Abstract
In this study, lycopene, was loaded on nanostructured lipid carrier and solid lipid nanoparticles using combination of high shear homogenization and ultrasonication method. Effect of applied lipids types, nanocarrier’s type and lycopene loading on physicochemical properties of developed nanocarriers were studied. Particle sizes of developed nanocarriers were between 74.93 and 183.40 nm. Encapsulation efficiency of nanostructured lipid carrier was significantly higher than solid lipid nanoparticles. Morphological study of developed nanocarriers using scanning electron microscopy showed spherical nanoparticles with smooth surface. Lycopene was entrapped in nanocarriers without any chemical interaction with coating material according to Fourier transform infrared spectroscopy spectrum and differential scanning calorimetry thermogram. Glycerol monostearate containing nanoparticles showed phase separation after 30 days in 6 and 25 °C, whereas this event was not observed in nanosuspensions that produced by glycerol distearate. Lycopene release in gastrointestinal condition was studied by the dialysis bag method. To evaluate nanocarrier’s potential for food fortification, developed lycopene-loaded nanocarriers were added to orange drink. Results of sensory analysis indicated that nanoencapsulation could obviate the poor solubility and tomato after taste of lycopene. Fortified sample with lycopene nanocarriers didn’t show significant difference with blank orange drink sample except in orange odor.
Keywords: Nanostructure lipid carriers, Solid lipid nanoparticles, Lycopene, Food fortification
Introduction
Nowadays, the tendency to use of functional foods has been increased due to health benefits of these foods. In this regard, the use of carotenoids as a functional component in these foods has attracted much attention.
Carotenoids are a group of pigmented compounds that made by plant and microorganism and are responsible for red, orange and yellow colors in fruits and vegetables (Rao and Rao 2007). Lycopene is an acyclic open-chain unsaturated carotenoid with 11 conjugated double bonds. This extended system of conjugated double bounds are responsible for red color and antioxidant activities of lycopene. This carotenoid is the most potent natural antioxidants among the carotenoids and frequently found in tomato (Shi and Maguer 2000).
Lycopene has no provitamin A activity, but epidemiological studies have shown that it has beneficial effects in the prevention and treatment of chronic diseases such as different types of cancer, especially prostate cancer, cardiovascular disease, osteoporosis and hypertension (Kun et al. 2006). Due to the antioxidant activity and functional effects of lycopene, there is a growing interest in the use of this carotenoid as a functional ingredient in food fortification, however, its sensitivity to oxidants, light and heat, insolubility in water and low solubility in oil at ambient temperature, low bioavailability of this compound (10–30% of dietary lycopene) (Rao and Rao 2007) and its crystallization during storage (because of high melting point) has limited the use of this bioactive in the food sector.
One of the best strategies to overcome mentioned problems is nanoencapsulation. This technique makes it possible to protect sensitive bioactive from undesirable conditions and improves the solubility and bioavailability of this compound (Fathi et al. 2012). In addition, nanoencapsulation allow controlling the rate of bioactive release and its targeting. Nanoencapsulation of bioactive compounds and drugs using lipid base systems and production of nanocarriers with different applications has been done for a long time in the pharmaceutical field and is growing in the food field.
Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) are new generations of lipid-base delivery systems that are very appropriate for food application; because they allow the use of biocompatible and biodegradable lipids in their production with no organic solvent use (Fathi et al. 2013).
SLN are an oil in water nanoemulsion that its liquid lipid (oil) has been completely replaced by a solid lipid (fat) or blend of several fats which remain solid at room and body temperature. These nanovehicles have been developed to eliminate disadvantages of polymeric nanoparticles (use of organic solvents, Inability to scale up), emulsions (uncontrolled rapid drug release) and liposome (drug degradation during storage). SLNs have provided new carrier systems with good stability, controlled release, ability to protect sensitive drug against degradation, and possibility of large scale production (Mehnert and Mäder 2001).
In contrast to SLN that made with solid lipid, NLC are produced by blend of solid and liquid lipids. Less ordered solid lipid matrix which is created by the use of liquid lipid in NLC, reduces drug leakage during storage and improves drug entrapment and release properties of NLC in comparison with SLN (Müller et al. 2002).
Despite the numerous advantages that have been stated for lycopene, few researches have been done on the encapsulation of these compounds with NLC and SLN as appropriate carriers for food application. In one of two related researches, Riangjanapatee and Okonogi (2012) used NLC for encapsulation of lycopene along with different surfactants and evaluated physical properties and chemical stability of these lycopene-loaded nanoparticles. Their results showed that NLC can enhance the chemical stability of entrapped lycopene compare to free lycopene. In another study, Riangjanapatee et al. 2013 entrapped the lycopene in NLC, SLN and nanoemulsion; that had been produced by natural lipids (rice oil and cholesterol). Particle size of produced nanocarriers was 405 nm for the SLN, 350 nm for the NLC without cholesterol and 287 nm for the NLC with cholesterol. Their results showed that the chemical stability profile of lycopene was unfavorable when cholesterol was used in NLC production.
SLN and NLC have used for other carotenoids such as: β-carotene (Hung et al. 2011) and lutein (Liu and Wu 2010).
In this study, lycopene free and lycopene-loaded SLN and NLC were produced using high energy methods and the effect of nanocarriers type (SLN and NLC), applied solid lipid (glycerol monostearate and glycerol distearate), and lycopene loading on different properties of developed nanocarriers were studied. In addition, the potential of developed nanocarriers for food fortification were investigated by using them in orange drink as a model food system.
Materials and methods
Materials
Lycopene was obtained from Unicorn Natural Products limited (India). Glycerol Distearate (GDS) (Precirol® ATO 5) and Caprylic/Capric triglyceride (MCT oil) (Labrafac™ lipophile WL 1349) were kindly donated by Gattefosse (France). Glycerol Monostearate (GMS) (BDH Laboratory supplies, Poole, England), soybean phosphatidylcholine (Lecithin granular, Across Organics, USA), tween 80 (Merck, Germany) and other chemicals and reagents were analytical grade.
Methods
Production of SLN and NLC
SLN and NLC were produced by a combination of high shear homogenization and ultrasound methods. This method is easy to use and widespread without any solvent use (Mehnert and Mäder 2001). GDS and GMS were used as solid lipid in the SLN and NLC production. Aqueous phase containing 3% (w/w) tween 80, lipid phases containing 0.6% (w/w) lecithin and 0.35% (w/w) lycopene (in final formulations) were separately prepared. The purpose of adding lecithin as lipophilic co-surfactant to formulate was improving the stability of nanoparticles by increasing the zeta-potential. On the other hand, combination of tween 80 and lecithin causing smaller particles with lower PDI in comparison to use of them separately. Lipid phase was stirred in 80 °C for 30 min to be solved lecithin and lycopene in melted lipid, completely. Lipid concentrations in all samples were fixed (5% w/w). In NLC formulations, 20% of solid lipid in SLN was replaced by MCT oil. Ingredients of developed nanocarriers are shown in Table 1.
Table 1.
Composition of NLC and SLN formulations (percent w/w)
| Formulation | GMS | GDS | MCT oil | Lycopene |
|---|---|---|---|---|
| T1 | 5 | – | – | – |
| T2 | 5 | – | – | 0.35 |
| T3 | – | 5 | – | – |
| T4 | – | 5 | – | 0.35 |
| T5 | 4 | – | 1 | – |
| T6 | 4 | – | 1 | 0.35 |
| T7 | – | 4 | 1 | – |
| T8 | – | 4 | 1 | 0.35 |
In all formulation 3% tween 80, 0.6% lecithin and up to 100% distilled water were added
The aqueous phase was reached to temperature of 80 °C, added to the lipid phase at same temperature and homogenized in 20,000 rpm for 60 s with high shear homogenizer (T 25 digital ULTRA-TURRAX®, IKA, Germany). The obtained pre-emulsion was ultrasonicated for 225 s (4 s on, 1 s off) with an ultrasound generator (XL2020, Misonix, USA) in power output of 25%. After this, another homogenization cycle was performed with pervious conditions. Obtained nanoemulsion was cooled in ice batch for 30 min to form lipid nanoparticles.
Freeze drying of SLNs and NLCs suspension
Nanosuspensions were freeze dried to obtain a powder that was used in subsequent experiments such as Differential Scanning Calorimetry (DSC) and Fourier Transform Infrared Spectroscopy (FTIR). 10 ml of dispersion was quickly frozen using appropriate volume of liquid nitrogen and then lyophilized for 24 h in Martin Christ Freeze dryer (Germany) at − 30 °C and 0.100 mBar (Nayak et al. 2010). The lyophilized samples were collected and stored in Polyethylene container at 4 centigrade.
Particle size, polydispersity index (PDI) and zeta-potential
Particle size and polydispersity index (PDI) were determined based on dynamic light scattering (DLS) using a VASCO particle size analyzer (Cordouan Technologies, France) in wavelength 657 nm at room temperature. DLS measures the volatility of scattered light intensity which is created by particle movement (Mehnert and Mäder 2001). The Z-average was reported as average hydrodynamic diameter in this study. The homogeneity of dispersion is characterized by PDI and its value is variable from 0 to 1. A PDI value greater than 0.3 shows high heterogeneity and values nearly 0 shows a homogenous dispersion (Rahman et al. 2013).
Zeta-potential as an indicator of particle surface charge was automatically calculated by Zeta-potential meter (Zeta Compact, CAD instrumentation, France) based on measuring mean electrophoretic mobility of particles and converting them to zeta-potential by Helmholtz–Smoluchowski.
In order to prevent multiple scattering effects, dispersion was diluted to a suitable concentration with deionized water before measuring these parameters (Tamjidi et al. 2013).
Encapsulation efficiency (EE) and drug loading (DL)
EE and DL of lycopene-loaded nanoparticles was calculated by measuring the amount of free lycopene in NLC and SLN dispersion according to Liu and Wu (2010) method with some modification. 0.3 ml of nanosuspension was carefully weighed and mixed with 10 ml hexane as extracting solvent for free lycopene. These researchers reported that NLC, SLN and entrapped bioactive remain intact in hexane for 30 min. The mixture of hexane and nanosuspension was shacked and then centrifuged for 3 min in 2000 rpm (Sigma, 3-30K; Germany). Upper phase (supernatant) which contains free lycopene in hexane were separated and amount of lycopene in this phase was determined by spectrophotometer (Shimadzu UV mini 1240, Duisburg, Germany) in 472 nm wavelength (λmax of lycopene in hexane). This procedure was also performed for lycopene-free nanoparticles as blank for spectroscopy. EE and EL were calculated by Eqs. (1) and (2):
| 1 |
| 2 |
where W total and W lipid are weights of applied lycopene and applied lipid in the formulation of dispersion, respectively and W F is amount of free lycopene that measured in supernatant. All measurements for EE and DL were at least done in triplicate.
Morphological properties
The surface morphology of formulations was examined by scanning electronic microscopy (SEM). 1 ml of nanosuspension was diluted with deionized water and a few drops of this diluted suspension were placed on stubs that were covered with aluminum slab and dried. These dried samples were coated with a thin layer of Au/Pd in a sputter coater (SC7620 Mini Sputter Coater, Polaron, England) and then observed by SEM (SEM, LEO 1450 VP, Leo, Germany) in different magnification.
Thermal analysis
Melting point and crystallization status of pure material and developed nanocarriers were investigated by differential scanning calorimetry (DSC) analysis using Mettler Toledo DSC (DSC 822, Switzerland). About 5 mg samples were placed in an aluminum pan and heated at the scanning rate of 10 °C/min from 20 to 210 °C under nitrogen purge 30 ml/min and empty aluminum pan as reference standard.
Fourier transform infrared spectroscopy
In order to identify possible interactions that may be occur during production of nanoparticles, the IR spectrum of nanoparticles and pure ingredients was measured by Fourier Transform Infrared (FTIR) spectrometer (Avatar, 370 FT-IR, Thermo Nicolet, USA) using KBr pellets technique at room temperature. In this technique, lyophilized samples were mixed with KBr and compressed to form a thin pellet that was used for testing. The measurements were recorded in the frequency range of 4000–400 cm−1.
Stability study
Physical stability of NLC and SLN dispersions was evaluated by keeping them in glass tubes for a month at a temperature of 6 ± 2 °C (refrigeration temperature) and 25 ± 1 °C (room temperature) (Fathi et al. 2013). Following the completion of this course, the particle size, PDI and zeta-potential of nanodispersions were determined in the same way described previous (part 3).
To investigate the leakage of lycopene from lycopene-loaded nanocarriers during storage, the amount of free lycopene in suspensions was spectroscopically determined at appropriate time intervals (0, 4, 15 and 30 days) (Fathi et al. 2013). In this test, lycopene free nanocarriers were used as control (blank) in each time interval.
In vitro drug release
Dialysis bag method was used for lycopene release study in gastric (pH of 1.2) and intestine (pH of 6.8) solutions. Dialysis tube with a 12-kDa cut-off (D0530, sigma-Aldrich, Canada) was soaked in the release media overnight. To ensure sink conditions, tween 80 (0.5%) was added to release media (Liu et al. 2011). 3 ml of dispersion was weighed accurately and poured into dialyses bag and sealed. The bag was placed into 50 ml gastric solution buffer for 120 min and then was immersed into 60 ml intestinal solution for 6 h. During the experiment, the solution temperature was kept at 37 °C and the stirring speed was 100 rpm. At certain time intervals 5 ml of the release medium was removed and replaced with fresh medium at the same temperature (Fathi et al. 2013). The amount of released lycopene was spectroscopically measured at wavelength of 472 nm.
Food application of developed nanoparticles
In order to investigate the possibility of applying produced nanocarriers in food and beverage fortification, orange drink (13° Brix orange drink; Takdaneh Company; Iran) was selected as a sample food system. It was fortified using lycopene-loaded nanoparticles (0.5% w/w) and the direct lycopene addition (at the same concentration provided by nanocarriers). A panel of eight trained panelists with experience in sensory evaluation was used for evaluation of these 3 type orange drinks. Before beginning, the terms of color, turbidity, orange odor, and particle remaining in the mouth were described fully and clearly base on Kitsawad and Win (2010) paper. Tomato tastes after taste, homogeneity and total acceptance along with above attributes were scored by panelist based on the intensity of each attribute in 15-cm unstructured line scale (1 for lowest and 15 for highest intensity).
Statistical analysis
All experiments were replicated at least three times and the average values of data with standard deviation (SD) were reported. In this research, the effect of applied solid lipid (GMS and GDS), lycopene loading (70 mg per gram of lipid), and carrier type (SLN, NLC) on properties of produced nanocarriers were studied. Data were statistically analyzed by one-way analysis of variance and “Duncan” test, at a 5% significance level using SPSS software (version 16).
Results and discussion
In this study, empty and lycopene-loaded solid lipid nanoparticles and nanostractured lipid carrier were produced by combination of high shear homogenization and ultrasound method without any organic solvent. Production of nanoparticles with higher PDI value is one disadvantage of batch process techniques such as probe ultrasonication (Tamjidi et al. 2014), therefore in this study, high shear homogenization was also done. Our preliminary tests showed that high shear homogenization after ultrasonication reduces the PDI value of nanoparticles significantly while reduction in particle size was not significant (data not shown). Therefore, in this study the purpose of the first homogenization cycle (before ultrasonication) was to produce a uniform mixture of ingredient while the second cycle (after ultrasonication) was mainly done for the production of nanoparticles with lower PDI. The concentration of lipid and emulsifiers was kept constant and effect of applied lipid (GMS, GDS), carrier type (SLN and NLC) and lycopene loading on different properties of developed nanoparticles were studied. It is recognized that the simultaneous use of lipophilic and hydrophilic nature surfactants produces a more stable disperse system and therefore in this study, tween 80 and lecithin were simultaneously used as a hydrophilic and lipophili surfactant respectively (Nerella et al. 2014). For NLC production, a part of solid lipids in SLN replaced by MCT oil as liquid oil. Thermodynamic stability of MCT oil and high solubility of many bioactive in this oil were the major reasons for choosing this oil.
Particle size and PDI
The results of average particle size (nm), PDI, and zeta potential (mV) of all formulation and encapsulation efficiency and drug loading of lycopene-loaded nanoparticles immediately after production have been shown in Table 2. The mean diameter of particles was below 200 nm ranging 74.93–183.40 nm and PDI value of them was lower than 0.32 indicating their relatively narrow size distribution (Tamjidi et al. 2013).
Table 2.
Particle size, PDI, Zeta potential, encapsulation efficiency, drug loading, melting temperature, and enthalpy of pure applied lipids and developed nanocarriers
| Formulation | Particle size (nm) | PDI | Zeta potential (mV) | Encapsulation efficiency (%) | Drug loading (%) | Melting point (°C) | Enthalpy (J/g) |
|---|---|---|---|---|---|---|---|
| GMS | – | – | – | – | – | 63.21 | 168.5 |
| GDS | – | – | – | – | – | 60.6 | 114.31 |
| T1 | 95.24 ± 2.91D | 0.22 ± 0.014D | − 6.17 ± 0.38D | – | – | 59.19 | 69.55 |
| T2 | 183.40 ± 3.03A | 0.32 ± 0.009A | − 12.85 ± 1.39B | 64.79 ± 2.95C | 4.54 ± 0.21C | 58.4 | 70.05 |
| T3 | 80.35 ± 2.77E | 0.23 ± 0.014CD | − 5.78 ± 0.60D | – | – | 56.22 | 76.88 |
| T4 | 121.79 ± 4.09C | 0.27 ± 0.011B | − 13.19 ± 0.76B | 73.79 ± 3.48B | 5.17 ± 0.24B | 55.93 | 77.62 |
| T5 | 83.03 ± 1.55E | 0.24 ± 0.021CD | − 10.23 ± 0.70C | – | – | 55.43 | 68.74 |
| T6 | 133.39 ± 2.71B | 0.28 ± 0.005B | − 15.87 ± 0.20A | 75.11 ± 3.72AB | 5.26 ± 0.26AB | 55.74 | 64.16 |
| T7 | 74.93 ± 1.39F | 0.24 ± 0.009C | − 10.65 ± 0.59C | – | – | 53.78 | 58.22 |
| T8 | 135.53 ± 3.37B | 0.28 ± 0.007B | − 14.03 ± 1.73B | 78.89 ± 1.74A | 5.52 ± 0.12A | 54.38 | 62.86 |
Values with different letters in same column are significantly different (p ≤ 0.05)
Particle size has a serious role in physical stability and gastrointestinal uptake of nanocarriers and since the produced nanoparticles in this study is below 300 nm, they are advisable for intestinal transport (Wang et al. 2013). The developed lycopene-loaded NLCs are smaller than those made by other researchers using different surfactants or different liquid oil (Riangjanapatee et al. 2013; Riangjanapatee and Okonogi 2012). The smaller particles move faster due to Brownian motion, and this leads to dispersion stability against gravity (Fathi et al. 2013). Particle size analysis showed that the size of lycopene-loaded nanocarriers were significantly (p < 0.05) higher than lycopene-free nanocarriers due to entrapment of lycopene in NLC and SLN structures (Hu et al. 2005). Difficulty of size reduction during homogenization and ultrasonication due to greater viscosity of the dispersed phase in the presence of the active substance can be another reason for larger particle size of lycopene-loaded nanoparticles (Tamjidi et al. 2014). On the other hand, applied lipids and nanocarriers type also have significant effects (p < 0.05) on particle size. GMS containing formulations and SLNs was bigger than GDS containing formulations and NLCs. This observation can be explained by melting point and crystalline characteristics of applied lipid and developed nanocarriers. DSC results (Table 2) showed that melting point and crystalline order (melting enthalpy) of GMS containing nanoparticles and SLNs is higher than nanoparticles that produced by GDS and NLCs. Higher melting point of dispersed phase in GMS containing nanosuspansion lead to an increase in the viscosity of formulation (Khalil et al. 2013), whereby the effective force applied during the production process of nanocarriers are reduced and therefore particle size of nanocarriers increases. In contrast, reduction in viscosity of dispersion by using lower melting lipids (GDS) or adding liquid oil (NLC production) cause facilitating dispersing, improvement of bioactive solubility in lipid matrix and so smaller particle production. Khalil et al. (2013) observed that adding liquid oil to lipid matrix, decrease the particle size of Meloxicam loaded NLC and explained that this result is due to the higher molecular mobility of the matrix after oil addiction and thus promote the formation of the small particle population. Hu et al. (2005) also reported that liquid oil addition to formulation reduces the surface tension to form smaller particles due to reduction of viscosity inside NLC.
Liquid oil that add for NLC production can be dispersed in a lipid matrix (Matrix type) and reduce particle size or produce core–shell type nanocarriers that in this case, particle size increases with oil addition. Since the particle size of nanocarriers decreased with oil addition, so developed NLCs in this study are likely matrix type.
PDI, shows the wide of particle size distribution and is an important parameter for physical stability of nanosuspension. For long-term stability of suspension this character should be as small as possible (Lakshmi and Kumar 2010). Applying lipid and nanocarriers type didn’t change PDI values of nanoparticles significantly (p ≥ 0. 05) but this parameter was significantly greater in lycopene-loaded nanoparticles (p < 0.05). Greater PDI in lycopene containing formulation can be attributed to unencapsulated lycopene or presence of small and large particles due to difficulty of size reduction in presence of lycopene (Tamjidi et al. 2014) as explained above.
Zeta potential
Zeta potential also is an important factor in physical stability of nanoparticles. The higher zeta potential shows better stability of the dispersion. Zeta potential of all formulations was negative in the range of − 5.78 mg for T3 and − 15.87 for T6 formulation that is not very desirable. Negative charge can be mainly attributed to the lecithin although hydroxyl groups of tween 80 also can create a slight negative charge. In this study, stabilization of nanosuspension occurs by combination of electrostatic (by lecithin) and steric force (by tween 80) and therefore a minimum Zeta-potential of ± 20 mV is sufficient for this nanosuspension stabilization (Tamjidi et al. 2013). Lycopene loading in the SLN and NLC increased the zeta potential of nanocarriers significantly, but the results don’t show any relation between applied lipids and Z-potential. In addition MCT addition to formulation (NLC production) produced more negative nanoparticles because of the negative charged MCT at their carboxylic groups.
Encapsulation efficiency (EE) and drug loading (DL)
EE is defined as the ratio between the amount of encapsulated lycopene and total amount of lycopene used in formulation and DL is referred to amount of encapsulated lycopene of total lipid used in formulation. Developed nanocarriers had EE and EL ranging from 64.79 to 78.89% and 4.54 to 5.52%, respectively. These two parameters are relatively high, because of the highly lipophilic nature of lycopene which cause very lower tendency of this molecule to enter the aqueous phase compared to lipid phase. Higher drug loading is a major advantage for nanocarriers because for delivering a predetermined bioactive level into food, lower amount of the NLC or SLN is required; but it should be noted that very high EL can damage the surface of nanoparticles (Fathi et al. 2013; Tamjidi et al. 2013). EE and Dl in GMS containing and SLNs were significantly lower than GDS containing and NLCs formulations (p < 0.05). Use of liquid oil in NLC formulation limits the recrystallization of lipid matrix and forms an amorphous or imperfect crystalline structures witch these structures provide more space for drug entrapment (increase in DL) and also reduces drug explosion or leakage during storage (Fathi et al. 2013).
Morphological properties
Particle shape could affect drug loading and release behavior of nanoparticles. To study the morphological properties of nanocarriers, lyophilized samples were used at first. Obtained images from these samples were not desirable due to lack of proper separation of particles from each other; Therefore in next step, diluted liquid nanosuspensions were used for morphological study of nanoparticles. Figure 1 shows the SEM image of T8 formulation. Science all formulations have similar morphology, this image is shown as an example. As evident from this picture, particles are spherical with a relatively smooth surface. Because of small specific surface area of spherical nanoparticles and therefore minimum contact with the aqueous environment, they have higher potential for controlled release and protection of encapsulated bioactive. In addition smallest emulsifier is required for stabilization of these nanoparticles (Bunjes 2005). Low density of nanoparticles in this picture has been created due to dilution of nanosuspension before preparing the SEM photographs.
Fig. 1.
SEM photograph of T8 formulation
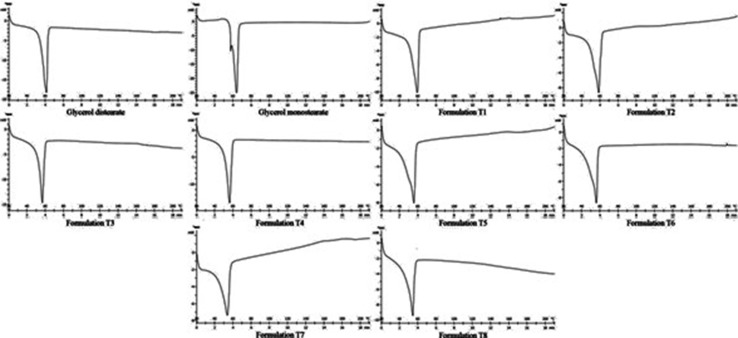
Differential scanning calorimetry
DSC is an effective tool to investigate the melting behavior and crystalline state of nanocarriers and pure materials (Madhusudhan et al. 2012). Science the crystallinity has a significant impact on some properties of lipid nanoparticles such as drug loading, drug release and stability of drugs during storage, therefore studying of it can be useful to judgment about nanocarriers features. Lycopene showed an endothermic peak at 163.3 °C related to its melting point. DSC thermograms of pure lipids, lycopene loaded and lycopene free (blank) NLCs and SLNs along with melting temperature and enthalpy data have been shown in Fig. 2 and Table 2. Melting temperature and enthalpy of pure GMS was higher than pure GDS and its peak wide was lower that these indicate the more crystalline nature of GMS in contrast to GDS. All developed nanocarriers are solid at body temperature (minimum onset point was 45.01 °C for t7 formulation) and their melting point and enthalpy is relatively lower than bulk lipids. Presence of surfactant in nanocarriers and changing the crystalline state, less ordered arrangement of nanoparticles in comparison to bulk lipid, and larger ratio of surface to volume in smaller particles are reasons of this melting temperature reduction (Rahman et al. 2013). The relation between the particle size and melting temperature has been expressed by Thomson as (Ln T/T0 = −2γVs/rΔH); Where T and T0 represent the melting point of particle and bulk material respectively, r is the radius of the particle, ∆H, γ and Vs are also molar melting enthalpy, interfacial energy and molar volume of desired material respectively (Siekmann and Westesen 1994). As evident from this equation, whatever the sizes of particles become smaller the melting temperature of particles decreases. Melting point of SLN and NLC after loading of lycopene was changed very little (less than 0.8 °C).
Fig. 2.
DSC thermographs of pure lipids and developed nanocarriers
NLCs also have a lower Fusion entropy and temperature than SLNs. The addition of MCT oil to the formulation of NLC, disturbs the discipline of lipid matrix and therefore energy requirements for overcoming lattice force and melting of NLC structure is lower than SLN or bulk lipids (Khalil et al. 2013). Free spaces that created by an imperfect lipid matrix of NLC can be occupied by lycopene and thus enhances lycopene loading and its remaining in the NLC (during storage). In the GMS containing formulation the difference between NLC and SLN melting enthalpy was very low.
As is apparent from DSC curves, there was no melting peak for lycopene around 163 °C in the thermograms of lycopene loaded formulations. This shows that lycopene has completely been trapped in the SLN and NLC structures or it is in amorphous state (not in crystalline state) in the homogenous matrix of lipid nanoparticles (Madhusudhan et al. 2012). X-ray diffraction (XRD) is a useful tool which can be used for identifying the physical state of lycopene in NLC and SLN.
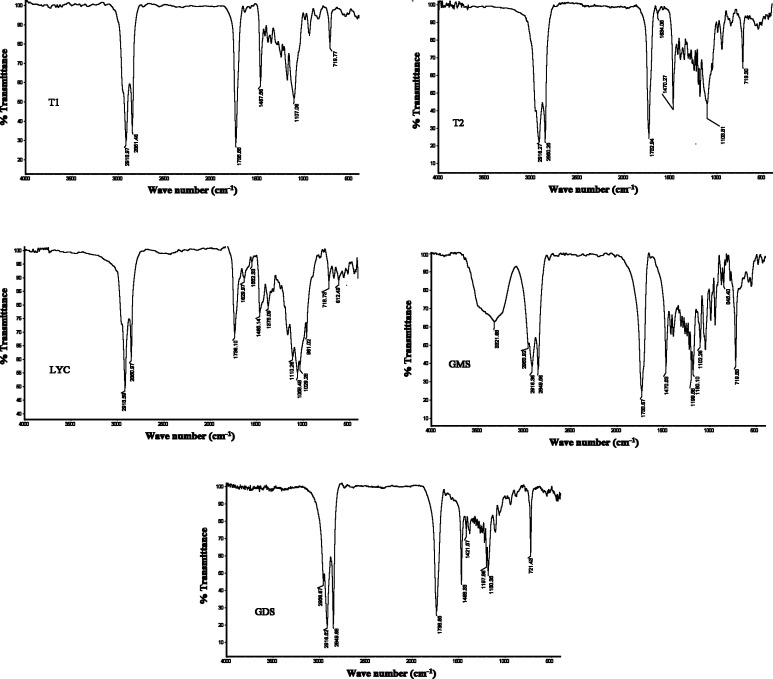
Fourier transform infrared spectroscopy
Infrared spectroscopy is a non-destructive analysis technique that were conducted for identifying of interactions that may occur between materials during production of nanoparticles (Madhusudhan et al. 2012). FTIR spectrums of lycopene, pure lipids (with their major peaks) and some of developed nanocarriers have been shown in Fig. 3. In order to investigate the interaction of lycopene with other materials that used for nanocarriers production, each spectrum of lycopene loaded nanocarriers were compared with same blank nanocarriers (for example T2 formulation spectrum compared with T1 formulation). The FTIR spectrum of lycopene showed the vibration bands of CH2/CH3 (symmetric and asymmetric stretching) at 2918.59 and 2850.97 cm−1 (between 2800 and 3000 cm−1) (De Nardo et al. 2009) with corresponding scissoring (bending) vibrations of CH2 at 1465.14 cm−1 and CH3 deformation at 1375.09 cm−1 (De Nardo et al. 2009), the R2C=CR at 612.49 cm−1(Qiu et al. 2006), trans CH out of plane bending at 961.02 cm−1(De Nardo et al. 2009), stretching vibration of C=C at 1629.97 cm−1 and 1552.33 cm−1, stretching band of C–C at 961–1170 cm−1(Wilkerson et al. 2013), and out of plane CH2 rocking vibration at 718.73 cm−1 (Buzoianu et al. 2014). The absence of new peak in the lycopene loaded nanocarriers spectrum compared with their blank indicates that no chemical reaction has been occurring between lycopene and other ingredient of nanoparticles and it has only dissolved in lipid matrix. This observation proves that lack of lycopene melting peak in DSC thermograms is not due to chemical reaction of lycopene with other ingredient, but indicating entrapment of lycopene in lipid matrix.
Fig. 3.
FTIR spectrum of lycopene, pure lipids and produced nanoparticles. Formulation T1 and T2, LYC Lycopene, GDS Glycerol Distearate, and GMS Glycerol Monostearate
Stability study
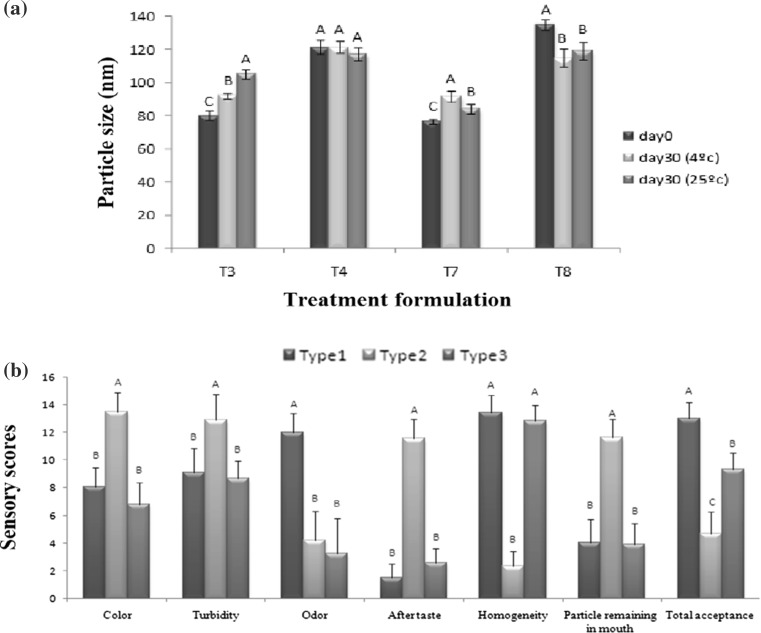
Generally, the particle size can be used as a tool for determine the stability of lipid nanoparticles. SLN and NLC formulations were stored in 6 and 25 °C for physical stability study. Particle size, zeta potential and PDI of formulations were determined after 1 month. At the end of this period, GMS containing formulation showed macroscopic phase separation (Detectable with the naked eye) in both temperatures and therefore the remaining formulation was analyzed for size, PDI and zeta potential. These observations indicate that under similar storage conditions, developed GMS containing nanoparticles are less physically stable than GDS containing nanoparticles while their initial zeta potentials was almost similar (p > 0.05). One of likely reason for phase separation of GMS containing formulations is change in their shapes during storage because of change in their polymorphism from higher energy modification to more-ordered modification. Nanoparticles was produced in high temperature and cooled rapidly to 0 °C in short time, therefore probabilities of alpha modification crystals forming is higher; while during storage, this crystal change to the more stable beta modification. As previously mentioned nanoparticles shape was spherical and any change in this shape increases their surface area so if sufficient surfactant for covering new created interfaces not be available, particle aggregation occurred. Using a blend of different solid lipid (mono, di or triglycerides with different chain fatty acid) can be useful for producing imperfect lipid matrix and decreasing change in polymorphic form during storage. Percirol ATO5 (GDS) is a mixture of C16 and C18 fatty acids therefore have lower tendency to transform to more-ordered modification and this can be the reason of its stability during storage.
PDI and zeta potential of all GDS containing formulations didn’t significantly change in 6 or 25 °C (p > 0.05). The particle size of these formulations after 1 month storage has been shown in Fig. 4a. As evident from this figure, particle size of T3 and T7 formulation were significantly increased (p < 0.05) in both temperatures. One of the reasons for this observation is the low zeta potential of these formulations that cause lower repulsion force between nanoparticles and therefore the particle size was increased (Fathi et al. 2013). In lycopene loaded case (T4 and T8), There was no increase in particle size because of higher zeta potential, even a significant reduction in particle size was observed in the T8 formulation (p < 0.05). This phenomenon can be explained that during production of nanoparticles, surfactant (tween 80) covers the new surfaces which are created by new formed nanoparticles but since the production time and time interval between particle production and determination of particle size is short, therefore it is possible that some of these surface not covered well with surfactant. During the storage period, the surfactant has enough time to penetrate these surfaces and cover them completely therefore nanoparticles size reduced.
Fig. 4.
a Particle size of glycerol distearate containing formulations after 1 month storage in 6 and 25 °C (different letters shows significant difference (p < 0.05) between each formulation in different storage temperature), b sensory analysis results of blank and fortified orange drink [in each attribute, different letters shows statically significant difference, (p < 0.05)]
To investigate lycopene leakage from nanoparticles during storage; free lycopene in dispersion was determined on days 4, 15 and 30. Results showed that lycopene is well incorporated into nanoparticles (expect T2 formulation) and maximum amount of its leakage at the end of period was only 2.3% in 6 °C and 1.7% in 25 °C. Lycopene leakage from T2 formulation only occurred in 25 °C and its leakage in refrigerant temperature was only 2.14%. After 4 days storage of this formulation in room temperature, 14.18% of entrapped lycopene leaked out from nanoparticles and this number increased to 22.02% at the end of storage period. As mentioned before, T2 formulation is a lycopene-loaded SLN that has been produced by GMS therefore it has a lot of potential for polymorphism change from higher energy modification to more-ordered modification that occurred during storage of nanoparticles and this can be the reason of drug leakage from T2 formulation nanoparticles.
Nerella et al. (2014) also have explained that monoglycerides and fatty acids can compete with surfactants that exist in formulation for positioning in the surface and constitute mixed micelles that might enhance the partitioning of hydrophobic drug out of the nanoparticles.
In connection with no leakage of lycopene in the refrigerator temperature during storage should be noted that, after production of SLN with hot homogenization the lipid structure of nanocarriers is present in β′ and α polymorphs and kinetic energy such as temperature causes a transformation to β polymorph (more ordered structure). Storing of SLN formulation in refrigerator condition, can prevent or minimize this transformation and therefore drug leakage from formulation reduced in 6 °C (Das and Chaudhury 2011; Freitas and Muller 1998).
In vitro drug release
The release behavior of lycopene loaded nanocarriers in simulated gastrointestinal conditions was investigated by the dialysis bag method in 37 °C. Shape, size, bioactive solubility and diffusivity in environmental media, polymorphism status of lipid nanocarriers, pH of the medium, DL, porosity, and the bioactive ratio between receiving media and carriers are different variables that may influence proactive release from nanocarriers (Fathi et al. 2012).
Lycopene doesn’t have any rules to receiving solution in all formulations, because of very low water solubility of this proactive and its tendency to remain associated with lipid nanoparticles. Tween 80 (0.5%) was added to release media for solving this problem and creating sink conditions, but under these conditions also, there was no satisfactory results and the maximum release of lycopene was only 3.48% (in the case of T8 formulation).
Ethanol addition to release media, using other release study methods such as external sink (Chorny et al. 2002) and dispersion method (Shazly et al. 2008) instead of dialyses bag method, and surfactant or hydrotropic addition to release media (Liu et al. 2011) are different strategies that used by other researchers for overcoming this problem. Ruktanonchai et al. (2009) studied the release behavior of alpha-lipoic acid in the release media containing 20% ethanol and reported that only 23% of alpha-lipoic acid was released from SLN after 72 h, because of its low water solubility.
Poor release of lycopene from developed nanocarriers in simulated gastrointestinal conditions is not indicative of undesirability of these nanocarriers for bioactive delivery, because as mentioned before these nanocarriers have small size (below 200 nm) and therefore they can be suitable for intestinal transport (Wang et al. 2013).
Food application of developed nanoparticles
Orange drink was selected as a sample food system to investigate the ability of developed nanocarriers for food fortification. T8 formulation was selected as the best formulation because of its higher stability and DL; lyophilized using sucrose as cryoprotect and added to orange drink for sensory analysis. Figure 4b shows the sensory results of blank (Type1), fortified sample using direct lycopene addition (Type2) and fortified orange drink with lycopene loaded nanocarriers (NLC) (Type3).
Allocated scores showed that direct addition of lycopene to orange drink, increases the color, turbidity, aftertaste, and particle remaining in the mouth and decrease the homogeneity, orange odor and total acceptance of the final product, significantly (p < 0.05). The low solubility of lycopene in aqueous medium increased the presence of undissolved particles of lycopene crystals and thus the homogeneity of final sample was sharply reduced. In addition, the presence of undissolved lycopene crystals in liquid sample enhanced the remaining of particles in the mouth and as a result, tomato taste after taste was also dramatically increased. This observation and low total acceptance of fortified sample showed that direct addition of lycopene to aqueous medium is not possible. In the case of fortifying by lycopene-loaded NLC, no significant differences were observed between blank and fortified samples in sensory attributes expected in orange odor. Nanoencapsulation eliminated some disadvantages such unpleasant aftertaste and low solubility of lycopene but total acceptance of sample that had been fortified by lycopene nanoparticles was significantly (p < 0.05) lower than blank sample. Encapsulated lycopene addition to orange drink was caused that orange odor of sample reduces significantly and this reduction in orange odor reduced the overall acceptability of the sample, according to panelists.
Conclusion
In this paper the possibility of lipid nanocarriers (SLN and NLC) using for encapsulation of lycopene as a beneficial carotenoid was investigated. Nanoparticles were produced by a combination of high shear homogenization and ultrasonication methods and different characteristics of them were investigated. Lycopene-loaded nanoparticles showed larger particles with higher PDI. Size of NLC samples was smaller than SLNs while their EE and DL was significantly higher. Zeta-potential of lycopene-loaded samples and NLCs was more negative than blank sample or SLNs. DSC analysis showed that all developed nanocarriers were solid at body temperature and their crystalline state were less ordered than pure material. FTIR results confirmed that lycopene incorporated into nanoparticles without any chemical reaction with coating materials. GDS containing formulation showed relatively good physical stability along with no remarkable leakage during storage term in 6 and 25 °C while in GMS containing formulation, the phase separation was observed. Lycopene release in gastrointestinal condition was not favorable because of its low solubility in aqueous media and this problem may be resolved by other method using such as external sink method. Result of orange drink fortification with lycopene showed that nanoencapsulation of this bioactive can facilitate its use in aqueous samples by improving solubility of this compound, masking its tomato taste aftertaste and increasing the homogeneity of fortified samples.
References
- Bunjes H. Lipospheres in drug targets and delivery: approaches, methods, and applications. Boca Raton: CRC PRESS; 2005. [Google Scholar]
- Buzoianu AD, Rati IV, Socaciu C. Untargeted metabolomics for Sea Buckthorn (Hippophae rhamnoides spp. carpatica) berries and leaves: Fourier transform infrared spectroscopy as a rapid approach for evaluation and discrimination. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2014;42(2):545–550. [Google Scholar]
- Chorny M, Fishbein I, Danenberg HD, Golomb G. Lipophilic drug loaded nanospheres prepared by nanoprecipitation: effect of formulation variables on size, drug recovery and release kinetics. J Controll Rel. 2002;83:389–400. doi: 10.1016/S0168-3659(02)00211-0. [DOI] [PubMed] [Google Scholar]
- Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12(1):62–76. doi: 10.1208/s12249-010-9563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nardo T, Shiroma-Kian C, Halim Y, Francis D, Rodriguez-Saona LE. Rapid and simultaneous determination of lycopene and β-carotene contents in tomato juice by infrared spectroscopy. J Agric Food Chem. 2009;57(4):1105–1112. doi: 10.1021/jf802920z. [DOI] [PubMed] [Google Scholar]
- Fathi M, Mozafari MR, Mohebbi M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci Technol. 2012;23(1):13–27. doi: 10.1016/j.tifs.2011.08.003. [DOI] [Google Scholar]
- Fathi M, Varshosaz J, Mohebbi M, Shahidi F. Hesperetin-loaded solid lipid nanoparticles and nanostructure lipid carriers for food fortification: preparation, characterization, and modeling. Food Bioprocess Technol. 2013;6(6):1464–1475. doi: 10.1007/s11947-012-0845-2. [DOI] [Google Scholar]
- Freitas C, Muller RH. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN™) dispersions. Int J Pharm. 1998;168:221–229. doi: 10.1016/S0378-5173(98)00092-1. [DOI] [Google Scholar]
- Hu FQ, Jiang SP, Du YZ, Yuan H, Ye YQ, Zeng S. Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloids Surf B. 2005;45(3):167–173. doi: 10.1016/j.colsurfb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Hung LC, Basri M, Tejo BA, Ismail R, Nang HLL, Hassan HA, May CY. An improved method for the preparations of nanostructured lipid carriers containing heat-sensitive bioactives. Colloids Surf B. 2011;87:180–186. doi: 10.1016/j.colsurfb.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Khalil RM, El-Bary AA, Kassem MA, Ghorab MM, Ahmed MB (2013) Solid lipid nanoparticles for topical delivery of meloxicam: development and in vitro characterization. Paper presented at the 1st annual international interdisciplinary conference, AIIC, Azores, Portugal 24–26 April
- Kitsawad K, Win DT. Sensory characteristics and consumer acceptance of fruit juice containing probiotics beads in Thailand. AU J Technol. 2010;14:33–38. [Google Scholar]
- Kun Y, Ssonko Lule U, Xiao-Lin D. Lycopene: its properties and relationship to human health. Food Rev Int. 2006;22(4):309–333. doi: 10.1080/87559120600864753. [DOI] [Google Scholar]
- Lakshmi P, Kumar GA. Nanosuspension technology: a review. Int J Pharm Pharm Sci. 2010;2(4):35–40. [Google Scholar]
- Liu C-H, Wu C-T. Optimization of nanostructured lipid carriers for lutein delivery. Colloids Surf A. 2010;353(2):149–156. doi: 10.1016/j.colsurfa.2009.11.006. [DOI] [Google Scholar]
- Liu D, Liu Z, Wang L, Zhang C, Zhang N. Nanostructured lipid carriers as novel carrier for parenteral delivery of docetaxel. Colloids Surf B. 2011;85(2):262–269. doi: 10.1016/j.colsurfb.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Madhusudhan A, Reddy GB, Venkatesham M, Veerabhadram G. Design and evaluation of efavirenz loaded solid lipid nanoparticles to improve the oral bioavailability. Int J Pharm Pharm Sci. 2012;2(4):84–89. [Google Scholar]
- Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47(2):165–196. doi: 10.1016/S0169-409X(01)00105-3. [DOI] [PubMed] [Google Scholar]
- Müller RH, Radtke M, Wissing S. Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm. 2002;242(1):121–128. doi: 10.1016/S0378-5173(02)00180-1. [DOI] [PubMed] [Google Scholar]
- Nayak AP, Tiyaboonchai W, Patankar S, Madhusudhan B, Souto EB. Curcuminoids-loaded lipid nanoparticles: novel approach towards malaria treatment. Colloids Surf B. 2010;81(1):263–273. doi: 10.1016/j.colsurfb.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Nerella A, Basava Raju D, Devi A. Formulation, optimization and in vitro characterization of letrozole loaded solid lipid nanoparticles. Int J Pharm Sci Drug Res. 2014;6(3):183–188. [Google Scholar]
- Qiu W, Jiang H, Wang H, Gao Y. Effect of high hydrostatic pressure on lycopene stability. Food Chem. 2006;97(3):516–523. doi: 10.1016/j.foodchem.2005.05.032. [DOI] [Google Scholar]
- Rahman HS, Rasedee A, How CW, Abdul AB, Zeenathul NA, Othman HH, Yeap SK. Zerumbone-loaded nanostructured lipid carriers: preparation, characterization, and antileukemic effect. Int J Nanomed. 2013;8:2769–2781. doi: 10.2147/IJN.S45313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res. 2007;55(3):207–216. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Riangjanapatee P, Okonogi S. Effect of surfactant on lycopene-loaded nanostructured lipid carriers. Drug Discov Ther. 2012;6(3):163–168. [PubMed] [Google Scholar]
- Riangjanapatee P, Müller RH, Keck CM, Okonogi S. Development of lycopene-loaded nanostructured lipid carriers: effect of rice oil and cholesterol. Pharmazie. 2013;68(9):723–731. [PubMed] [Google Scholar]
- Ruktanonchai U, Bejrapha P, Sakulkhu U, Opanasopit P, Bunyapraphatsara N, Junyaprasert V, Puttipipatkhachorn S. Physicochemical characteristics, cytotoxicity, and antioxidant activity of three lipid nanoparticulate formulations of alpha-lipoic acid. AAPS PharmSciTech. 2009;10(1):227–234. doi: 10.1208/s12249-009-9193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shazly G, Nawroth T, Langguth P. Comparison of dialysis and dispersion methods for in vitro release determination of drugs from multilamellar liposomes. Dissolution Technol. 2008;15(2):7–10. doi: 10.14227/DT150208P7. [DOI] [Google Scholar]
- Shi J, Maguer ML. Lycopene in tomatoes: chemical and physical properties affected by food processing. Crit Rev Food Sci Nutr. 2000;40(1):1–42. doi: 10.1080/10408690091189275. [DOI] [PubMed] [Google Scholar]
- Siekmann B, Westesen K. Thermoanalysis of the recrystallization process of melt-homogenized glyceride nanoparticles. Colloids Surf B. 1994;3(3):159–175. doi: 10.1016/0927-7765(94)80063-4. [DOI] [Google Scholar]
- Tamjidi F, Shahedi M, Varshosaz J, Nasirpour A. Nanostructured lipid carriers (NLC) A potential delivery system for bioactive food molecules. Innov Food Sci Emerg Technol. 2013;19:29–43. doi: 10.1016/j.ifset.2013.03.002. [DOI] [Google Scholar]
- Tamjidi F, Shahedi M, Varshosaz J, Nasirpour A. Design and characterization of astaxanthin-loaded nanostructured lipid carriers. Innov Food Sci Emerg Technol. 2014;26:366–374. doi: 10.1016/j.ifset.2014.06.012. [DOI] [Google Scholar]
- Wang Q, Cheng H, Zhou K, Wang L, Dong S, Wang D, Chen W. Nanostructured lipid carriers as a delivery system of biochanin A. Drug Deliv. 2013;20(8):331–337. doi: 10.3109/10717544.2013.838716. [DOI] [PubMed] [Google Scholar]
- Wilkerson ED, Anthon GE, Barrett DM, Sayajon GFG, Santos AM, Rodriguez-Saona LE. Rapid assessment of quality parameters in processing tomatoes using hand-held and benchtop infrared spectrometers and multivariate analysis. J Agric Food Chem. 2013;61(9):2088–2095. doi: 10.1021/jf304968f. [DOI] [PubMed] [Google Scholar]