and food products (Sadeghi et al. 2016). Malt is a germinated cereal grain that has been dried in a process known as “malting”. The grains (generally barley) were left to germinated after soaking in water, and then they are dried with hot air to stop the germination (Liu et al. 1975; Gupta et al. 2010). The most important use of barley throughout the world is as malt for manufacturing beverages or malt enriched food products. It is also used for industrial purposes, such as medicine and manufacturing baby food (Alam et al. 2007; Carvalho et al. 2016). Barley, malt extracts and syrups are used in small amounts in food products to give bitter flavour and colour, for example in breakfast cereals and baked foods (Goplan et al. 1989; Arif et al. 2011). The compounds of barley and malt grains show a change with germination process. Germination results in structural modification and synthesis of new compounds and improves the nutritional value and stability of grains (Ha et al. 2016). Free and bound phenolic compounds of barley grains are found in the husk and aleurone layer (Marecek et al. 2017). The phenolic compounds of barley change due to germinating and heating during malting process (Carvalho et al. 2015). Cai et al. (2015) to sdudied on antioxidant activity and polyphenol contents of some barley genotypes. The objective of this study was to determine the effect of malting process on the phenolic compounds, antioxidant activity, carotenoid and oil contents and fatty acid compositions of barley, green malt and malt grain and oils.
Materials and methods
Samples
Barley
Barley sample was provided from a barley farm in Konya (Çumra) province. Barley grains on 2.5 and 2.8 mm oblong sieves were used in this study. Raw grains were soaked to begin germination. The cleaned and classified barley (about 500 g) was steeped in tap water until the moisture content was reached to 45% (about 48 h) at 16 °C. The amount of water was 1.5 L for each period. During steeping, the water was changed every 12 h. The grains were turned periodically to help prevent bacterial growth.
Green malt
After steeping, the grains were removed from water and placed in malting chambers to germinate at 16 °C for a week. During germination, water was sprayed on the grain twice a day for the first 3 days and then three times per day for the remainder of the germination period (Kim et al. 1993). Germination was maintained until the green acrospire (sprout) reaches a length approximately the length of the grain. The germinated barley is called as gren malt.
Malt
It was dried in the oven to stop the germination of the green malt. At the end of germination, green malt was gradually dried at 80 °C in oven for 13 h. Then, the rootlets were removed by hand. Dried malt was kept in a hermetic glass jar at + 4 °C till analyses. All experiment was conducted in laboratory conditions.
Methods
Moisture content
Before analysis, the barley and malt grains were ground on a mill (Retsch Model, Type ZM100, power 220–240 v 50/60 Hz, speed 14,000–18,000) to pass a 20-mesh sieve. Moisture content of materials was measured by drying in an oven (Nüve FN055 Ankara, Turkey) at 135 °C according to AACC (1990) method.
Sample extraction
Phenolic compounds and antioxidants of samples were extracted according to Carvalho et al. (2015) with some modifications. The samples ground on a mill (Retsch Model, Type ZM100, power 220–240 v 50/60 Hz, speed 14,000–18,000), then about (about 3 g) were added to 20 mL of methanol (Merck, Darmstadt-Germany). The mixture was shaken by vortex (Labart mult-mixer MVS-1 50 Hz) for 1 min and sonicated (Bendelin Heidolph Laborota 4001, Germany) for 10 min, followed by centrifugation (Hermle Z 200 A, Germany) at 6000 rpm for 10 min. These steps were repeated twice and the supernatants were collected. After the extract was concentrated at 45 °C in a rotary evaporator (Rotary Heidolph Laborota 4001, Germany) under vacuum, extract was added into a flask. Then, 10 mL methanol/water (50/50, v/v) was added on extracts. The final volume was completed to 25 mL.
Total phenolic content
Total phenolic content of extracts (100 µL) were determined with the Folin–Ciocalteu (FC) reagent according to Yoo et al. (2004). 1 mL of Folin–Ciocalteu was added into samples, and shaked by vortex for 5 min. After 10 mL of 7.5% Na2CO3 was added into mixture, the final volume was completed to 25 mL with distilled water. At the end of 60 min., absorbance were measured at 750 nm in spectrophotometer (Shimadzu UV–Vis spectrophotometer, UV mini 1240). The results were given as mg GAE/100 g.
Antioxidant activity
The antioxidant activities of samples were determined with 0.004% DPPH (1,1-diphenyl-2-picrylhydrazyl) method (Lee et al. 1998). The extract (0.1 mL) was mixed with 2 mL methanolic DPPH, and the mixture was shaken, and kept at room temperature for 30 min. The absorbance was measured at 517 nm. Antioxidant activity (%) was calculated according to formula given below.
Determination of phenolic compounds
Phenolic compounds of barley, green malt and malt samples were determined by Shimadzu-HPLC equipped with PDA detector and Inertsil ODS-3 (5 µm; 4.6 × 250 mm) column. As mobile phases, 0.05% acetic acid in water (mobile phases A) and acetonitrile (mobile phases B) mixture were used. The gradient program was as follows: 0–0.10 min 8% B; 0.10–2 min 10% B; 2–27 min 30% B; 27–37 min 56% B; 37–37.10 min 8% B; 37.10–45 min 8% B. The flow rate of the mobile phase and the injection volume were 1 mL/min at 30 °C and 20 µL, respectively. The peak records were carried out at 280 and 330 nm. The total running time for each sample was 60 min.
Carotenoid content
Extraction of carotenoids was performed according to Silva da Rocha et al. (2015). 2 g of ground samples were added to 25 mL of acetone. The mixture was shaken by vortex (Labart mult-mixer MVS-1 50 Hz) for 10 min and filtrated using filter paper (Whatman No. 1), followed by taking in a separation funnel. The filtrate was fractionated with 20 mL of petroleum ether and washed with 100 mL of distilled water in order to remove the acetone. These steps were repeated twice. Whatman No. 1 covered with anhydrous sodium sulfate (5 g) for removing residual water was used to filtrate the petroleum ether layer. The volume of the extracts was completed to 25 mL by petroleum ether. After these procedures, the absorbance was measured at 450 nm.
Lipids content
Lipids content of samples was determined according to AOAC (1990) method. After lipids of samples was extracted with petroleum benzine in Soxhlet Apparatus for 5 h, solvent was evaporated at 50 °C.
Fatty acid composition
Oil was esterified according to ISO-5509 (1978) method. Fatty acid methyl esters of samples were analysed by gas chromatography (Shimadzu GC-2010) equipped with flame-ionization detector (FID) and capillary column (Tecnocroma TR-CN100, 60 m × 0.25 mm, film thickness: 0.20 µm). The temperature of injection block and detector was 260 °C. Carrier gas was nitrogen with 1.51 mL/min flow rate. Total flow rate was 80 mL/min and split ratio was 1/40. Column temperature was programmed as follows: 120 °C for 5 min and increased 240 °C at 4 °C/min and held 25 min at 240 °C.
Statistical analysis
Minitab Version 16.2.2 (Minitab Ltd, Coventry, UK) was used for statistical analysis. Results of the research were analysed for mean ± SD and statistical significance by analysis of variance (Püskülcü and Filiz 1989).
Results and discussion
Moisture content, antioxidant activities, total phenolic, carotenoid and content of barley, green malt and malt grains are shown in Table 1. Moisture content of barley, green malt and malt grains was 12.7, 34.2 and 6.3%, respectively. Antioxidant activities and total phenolic content ranged from 66.48 to 79.80% and from 101.88 to 122.43 mg/100 g, respectively. The activities of antioxidants and total phenolic content of barley and malt were similar, while the highest value was observed for in green malt (79.80%, 122.43 mg/100 g). In the experiments reported by Ha et al. (2016), total phenolic content of un-germinated and germinated (48 h) barley extract were reported as 1.06 and 3.37 mg/g, respectively. After 48 h, total phenolic content decreased may be because of initiation of lignification. Additionally, antioxidant activity of barley increased during 24 h germination. The reason of reduction in total phenolic content was conversion of the phenolic compounds into lignans or lignin when lignification process was initiated (Andarwulan et al. 1999). Carotenoid contents of samples were found between 1.16 (malt) and 1.71 µg/g (green malt). Goupy et al. (1999) reported that carotenoid contents of Clarine, Esterel, Plaisant varieties increased, while a decrease was observed in Caminant and Labea varieties after malting process. The highest oil content was found in green malt (2.13%), followed by malt (1.94%) and barley (1.73%). Cozzolino and Degner (2016) informed that oil content of barley was between 1 and 3%. Bravi et al. (2012) reported a significant decrease was observed in total lipid content during malting process in contrast to our results. Malting conditions such as temperature, moisture and germination time, effect the level of lipid degradation (Frank et al. 2011).
Table 1.
Physicochemical properties of barley, green malt and malt samples
| Moisture content (%) | Antioxidant activity (%) | Total phenolic content (mg/100 g) | Carotenoid content (µg/g) | Oil content (%) | |
|---|---|---|---|---|---|
| Barley | 12.7 ± 0.53*b | 66.48 ± 0.00c | 101.88 ± 0.01c | 1.49 ± 0.09b | 1.73 ± 0.02c |
| Green malt | 34.2 ± 0.04a** | 79.80 ± 0.00a | 122.43 ± 0.01a | 1.71 ± 0.02a | 2.13 ± 0.02a |
| Malt | 6.3 ± 0.12c | 67.31 ± 0.00b | 107.78 ± 0.00b | 1.16 ± 0.00c | 1.94 ± 0.03b |
* Mean ± SD; ** values within each row followed by different letters are significantly different (p < 0.05)
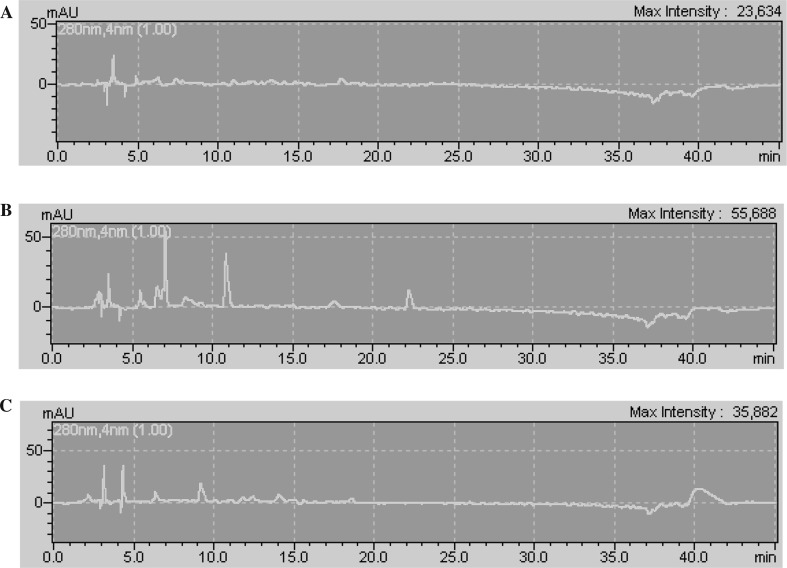
Phenolic compounds of barley, green malt and malt samples are presented in Table 2. The chromatograms of barley, green malt and malt extracts are displayed in Fig. 1a–c, respectively. The main phenolic compounds of barley were (+)-catechin (52.16 mg/100 g), 1,2-dihydroxybenzene (36.05 mg/100 g), 3,4-dihydroxybenzoic acid (26.81 mg/100 g), and gallic acid (19.66 mg/100 g) (p < 0.05). Germination process resulted in an increase in phenolic contents. The major increase was observed in quercetin (from 7.27 to 30.78 mg/100 g), followed by (+)-catechin (from 52.16 to 69.06 mg/100 g) and isorhamnetin (from 6.35 to 22.44 mg/100 g) contents (p < 0.05). Also, gallic acid, 3,4-dihydroxybenzoic acid, (+)-catechin, 1,2-dihydroxybenzene, caffeic acid, quercetin and isorhamnetin content of green malt extract were found as 21.36, 27.38, 69.06, 37.21, 17.38, 30.78 and 22.44 mg/100 g, respectively. In addition, malt extract contained 15.47 mg/100 g gallic acid, 12.95 mg/100 g 3,4-dihydroxybenzoic acid, 21.30 mg/100 g (+)-catechin, 37.34 mg/100 g 1,2-dihydroxybenzene, 8.32 mg/100 g apigenin 7-glucoside and 8.10 mg/100 g quercetin. The results demonstrated that malt had the lowest phenolic contents in comparison to barley and dried green malt. Phenylalanine ammonia lyase (PAL) plays an important role in the biosynthesis of phenolics and this enzyme is detected in barley. In addition kilning temperatures the stability of this enzyme (Maillard and Berset 1995). The phenolic compounds of green malt were found higher than phenolic content of malt. The reason why the green malt contains more phenolic substances may be probably due to enzyme activity in germination stage and changes in extractibility of samples (Maillard et al. 1996). Consequently, green malt is rich in phenolic compounds, followed by malt and barley. Also, green malt had high antioxidant activity. According to study of Langos et al. (2015), the content of ferulic, p-coumaric and caffeic acids in mg/kg were 0.59 in barley, 2.76 in green malt and 3.37 in dried malt; 0.28 in barley, 1.31 in green malt and 0.98 in dried malt; 0.42 in barley, under the LOD value in green malt and dried malt, respectively. Results showed some differences compared to literature. These differences can be probably due to barley type, malting process and analytical conditions.
Table 2.
Phenolic compounds of barley, green malt and malt samples (mg/100 g)
| Phenolic compounds | Barley | Green malt | Malt |
|---|---|---|---|
| Gallic acid | 19.66 ± 0.73*b | 21.36 ± 1.38a | 15.47 ± 7.60c |
| 3,4-Dihydroxybenzoic acid | 26.81 ± 1.10a** | 27.38 ± 0.33a | 12.95 ± 0.02b |
| (+)-Catechin | 52.16 ± 6.64b | 69.06 ± 0.97a | 21.30 ± 9.99c |
| 1,2-Dihydroxybenzene | 36.05 ± 0.80b | 37.21 ± 4.28a | 37.34 ± 0.32a |
| Syringic acid | 9.16 ± 2.00a | 7.63 ± 0.26b | 6.84 ± 0.72c |
| Caffeic acid | 9.28 ± 0.03b | 17.38 ± 2.39a | 7.52 ± 0.25c |
| Rutin trihydrate | 7.60 ± 1.51b | 5.63 ± 1.25c | 8.02 ± 5.67a |
| p-coumaric acid | 1.25 ± 0.48a | 1.08 ± 0.47a | 0.90 ± 0.47b |
| Trans-ferulic acid | 5.51 ± 0.95a | 0.92 ± 0.14b | 5.42 ± 0.45a |
| Apigenin 7-glucoside | 8.32 ± 2.66a | 6.78 ± 2.10b | 8.32 ± 1.39a |
| Resveratrol | 2.84 ± 1.06a | 2.64 ± 1.26b | 2.63 ± 0.47b |
| Quercetin | 7.27 ± 1.64bc | 30.78 ± 0.62a | 8.10 ± 2.40b |
| Trans-cinnamic acid | 1.06 ± 0.37b | 3.78 ± 1.20a | 0.95 ± 0.36c |
| Naringenin | –*** | – | – |
| Kaempferol | 1.99 ± 0.05c | 7.89 ± 3.06a | 2.15 ± 0.37b |
| Isorhamnetin | 6.35 ± 1.85b | 22.44 ± 1.47a | 6.19 ± 1.85b |
* Mean ± SD; ** values within each row followed by different letters are significantly different (p < 0.05), *** not dedected
Fig. 1.
Chromatograms of phenolic compounds of barley (a), dried green malt (b) and malt (c) methanol extracts. a Chromatogram of phenolic compounds of barley extract. b Chromatogram of phenolic compounds of dried green malt extract. c Chromatogram of phenolic compounds of malt extract
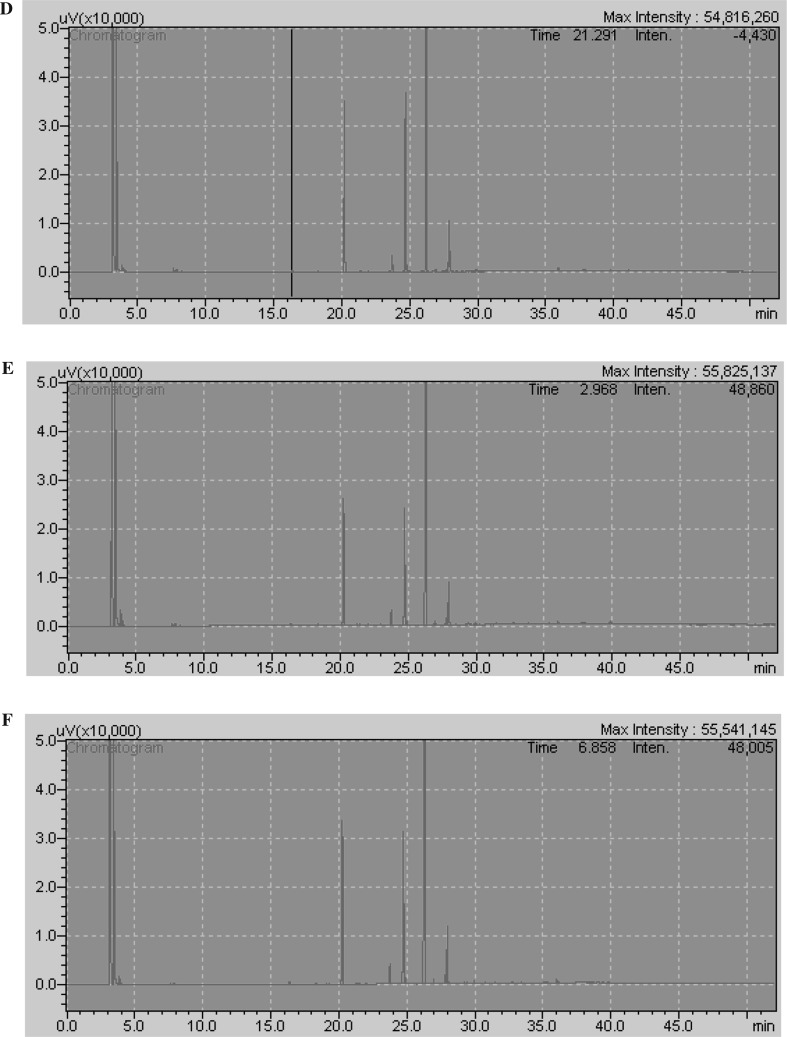
Fatty acid composition of barley, green mallt and malt is given in Table 3. The chromatograms of fatty acids of barley, green malt and malt grain oils are given in Fig. 2d–f, respectively. The dominant fatty acids of barley were linoleic (51.74–56.73%), oleic (15.62–19.94%) and palmitic (17.05–18.53%) acids (p < 0.05). The fatty acid profiles of lipids showed a significant change with malting process. While barley contain 18.53% palmitic, 19.94% oleic and 51.74% linoleic acids, malt oil contained 17.33% palmitic, 15.62% oleic and 56.56% linoleic acids. Linoleic acid content increased from 51.74 to 56.73% in green malt while, oleic acid content decreased from 19.94 to 15.79% green malt; to 15.62% in malt (p < 0.05). Additionally, the highest palmitic acid content was observed in barley with the value of 18.53%. While linoleic acid content increased during malting process whereas oleic and palmitic acid content decreased. According to the study of Bravi et al. (2012), the linoleic acid content of different barley varieties increased from 56.09–57.81 to 56.90–60.65%, while oleic acid content decreased from 12.93–13.97 to 10.49–12.01% during malting process.
Table 3.
Fatty acid compositions of barley, green malt and malt sample oils (%)
| Fatty acids | Barley | Green malt | Malt |
|---|---|---|---|
| Myristic | 0.22 ± 0.00*b | 0.27 ± 0.01a | 0.23 ± 0.01b |
| Palmitic | 18.53 ± 0.27a** | 17.05 ± 0.18b | 17.33 ± 0.44b |
| Stearic | 1.85 ± 0.02b | 2.02 ± 0.00a | 2.13 ± 0.02a |
| Oleic | 19.94 ± 0.07a | 15.79 ± 0.11b | 15.62 ± 0.11b |
| Linoleic | 51.74 ± 0.22b | 56.73 ± 0.30a | 56.56 ± 0.28a |
| Arachidic | 0.31 ± 0.01c | 0.47 ± 0.02a | 0.45 ± 0.01b |
| Linolenic | 0.97 ± 0.04a | 0.95 ± 0.00b | 0.86 ± 0.02c |
| Behenic | 0.18 ± 0.01c | 0.33 ± 0.01a | 0.25 ± 0.01b |
| Arachidonic | 0.14 ± 0.01b | 0.18 ± 0.01a | 0.17 ± 0.01a |
* Mean ± SD; ** values within each row followed by different letters are significantly different (p < 0.05)
Fig. 2.
Chromatograms of fatty acid compositions of barley (d), dried green malt (e) and malt (f) grain oils. d Chromatogram of fatty acid profile of barley grain oil. e Chromatogram of fatty acid profile of dried green malt grain oil. f Chromatogram of fatty acid profile of malt grain oil
Conclusion
Antioxidant activity, total phenolic content, phenolic compounds and carotenoid content of green malt was the highest when compared with barley and malt. Many changes occured in the bioactive components, and fatty acid composition of barley during malting process. (+)-Catechin, caffeic acid and quercetin content showed the major increase during germination. Accordingly, germination process has an important role to increase the content of bioactive compounds. Results showed that lipids content of green malt increased with germination compared to barley and malt grains. Moreover, linoleic acid content increased during malting process while oleic and palmitic acid content decreased.
Acknowledgement
The authors extend their appreciation to the International Scientific Partnership Program ISPP at King Saud University for funding this research work through ISPP# 0015.
References
- AACC . American Association of Cereal Chemists, Approved methods of the AACC. St. Paul: The Association; 1990. [Google Scholar]
- Alam MZ, Haider SA, Paul NK. Yield and yield components of barley (Hordeum vulgare L.) cultivars in relation to nitrogen fertilizer. J Appl Sci Res. 2007;3:1022–1026. [Google Scholar]
- Andarwulan N, Fardiaz D, Wattimena GA, Shetty K. Antioxidant activity associated with lipid and phenolic mobilization during seed germination of Pangium edule Reinw. J Agric Food Chem. 1999;47:3158–3163. doi: 10.1021/jf981287a. [DOI] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis. 15. Washington: Association of Official Analytical Chemists; 1990. [Google Scholar]
- Arif M, Abbas Bangash J, Khan F, Abid H. Effect of soaking and malting on the selected nutrient profile of barley. Pak J Biochem Mol Biol. 2011;44:18–21. [Google Scholar]
- Bravi E, Marconi O, Perretti G, Fantozzi P. Influence of barley variety and malting process on lipid content of malt. Food Chem. 2012;135:1112–1117. doi: 10.1016/j.foodchem.2012.06.041. [DOI] [PubMed] [Google Scholar]
- Cai S, Han Z, Huang Y, Chen ZH, Zhang G, Dai F. Genetic diversity of individual phenolic acids in barley and their correlation with barley malt quality. J Agric Food Chem. 2015;63:7051–7057. doi: 10.1021/acs.jafc.5b02960. [DOI] [PubMed] [Google Scholar]
- Carvalho DO, Curto AF, Guido LF. Determination of phenolic content in different barley varieties and corresponding malts by liquid chromatography-diode array detection-electrospray ionization tandem mass spectrometry. Antioxidants. 2015;4:563–576. doi: 10.3390/antiox4030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho DO, Goncalves LM, Guido LF. Overall antioxidant properties of malt and how they are influenced by the individual constituents of barley and the malting process. Compr Rev Food Sci Food Saf. 2016;15:927–943. doi: 10.1111/1541-4337.12218. [DOI] [PubMed] [Google Scholar]
- Cozzolino D, Degner S. An overview on the role of lipids and fatty acids in barley grain and their products during beer brewing. Food Res Int. 2016;81:114–121. doi: 10.1016/j.foodres.2016.01.003. [DOI] [Google Scholar]
- Frank T, Scholz B, Peter S, Engel KH. Metabolite profiling of barley: influence of the malting process. Food Chem. 2011;124:948–957. doi: 10.1016/j.foodchem.2010.07.034. [DOI] [Google Scholar]
- Goplan C, Ramashastri BV, Balsubramanian SC, Narasiga Rao BS, Deosthale YG, Pant KC. Nutritive value of Indian foods. Hydrabad: National Institute of Nutrition, ICMR; 1989. [Google Scholar]
- Goupy P, Hugues M, Boivin P, Amiot MJ. Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts and of isolated phenolic compounds. J Sci Food Agric. 1999;79:1625–1634. doi: 10.1002/(SICI)1097-0010(199909)79:12<1625::AID-JSFA411>3.0.CO;2-8. [DOI] [Google Scholar]
- Gupta M, Abu-Ghannam N, Gallaghar E. Barley for Brewing: characteristic changes during maltig, brewing and applications of its by-products. Compr Rev Food Sci Food Saf. 2010;9:318–328. doi: 10.1111/j.1541-4337.2010.00112.x. [DOI] [PubMed] [Google Scholar]
- Ha KS, Jo SH, Mannam V, Kwon YI, Posyolidis E. Stimulation of phenolics, antioxidant and α-glucosidase inhibitory activities during barley (Hordeum vulgare L.) seed germination. Plant Foods Hum Nutr. 2016;71:211–217. doi: 10.1007/s11130-016-0549-2. [DOI] [PubMed] [Google Scholar]
- International Organization For Standardization (ISO) Animal and vegetable fats and oils preperation of methyl esters of fatty acids, ISO. Geneve: Method ISO; 1978. pp. 1–6. [Google Scholar]
- Kim KO, Kim MK, Kang YY, Lee YC. Effects of malting conditions on quality characteristics of malt and roasted malt extract. Cereal Chem. 1993;70(4):440–442. [Google Scholar]
- Langos D, Gganvogl M, Meitinger M, Schieberle P. Development of stable isotope dilution assays for the quantitation of free phenolic acids in wheat and barley and malts produced thereof. Eur Food Res Technol. 2015;241:637–645. doi: 10.1007/s00217-015-2492-0. [DOI] [Google Scholar]
- Lee SK, Mbwambo ZH, Chung HS, Luyengi L, Games EJC, Mehta RG. Evaluation of the antioxidant potential of natural products. Comb Chem High Throughput Screen. 1998;1:35–46. [PubMed] [Google Scholar]
- Liu DJ, Pomeranz Y, Robbins GS. Mineral content of developing and malted barley. Cereal Chem. 1975;5:678–686. [Google Scholar]
- Maillard MN, Berset C. Evolution of antioxidant activity during kilning: role of insoluble bound phenolic acids of barley and malt. J Agric Food Chem. 1995;43(7):1789–1793. doi: 10.1021/jf00055a008. [DOI] [Google Scholar]
- Maillard MN, Soum MH, Boivin P, Berset C. Antioxidant activity of barley and malt: relationship with phenolic content. Lebensm Wiss Technol. 1996;29(3):238–244. doi: 10.1006/fstl.1996.0035. [DOI] [Google Scholar]
- Marecek V, Mikyska A, Hampel D, Cejka P, Neuwırthova J, Malachova A, Cerkal R. ABTS and DPPH methods as a tool for studying antioxidant capacity of spring barley and malt. J Cereal Sci. 2017;73:40–45. doi: 10.1016/j.jcs.2016.11.004. [DOI] [Google Scholar]
- Püskülcü H, Filiz F. Introduction to statistic. İzmir: Bilgehan Press; 1989. p. 333. [Google Scholar]
- Sadeghi N, Oveisi MR, Jannat B, Hjimahmoodi M, Malayeri N, Behzad M. Assessment of some heavy metals concentration and antioxidant activity in barley grain cultivars and their malts from Iran. J Agric Chem Environ. 2016;5:121–131. [Google Scholar]
- Silva da Rocha A, Rozha EK, Aalves LM, Amaral de Moraes B, Carvalho de Castro T, Albarello N, Simoes-Gurgel C. Production and optimization through elicitation of carotenoid pigments in the in vitro cultures of Cleome rosea Vahl (Cleomaceae) J Plant Biochem Biotechnol. 2015;24:105–113. doi: 10.1007/s13562-013-0241-7. [DOI] [Google Scholar]
- Yoo KM, Lee KW, Park JB, Lee HJ, Hwang IK. Variation in major antioxidants and total antioxidant activity of Yuzu (Citrusjunos SiebexTanaka) during maturation and between cultivars. J Agric Food Chem. 2004;52:5907–5913. doi: 10.1021/jf0498158. [DOI] [PubMed] [Google Scholar]