Abstract
Enzymatic mungbean meal protein hydrolysate (eb-MPH) is a novel natural flavour/antioxidant source. A 15% bromelain (w/w) concentration with a hydrolysis time of 12 h was the optimum conditions to produce eb-MPH, which showed the greatest antioxidant activities and sensory characteristics. eb-MPH was composed of oligopeptides that had low molecular weight (< 10 kDa) as well as surface hydrophobicity and high content of hydrophobic amino acids. eb-MPH contributed to DPPH (80) and ABTS (95%) scavenging activities and to savoury/sweet flavour volatile compounds (3-methyl-butanal, furfural and benzaldehyde), bouillon odour, sweet odour, chicken odour, meaty odour, moderate bitter taste and umami. In addition, principal component analysis (PCA) showed that 72.87% of the total variance confirmed the correlation between DH, S0, DPPH, ABTS, sensory characteristics and volatile flavour compounds. These results suggested that eb-MPH can be used as a natural food flavouring agent and antioxidant.
Keywords: Flavour/antioxidant peptide, Bromelain, Protein hydrolysate, Mungbean meal
Introduction
Hydrolyzed vegetable protein (HVP) or protein hydrolysate, which is prepared from the hydrolysis of plant proteins, i.e., soybean, wheat and maize, is hydrolyzed by acid or enzymes (Flavia and Maria 1998). Acid hydrolysis generates an intense flavour, but this process produces carcinogens, which are a major concern of the World Health Organization. Enzyme hydrolysis can prevent the generation of these carcinogens. The conditions of enzyme hydrolysis are mild to obtain the desired product and non-carcinogenic compounds. Enzymes are also more specific for controlling the end product (Adler-Nissen 1986). After endogenous or exogenous enzyme hydrolysis, peptides can release biological properties and flavour characteristics, and these peptides are inactive in their parent protein sequences (Chi et al. 2014).
In terms of flavour, amino acids and peptides are necessary for flavour formation in the protein hydrolysate (Izzo and Ho 1992). Different types of amino acids and peptides contribute to different taste properties (Shahidi 1998). Although protein hydrolysate produces good aroma, its bitter taste is a major problem in all protein hydrolysates. The bitter taste is due to the presence of hydrophobic amino acids in the peptide chains and the low molecular weight of the peptide (lower than 6 kDa) (Tanford 1961). However, these amino acids and peptides do not only possess to flavour or taste but also biological activities. Hydrophobic amino acids in the peptide chain and small peptide size contribute to antioxidant activity. From previous research, hydrophobic amino acids and one or more residues of His, Pro, Met, Cys, Tyr, Trp and Phe are believed to enhance the activities of the antioxidant peptides (Chen et al. 1998). Most bioactive peptides contain 2–20 amino acid residues in their chain. The molecular weight, amino acid composition and sequence influence the characteristic biological effect or functional properties, as do the source in proteins, type of enzyme and the degree of hydrolysis (Chi et al. 2014).
Mungbean meal, which is a rich source of protein, is a by-product derived from the mungbean noodle industry. Mungbean meal is excellent to use as a protein source for protein hydrolysate production because its protein content is approximately 70% dry weight. Mungbean meal protein contains high levels of hydrophobic amino acids, and these amino acids exhibit antioxidant activity in the active form as free amino acids or oligopeptides. However, the biological activity of oligopeptides usually relies on their specific amino acid sequences or molecular weights, which can be obtained from the protein via enzymatic hydrolysate (Chi et al. 2014). Recently, there were reports of using Alcalase, Neutrase and Virgibacillus sp. SK37 protease hydrolysed mungbean protein for producing bioactive peptides, which possess antioxidant activity and angiotensin I converting enzyme (ACE) inhibitor activity (Lapsongphon and Yongsawatdigul 2013; Li et al. 2006). The mungbean meal protein hydrolysate contributed to the antioxidant activity and the flavour characteristics. From a previous study, mungbean meal protein hydrolysate by Bromelain (eb-MPH) could generate flavour peptides which had a great flavour and taste such as high bouillon, high meaty and a moderate bitter taste. All enzymes for producing flavour peptides and bioactive peptides are endoproteases. Among endoproteases, bromelain has a specific cleavage amino acid position close to Arg, Lys and Phe (Adler-Nissen 1986). However, these amino acids are present in high amounts in mungbean meal, resulting in bromelain having high enzyme activity during hydrolysis (Sonklin et al. 2011). Although mungbean meal protein hydrolyzed by some proteases has been reported, in terms of the biological-antioxidant activities and flavour characteristics, the antioxidant activity and flavour characteristics of mungbean meal hydrolyzed by bromelain have been limited. Therefore, the objectives of this study were to characterize a novel potential flavour/antioxidant oligopeptide in mungbean meal protein hydrolyzed by bromelain. In addition, the correlations of the degree of hydrolysis, surface hydrophobicity, volatile flavour compounds, sensory characteristics and antioxidant activity were determined by principal component analysis (PCA).
Materials and methods
Materials
Defatted mungbean meal (DMM) was prepared from mungbean meal obtained from Sittinan Co., Ltd., Thailand, and was extracted twice with hexane. Bromelain (enzymatic activity = 97,540 CDU) was provided by K-Much Industry Co., Ltd. (Thailand). All chemical reagents were of analytical grade (Merck and Co., USA).
Preparation of mungbean protein hydrolysate by bromelain (eb-MPH)
DMM was dispersed with sterile water (10 g/100 mL) in 500-mL flasks equipped with a condenser. The pH was adjusted to 6.0 with 2 M NaOH or 2 M HCl. Bromelain was added at concentrations of 5, 10, 15 and 20% w/w. Bromelain hydrolysis was performed at 50 °C, and the hydrolysis time was 6, 12, 18 and 24 h. The reaction was stopped by heating at 95 °C for 15 min. Enzymatic bromelain mungbean meal protein hydrolysates (eb-MPH) were filtered through Whatman No. 1 filter paper. The filtrated eb-MPH was concentrated by evaporation and was collected for further analysis.
Degree of hydrolysis (% DH)
The degree of hydrolysis was determined using trichloroacetic acid (TCA), as described by Flavia and Maria (1998). Total protein (N = 6.25) was measured by the Kjeldahl method. The DH values were calculated by the following equation
Molecular weight distribution
The enzymatic mungbean protein hydrolysates (eb-MPHs) were analyzed for their molecular weight distribution using gel electrophoresis. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) was conducted using 17% separating gel in 4% stacking gel. The 25% protein of each sample was mixed with sample buffer, which consisted of distilled water, 0.5 M Tris–HCl pH 6.8, glycerol, 10% (w/v) SDS, 2-mercaptoethanol and 1% (w/v) bromophenol blue. Samples were heated at 95 °C for 40 min and were cooled to room temperature. The protein solutions (15 mL) were prepared and loaded onto the gel wells. The gels were run in an electrode buffer (pH 8.3) containing 0.3% (w/v) Tris base, 1.44% (w/v) glycine and 0.1% (w/v) SDS in a Bio-Rad vertical slab gel electrophoresis unit model Mini-Protean™ II equipped with power supply model 3000 XI (Bio-Rad Laboratory, USA). After running the electrophoresis, the gels were fixed and stained with 0.1% Coomassie Brilliant Blue R-250 in a mixture of 10% acetic acid and 40% methanol and were then destained using a mixture of 10% acetic acid and 40% methanol (Tang et al. 2003). The molecular weight standard was the Precision PlusProtein™ Dual Xtra Standards (10–250 kDa) (Bio-Rad-Laboratory, USA).
Total and free amino acid composition by HPLC
The amino acid composition of eb-MPH was determined by the method of Li et al. (2008) with slight modification. An appropriate pretreatment of the sample was performed before the amino acid analysis. For the determination of free amino acids, an equivalent volume of trichloroacetic acid (TCA) was added to the sample to precipitate peptides or proteins, with an incubation time of 2 h. The solution was then filtered through Whatman filter paper No 4. The filtrate was centrifuged at 6000 g for 10 min, and the supernatant was stored at 4 °C before injection. For the total amino acid determination, the sample was hydrolyzed at 110 °C for 22 h with 6 M HCl. After finishing the hydrolysis, the sample was transferred into a 25 mL volumetric flask, shaken vigorously and then filtered. The filtrate was then transferred into a 10-mL beaker and was placed in a vacuum desiccator to remove hydrochloric acid. The dried sample was then dissolved in 1 mL of 0.02 M hydrochloric acid and stored at 4 °C before injection. The amino acid in peptide form was the difference between the total amino acids and the free amino acids.
The amino acids of each sample were analyzed by reversed-phase, high-performance liquid chromatography (RP-HPLC) (Agilent Technologies, Agilent1100, USA). After precolumn derivatization with o-phthaldialdehyde (OPA), 1 µL of sample was injected on a Zorbax 80 A C18 column (4.6 i.d. × 180 mm, Agilent Technologies, Palo Alto, USA) at 40 °C with detection at 338 and 262 nm. Mobile phase A was 7.35 mmol/L sodium acetate/triethylamine/tetrahydrofuran (500:0.12:2.5, v/v/v) adjusted to pH 7.2 with acetic acid, and mobile phase B (pH 7.2) was 7.35 mmol/L sodium acetate/methanol/acetonitrile (1:2:2, v/v/v). External standards were used for quantification. The amino acid standards included Ala, Arg, Asp, Cys, Glu, Gly, His, Ile, Leu, Lys, Met, Phe, Pro, Ser, Thr, Tyr and Val.
Surface hydrophobicity (So)
Protein surface hydrophobicity was determined using an apolar fluorescent dye, 1-anilino-8-naphthalenesulfonate (ANS) (Tang et al. 2008). The eb-MPHs were prepared as 1% w/v protein solutions followed by five dilutions in phosphate buffer to obtain final concentrations ranging from 0.00125 to 0.03% (w/v). Twenty millilitres of ANS solution (8.0 mM in 0.l M phosphate buffer, pH 7.4) was added to 4 mL of sample. The fluorescence intensity (FI) was determined using a fluorescence spectrophotometer (JascoFP-6200 spectrofluorometer, Japan) set at 390 and 470 nm as the excitation and emission wavelengths, respectively, with a constant excitation and emission slit of 5 nm. The slope of the FI versus protein concentration plot was calculated by linear regression analysis and was used as an index of S0.
Volatile flavour compounds
The volatile flavour compounds in eb-MPHs were analyzed using dynamic headspace, solid-phase micro-extraction gas chromatography mass spectrometry (HS–SPME–GC–MS). Five millilitres of eb-MPHs were prepared in a 20 mL vial for the headspace technique. Samples were heated at 80 °C for 10 min in a GC–MS heating block. The auto-injector (CTC-CombiPal, Agilent Technologies, Switzerland) for the GC–MS system was equipped with an SPME 50/30 µm DVB/CAR/PDMS coated (2 cm) fibre (Supelco, USA). Volatile flavour compounds were absorbed by the fibre for 30 min and were desorbed for 15 min in an injector port. Volatile compounds were separated using an Agilent Technologies GC-7890A (China) DB-WAX capillary column (30 m × 0.25 mm i.d.; coated film thickness: 0.25 μm). Five hundred microliters of the headspace volume of the samples was introduced in the splitless mode. The temperature of the injection port was 240 °C. The oven temperature was maintained at 55 °C for 5 min, then increased to 180 at 10 °C/min, from 180 to 200 at 20 °C/min, and was finally held at 200 °C for 10 min. A mass spectrometer (5975C-inert-XL-EI/CI-MSD with a triple-axis detector, Agilent Technology, USA) was interfaced with the chromatographic system at an interface temperature of 250 °C using a mass scan range of 35–450 amu. The scan rate was 3.46 scans per second. Electron impact ionization was used at energy of 70 eV. Helium was used as the carrier gas at a flow rate of 2 mL/min, and the pressure was 3.32 × 104 psi. The compounds were identified by first comparing their mass spectrum (MS) with those in Wiley 275 and the NIST library at a percentage of quality matches over 85%. After that MS of each compound was compared to standard ion spectrum in Wiley 275 and the NIST library database using ChemStation Data Analysis software. The results were compared with previously published literature, followed by retention index (RI) values. RI obtained from the comparison of eb-MPH fractions with alkane standards (C8–C20).
Sensory analysis
Descriptive sensory analysis of the taste was obtained using 15 semi-trained panellists. All semi-trained panellists had previously received training in descriptive sensory analysis with more than 20 h of experience in sensory analysis of various food samples. Their personal attributes were verified according to sensory standards, and they were trained before providing the assessment score results. Training involved a questionnaire screening and taste discrimination of five standards. The concentration gradients of the five standard taste samples were: umami (0.1, 0.2, 0.4, 0.6, 0.8 and 1.0% monosodium glutamate), salty taste (0.001, 0.005, 0.01, 0.05, 0.1 and 0.2% NaCl), sour taste (0.02, 0.04, 0.08, 0.12, 0.16 and 0.2% citric acid), bitter taste (0.025, 0.05, 0.1, 0.15, 0.2 and 0.25% tannic acid) and sweet taste (0.25, 0.5, 1, 1.5, 2 and 2.5% sugar). One millilitre of eb-MPH paste was prepared by dissolving in 10 mL of water at room temperature (30 °C). The scores of eb-MPH were a quantitative descriptive analysis, with a five-point scoring test of odour: meaty, bouillon, chicken, pork, mungbean; taste: bitter, salty, sweet, sour and umami and overall acceptance. Scores ranging from none (0) to strong (5) odour or taste were defined and used for the evaluation. Because all tastes are a persistent sensation, a 3 min break was taken between the samples, during which the panellists were asked to eat unsalted biscuits as a neutralizer and to rinse their mouths thoroughly with spring water. The assessments were conducted in a sensory laboratory room fulfilling the requirements of the ISO standards (ISO 8589, 1998).
Antioxidant activities
1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging activity
The DPPH radical-scavenging activity of the hydrolysates was determined as described by Bersuder et al. (1998). Samples (300 µL) were mixed with 900 µL of a 0.1 mM DPPH in 95% of ethanol. The mixtures were incubated for 30 min in the dark at 25 °C, and the reduction of DPPH radicals was measured at 517 nm by a spectrophotometer (Orion Aquamate 800, Thermo Scientific). The control was conducted in the same manner, except distilled water was used in place of the sample. The blank was the value of 300 µL of sample solution mixed with 900 µL ethanol. The DPPH radical-scavenging activity was calculated as:
2,2′-Azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) radical-scavenging activity
The ABTS radical-scavenging activity was determined by the ABTS assay, modified from Binsan et al. (2008). The stock solutions were 7.4 mM ABTS solution and 2.6 mM potassium persulfate solution. The working solution was prepared by mixing the two stock solutions in equal quantities and allowing them to react for 12 h at room temperature in the dark. The solution was then diluted by mixing 1 mL ABTS solution with 50 mL ethanol to obtain an absorbance of 1.1 ± 0.02 units at 734 nm using a spectrophotometer (Orion Aquamate 800, Thermo Scientific). Fresh ABTS solution was prepared for each assay. Sample (50 µL) was mixed with 950 µL of ABTS solution, and the mixture was left at 25 °C for 2 min in the dark. The absorbance was then measured at 734 nm using a spectrophotometer. The control was prepared in the same manner, except distilled water was used instead of sample. The blank was the 50 µL of sample solution mixed with 950 µL ethanol. The ABTS radical-scavenging activity was calculated as:
Statistical analysis
All analyses were conducted based on a 4 × 4 factorial in a completely randomized design (CRD) with three replicates. Randomized complete block design (RCBD) with three replications was used for the sensory analysis. The results obtained were subjected to one-way analysis of variance (ANOVA). Duncan’s New Multiple Range Test was performed to determine the means of the significant difference (p ≤ 0.05) using the SAS Program Version 6.0 (SAS Institute, 1997, USA). Principal component analysis (PCA) was applied to observe the relationships between the degree of hydrolysis, ABTS, DPPH, surface hydrophobicity, sensory characteristics and volatile flavour compounds based on the Pearson correlation matrix. The correlations among variables were assessed by the means of the Pearson’s correlation test using the Principal Component Analysis (PCA) using the SPSS software packages version 7.5.
Results and discussion
Degree of hydrolysis of eb-MPHs
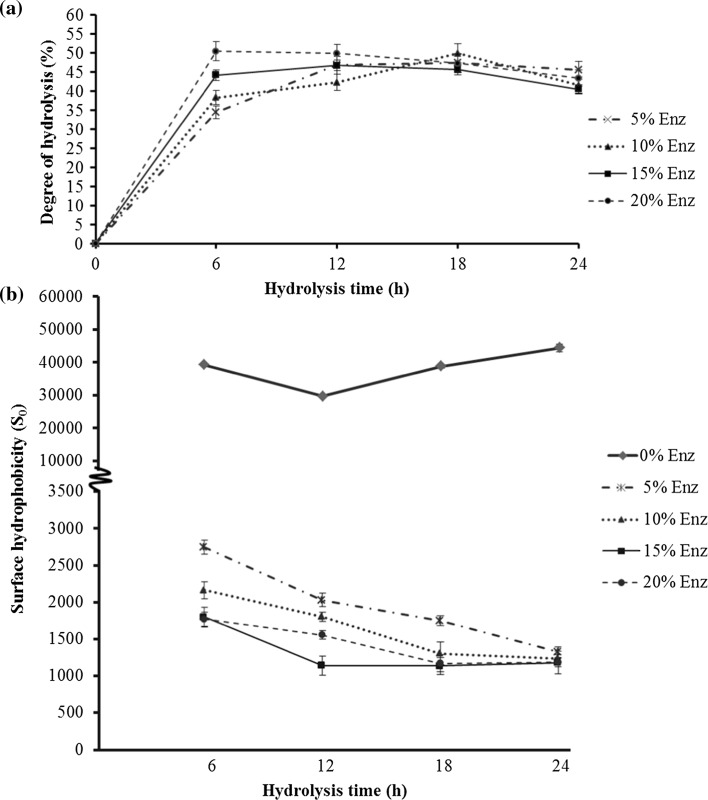
The extent of protein degradation by proteolytic enzyme was measured by assessing the degree of hydrolysis (DH), which is defined as the proportion of broken peptide bonds and total protein in a protein hydrolysate (Rutherfurd 2010). The interaction of the enzyme concentration and hydrolysis time directly influenced the degree of hydrolysis (p ≤ 0.05). The degree of hydrolysis at hydrolysis times 0–6 h for all enzyme concentrations rapidly increased and slightly increased at 6–12 h, before becoming stable. The eb-MPH of the 20% (w/w) enzyme concentration at a hydrolysis time of 6 h had the highest DH (50.4%), but its DH was not significantly different from the 15% enzyme concentration at hydrolysis times of 12, 18 and 24 h (Fig. 1a). It implied that the enzyme can completely hydrolyse mungbean meal at this condition. Even through enzyme concentration and hydrolysis time were increased, DH could not increase. This evidence might be a result of product inhibition (Kanu et al. 2009). Considering the increasing DH, bromelain can cleave the peptide bonds of mungbean meal protein and change the protein in mungbean meal from insoluble protein to soluble protein because mungbean protein consists of globulin (Fig. 2, lane 2), which can dissolve in water at pH 7–7.6 and has high amounts of Arg, Lys and Phe. This pH was in accordance with the pH optimum of bromelain, and Arg, Lys and Phe within globulin’s sequence were the specific cleavage sites of bromelain (Adler-Nissen 1986), which implied that mungbean protein was a good substrate for bromelain hydrolysis.
Fig. 1.
Degree of hydrolysis (a), surface hydrophobicity (b) of eb-MPHs from the different enzyme concentration (%w/w) and hydrolysis time (h)
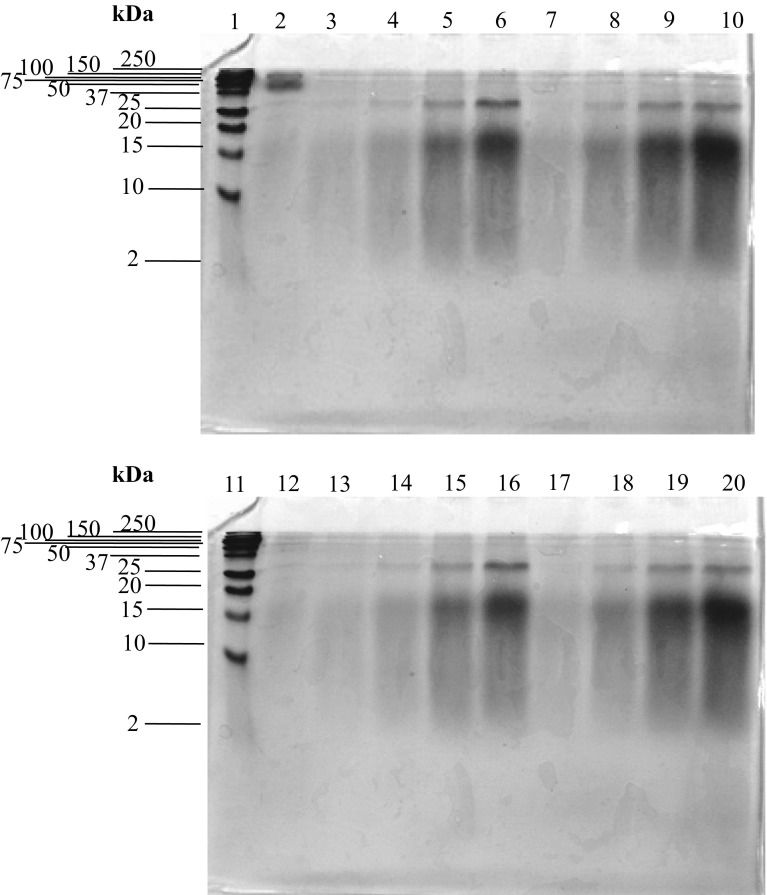
Fig. 2.
SDS–PAGE patterns of eb-MPHs, (1) and (11) molecular weight protein marker, (2) native mungbean meal protein, (3), (4), (5), (6) eb-MPH from 5, 10, 15 and 20% enz. at hydrolysis time of 6 h, respectively. (7), (8), (9) and (10) eb-MPH from 5, 10, 15 and 20% enz. at 12 h, respectively. (12) eb-MPH from 0% enz. 24 h, (13), (14), (15) and (16) eb-MPH from 5, 10, 15 and 20% enz. at hydrolysis time of 18 h, respectively. (17), (18), (19) and (20) eb-MPH from eb-MPH from 5, 10, 15 and 20% enz. at hydrolysis time of 24 h, respectively
Molecular weight distribution of eb-MPHs
The molecular weight (MW) distribution of peptides in eb-MPHs was estimated by SDS–PAGE (Fig. 2) and the MW was referenced to the 2–250 kDa standard protein markers (lane 1 and lane 11). The MW of mungbean meal protein before hydrolysis was 150 and 64 kDa (lane 2), which was consistent with the report of Medoza et al. (2001). After hydrolysis with different concentrations of bromelain for different hydrolysis times, the intensity of the bands at 150 and 64 kDa decreased, whereas the intensity of the bands at 32, 15–20 and less than 10 kDa increased with increasing of enzyme concentration and hydrolysis time (lanes 3–20). Especially, the bands at 15–20 and < 10 kDa have high intensity, which indicated that the major peptides in eb-MPHs were 15–20 and < 10 kDa. These peptides increased with the increasing of the enzyme concentration and hydrolysis time because the large MW portion of mungbean meal protein was hydrolyzed and changed to low MW peptides.
Amino acid compositions of eb-MPH
The total amino acid compositions showed that Glu, Asp, Lys, Arg, Leu, Ser, Pro and Phe were the major amino acids of the eb-MPH at 325.249, 208.780, 144.235, 134.018, 125.845, 108.537, 96.036 and 91.228 mg/g protein, respectively (Table 1). Bromelain cleaved the protein at the amino acids positions of Arg, Lys, Phe and hydrophobic amino acids (Adler-Nissen 1986; Sonklin et al. 2011), resulting in a high amount of these amino acids in eb-MPHs. Moreover, the results (Table 1) showed that most amino acids were in the peptide form because bromelain is an endoprotease, which cleave internally in the protein sequence (Jenkins 2002). Based on result of the molecular weight and amino acids in eb-MPH, eb-MPH was identified as peptide chains composed of a high amount of hydrophobic amino acids, and its molecular weight was less than 10 kDa.
Table 1.
Amino acid compositions and predicted taste of eb-MPH
| Amino acids | Content of amino acids in eb-MPHa (mg/g Protein) | ||
|---|---|---|---|
| Total amino acids | Free amino acids | Constituent amino acids of peptides | |
| Aspartic acid (Asp) | 208.78 ± 7.86 | 2.12 ± 0.08 | 206.66 ± 7.78 |
| Serine (Ser) | 108.54 ± 4.08 | 27.41 ± 1.03 | 81.13 ± 3.05 |
| Glutamic acid (Glu) | 325.25 ± 12.24 | 33.85 ± 1.27 | 291.40 ± 10.97 |
| Glycine (Gly) | 73.56 ± 2.77 | 10.99 ± 0.41 | 62.57 ± 2.35 |
| Histidine (His) | 40.03 ± 1.51 | 23.15 ± 0.87 | 16.88 ± 0.64 |
| Arginine (Arg) | 134.02 ± 5.04 | 44.21 ± 1.66 | 89.81 ± 3.38 |
| Threonine (Thr) | 66.83 ± 2.51 | 11.85 ± 0.45 | 54.98 ± 2.07 |
| Alanine (Ala) | 80.05 ± 3.01 | 20.72 ± 0.78 | 59.33 ± 2.23 |
| Proline (Pro) | 96.04 ± 3.61 | 7.14 ± 0.27 | 88.90 ± 3.35 |
| Cysteine (Cys) | 7.93 ± 0.30 | 0.39 ± 0.01 | 7.55 ± 0.28 |
| Tyrosine (Tyr) | 58.05 ± 2.18 | 10.48 ± 0.39 | 47.57 ± 1.79 |
| Valine (Val) | 72.96 ± 2.75 | 13.37 ± 0.50 | 59.59 ± 2.24 |
| Methionine (Met) | 17.79 ± 0.67 | 9.21 ± 0.35 | 8.58 ± 0.32 |
| Lysine (Lys) | 144.24 ± 5.43 | 57.89 ± 2.18 | 86.35 ± 3.25 |
| Isoleucine (Ile) | 73.80 ± 2.78 | 7.26 ± 0.27 | 66.54 ± 2.50 |
| Leucine (Leu) | 125.85 ± 4.74 | 44.59 ± 1.68 | 81.25 ± 3.06 |
| Phenylalanine (Phe) | 91.23 ± 3.43 | 14.11 ± 0.53 | 77.12 ± 2.90 |
| Taste from amino acidsb | |||
| Bitter taste | 555.68 ± 15.35 | 155.90 ± 5.8 | 399.77 ± 15.04 |
| Umami | 534.03 ± 9.41 | 35.96 ± 1.35 | 498.07 ± 18.74 |
| Sweetness | 328.98 ± 12.38 | 70.96 ± 2.67 | 258.01 ± 9.71 |
| Tasteless | 202.29 ± 7.61 | 68.37 ± 2.57 | 133.92 ± 5.04 |
aEach value is expressed as the mean ± SD (n = 3)
bTaste from amino acids: bitter taste = Arg + His + Ile + Leu + Met + Phe + Val; umami (monosodium glutamate-like) = Asp + Glu; Sweetness = Ala + Gly + Ser + Thr; tasteless = Lys + Tyr (Tsai et al. 2009)
However, free amino acids and peptides are necessary for odour and taste formation in the protein hydrolysate. The taste of peptides depends on their amino acid compositions. Each amino acid has a unique taste, which are divided into several classes on the basis of their taste characteristics. Arg, His, Ile, Leu, Met, Phe and Val possess bitter tastes. Asp and Glu are umami or monosodium glutamate-like. Ala, Gly, Ser and Thr have a sweet taste, and Lys and Tyr are tasteless (Izzo and Ho 1992). Based on amino acids compositions, the taste of eb-MPH could be determined by the amino acid accumulation. eb-MPH had distinct contributions of umami and bitter tastes, followed by sweet taste and a little salty taste (Table 1).
Surface hydrophobicity (So)
Changes in surface hydrophobicity affected the stability, conformation and the functional properties of protein, especially the interfacial properties of the protein hydrolysates. In Fig. 1b, the So of eb-MPH was significantly reduced with increasing enzyme concentration and hydrolysis time. The surface hydrophobicity of mungbean meal protein rapidly decreased from 45,000 to 3000 (15-folds) at the beginning to 6 h of hydrolysis time and further slightly decreased until 12 h, thereafter reaching a constant value. The So of mungbean meal proteins was decreased by bromelain hydrolysis. The structure of the native mungbean meal protein had a high content of hydrophobic amino acids and aromatic amino acids, especially Phe and Tyr (Sonklin et al. 2011). Phe and Tyr are not always embedded in the protein molecule due to their bulky structure (Akita and Nakai 1990). They may be situated at the surface position, where they contribute to the high So of mungbean meal protein molecules. After bromelain hydrolysis, the So of mungbean meal protein was reduced because the molecules were changed from high to low molecular weight, resulting in decreased surface hydrophobicity. The decreasing of So in mungbean meal protein is due to the unique structure. The structure is a hydrophilic core covered by a hydrophobic surface. In the bromelain hydrolysis of proteins, initial conformational changes occur due to cleavages in the polypeptide chain, unfolding of embedded sections and exposure of hydrophilic sections. In addition, eb-MPH comprised high contents of low molecular weight peptides, which have a high charge density. These peptides have few hydrophobic binding sites for the ANS probe (Wu et al. 1998), leading to a decrease in So. Moreover, the high charge density of eb-MPH peptides can interact with water molecules through hydrogen bonds and electrostatic interactions, resulting in increased protein solubility (Kim et al. 1990), which is related to the degree of hydrolysis. Although the So from this study is opposite to the previous studies of Ju et al. (2001), who reported that a higher DH had a higher So, there are other studies that observed a decrease in surface hydrophobicity of smaller peptides (Wu et al. 1998). Loss or gain in the hydrophobicity of peptides depends on the nature of the substrate, the specificity of the protease and the molecular weight of the produced peptides (Liu et al. 2010). However, this research is the first to report that the So of mungbean meal protein decreased after changing its structure by bromelain hydrolysis.
Volatile flavour compounds and sensory characteristic of eb-MPHs
All eb-MPH showed the same profile of volatile flavour compounds, which consisted of 19 compounds (Table 2). The volatile flavour compounds increased with increasing enzyme concentration and hydrolysis time. The volatile flavour compounds of eb-MPH (15% of bromelain and hydrolysis time for 12 h) had the highest abundance when compared to other condition (data not shown). The 3-methyl-butanal, 2-pentyl-furan, furfural and benzaldehyde were the major volatile compounds. Most compounds had aldehyde and furan group, which were generated from the Maillard reaction. Furan was generated by amino acid degradation of Ser, Cys, Ala, Thr and Asp, reaction with sugar by the Maillard reaction, sugar cyclization or lipid oxidation (Varanová and Ciesarová 2009). The major volatile compounds are an important factor of the odour characteristics in food. Each volatile had a specific odour description, resulting in different odour characteristics in different foods. Considering the odour description of the major volatile compounds in eb-MPH, 3-methyl-butanal contributed to the meat and bone meal and poultry odour. The odour description of 2-pentyl-furan was green bean and butter flavour (Elmore et al. 2002). Fufural had bouillon, sweet and caramel odour (Hierro et al. 2004). Benzaldehyde contributed to sweet-like and oily odour, and it was found in meat, bone meal and sukiyaki flavour (Shibamoto et al. 1981). From all odour descriptions of the major volatile compounds, eb-MPH contributed to a strong meaty and sweet odour, which was associated with the results from the sensory characteristics of eb-MPHs.
Table 2.
Retention time and characteristic mass fragment ion of volatile compounds from eb-MPH
| No. | Compounds | Retention time (min) | RIa | Relative peak area (%) | Fragment ion (m/z) | |
|---|---|---|---|---|---|---|
| Compounds | Standard ion spectrum from databasec | |||||
| 1 | 3-Methyl-butanal | 2.052 | 643 | 41.26 | 39(50)b, 41(100), 42(18), 44(85), 45(15), 57(37), 58(60), 86(10) | 39(50), 41(98), 42(17), 44(100), 45(12), 57(24), 58(50), 86(9) |
| 2 | 2-Heptanone | 5.946 | 853 | 4.74 | 39(11), 43(100), 58(60), 59(10), 71(20), 114(14) | 39(10), 43(100), 58(55), 59(9), 71(25), 114(12) |
| 3 | 2-Pentyl-furan | 7.153 | 951 | 2.17 | 39(16), 41(16), 43(7), 53(25), 81(100), 82(28), 95(19), 109(15), 123(7), 138(25) | 39(16), 41(16), 43(7), 53(25), 81(100), 82(37), 95(19), 109(15), 123(7) 138(18) |
| 4 | 3-Hydroxy-2-butanone | 8.875 | 994 | 1.43 | 42(7), 43(80), 44(10), 45(100), 73(7), 88(12) | 42(8), 43(88), 44(5), 45(100), 73(5), 88(8) |
| 5 | 2-Ethyl-5-methyl-pyrazine | 10.233 | 1002 | 6.04 | 39(20), 40(5), 43(24), 52(6), 53(8), 54(3), 56(25), 79(8), 85(7), 94(20), 121(100), 122(68) | 39(28), 40(8), 43(25), 52(6), 53(8), 54(9), 56(23), 79(8), 85(5), 94(15), 121(100), 122(67) |
| 6 | Trimethyl-pyrazine | 12.604 | 1035 | 0.39 | 39(28), 40(13), 42(40), 52(7), 53(12), 54(10), 81(15), 122(95) | 39(28), 40(13), 42(27), 52(6), 53(7), 54(9), 81(13), 122(75) |
| 7 | Acetic acid | 14.768 | 1079 | 1.72 | 41(6), 42(23), 43(100), 45(98), 60(70), 61(4) | 41(6), 42(24), 43(93), 45(94), 60(71), 61(2) |
| 8 | Furfural | 17.151 | 1103 | 22.80 | 38(12), 39(47), 42(4), 67(8), 95(92), 96(100), 97(5) | 38(14), 39(67), 42(5), 67(6), 95(89), 96(100), 97(6) |
| 9 | Benzaldehyde | 18.391 | 1136 | 6.04 | 39(8), 50(25), 51(30), 52(12), 74(10), 77(82), 78(20), 104(97), 105(97) | 39(10), 50(30), 51(50), 52(17), 74(15), 77(98), 78(16), 104(100), 105(97) |
| 10 | 2,3-Butanediol | 21.546 | 1178 | 3.74 | 43(17), 44(6), 45(100), 46(4), 47(8), 57(10), 90(2) | 43(17), 44(5), 45(100), 46(4), 47(4), 57(10), 90(2) |
| 11 | Benzeneacetaldehyde | 22.877 | 1211 | 3.64 | 51(10), 63(12), 65(20), 91(100), 92(34), 120(24) | 51(9), 63(10), 65(25), 91(100), 92(26), 120(16) |
| 12 | 3-Methyl-butanoic acid | 24.626 | 1281 | 0.35 | 39(23), 41(44), 42(12), 43(28), 45(25), 60(100), 87(28), 102(2) | 39(13), 41(33), 42(7), 43(32), 45(16), 60(100), 87(17), 102(2) |
| 13 | 3-Methylthio-1-propanol | 25.354 | 1299 | 0.58 | 41(23), 47(31), 48(22), 49(23), 57(50), 61(48), 73(29), 106(100) | 41(30), 47(35), 48(33), 49(35), 57(58), 61(75), 73(24), 106(100) |
| 14 | Pentanoic acid | 26.949 | 1368 | 1.82 | 39(12), 41(22), 42(12), 43(10), 45(14), 60(100), 73(50), 102(2) | 39(12), 41(21), 42(12), 43(17), 45(14), 60(100), 73(34), 102(2) |
| 15 | Benzene-ethanol | 30.828 | 1437 | 1.06 | 39(8), 63(8), 65(19), 77(8), 91(100), 92(48), 122(30) | 39(1), 63(7), 65(23), 77(5), 91(100), 92(56), 122(32) |
| 16 | Quinoline | 34.164 | 1525 | 0.71 | 51(7), 75(4), 77(9), 101(5), 129(100) | 51(7), 75(5), 77(10), 101(8), 129(100) |
| 17 | Pyrrorole-2-carboxaldehyde | 34.700 | 1588 | 0.87 | 37(8), 38(12), 39(34), 40(9), 66(45), 94(61), 95(100) | 37(5), 38(11), 39(31), 40(7), 66(48), 94(55), 95(100) |
| 18 | 2-Pyrrolidinone | 35.439 | 1643 | 0.27 | 39(15), 41(62), 42(48), 43(25), 55(10), 56(12), 85(97), 86(4) | 39(10), 41(69), 42(70), 43(30), 55(5), 56(9), 85(100), 86(7) |
| 19 | 2-Methoxy-4-vinylphenol | 40.698 | 1792 | 0.37 | 39(12), 51(15), 77(45), 78(10), 79(15), 107(50), 135(95), 150(97) | 39(21), 51(29), 77(67), 78(17), 79(24), 107(67), 135(100), 150(97) |
aRI (retention index) calculated with a DB-Wax stationary phase using a series of alkanes between C8 and C20
bNumber in brackets are the abundance (%) of fragment ion
cStandard ion spectrum from Wiley 275 and the NIST library database
The sensory characteristics of eb-MPHs were quantitatively analyzed with a five-point scoring test. The odour characteristics of eb-MPH showed that bouillon odour (score = 3.3) had the highest score, followed by meaty (score = 3.0), chicken (score = 2.3), sweet (score = 2.3) and mungbean (score = 1.0) odour. For the taste characteristics, eb-MPH contributed mostly to umami (score = 3.3), followed by moderate bitter taste (score = 3.0), sour taste (score = 2.3), salty taste (score = 2.0) and sweet taste (score = 2.0). The eb-MPH received a high score of overall acceptance from semi-trained panellists (score = 4.0). The tastes of eb-MPH resulted from free amino acids and/or amino acids within peptides, which are important factors contributing to flavour in foodstuff (Kirimura et al. 1969). The taste of eb-MPH from the sensory analysis was consistent with the predicted taste from the amino acid compositions of the protein hydrolysate. The strong umami taste of eb-MPH is due to the high content of Glu and Asp in the eb-MPH peptides. In addition to umami, Glu and Asp have a moderately sour taste (Kirimura et al. 1969), and the sensory characteristics of eb-MPH had a sour taste. For the bitter taste, the eb-MPH peptides consisted of a high amount of hydrophobic amino acids, such as Leu, Pro, Phe, Tyr, Ile and Trp, which are important factors in bitter taste (Matoba and Hata 1972). Bitter taste results from low molecular weight oligopeptides (Tanford 1961), and the molecular weight of the eb-MPH peptides was less than 10 kDa. From previous research, low molecular weight oligopeptides containing large amounts of hydrophobic amino acids resulted in bitter taste in peptides (Matoba and Hata 1972). Leu residues at the C-terminus contribute to the strongest bitter taste (Ishibashi et al. 1987). However, eb-MPH in this study had low molecular weight oligopeptides and contained large amounts of hydrophobic amino acid, but it had a moderately bitter taste. These results indicated that not only the type of amino acids and molecular weight but also the position of the amino acids in the peptide chain are important factors in the bitter taste in eb-MPH. Considering the results of the amino acid compositions and the sensory analysis in eb-MPH and the results from literature reviews, the eb-MPH peptide chains consisted of Glu, Asp and hydrophobic amino acids, and the C-terminus is not Leu. Although eb-MPH had a moderately bitter taste, it also had umami flavour and good flavour characteristics. These results indicate that the flavour characteristics of mungbean meal protein can be improved by bromelain hydrolysis.
Antioxidant activities of eb-MPHs
The antioxidant activities of eb-MPHs were determined using different enzyme concentrations and hydrolysis times by the DPPH and ABTS radical-scavenging activity. DPPH and ABTS are the stable radicals mainly used in ‘in vitro’ assays as they provide easily comparable results because of its rapidity, sensitivity and reproducibility (Shalaby and Shanab 2013). However the reaction mechanism of DPPH is Single Electron Transfer (SET) and ABTS is Hydrogen Atom Transfer (HAT) (Badarinath et al. 2010). These two radicals are foreign to biological systems. DPPH is used more often to determine antioxidant activity of natural plant extracts than ABTS. Moreover, ABTS is applicable for hydrophilic and lipophilic antioxidants because it is soluble in aqueous and organic solvent (Shalaby and Shanab 2013). According to the result of amino acid composition, eb-MPH composed of many amino acids which have the different characteristics. Some of amino acids can react with radical via SET, other amino acids via HAT or SET/HAT. Therefore, DPPH and ABTS were the method chosen to determine the in vitro antioxidant activity of eb-MPH. Results were expressed as a percentage of scavenging activity and revealed that the enzyme concentration and hydrolysis time had a significant effect on the DPPH and ABTS radical-scavenging activity of eb-MPH (Fig. 3a, b). The DPPH radical-scavenging activities of eb-MPHs were significantly higher than the native protein (0% bromelain). The eb-MPHs at the same enzyme concentration exhibited rapidly increased scavenging activity during the first 6 h of hydrolysis and slightly increased activity during 6–12 h before reaching constant activity (Fig. 3a). The eb-MPH derived from 15% bromelain at a hydrolysis time of 12 h had the highest DPPH radical scavenging activity (82.31%), but it was not significantly different from the 18 and 24 h and eb-MPH from 10% bromelain at 12, 18 and 24 h. In addition, the DPPH scavenging activity of eb-MPH was compared with antioxidant standards: vitamin C (water-soluble), tocopherol (water-insoluble) and trolox (water-insoluble antioxidant which was synthesized as a vitamin E derivative). The antioxidant activity of eb-MPH at 80% scavenging activity was equivalent with 600 ppm of vitamin C, 1000 ppm of Tocopherol and 600 ppm of Trolox.
Fig. 3.
a DPPH radical-scavenging activity, b ABTS radical-scavenging activity of eb-MPHs
Similarly, the ABTS radical-scavenging activities of eb-MPH are shown in Fig. 3b. The ABTS radical-scavenging activity of eb-MPH at all enzyme concentrations was increased from the initial hydrolysis time to 6 h and was unchanged after 12 h. The eb-MPH, hydrolyzed with 15% bromelain at a hydrolysis time of 12 h had the highest ABTS radical scavenging activity (94.93%) at the same conditions as the highest DPPH scavenging activity. This result indicated that a critical molecular weight peptide was important for the optimal scavenging activities. The increased DPPH and ABTS scavenging activity of eb-MPH is caused by low molecular weight peptides of less than 10 kDa. Molecular weight peptides of less than 2 kDa were rich in eb-MPH. The extension of hydrolysis time by bromelain resulted in a higher content of low molecular weight peptides. The antioxidant activity of eb-MPH is a result of the low molecular weight, low surface hydrophobicity and high protein solubility in water, resulting in easy reactivity with free radicals (Kim et al. 1990). Generally, antioxidant peptides consist of 2–20 amino acids or have molecular weights less than 6 kDa (Meisel and FitzGerald 2003). Moreover, eb-MPH peptides are composed of Phe, His, Pro, Cys, Tyr, Phe and Met, which enhance the antioxidant activity of peptides (Chen et al. 1998). The antioxidant mechanism of Phe and Tyr is explained by the special capability of phenolic groups to serve as hydrogen donors, which is one mechanism of inhibiting the radical-mediated peroxidizing chain reaction (Jung et al. 1995). The imidazole group, which is a special structure of His, Met and Cys, is very important to the radical-scavenging activity of peptides. His has proton-donation ability (Tsuge et al. 1991). Met is prone to oxidation of Met sulfoxide. Cys donates a sulfur hydrogen to the free radical (Hernández-Ledesma et al. 2005). These results suggest that both optimum molecular weight and amino acids in the peptide chain are necessary for the antioxidant activities of peptides.
According the result of amino acid composition, protein hydrolysate composed of many amino acids which have the different characteristics. Some of them can react with radical via SET, Some of them via HAT or both of two mechanisms. Therefore, DPPH and ABTS method can cover to preliminary determine the in-vitro antioxidant activity of our sample.
Correlations of DH, So, volatile flavour compounds, sensory characteristic and antioxidant activity
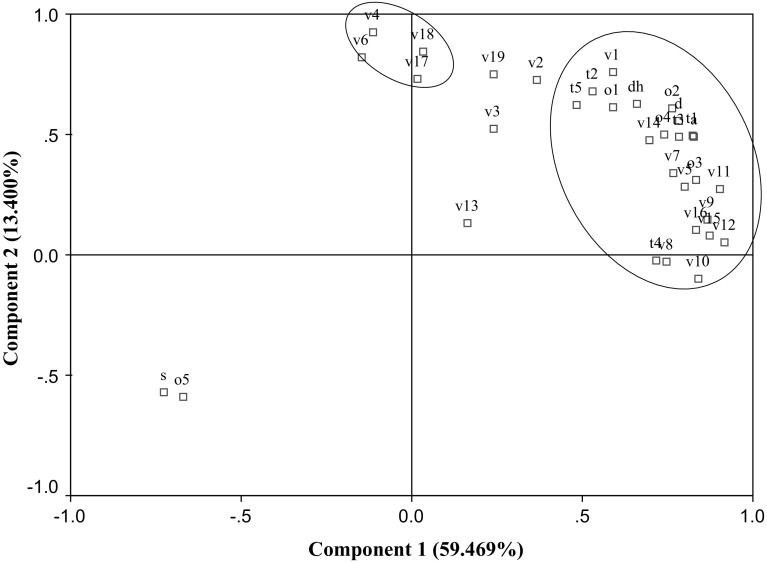
Principal component analysis (PCA) was used to determine and confirm the relationships between DH, So, volatile flavour compounds, sensory characteristic and antioxidant activity (DPPH and ABTS) of eb-MPH. Two principal components (PC) with 72.876% of the total variance data were obtained (Fig. 4). The first principal factor (PC1) explained 59.469% of the variation across samples, whereas the second principal factor (PC2) explained 13.400% of the variance. A highly significant correlation (p ≤ 0.05) was found between the DH, So, DPPH, ABTS, bouillon odour, bitter taste, sweet taste, sweet odour, mungbean odour, chicken odour, meaty odour, salty taste, sour taste, umami, 3-methyl-butanal (v1), 2-ethyl-5-methyl-pyrazine (v5), acetic acid (v7), furfural (v8), benzaldehyde (v9), 2, 3-butanediol (v10), benzeneacetaldehyde (v11), 3-methy-butanoic acid (v12), pentanoic acid (v14), benzene-ethanol (v15) and quinoline (v16). All correlated variables showed direct relationships, but So and mungbean odour showed an inverse relation because high DH exposed to the low MW peptides (< 10 kDa) resulted in decreased So, contributed to good antioxidant activity, increased the bitter taste, decreased mungbean odour, improved the odour/taste characteristics and contributed to some volatile flavour compounds. Moreover, these correlations indicated the relationship of the volatile flavour compounds and the odour characteristics of eb-MPH because some volatile flavour compounds, especially the major compounds were highly correlated to bouillon odour, sweet odour, chicken odour and meaty odour from sensory evaluation.
Fig. 4.
The correlations of DH, S0, volatile flavour compounds, sensory characteristics and antioxidant activity. The name of the specific variables corresponding to code are 3-methyl-butanal (v1), 2-heptanone (v2), 2-pentyl-furan (v3), 3-hydroxy-2-butanone (v4), 2-ethyl-5-methyl-pyrazine (v5), trimethyl-pyrazine (v6), acetic acid (v7), furfural (v8), benzaldehyde (v9), 2,3-butanediol (v10), benzeneacetaldehyde (v11), 3-methy-butanoic acid (v12), 3-methylthio-1-propanol (v13), pentanoic acid (v14), benzene-ethanol (v15), quinolone (v16), pyrrorole-2-carboxaldehyde (v17), 2-pyrrolidinone (v18), 2-methoxy-4-vinylphenol (v19), meaty odour (o1), bouillon odour (o2), chicken odour (o3), pork odour (o4), smoky odour (o5), bitter taste (t1), salty taste (t2), sweet taste (t3), sour taste (t4), umami (t5), DPPH (d), ABTS (a), DH (dh) and surface hydrophobicity (s)
Conclusion
Mungbean protein was a good substrate of bromelain hydrolysis to produce eb-MPH. The eb-MPH contained oligopeptides that had both antioxidant activities and good flavour characteristics. These oligopeptides had molecular weights < 10 kDa; high contents of Glu, Asp, Lys, Arg, Leu, Ser, Pro and Phe; DPPH and ABTS radical scavenging activity; and surface hydrophobicity of 3000. This is the first report on the changing surface hydrophobicity of mungbean meal protein hydrolyzed by bromelain. Bromelain reduced the So of mungbean meal protein (15-fold from native protein), and hydrophobic amino acids in the oligopeptide chain were exposed, resulting in enhanced antioxidant activities. The eb-MPH contributed to the antioxidant activity and the flavour characteristics. They possessed high meaty, bouillon, chicken, sweet and non-mungbean odour, strong umami, moderate bitter taste, little sour taste and good overall acceptance. The principal component analysis (PCA) indicated that the DH, DPPH, ABTS, sensory characteristics and volatile flavour compounds had strong correlations, and the correlated variables showed direct proportions. Finally, the development of bioactive peptides from mungbean meal protein may open new economic opportunities for increased value-added applications of mungbean meal.
Acknowledgements
This study was financially supported by The Royal Golden Jubilee Ph.D. Program (PHD/0107/2553:2.J.KT/53.I.2).
References
- Adler-Nissen J. Enzymic hydrolysis of food protein. New York: Elsevier Applied Science Publishers; 1986. pp. 110–169. [Google Scholar]
- Akita EM, Nakai S. Lipophilization of β-lactoglobulin: effect on hydrophobicity, conformation and surface functional properties. J Food Sci. 1990;55:711–717. doi: 10.1111/j.1365-2621.1990.tb05213.x. [DOI] [Google Scholar]
- Badarinath AV, Rao MK, Chetty MSC, Ramkanth S, Rajan TVS, Gnanaprakash K. A review on in-vitro antioxidant methods: comparisions, correlations and considerations. Int J PharmTech Res. 2010;2(2):1276–1285. [Google Scholar]
- Bersuder P, Hole M, Smith G. Antioxidants from a heated histidine–glucose model system. I. Investigation of the antioxidant role of histidine and isolation of antioxidants by high performance liquid chromatography. J Am Oil Chem Soc. 1998;75:181–187. doi: 10.1007/s11746-998-0030-y. [DOI] [Google Scholar]
- Binsan W, Benjakul S, Visessanguan W, Roytrakul S, Tanaka M, Kishimura H. Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei) Food Chem. 2008;106:185–193. doi: 10.1016/j.foodchem.2007.05.065. [DOI] [Google Scholar]
- Chen HM, Muramoto K, Yamauchi F, Fujimoto K, Nokihara K. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of soybean proteins. J Agric Food Chem. 1998;46:49–53. doi: 10.1021/jf970649w. [DOI] [PubMed] [Google Scholar]
- Chi C, Wang B, Deng Y, Wang Y, Deng S, Ma J. Isolation and characterization of three antioxidant pentapeptides from protein hydrolysate of monkfish (Lophius litulon) muscle. Food Res Int. 2014;55:222–228. doi: 10.1016/j.foodres.2013.11.018. [DOI] [Google Scholar]
- Elmore JS, Campo MM, Enser M, Mottram DS. Effect of Lipid composition on meat-like model systems containing cystein, ribose and polyunsaturated fatty acids. J Agric Food Chem. 2002;50:1126–1132. doi: 10.1021/jf0108718. [DOI] [PubMed] [Google Scholar]
- Flavia MN, Maria A. Production and characterization of enzymatic hydrolysate from soy protein isolate. LWT-Food Sci Technol. 1998;31:624–631. doi: 10.1006/fstl.1998.0396. [DOI] [Google Scholar]
- Hernández-Ledesma B, Dávalos A, Bartolomé B, Amigo L. Preparation of antioxidant enzymatic hydrolysates from clactalbumin and a-lactoglobulin, identification of active peptides by HPLC–MS/MS. J Agric Food Chem. 2005;53:588–593. doi: 10.1021/jf048626m. [DOI] [PubMed] [Google Scholar]
- Hierro E, De la Hoz L, Ordónez JA. Headspace volatile compounds from salted and occasionally smoked dried meats (cecinas) as affected by animal species. Food Chem. 2004;85:649–657. doi: 10.1016/j.foodchem.2003.07.001. [DOI] [Google Scholar]
- Ishibashi N, Arita Y, Kanehisa H, Kouge K, Okai H, Fukui S. Bitterness of leucine containing peptides. Agric Biol Chem. 1987;51:2389–2394. [Google Scholar]
- Izzo HV, Ho CT. Peptide-specific maillard reaction products: a new pathway for flavor chemistry. Trends Food Sci. 1992;3:253–257. doi: 10.1016/S0924-2244(10)80004-4. [DOI] [Google Scholar]
- Jenkins RO (2002) Proteins biochemistry and biotechnology. Walsh G (ed) Wiley, London, p 560
- Ju ZY, Hettiarachchy NS, Rath N. Extraction, denaturation and hydrophobic properties of rice flour proteins. J Food Sci. 2001;66:229–232. doi: 10.1111/j.1365-2621.2001.tb11322.x. [DOI] [Google Scholar]
- Jung MY, Kim SK, Kim SY. Riboflavin-sensitized photo oxidation of ascorbic acid: kinetics and amino acid effects. Food Chem. 1995;53:397–403. doi: 10.1016/0308-8146(95)99834-M. [DOI] [Google Scholar]
- Kanu PJ, Jestina BK, Edward HS, Joseph BAK, Philip MPM, Zhou H. Optimization of enzymatic hydrolysis of defatted sesame flour by different proteases and their effect on the functional properties of the resulting protein hydrolysate. J Food Technol. 2009;4:226–240. doi: 10.3923/ajft.2009.226.240. [DOI] [Google Scholar]
- Kim SY, Park PSW, Rhee KC. Functional properties of proteolytic enzyme modified soy protein isolate. J Agric Food Chem. 1990;38:651–656. doi: 10.1021/jf00093a014. [DOI] [Google Scholar]
- Kirimura J, Shimizu A, Kimizuka A, Ninomiya T, Katsuya N. The contribution of peptides and amino acids to the taste of foodstuffs. J Agric Food Chem. 1969;17:689–695. doi: 10.1021/jf60164a031. [DOI] [Google Scholar]
- Lapsongphon N, Yongsawatdigul J. Production and purification of antioxidant peptides from a mungbean meal hydrolysate by Virgibacillus sp. SK37 proteinase. Food Chem. 2013;141:992–999. doi: 10.1016/j.foodchem.2013.04.054. [DOI] [PubMed] [Google Scholar]
- Li Gh, Jz Wan, Gw Le, Shi Yh. Novel angiotensin I-conventing enzyme inhibitory peptides isolatied from Alcalase hydrolysate of mungbean protein. J Pept Sci. 2006;12:509–514. doi: 10.1002/psc.758. [DOI] [PubMed] [Google Scholar]
- Li Y, Jiang B, Zhang T, Mu W, Liu J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH) Food Chem. 2008;106:444–450. doi: 10.1016/j.foodchem.2007.04.067. [DOI] [Google Scholar]
- Liu Q, Kong B, Xiong YL, Xia X. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010;118:403–410. doi: 10.1016/j.foodchem.2009.05.013. [DOI] [Google Scholar]
- Matoba T, Hata H. Relationship between bitterness of peptides and their chemical structures. Agric Biol Chem. 1972;36:1423–1431. doi: 10.1080/00021369.1972.10860410. [DOI] [Google Scholar]
- Medoza E, Adachi M, Bernardo A, Utsumi S. Mungbean [Vignaradiata (L.) Wilczek] Globulins: purification and Characterization. J Agric Food Chem. 2001;49:1552–1558. doi: 10.1021/jf001041h. [DOI] [PubMed] [Google Scholar]
- Meisel H, FitzGerald RJ. Biofunctional peptides from milk proteins: mineral binding and cytomodulatory effects. Curr Pharm Des. 2003;9:1289–1295. doi: 10.2174/1381612033454847. [DOI] [PubMed] [Google Scholar]
- Rutherfurd SM. Methodology for determining degree of hydrolysis of proteins in hydrolysates: a review. J AOAC Int. 2010;93:1515–1522. [PubMed] [Google Scholar]
- Shahidi F. Flavor of meat, meat products, and sea foods. 2. London: Blackie Academic and Professional; 1998. [Google Scholar]
- Shalaby AE, Shanab MMS. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J Mar Sci. 2013;42(5):556–564. [Google Scholar]
- Shibamoto T, Kamiya Y, Mihara S. Isolation and identificationof volatile compounds in cooked meat: sukiyaki. J Agric Food Chem. 1981;29:57–63. doi: 10.1021/jf00103a015. [DOI] [Google Scholar]
- Sonklin C, Laohakunjit N, Kerdchoechuen O. Physicochemical and flavor characteristics of flavoring agent from mungbean protein hydrolyzed by bromelain. J Agric Food Chem. 2011;59:8475–8483. doi: 10.1021/jf202006a. [DOI] [PubMed] [Google Scholar]
- Tanford C. Physical chemistry of macromolecules. London: Wiley; 1961. [Google Scholar]
- Tang S, Hettiarachchy NS, Horax R, Eswaranandam S. Physicochemical properties and functionality of rice bran protein hydrolysate prepared from heat-stabilized defatted rice bran with the aid of enzymes. J Food Sci. 2003;68:152–157. doi: 10.1111/j.1365-2621.2003.tb14132.x. [DOI] [Google Scholar]
- Tang CH, Sun X, Yin SW, Ma CY. Transglutaminase-induced crosslinking of vicillin-rich kidney protein isolate: influence on functional properties and in vitro digestion. Food Res Int. 2008;41:941–947. doi: 10.1016/j.foodres.2008.07.015. [DOI] [Google Scholar]
- Tsai SY, Huang SJ, Lo SH, Wu TP, Lian PY, Mau JL. Flavour components and antioxidant properties of several cultivated mushrooms. Food Chem. 2009;113:578–584. doi: 10.1016/j.foodchem.2008.08.034. [DOI] [Google Scholar]
- Tsuge N, Eikawa Y, Nomura Y, Yamamoto M, Sugisawa K. Antioxidant activity of peptides prepared by enzymatic hydrolysis of egg-white albumin. Nippon NogeikagakuKaishi JSBBA. 1991;65:1635–1641. [Google Scholar]
- Varanová J, Ciesarová Z. Furan in food—a review. Czech J Food Sci. 2009;27:1–10. [Google Scholar]
- Wu W, Hettiarachchy N, Qi M. Hydrophobicity, solubility, and emulsifying properties of soy protein peptides prepared by papain modification and ultrafiltration. J Am Oil Chem Soc. 1998;75:845–850. doi: 10.1007/s11746-998-0235-0. [DOI] [Google Scholar]