Abstract
The aim of this work was to evaluate the incorporation of a freeze-dried probiotic strain (Lactobacillus plantarum CIDCA 83114) into zeolites. The bacteria-zeolite mixture was added to poultry feed together with thyme, and the obtained product was stored for 60 days at 25 °C and 60–70% relative humidity. The ability of the obtained product to remove aflatoxin B1 (AFB1) was studied. The highest bacterial viability was observed when 50% w/w bacteria were added to zeolites. The bacterial:zeolite mixtures were then incorporated into poultry feed containing or not thyme. Initial counts of L. plantarum were in the range of 1–2 × 108 CFU/g for all samples. In all cases, bacterial viability decreased one logarithmic order after 20 days of storage, and three logarithmic orders after 60 days. No significant viability loss was observed after exposure of the poultry feed to gastro-intestinal conditions. Freeze-dried L. plantarum and zeolite were able to remove AFB1, with an average reduction of 20 and 80%, respectively. Moreover, the freeze-dried bacteria-zeolite mixture was capable to remove up to 90% AFB1. This work contributes to enhance the nutritional quality of poultry feed, with a strong impact in production.
Keywords: Lactobacillus plantarum, Poultry feed, Zeolite, Aflatoxin B1
Introduction
The nutritional quality of poultry feed is crucial for many aspects of production, including productivity, health, protection against pathogens or detoxification and modulation of the immune system among others (Chaucheyras-Durand and Durand 2010; Stanley et al. 2014). Feedstuffs containing additives promoting optimal gastrointestinal environment may contribute to decrease infections by enhancing the concentration of beneficial bacteria (i.e.: lactic acid bacteria) (Mookiah et al. 2014).
Since 2006, the European Union and other countries have prohibited the use of antibiotics as growth promoters in poultry. As alternatives to prevent the proliferation of pathogenic bacteria and maintain digestive health and production levels, a wide range of available products (i.e., probiotics, prebiotics, phytogenic compounds, exogenous enzymes and acidification) have demonstrated a good performance. Using probiotics promotes an adequate balance on microbial populations and exerts a positive influence on the immune system of animals (Brisbin et al. 2011). At present, many studies have been addressed to implement probiotics as health promoting agents in replacement of antibiotics (Salim et al. 2013; Zhang and Kim 2014). Their benefits include a significant increase in the resistance of Escherichia coli, Salmonella or Clostridium infections in broilers (Carter et al. 2017). In turn, phytogenic additives can modulate microflora and immune response and their antimicrobial, antioxidant, anti-stress properties (Windisch et al. 2008) when added to feedstuffs. In particular, the thyme leaves and flowers contain considerable amounts of essential oils (1.0–2.5%), flavonoids, tannins, phenolic acids, carbohydrates and triterpenes and have antimicrobial effects against a wide variety of potential animal pathogens (Haselmeyer et al. 2015).
It must also be considered that poultry feed is usually contaminated with mycotoxins, generating a great risk for animal health. This contamination may cause severe economic losses as a consequence of the lower efficacy of animal husbandry. Aflatoxins, potent mycotoxins produced mainly by Aspergillus flavus and Aspergillus parasiticus, represent an important concern in poultry production. Their carcinogenic, mutagenic, teratogenic, and growth inhibitory effects have been extensively proved (Yunus et al. 2011). To reduce the presence of mycotoxins in poultry feed, several types of mineral adsorbents (aluminosilicates, bentonites, zeolites, sepiolite, diatomite and activated carbons) have been used (Di Gregorio et al. 2014). In particular, clinoptilolite (a natural zeolite) has proved to adsorb aflatoxins as result of the interaction of its surface with calcium. Zeolites contain most of the major and trace minerals that are essential for the growth of chicken, livestock, and aquatic animals (Khadem et al. 2012). Moreover, it has been reported that the presence of probiotics can bind mycotoxins in food (Biernasiak et al. 2006). Therefore, the presence of zeolite and probiotics in chicken feed may enhance removal of mycotoxins and avoid injury during poultry breeding.
Considering that up to our knowledge no studies dealing with probiotic, phytogenic and zeolites incorporated altogether in poultry feed have been carried out, the aim of this study was to evaluate the stability of L. plantarum CIDCA 83114 mixed with zeolite when incorporated in poultry feed containing thyme. The ability of the obtained product to bind aflatoxins was also assessed.
Materials and methods
Bacteria and growth conditions
A fresh culture of L. plantarum CIDCA 83114 (approximately 2 × 1010 CFU/mL) was suspended in milk and freeze-dried on a Heto FD4 equipment (Heto Lab Equipment, Denmark) operating with the condenser at − 45 °C for 48 h. Freeze-dried microorganisms were stored at 4 °C. Lactobacilli counts were performed on MRS agar and the plates, incubated at 37 °C in aerobic conditions. Viable counts were performed in duplicate and results, expressed as logarithmic colony forming units per gram (log CFU/g).
Poultry feed composition and zeolite microbiological analysis
A corn-based feed containing 77% w/w corn, 18% w/w soy expeller HI pro, 1% w/w CaCo3, 1% w/w NaCl, 1% w/w meat meal, 1% w/w blood meal and 1% w/w dried crushed thyme was used. Corn-based feed without crushed thyme was used as control.
A natural zeolite (puesto Calchaquí, Salta, Argentina) was used in the experiments. Lactobacilli, total mesophyll bacteria and mold concentrations were determined by plate counting in MRS agar (Difco, Beauvais, France), Plate Count Agar (Merck, Darmstadt, Germany) Dichloran Rose-Bengal Chloramphenicol Agar (BIOKAR, Oise, France), respectively. Lactobacilli were incubated at 37 °C and aerobic mesophylls and molds, at 30 °C. Viable counts were performed in duplicate and results were expressed as log CFU/g.
Incorporation of freeze-dried Lactobacillus plantarum CIDCA 83114 into zeolite
An amount of 10, 30 and 50% w/w of freeze-dried L. plantarum were added into the zeolite. The mixtures were stored for 40 days at 25 ± 2 °C and 60–70% relative humidity. Lactobacilli counts were performed every 20 days.
Viability during storage
The feed mixtures assayed were prepared as follows: (A) poultry feed (99.0 g/100 g) + freeze-dried L. plantarum CIDCA 83114 (0.5 g/100 g) + zeolite (0.5 g/100 g) + thyme (0.5 g/100 g); (B) poultry feed (99.0 g/100 g) + freeze-dried L. plantarum CIDCA 83114 (0.5 g/100 g) + zeolite (0.5 g/100 g); (C) poultry feed (99.5 g/100 g), freeze-dried L. plantarum CIDCA 83114 (0.5 g/100 g) + thyme (0.5 g/100 g); (D) poultry feed (99.5 g/100 g) + freeze-dried L. plantarum CIDCA 83114 (0.5 g/100 g).
Samples were stored for 60 days at 25 ± 2 °C and 60–70% relative humidity. Lactobacilli were plate counted every 20 days.
Resistance to simulated gastric and intestinal conditions
The resistance to gastric and intestinal digestions was assessed according to Musikasang et al. (2009). Briefly, a simulated gastric juice (125 mM NaCl, 7 mM KCl, and 45 mM NaHCO3) was prepared by suspending 3 mg/mL pepsin (Sigma-Aldrich, Germany) and adjusting the pH to 3.0 with 1.0 M HCl. The intestinal fluid contained 22 mM NaCl, 3.2 mM KCl, 7.6 mM NaHCO3, 1 mg/mL pancreatin (Sigma- Aldrich, Germany), 7% v/v fresh chicken bile. The pH was adjusted to 8.0 with 1.0 M NaOH.
A 0.5 g mass of freeze-dried L. plantarum CIDCA 83114 or 1 g of feed mixture was suspended in simulated gastric juice. After 2 h of digestion at 41 °C, cells were harvested (1500×g for 10 min) and suspended in simulated intestinal juice. The suspension was incubated at 41 °C for 6 h. Resistance was assessed by plate counting lactobacilli after exposure to gastric and intestinal conditions.
AFB1 binding assay and quantitation
The capacity of freeze-dried L. plantarum CIDCA 83114 (2 × 108 CFU/mL) and zeolite suspensions (0.5 g/100 mL), and bacteria:zeolite mixtures (1:1) to bind AFB1 was investigated according to Peltonen et al. (2001). Briefly, 1 mL of culture was centrifuged (1500×g, 15 min), the pellet was washed twice with 1 mL of sterile double-distilled water, and suspended in 1 mL of the working solution of AFB1 (500 ppb) and incubated at 30 °C for 1 h. Subsequently, the microorganisms and/or zeolite were removed by centrifugation. The supernatants containing unbound AFB1 were collected and analyzed by HPLC, as described by Peltonen et al. (2001). Lactobacillus plantarum suspended in phosphate buffered saline (PBS) [K2HPO4 0.144 g/L; NaCl 9.00 g/L; Na2HPO4 0.795 g/L], and in 500 ppb solution of AFB1 (working solution) were used as controls.
Lactobacilli counts were performed as described above. The percentage of aflatoxin bound to bacteria was calculated using the following equation:
where A is the concentration of aflatoxin in the supernatant of the control (not bound) and B is the concentration of aflatoxin in the supernatant of the treated samples.
Supernatants were also analyzed using a commercial kit (Agraquant® ELISA Aflatoxin B1, Romer Labs, USA).
Statistical analysis
Results were expressed as mean ± SD of at least two independent duplicate trials. For statistical comparisons, ANOVA and Fisher’s test was performed and if p < 0.05 the differences were considered statistically significant.
Results and discussion
Viability of freeze-dried Lactobacillus plantarum CIDCA 83114 in zeolite with thyme
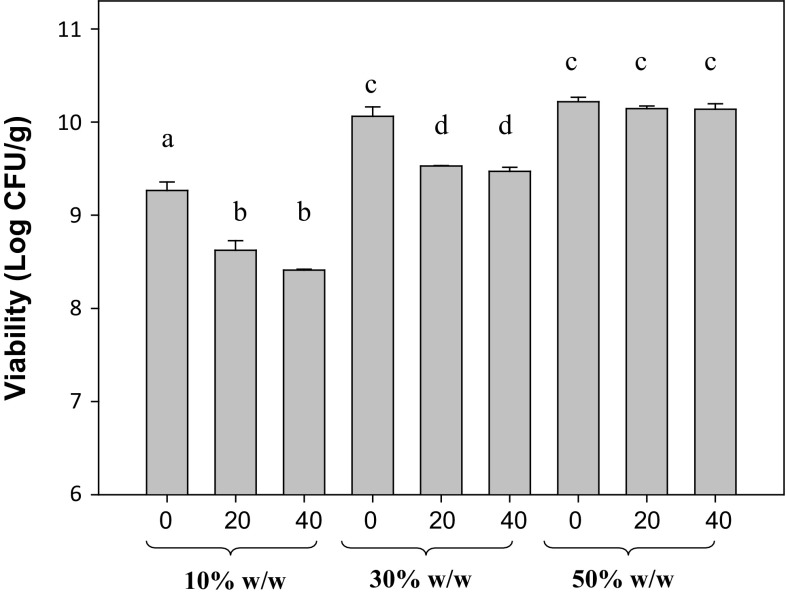
To evaluate the feasibility of incorporating freeze-dried L. plantarum CIDCA 83114 into zeolites, different bacterial concentrations (10, 30 and 50% w/w) were added to the zeolite and viability was assessed during storage at 25 °C (Fig. 1). When 10% w/w microorganisms were added to zeolite, the initial count (time 0) was 9 log CFU/g and after 20 and 40 days of storage, a significant viability decrease was observed (p < 0.05). When 30% w/w bacteria were added, the initial count was 10 log CFU/g and after 20 and 40 days of storage, a significant decrease of viability was also observed (p < 0.05). In turn, adding 50% w/w microorganisms to zeolite did not lead to a significant viability decrease during storage (p > 0.05). This result suggests that natural zeolite did not affect the L. plantarum CIDCA 83114 viability during the storage.
Fig. 1.
Viability of freeze-dried Lactobacillus plantarum CIDCA 83114 when mixed with zeolite. Different concentrations of bacteria were used: 10, 30 and 50% w/w. Microorganisms plus zeolites were stored for 0, 20 and 40 days at 25 ± 2 °C and 60–70% relative humidity. Results are expressed as log CFU/g mixture and they are presented as means and standard deviations. Different letters indicate significant differences (p < 0.05)
Zeolites are inorganic compounds whose non-hygroscopic properties preclude spoilage or caking during storage. In this regard, Bintas et al. (2014) reported that low amounts of zeolites (less than 0.8% w/w) may be included in broiler diets to prevent adversely effects in production. Incorporation of up to 10% of zeolites in animal feed results in changes in the energy, proteins and amino acid contents of the diets and this could be detrimental for the optimal development of the animals. Freeze-dried L. plantarum CIDCA 83114 was added in poultry feed and its survival rate was evaluated for up to 60 days (Table 1). Immediately after incorporation in different mixtures (time equal to 0), bacterial counts were around 8 log CFU/g. After 20 days of storage, the viability decreased one logarithmic order in all cases. Finally, after 60 days of storage a decrease between 3 and 4 logarithmic orders was observed in all cases.
Table 1.
Lactobacillus plantarum CIDCA 83114 viability in the feed mixture during storage
| Mixture | Composition | Storage day (log CFU/g)* | ||
|---|---|---|---|---|
| 0 | 20 | 60 | ||
| A | Poultry feed + strain + zeolite + thyme | 8.33 ± 0.12a | 7.76 ± 0.11b | 4.38 ± 0.12c |
| B | Poultry feed + strain + zeolite | 8.16 ± 0.10a | 7.53 ± 0.12b | 4.38 ± 0.15c |
| C | Poultry feed + strain + thyme | 8.21 ± 0.14a | 7.63 ± 0.18b | 4.70 ± 0.05c |
| D | Poultry feed + strain | 8.08 ± 0.08a | 7.66 ± 0.11b | 5.00 ± 0.32c |
*Different letters indicate significant differences
In a further step, the probiotic strain plus zeolite was added to poultry feed containing thyme. It was reported that thyme improves the broilers performance when added in chicken feed in concentrations of 15–20 g/kg (Abdel-Wareth et al. 2012). Given that thyme exerts antimicrobial activity, we assessed the strain viability in the presence of this additive. Lactobacillus plantarum CIDCA 83114 plus zeolite was added to poultry feed with or without thyme and probiotic counts did not show significant differences at a given time of storage (Table 1). These results indicate that the different components of the mixtures did not affect strain viability.
Resistance to simulated gastric juice
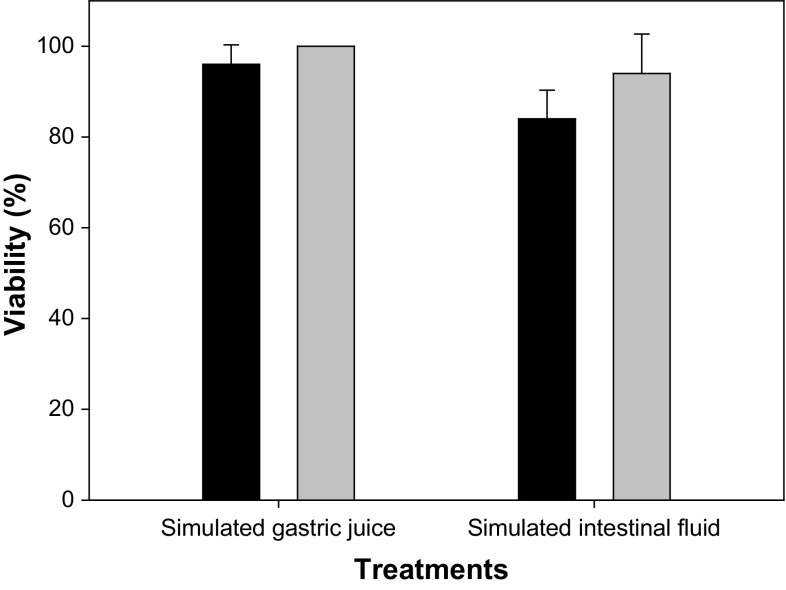
Functionality of probiotics depends on their ability to overcome the harmful gastrointestinal environment and arrive viable in large amounts to the gut. Therefore, bacterial resistances to the acidic stomach environment and to bile acids are important properties (Musikasang et al. 2009). Pure L. plantarum CIDCA 83114 is highly resistant to bile and sensible to pH ranges of 2.3 ± 0.1 when exposed to human simulated gastrointestinal conditions (Hugo et al. 2016). In this work, simulated gastrointestinal conditions were defined according to those found in chickens. The viability of freeze-dried L. plantarum CIDCA 83114 did not decrease in simulated gastric juice (black bars in Fig. 2). After exposure to simulated intestinal fluid for 6 h, bacterial viability decreased 12%. In turn, when microorganisms were incorporated into the poultry feed + zeolite + thyme, the viability of L. plantarum CIDCA 83114 did not decrease either after exposure to gastric or after exposure to intestinal conditions (gray bars in Fig. 2). These results show the protective effect of the poultry feed + zeolites when exposed to gastrointestinal conditions, thus enabling the safe arrival of bacteria to the gut, where probiotics exert their beneficial effect on the host health (Chaucheyras-Durand and Durand 2010).
Fig. 2.
Survival of freeze-dried Lactobacillus plantarum CIDCA 83114 (black bars) and feed mixture containing poultry feed + zeolite + freeze-dried L. plantarum CIDCA 83114 [99/0.5/0.5 w/w/w] (gray bars) to simulated gastrointestinal conditions. Samples were incubated in simulated gastric juice. Initial counts of freeze-dried L. plantarum CIDCA 83114 in MRS agar were 1.05 ± 0.21 × 1010 CFU/mL. Results are presented as means and standard deviations of the viability percentage of the strain after each treatment. The viability percentage was calculated as the ratio between the initial counts and the final counts after each treatment
AFB1 binding ability
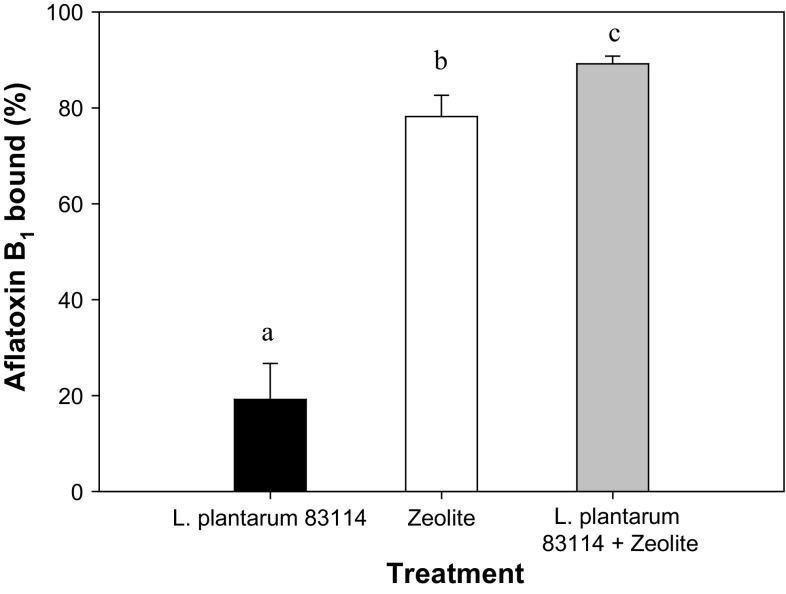
The AFB1 binding ability of L. plantarum CIDCA 83114 and zeolite was assessed individually and in mixtures containing 50% w/w bacteria. Probiotic bacteria are able to bind specific dietary contaminants, although the extent of binding differs depending on the bacterial strain, ranging from 5.8 to 31.3% (Peltonen et al. 2001). Freeze-dried L. plantarum CIDCA 83114 was capable to remove 20 ± 4% AFB1 throughout the incubation period (Fig. 3). Additionally, zeolite was capable to remove AFB1 four times more than freeze-dried L. plantarum. In turn, the freeze-dried L. plantarum CIDCA 83114 + zeolite mixture was able to remove significantly higher amounts of AFB1 (p < 0.05), reaching a maximum average of 90% (Fig. 3). The results show that freeze-dried L. plantarum CIDCA 83114 binds AFB1 immediately after having interacted with the toxin. Different studies have shown the ability of different strains of lactic acid bacteria to bind AFB1 under different conditions. Some of these conditions include viable and non-viable microorganisms, different pH values, presence of bile salts, or bacteria grown in different media, among others (Peltonen et al. 2001; Gratz et al. 2004).
Fig. 3.
Aflatoxin B1 binding by freeze-dried L. plantarum CIDCA 83114, zeolite and the combination of both. Results are presented as means and standard deviations. Different letters indicate significant differences (p < 0.05)
It was reported that the natural zeolite (clinoptilolite) adsorbs damaging toxins that potentially reduce the animals’ growth, affects gut morphology, decreases pH, and reduces pathogenic bacteria counts, suggesting an improvement of the intestinal health (Al-Nasser et al. 2011; Wu et al. 2013). Moreover, the addition of yeast, zeolite and active charcoal, alone or in combination significantly reduce the symptoms arising from AFB1 intoxication in broilers fed with contaminated feed (Khadem et al. 2012). In addition, the in vivo efficacy of zeolites to ameliorate the consequences of aflatoxicosis, mainly in poultry, has also been reported (Wawrzyniak et al. 2017).
In this work, zeolita (clinoptilolite) alone bound four times more AFB1 (81%) than freeze-dried strain, indicating its high ability to bind this mycotoxin. The percentage of bound AFB1 was higher than that earlier reported by Dvorák (1989) (0.3–27% for zeolite added at 5% w/v in water and saline solution). Other studies compared the ability of zeolite, bentonite and diatomite to bind different mycotoxins in electrolyte solutions at pH 3.0 and 6.9, reporting more than 95% binding for AFB1 (Bočarov-Stančić et al. 2011). Our results were close to those previously reported, thus confirming the applicability of this additive to bind mycotoxins. These results are also consistent with other studies, which proved that zeolite channels allow the diffusion of AFB1 to the intracrystalline structure. Moreover, clinoptilolite exhibits adsorption indexes over 80% for AFB1 (Tomašević-Čanović et al. 2001) and the adsorption process takes place within the first few minutes of contact with zeolite. Our experiments were conducted between the additive and the aflatoxin, followed by the separation steps, which confirmed that binding took place immediately.
Mixtures containing zeolite and freeze-dried L. plantarum CIDCA 83114 significantly improved the capacity to bind AFB1 relatively to that of each component separately. These results support the conjoint use of both additives in the future.
Conclusion
Using probiotics in poultry feed is a useful approach to reduce infections in poultry and derivatives. The mixture of probiotics and zeolite used in this work allowed an optimal homogenization in poultry feed, with suitable bacterial survival during non-refrigerated storage (60 days at 25 °C). Suitable bacterial survival during storage and a high AFB1 binding capacity were other benefits of the developed poultry feed. Our results confirm that L. plantarum CIDCA 83114 overcame the exposure to in vitro gastrointestinal conditions, zeolites having a protective effect. Mixtures containing probiotic L. plantarum CIDCA 83114, zeolite and thyme can be used to increase poultry nutritional quality, thus improving the poultry production.
Acknowledgements
This work was supported by the Argentinean Agency for the Promotion of Science and Technology (Projects PID/2014/0049, PICT/2014/0912), the Argentinean National Research Council (CONICET) and CYTED Program (Network 115 RT0488). A.F.M is fellow from the Commission for Scientific Research (CICPBA, Argentina), A.G.-Z and M.G are members of the research career from the Argentinean National Research Council (CONICET).
References
- Abdel-Wareth A, Kehrausa S, Hippenstiela F, Südekuma KH. Effects of thyme and oregano on growth performance of broilers from 4 to 42 days of age and on microbial counts in crop, small intestine and caecum of 42-day-old broilers. Anim Feed Sci Technol. 2012;178:198–202. doi: 10.1016/j.anifeedsci.2012.10.006. [DOI] [Google Scholar]
- Al-Nasser A, Al-Saffar A, Abdullah F, Al-Bahouh M, Ragheb G, Mashaly M. Effect of adding flaxseed in the diet of laying hens on both production of omega-3 enriched eggs and on production performance. Int J Poult Sci. 2011;10:825–883. doi: 10.3923/ijps.2011.825.831. [DOI] [Google Scholar]
- Biernasiak J, Piotrowska M, Libudzisz Z. Detoxification of mycotoxins by probiotic preparation for broiler chickens. Mycotoxin Res. 2006;22:230–235. doi: 10.1007/BF02946747. [DOI] [PubMed] [Google Scholar]
- Bintaş E, Bozkurt M, Küçükyılmaz K, Konak R, Çınar M, Akşit H, Seyrek K, Uğur Çatlı A. Efficacy of supplemental natural zeolite in broiler chickens subjected to dietary calcium deficiency. Ital J Anim Sci. 2014;13:275–283. [Google Scholar]
- Bočarov-Stančić A, Adamović M, Salma N, Bodroža-Solarov M, Vučković J, Pantić V. In vitro efficacy of mycotoxins’s adsorption by natural mineral adsorbents. Biotechnol Anim Husb. 2011;27:1241–1251. doi: 10.2298/BAH1103241B. [DOI] [Google Scholar]
- Brisbin J, Gong J, Orouji S, Esufali J, Mallick A, Parvizi P, Sharif S. Oral treatment of chickens with Lactobacilli influences elicitation of immune responses. Clin Vaccine Immunol. 2011;18:1447–1455. doi: 10.1128/CVI.05100-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A, Adams M, La Ragione RM, Woodward MJ. Colonisation of poultry by Salmonella Enteritidis S1400 is reduced by combined administration of Lactobacillus salivarius 59 and Enterococcus faecium PXN-33. Vet Microbiol. 2017;199:100–107. doi: 10.1016/j.vetmic.2016.12.029. [DOI] [PubMed] [Google Scholar]
- Chaucheyras-Durand F, Durand H. Probiotics in animal nutrition and health. Benef Microbes. 2010;1:3–9. doi: 10.3920/BM2008.1002. [DOI] [PubMed] [Google Scholar]
- Di Gregorio M, Valganon de Neeff D, Vincenzi Jager A, Corassin C, de Pinho Carão A, de Albuquerque R, de Azevedo A, Fernandes Oliveira C. Mineral adsorbents for prevention of mycotoxins in animal feeds. Toxin Rev. 2014;33:125–135. doi: 10.3109/15569543.2014.905604. [DOI] [Google Scholar]
- Dvorák M. Ability of bentonite and natural zeolite to adsorb aflatoxin from liquid media. Vet Med (Prague) 1989;34:307–316. [PubMed] [Google Scholar]
- Gratz S, Mykkiinen H, Ouwehand A, Juvonen R, Salminen S, El-Nezami H. Intestinal mucus alters the ability of probiotic bacteria to bind aflatoxin B1 in vitro. Appl Environ Microbiol. 2004;70:6306–6308. doi: 10.1128/AEM.70.10.6306-6308.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselmeyer A, Zentek J, Chizzola R. Effects of thyme as a feed additive in broiler chickens on thymol in gut contents, blood plasma, liver and muscle. J Sci Food Agric. 2015;95:504–508. doi: 10.1002/jsfa.6758. [DOI] [PubMed] [Google Scholar]
- Hugo A, Bruno F, Golowczyc M. Whey permeate containing galacto-oligosaccharides as a medium for biomass production and spray drying of Lactobacillus plantarum CIDCA 83114. LWT Food Sci Technol. 2016;69:185–190. doi: 10.1016/j.lwt.2016.01.031. [DOI] [Google Scholar]
- Khadem A, Sharifi S, Barati M, Borji M. Evaluation of the effectiveness of yeast, zeolite and active charcoal as aflatoxin absorbents in broiler diets. Glob Vet. 2012;8:426–432. [Google Scholar]
- Mookiah S, Sieo C, Ramasamy K, Abdullah N, Ho Y. Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. J Sci Food Agric. 2014;94:341–348. doi: 10.1002/jsfa.6365. [DOI] [PubMed] [Google Scholar]
- Musikasang H, Tani A, H-kittikun A, Maneera S. Probiotic potential of lactic acid bacteria isolated from chicken gastrointestinal digestive tract. World J Microbiol Biotechnol. 2009;25:1337–1345. doi: 10.1007/s11274-009-0020-8. [DOI] [Google Scholar]
- Peltonen K, El-Nezami H, Haskard C, Ahokas J, Salminen S. Aflatoxin B1 binding by dairy strains of lactic acid bacteria and bifidobacteria. J Dairy Sci. 2001;84:1256–2152. doi: 10.3168/jds.S0022-0302(01)74660-7. [DOI] [PubMed] [Google Scholar]
- Salim H, Kang H, Akter N, Kim D, Kim J, Kim M. Supplementation of direct-fed microbials as an alternative to antibiotic on growth performance, immune response, cecal microbial population, and ileal morphology of broiler chickens. Poult Sci. 2013;92:2084–2090. doi: 10.3382/ps.2012-02947. [DOI] [PubMed] [Google Scholar]
- Stanley D, Hughes R, Moore R. Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appl Microbiol Biotechnol. 2014;98:4301–4310. doi: 10.1007/s00253-014-5646-2. [DOI] [PubMed] [Google Scholar]
- Tomašević-Čanović M, Daković A, Markovic V, Stojcic D. The effect of exchangeable cations in clinoptilolite and montmorillonite on the adsorption of aflatoxin B1. J Serb Chem Soc. 2001;66:555–561. [Google Scholar]
- Wawrzyniak A, Kapica M, Stępień-Pyśniak D, Łuszczewska-Sierakowska I, Szewerniak R, Jarosz Ł. The effect of dietary supplementation of transcarpathian zeolite on intestinal morphology in female broiler chickens. J Appl Poultry Res. 2017;26:421–430. doi: 10.3382/japr/pfx011. [DOI] [Google Scholar]
- Windisch W, Schedle K, Plitzner C, Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J Anim Sci. 2008;86(14 Suppl):140–148. doi: 10.2527/jas.2007-0459. [DOI] [PubMed] [Google Scholar]
- Wu Q, Wang L, Zhou Y, Zhang J, Wang T. Effects of clinoptilolite and modified clinoptilolite on the growth performance, intestinal microflora, and gut parameters of broilers. Poult Sci. 2013;92:684–692. doi: 10.3382/ps.2012-02308. [DOI] [PubMed] [Google Scholar]
- Yunus AW, Razzazi-Fazeli E, Bohm J. Aflatoxin B1 in affecting broiler’s performance, immunity, and gastrointestinal tract: a review of history and contemporary issues. Toxin. 2011;3:566–590. doi: 10.3390/toxins3060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Kim I. Effects of multistrain probiotics on growth performance, apparent ileal nutrient digestibility, blood characteristics, cecal microbial shedding, and excreta odor contents in broilers. Poult Sci. 2014;93:364–370. doi: 10.3382/ps.2013-03314. [DOI] [PubMed] [Google Scholar]