Abstract
Biofortification of crops with exogenous iodine is a novel strategy to control iodine deficiency disorders (IDD). The bioaccessibility of iodine (BI) in the biofortified vegetables in the course of soaking, cooking and digestion, were examined. Under hydroponics, the concentration of iodine in leafstalks of the celery and pakchoi increased with increasing exogenous iodine concentration, 54.8–63.9% of the iodine absorbed by pakchoi was stored in the soluble cellular substance. Being soaked in water within 8 h, the iodine loss rate of the biofortified celery was 3.5–10.4% only. More than 80% of the iodine in the biofortified celery was retained after cooking under high temperature. The highest BI of the biofortified vegetables after digestion in simulated gastric and intestinal juice amounted to 74.08 and 68.28%, respectively. Factors influencing BI included pH, digestion duration, and liquid-to-solid ratio. The high BI of the biofortified vegetables provided a sound reference for the promotion of iodine biofortification as a tool to eliminate the IDD.
Keywords: Biofortification, Bioaccessibility of iodine, Iodine-rich vegetable, Iodine deficiency disorders (IDD), Iodized salt
Introduction
Iodine is an essential microelement for the synthesis of thyroid hormone that has multiple functions such as enhancing protein synthesis, promoting growth and development, maintaining the structure of central neural system and metabolism (Hetzel 1983; Delange 2007; WHO 2007). Iodine deficiency will cause a series of physiological disorders, biological function abnormalities and even iodine deficiency disorder (IDD).
Iodine deficiency has been identified as a significant public health problem in 130 countries. It is endemic but diffused in many areas of the world. In 1990, the United Nations World Summit for Children established the goal of eliminating iodine deficiency worldwide. Since then, considerable progress has been achieved, largely through programs of universal salt iodization (Mosha et al. 2004; Andersson et al. 2005; Wegmuller et al. 2006). Nevertheless, in 2013, as defined by a national or sub-national median urinary iodine concentration of 100–299 μg/L in school-aged children, thirty countries remained iodine-deficient; 9 were moderately deficient, 21 were mildly deficient (Pearce et al. 2013; Fuge and Johnson 2015). Furthermore, iodized salt is not a perfect supplementation and even causes some negative effects because of the disadvantages associated with its inorganic form of iodine (I− or IO3 −). First, the iodine in iodized salt is chemically unstable, thus is likely to loss significantly during its production, storage, transport and cooking, e.g. the iodine level decreased by c. 45% within 150 days of table salt storage at medium humidity and limited air access (Waszkowiak and Szymandera-Buszka 2000), loss of iodine from iodized salt during boiling, roasting, deep frying and microwave cooking were found to be 40.23, 10.57, 10.40 and 27.13% respectively (Rana and Raghuvanshi 2013). Second, iodized salt may result in excessive iodine intake for some populations (Stimec et al. 2009; Shan et al. 2016), and more than adequate iodine intake may lead to hypothyroidism, autoimmune thyroiditis, or even thyroid cancer (Teng et al. 2006; Feldt-Rasmussen 2001; Bürgi 2010).
Previous studies revealed that the iodine absorbed by human is mainly from phytogenic foods (Graham et al. 1999; Welch and Graham 2005). It is reported that plants can absorb and transform inorganic iodine from soil/water into bioactive organic iodine by a series of biochemistry reactions (Weng et al. 2008, 2009). Such organic iodine can be readily assimilated by human body without any side effects (Yu 2000). Therefore, agronomic biofortification, i.e. to biofortify crops with iodine through fertilization, is an effective way to improve the iodine content of plant-based foods (Weng et al. 2013). However, the stability and bioaccessibility of iodine in the biofortified vegetables were still poorly discussed in literature (Comandini et al. 2013). This may hinder stakeholder’s interest in them (Mogendi et al. 2016). Using celery and pakchoi as the experiment vegetables, and KI or KIO3 as exogenous iodine, this paper aims to study the bioaccessibility of iodine in the biofortified vegetables throughout their lifecycle, i.e. to explore the iodine absorption characteristics of vegetables under hydroponics in which KI or KIO3 were added into the nutrient solution, to reveal the distribution of iodine among cellular structural components (insoluble organelle, cytoderm, and soluble cellular substance) of the biofortified crops, to make clear the stability of iodine in the biofortified vegetables during cooking, and to clarify the bioaccessibility of iodine during digestion.
Materials and methods
Cultivation of iodine-rich vegetables
Celery (Apium graveolens L.) and pakchoi (Brassica chinensis var chinensis), two species of the most common vegetables all over the world, especially in Asia, were chosen as the target crops. Their seeds, which were procured from Zhejiang Academy of Agricultural Sciences, were disinfected in 1% potassium permanganate (KMnO4) solution for 20 min, soaked in deionized water over night, and germinated at 25 °C in an incubator (The Ministry of Agriculture of the People’s Republic of China 1996; International Seed Testing Association 2017). After initial growth, seedlings were moved to the substrate when 80% of the seeds sprouted, after growth for 3–4 days, proper volume of 1/2 diluted Hoagland solution were regularly supplied (Table 1; Lian 1992; Li et al. 2017). When two true leaves expanded, the seedlings with similar size were placed into nutrient solution and fixed on a piece of sponge. Their roots were suspended in the upper section of the solution. Then, the seedlings were cultivated successively in tap water, 1/4 diluted Hoagland solutions, 1/2 diluted Hoagland solutions, and complete Hoagland solution respectively for every 3 days. At last, the seedlings were cultivated continuously in Hoagland solution adding iodine.
Table 1.
Components and concentrations of Hoagland nutrient solution.
| Macro elements | Trace elements | ||
|---|---|---|---|
| Constituent | Concentration (mmol L−1) | Constituent | Concentration (μmol L−1) |
| KNO3 | 6.00 | H3BO3 | 10.00 |
| Ca(NO3)2 | 3.50 | MnSO4·H2O | 0.50 |
| KH2PO4 | 1.33 | ZnSO4·7H2O | 0.50 |
| MgSO4·7H2O | 0.50 | CuSO4·5H2O | 0.20 |
| NaCl | 0.48 | (NH4)6Mo7O24 | 0.01 |
| Fe-EDTA | 200.00 | ||
To know the variation in iodine absorption rates of the plants growing in various concentrations of KI or KIO3 solution, as the controlled vegetables grew up, the celery and pakchoi were took out of nutrient solution and placed it in deionized water for 5 days. Plants were then all grown in iodine-added nutrient solution for another 4 h. The cultivating was conducted in artificial climate boxes (25 ± 0.5 °C; light intensity, 7100 lx). The iodine-added solutions were prepared by solely adding KI or solely adding KIO3 to the Hoagland solution. In our experiment, nine iodine solution sets (0.05, 0.1, 0.2, 0.3, 0.5, 1.0, 2.5, 5.0 and 10.0 mg/L) were prepared. Totally, there were 36 treatments (9 iodine concentration multiply 2 iodine forms and 2 vegetable species), each treatment had three replicates.
To investigate the iodine absorption rates under various biofortification duration, an experiment was conducted by replacing the nutrition solution every 10 min for the first hour and every 1 h in the following 12 h. Celery or pakchoi were cultivated in 400 ml KI or KIO3 solutions at the iodine concentration of 0.2 and 2.5 mg/L,) respectively. Totally, there were 8 treatments (2 iodine concentrations multiply 2 iodine forms and 2 crop species), each treatment had three replicates. The iodine concentration in the nutrient solution was determined before every replacement.
Tissue isolation and its iodine content measurement
The fresh tissues including roots, stalks and leaves of pakchoi under two treatments (0.5 and 5.0 mg/L iodine solution, respectively) were sampled. They were separated into insoluble cytoderm, organelles and soluble cellular substance by differential centrifugation method (Weigel and Jäger 1980). 5 g sample was added into 10 g homogenate which consisted of sucrose (250 mmol/L), dithioerythritol (1 mmol/L) and Tris buffer (50 mmol/L; pH = 7.5). The iodine in the cell tissues of stalks and leaves were extracted by adding 5% KOH solution at 105 °C for 1 h. The filtered solution was diluted 10-fold with 0.5% tetra methyl ammonium hydroxide (TMAH) prior to further analysis. Then the iodine was determined by ICP-MS, and 128Te was added as internal standard (Zheng et al. 2012; Li et al. 2016). Three replicates were measured for every cellular tissue.
Soaking experiment
Before cooking, cleaning is a necessary provision for cooking. In China, people often soak the vegetables in water for tens of minutes even several hours to remove their pesticide residues. An experiment was conducted to investigate the stability of iodine in leafstalks (the edible part) of the celery during soaking. 10 g iodine-biofortified celery leafstalk was soaked in 100 ml deionized water. The soaking duration was 1 h, 2 h, 3 h, 5 h, 8 h, 12 h, 18 h, 24 h and 36 h, respectively, for each treatment. Iodine concentrations in the solution were determined. Each treatment had three replicates.
Cooking experiment
In order to know the stability of iodine in the biofortified vegetable during cooking, the iodine-rich celery derived from IO3 − biofortification were cooked with non-iodized salt. As a comparison, the ordinary celery was cooked with iodized salt (NaCl–KIO3). The other ingredient used in the cooking experiments was vegetable oil.
To minimize systemic error, 50.0 g of iodized or non-iodized salt were dissolved into 250 ml deionized water. Iodine concentration in the iodized salt solution was also determined.
Usually, in China particularly, people mainly choose the leafstalks of the celery to be cooked into dishes, in the ways varying from stir-frying for a few minutes to stewed/boiled for dozens of minutes, while the celery leaves are discarded.
To know the effects of cooking duration, 10 g celery leafstalk sections, biofortified versus non-biofortified, were boiled with 200 ml deionized water and 5 ml saline solution at 100 °C for various duration (1 min, 2 min, 5 min, 10 min, 20 min and 30 min, respectively). Then, the iodine content in celery and soup were determined separately.
Under various cooking temperature (100 °C, 130 °C and 160 °C, respectively), 40 g celery leafstalk, biofortified versus non-biofortified, was cut into sections of 2 cm and fried with 2 ml vegetable oil for 90 s, then 5 ml saline solution was added. Furthermore, at the cooking temperature of 130 °C, more treatments were conducted, including further frying for another 2 min, or adding the saline solution at the beginning of the cooking.
The experiment was conducted on a temperature control induction cooker, and a matching iron pan. The pan was under cover during boiling experiments, and it was without cover during frying.
In-vitro simulated digestion
An in vitro simulated digestion experiment was conducted according to the static SHIME (Laird et al. 2007). The simulated gastric juice was prepared by dissolving the constituents including 0.5 g arabinogalactan, 1.0 g pectin, 0.5 g xylan, 1.5 g soluble starch, 0.4 g glucose, 3.0 g yeast extract, 1.0 g peptone, 4.0 g mucin, 0.5 g l-cysteine, 1.0 g pepsin, 3.0 g sodium chloride, and 5.0 g potassium bicarbonate in a bottle filling with 1 L distilled water. Then, HCl solution was added to acidify the reagent, and 1 g pepsase was dissolved to adjust the pH to various values including 1.50, 1.74, 2.00, 2.16, 2.54, 3.00, and 3.56. The simulated intestine juice was produced by dissolving the constituents including 1.5 g pancreatin, 6.0 g bile salt, and 15 g sodium bicarbonate in a bottle filled with 1 L distilled water.
In the course of the simulated in vitro gastric digestion, 40 g pakchoi fragments were mixed with 100 ml simulated gastric juice in a reaction tube. Then the tube was sealed using a rubber stopper. The air in tube was then pumped out to form an anaerobic condition. The reaction tube was kept away from light for 2 h at 37 °C. After that, the iodine content in solid part of the pakchoi was determinated after separating the liquid in the tube by centrifugation.
The simulated intestine digestion was conducted following the above mentioned 2 h gastric digestion. The pH of the 100 ml post-gastric-digestion solution was adjusted to 8.0 by adding saturated NaHCO3 solution. Then, 50 ml simulated intestine juice was added into a reaction tube. The air in the tube was pumped out as before. At the temperature of 37 °C, the reaction tube was kept away from light for 0.5 h, 1 h, 2 h, 4 h, 6 h or 8 h, respectively. At the end, the iodine content in solid pakcoi was determinated after separating the liquid by centrifugation.
Iodine in foods usually forms complex compounds with other ingredients. The iodine still in the solid part of the vegetable after digestion is considered as unavailable to the human body. Therefore, we define the bioaccessibility of iodine (BI) as the percentage of the biologically accessible iodine to the initial iodine in the vegetable before digestion:
| 1 |
where BI is the bioaccessibility of iodine (%), Cv and C1 are the iodine content of the vegetable before and after digestion respectively (mg/kg), while Mv and M1 are the weight of the vegetable before and after digestion (kg).
Iodine concentrations of all samples were determined by ICP-MS with test error smaller than 5%.
Results and discussions
Characteristics of iodine absorption
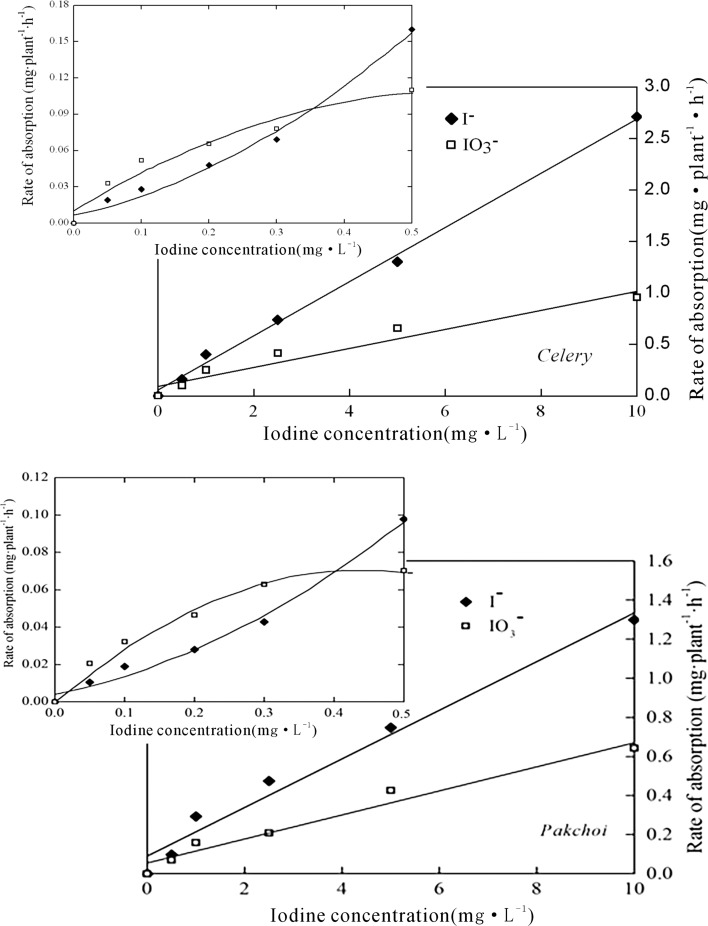
In general, the uptake of iodine increased with increasing exogenous iodine concentration (Fig. 1). Furthermore, under high iodine treatments (≥ 0.5 mg/L), the absorption rates of the crops for both IO3 − and I− were positively correlated to the iodine concentration of the solution (P < 0.01), showing characteristics of passive absorption, in addition, the absorption rate of I− was higher than that of IO3 − for the crops growing in the solution at the same iodine concentration. However, under low iodine treatments (< 0.5 mg/L), the absorption of IO3 − by celery was clearly greater than that of I−, indicating the domination of active absorption of IO3 −.
Fig. 1.
The iodine absorption rates of celery and pakchoi under various exogenous iodine concentrations
According to the widely used Michaelis–Menten equation for describing the kinetic of active absorption (Michaelis and Menten 1913):
| 2 |
The reaction rate (ion absorbed by plant cell) V, may reach the maximum Vmax as the substrate concentration [S] reached a certain level. After that, the uptake of iodine by the vegetable seemed to be dominated by the passive absorption.
The absorption of iodine can be explained by the Michaelis–Menten equation. As shown in Fig. 1, the reaction rate and substrate concentration was positively correlated if the iodine level was low (first order reaction). However, the reaction rate failed to increase proportionally with increasing iodine level, suggesting a mixed order reaction. As the iodine concentration continued to increase (≥ 0.5 mg/L), the enzyme had been saturated and the reaction turned into a zero order.
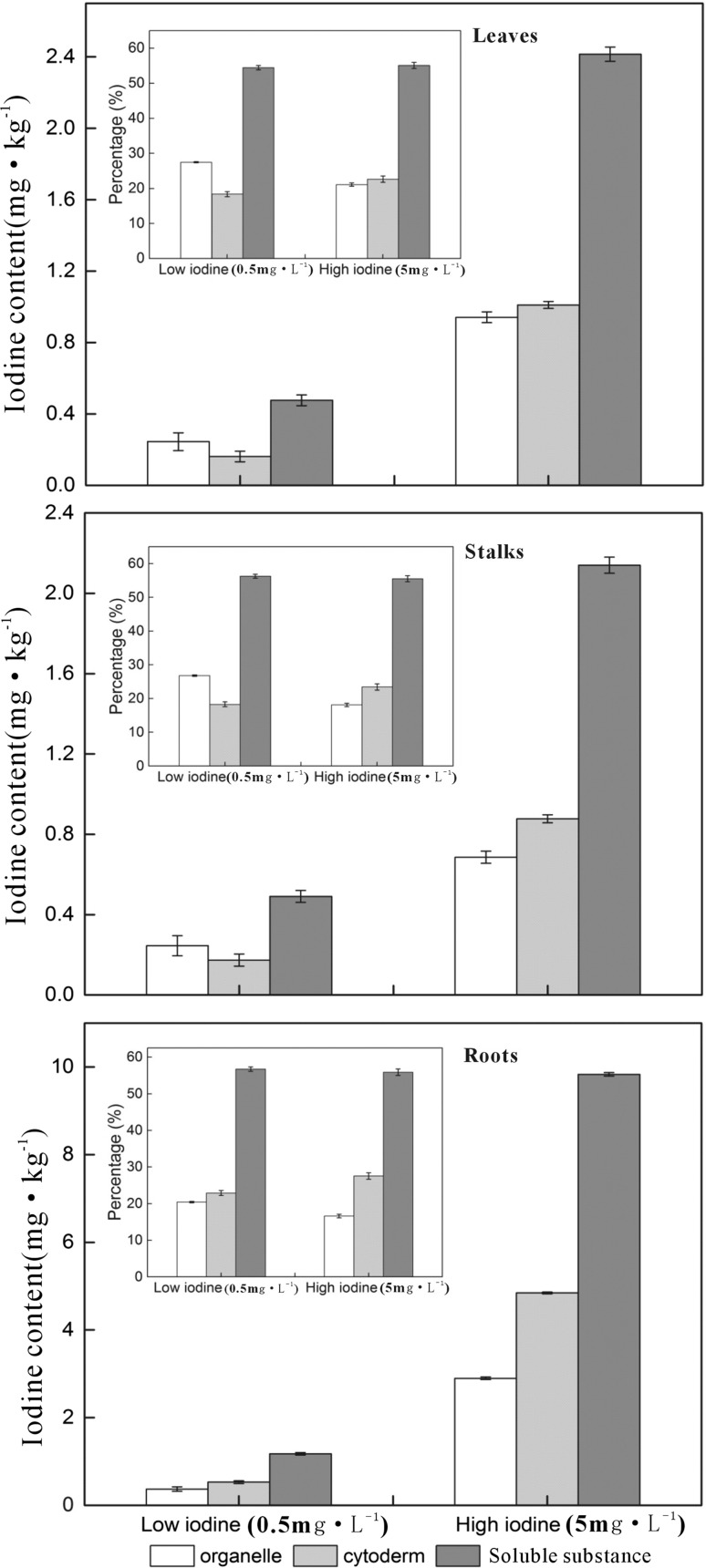
The iodine contents in various organs of pakchoi, including roots, stalks and leaves, increased with increasing iodine concentration of the nutrient solution (Fig. 2). More than half (54.8–63.9%) of the iodine absorbed by pakchoi was stored in the soluble cellular substance, while the rest iodine was stored in insoluble organelles or cytoderm, accounting for 16.3–23.8% in each component. However, in the stalks and leaves of pakchoi, cytoderm seemed to contain less iodine than organelle at low iodine solution (0.5 mg/L), but stored more iodine under high iodine solution (5 mg/L). This indicated that the cytoderm, as an inactive part of plant cell, may prevent or alleviate the cell from the toxicity of excessive exogenous iodine. This was consistent with the finding that higher percentage of iodine in the cytoderm contributed to the tolerance of iodine toxicity (Weigel and Jäger 1980).
Fig. 2.
The iodine content in organelle, cytoderm and soluble cellular substance of various Pakchoi organs (roots, stalks and leaves; n = 3)
It has been reported that by using similar strategy the iodine biofortification can be achieved also for crops grown under conventional field conditions (Smolen, et al. 2011; Cakmak et al. 2017).
Dynamic of iodine absorption by plants
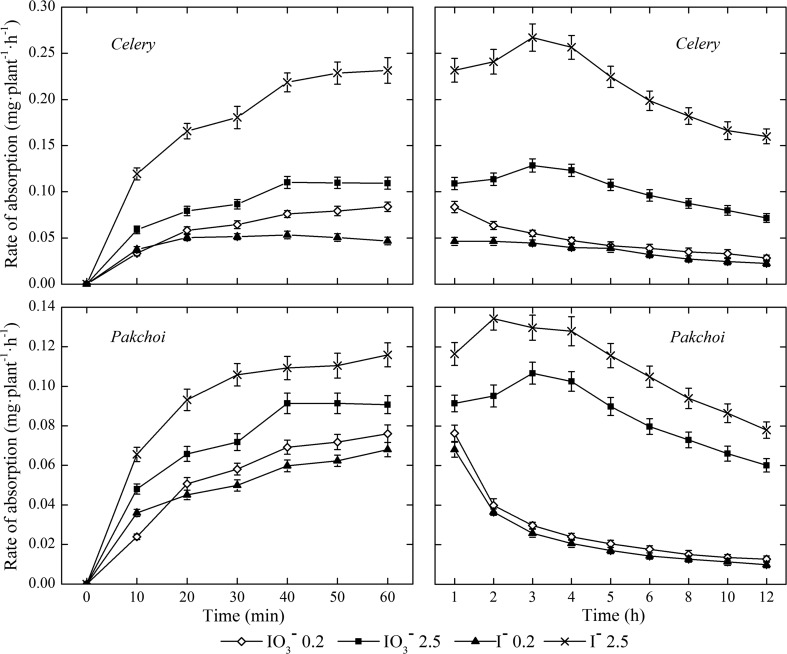
The absorption rate of iodine increased rapidly at the beginning, and then decreased gradually after the maximum absorption rate has reached (Fig. 3). Under low iodine treatment (0.2 mg/L), the peak of the I− absorption rate of celery appeared at the 20th min, and then decreased slightly. While, that of the IO3 − occurred at the 60th min. Under high iodine treatment (2.5 mg/L), the maximum iodine (both IO3 − and I−) absorption rates of celery occurred at about the 3rd h, and the absorption rate of the I− decreased faster than that of the IO3 − thereafter. Pakchoi showed similar iodine uptaking pattern, and the maximum absorption rate of IO3 − and I− occurred at about the 2nd h and the 3rd h, respectively. These results indicated that there was a upper limit for the iodine absorption and accumulation of crops. Due to the increasing storage of iodine in the plant tissues, the iodine absorption rate of the crops decreased. Furthermore, the absorption rate of iodine was also influenced by exogenous iodine concentration and iodine forms. Under low iodine treatment, the absorption rate of IO3 − iodine was higher than that of I− due to the active absorption of IO3 − (Fig. 3). Under high iodine treatment, however, the order is reversed probably because the passive absorption was dominated and I− was much mobile than IO3 −.
Fig. 3.
The iodine absorption rates of celery and pakchoi under various biofortification duration (n = 3)
Stability of iodine in the biofortified vegetable during washing and cooking
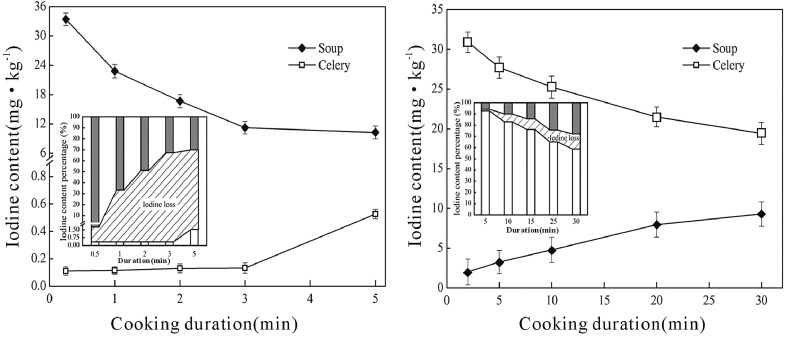
The initial iodine concentrations were respectively 38.84 mg/kg DW (1.94 mg/kg FW) in leafstalks and 55.89 mg/kg DW (2.52 mg/kg FW) in the leaves of the celery biofortified in the 0.5 mg/L IO3 − solution. As a comparison, the initial iodine concentration of the IO3 − iodized salt was set as 34.05 mg/kg.
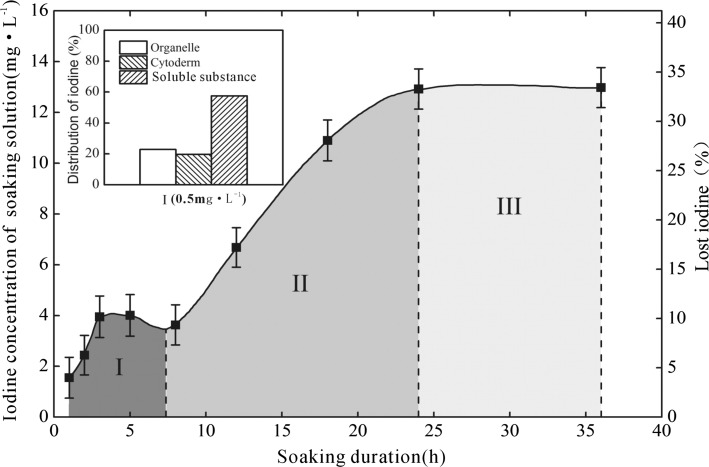
Figure 4 showed the cumulatively dissolved or lost iodine as the biofortified celery soaked in deionized water for various duration under room temperature. During stage I (the first 8 h), the iodine loss was low, being 1.36–4.05 mg/kg or 3.5–10.4% of the biofortified celery leafstalks. During stage II (the 8th–24th h), the iodine loss increased significantly, and reached a maximum of 12.89 mg/kg or 33.2% of the biofortified celery leafstalks. During stage III (From the 24th h on), the iodine loss of the soaked vegetable stopped, showing obvious equilibrium (Fig. 4). In most cases, the vegetable cleaning process is actually finished within a few minutes, therefore, the iodine loss of the biofortified vegetables is very low during cleaning.
Fig. 4.
The iodine dissolved from the iodine-biofortified celery into the soaking water under various soaking duration (n = 3), the left top subgraph showed the distribution of iodine in organelle, cytoderm and soluble cellular substance
Figure 5 showed the concentration of iodine in the non-biofortified celery and soup during cooking with iodized salt at 100 °C. In the first 3 min, iodine in the non-biofortified celery was low (0.1–0.14 mg/kg). It increased to about 0.53 mg/kg (1.56% of the initially iodized salt) after boiling for 5 min. Meanwhile, the iodine concentration in the soup decreased significantly during boiling. At the beginning, about 99.13% of the iodine in the iodized salt dissolved in the soup, resulting in an iodine concentration of 33.75 mg/kg. After boiling for 5 min, only 29.03% of the iodine in the iodized salt left in the soup, and the iodine concentration was as low as 9.88 mg/kg. Most iodine in the soup was volatilized with water, and only a very limited iodine eventually permeated into the celery.
Fig. 5.
The iodine concentration in the celery and soup after boiling for various time at 100 °C (n = 3). left, non-biofortified celery boiling in iodized salt solution; right, iodine-biofortified celery boiling in non-iodized salt solution
Furthermore, higher cooking temperature enhanced such loss. As the iodized salt was added at the end of cooking, after the non-biofortified celery was fried for 90 s at 100 °C, the iodine loss rate was 55%, however, the iodine loss rate had increased to 61% and 66% once the cooking temperature was set to 130 °C and 160 °C respectively. Cooking at 130 °C, if the iodized salt was added before cooking, the iodine loss rate increased to 77%; continuing to fry for 2 min more the iodine loss rate increased to 80%.
On the contrary, as the biofortified celery was boiled with the ordinary (non-iodized) salt at 100 °C, the iodine concentration in celery was 36.08 mg/kg after boiling for 2 min, and 22.32 mg/kg for 30 min (Fig. 5). The corresponding iodine concentration in the soup was 2.46 and 10.82 mg/kg respectively. As a result, the iodine loss rate in the biofortified celery was much lower, accounting for only 0.78% and 14.67% respectively after cooking for 2 min and 30 min, while, for the non-biofortified celery + iodized salt treatment, corresponding iodine loss rate amounted to 50% and 55% respectively. The biofortified celery can retain 92.89% and 85.62% of the initial iodine after boiling with non-iodized salt for 2 min and 5 min respectively, being 232 times and 55 times higher than that of the non-biofortified celery + iodized salt under the same treatment. This result demonstrated that the organic iodine in the biofortified celery was more stable than the inorganic iodine in the iodized salt during cooking.
In addition, the loss of iodine from the biofortified celery during cooking was found to be also sensitive to the cooking temperature, although the overall loss rates were still lower than that of the control (non-biofortified celery + iodized salt cooking). The iodine loss rate increased from 3% at 100 °C to 20% and 41% at 130 °C and 160 °C respectively after frying for 90 s. Further frying for another 2 min at 130 °C, the iodine loss rate increased to 39%. This may resulted from more severe damage of celery cells under increasing cooking temperature.
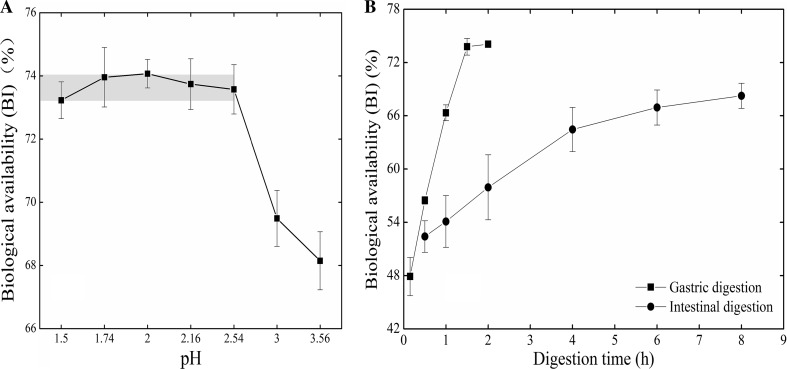
Bioaccessibility of iodine (BI) in the biofortified vegetable during digestion
As one of the key factors influencing the bioaccessibility of essential microelements (Laird et al. 2007), the pH of the gastric juice generally ranges 1.5–2.0 under starving condition and 3.0–7.0 when the stomach is full (Tandy et al. 2000). The BI in pakchoi varied with different pH of the simulated gastric juice (Fig. 6). It was relatively high if the pH of the simulated gastric juice was below 2.54, ranging 73.3–74.08% on average, and reaching the highest value (74.08%) at the pH of 2.00. However, the BI decreased quickly as the pH was above 2.54, which might be resulted from decreasing activity of pepsase, because the catalyze ability of pepsase can be maintained only under strong acid condition, and it generally decreases with increasing pH. Further increase of pH to above 6 may cause irreversible protein denaturation and destroy the catalyze ability (Lu et al. 2010; Yu et al. 2009). The overlap of the optimum pH range for the pepsase’s activity and that of high BI suggested that the BI of the biofortified pakchoi was associated with the activity of pepsase.
Fig. 6.
The bioaccessibility of iodine (BI) under various pH or digestion duration (n = 3)
The BI of pakchoi in the simulated gastric juice increased quickly with increasing digesting duration (Fig. 6). It reached 47.99% at the 10th min and 73.79% at the 90th min under digestion. As the digesting duration exceeded 90 min, the BI became relatively constant, indicating that the exchange of iodine between the pakchoi and the simulated gastric juice had reached the balance. Previous studies suggested that the digestion and absorption was a dynamic process and occurred simultaneously (Yu et al. 2009). However, our experiment did not include the absorption process, i.e. transferring iodine from gastric juice to other body tissues, then, the saturation phenomenon was expected after long time digestion in the gastric-juice-only system.
Generally, foods stay in the stomach only for a short period, and the absorption mainly occurs in small intestine. Therefore, it is critical to evaluate the BI in the biofortified vegetable during intestine digestion. The BI in the biofortified pakchoi increased from 52.46 to 68.28% as the digesting duration increased from 0.5 to 8 h. The increase of BI slowed down after digesting for 4 h (Fig. 6). Aquaron et al. (2002) had investigated the BI of two marine algae species using living Laminaria hyperborean and Gracilaria verucosa, and they reported higher BI values (61.5–85%). This is partly, besides the difference between marine algae and pakchoi, because the living organisms in their experiments can absorb iodine during digestion and remove iodine from the digestion juice.
Conclusions
The bioaccessibility of iodine in the biofortified vegetables throughout their lifecycle was evaluated in this study. Most of the iodine absorbed by pakchoi was stored in the soluble cellular substance, amounting to 54.8–63.9%. The iodine loss of the biofortified celery leafstalks was very low during soaking for less than 8 h, being 3.5–10.4% only. The iodine in the biofortified celery was more stable than that in the iodized salt during cooking. After cooking for 5 min, about 85% of the initial iodine was retained still in the biofortified celery, whereas only about 30% of the initial iodine remained in iodized salt cooked with the non-biofortified celery. The bioaccessibility of iodine of the biofortified celery after digestion in simulated gastric and intestinal juice amounted to 74.08 and 68.28%, respectively. During gastric digestion, the bioaccessibility of iodine the biofortified celery was the highest as the pH was 2.00, it increased with increasing digestion duration, reaching the upper limit as the digestion duration exceeded 90 min. During intestinal digestion, the bioaccessibility of iodine the biofortified celery increased with increasing digestion duration and liquid-to-solid ratio. As the digestion duration exceeded 4 h and the ratio was higher than 10, the increasing rate of the bioaccessibility of iodine slowed down.
In general, the bioaccessibility of iodine in the biofortified vegetable was high during cooking and digestion. Therefore, to supply iodine through agronomic biofortification of crops is an excellent and worth advocating way to eliminate iodine disorder.
Acknowledgements
This study was funded by the geological exploration project of Zhejiang Provincial Department of Land Resources (Grant No. 2014002), and National Natural Science Foundation of China (Grant No. 40873058 4033043).
References
- Andersson M, Takkouche B, Egli I, Allen HE, de Benoist B. Current global iodine status and progress over the last decade towards the elimination of iodine deficiency. Bull World Health Organ. 2005;83(7):518–525. [PMC free article] [PubMed] [Google Scholar]
- Aquaron R, Delange F, Marchal P, Lognoné V, Ninane L. Bioavailability of seaweed iodine in human beings. Cell Mol Biol. 2002;48(5):563–569. [PubMed] [Google Scholar]
- Bürgi H. Iodine excess. Best Parct Res Clin Endronical Metab. 2010;24(1):107–115. doi: 10.1016/j.beem.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Cakmak I, Prom-u-thai C, Guilherme LRG, Rashid A, Hora KH, Yazici A, Savasli E, Kalayci M, Tutus Y, Phuphong P. Iodine biofortification of wheat, rice and maize through fertilizer strategy. Plant Soil. 2017;418(1–2):319–335. doi: 10.1007/s11104-017-3295-9. [DOI] [Google Scholar]
- Comandini P, Cerretani L, Rinaldi M, Cichelli A, Chiavaro E. Stability of iodine during cooking: investigation on biofortified and not fortified vegetables. Int J Food Sci Nutr. 2013;64(7):857–861. doi: 10.3109/09637486.2013.798270. [DOI] [PubMed] [Google Scholar]
- Delange F. Iodine requirements during pregnancy, lactation and the neonatal period and indicators of optimal iodine nutrition. Public Health Nutr. 2007;10(12):1571–1580. doi: 10.1017/S1368980007360941. [DOI] [PubMed] [Google Scholar]
- Feldt-Rasmussen U. Iodine and cancer. Thyroid. 2001;11(5):483–486. doi: 10.1089/105072501300176435. [DOI] [PubMed] [Google Scholar]
- Fuge R, Johnson CC. Iodine and human health, the role of environmental geochemistry and diet, a review. Appl Geochem. 2015;63(2):282–302. doi: 10.1016/j.apgeochem.2015.09.013. [DOI] [Google Scholar]
- Graham R, Senadhira D, Beebe S, Iglesias C, Monasterio I. Breeding for micronutrient density in edible portions of staple food crops: conventional approaches. Field Crop Res. 1999;60(1):57–80. doi: 10.1016/S0378-4290(98)00133-6. [DOI] [Google Scholar]
- Hetzel B. Iodine deficiency disorders (IDD) and their eradication. Lancet. 1983;2(8359):1126–1129. doi: 10.1016/S0140-6736(83)90636-0. [DOI] [PubMed] [Google Scholar]
- International Seed Testing Association . International rules for seed testing rules. Bassersdorf: ISTA; 2017. [Google Scholar]
- Laird BD, Van de Wiele TR, Corriveau MC, Jamieson HE, Parsons MB, Verstraete W, Siciliano SD. Gastrointestinal microbes increase arsenic bioavailability of ingested mine tailings using the simulator of the human intestinal microbial ecosystem. Environ Sci Technol. 2007;41(15):5542–5547. doi: 10.1021/es062410e. [DOI] [PubMed] [Google Scholar]
- Li S, He S, Li JL, Li YJ, Fang J, Xian YP, Huang JF, Guo XD. Determination of total iodine in infant formula by KOH digestion and inductively coupled plasma mass spectrometry. J Instrum Anal. 2016;35(7):864–868. [Google Scholar]
- Li R, Liu HP, Hong CL, Dai ZX, Liu JW, Zhou J, Hu CQ, Weng HX. Iodide and iodate effects on the growth and fruit quality of strawberry. J Sci Food Agric. 2017;97:230–235. doi: 10.1002/jsfa.7719. [DOI] [PubMed] [Google Scholar]
- Lian ZH. Theories and techniques of soilless culture. Beijing: National Agricultural Press; 1992. [Google Scholar]
- Lu M, Yu Y, Zhang D, Han S, Wu M, Sheng G, Fu J. Factors affecting bioavailability of DDT and its metabolites in carrot. J Shanghai Univ (Nat Sci) 2010;16(2):189–195. [Google Scholar]
- Michaelis M, Menten L. The kinetics of inverting action. Biochem Z. 1913;49:333–369. [Google Scholar]
- Mogendi JB, De Steur H, Gellynck X, Makokha A. A novel framework for analysing stakeholder interest in healthy foods: a case study on iodine biofortification. Ecol Food Nutr. 2016;55(2):182–208. doi: 10.1080/03670244.2015.1112283. [DOI] [PubMed] [Google Scholar]
- Mosha TCE, Tarimo F, Tuzie E. Towards sustainable elimination of iodine deficiency disorder: a case study of selected villages in the endemic Southern Highlands, Tanzania. Ecol Food Nutr. 2004;43(5):375–408. doi: 10.1080/03670240490500307. [DOI] [Google Scholar]
- Pearce EN, Andersson M, Zimmermann MB. Global iodine nutrition: Where do we stand in 2013? Thyroid. 2013;23(5):523–528. doi: 10.1089/thy.2013.0128. [DOI] [PubMed] [Google Scholar]
- Rana R, Raghuvanshi RS. Effect of different cooking methods on iodine losses. J Food Sci Technol Mys. 2013;50(6):1212–1216. doi: 10.1007/s13197-011-0436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Z, Chen L, Lian X, Liu C, Shi B, Shi L, Tong N, Wang S, Weng J, Zhao J, Teng X. Iodine status and prevalence of thyroid disorders after introduction of mandatory universal salt iodization for 16 years in China: a cross-sectional study in 10 cities. Thyroid. 2016;26(8):1125–1130. doi: 10.1089/thy.2015.0613. [DOI] [PubMed] [Google Scholar]
- Smolen S, Rozek S, Strzetelski P, Ledwozyw-Smolen I. Prelimilary evaluation of the influence of soil fertilization and foliar nutrition with iodine on the effectiveness of iodine biofortification and mineral composotion of carrot. J Elementol. 2011;16(1):103–113. [Google Scholar]
- Stimec M, Kobe H, Smole K, Kotnik P, Sirca-Campa A, Zupancic M, Battelino T, Krzisnik C, Mis NF. Adequate iodine intake of Slovenian adolescents is primarily attributed to excessive salt intake. Nutr Res. 2009;29(12):888–896. doi: 10.1016/j.nutres.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Tandy S, Williams M, Leggett A, Lopezjimenez M, Dedes M, Ramesh B, Srai SK, Sharp P. Nramp2 expression is associated with pH-dependent iron uptake across the apical membrane of human intestinal Caco-2 cells. J Biol Chem. 2000;275(2):1023–1029. doi: 10.1074/jbc.275.2.1023. [DOI] [PubMed] [Google Scholar]
- Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, Jin Y, Yu X, Fan C, Chong W. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006;354(26):2783–2793. doi: 10.1056/NEJMoa054022. [DOI] [PubMed] [Google Scholar]
- The Ministry of Agriculture of the People’s Republic of China . Rules for agricultural seed testing-Germination test (GB/T 3543.4-1995) Beijing: China Standards Press; 1996. [Google Scholar]
- Waszkowiak K, Szymandera-Buszka K. Effect of storage conditions on potassium iodide stability in iodised table salt and collagen preparations. Int J Food Sci Technol. 2000;43(6):895–899. [Google Scholar]
- Wegmuller R, Zimmermann MB, Buhr VG, Windhab EJ, Hurrell RE. Development, stability, and sensory testing of microcapsules containing iron, iodine, and vitamin a for use in food fortification. J Food Sci. 2006;71(2):S181–S187. doi: 10.1111/j.1365-2621.2006.tb08923.x. [DOI] [Google Scholar]
- Weigel HJ, Jäger HJ. Subcellular distribution and chemical form of cadmium in bean plants. Plant Physiol. 1980;65(3):480–482. doi: 10.1104/pp.65.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch RM, Graham RD. Agriculture: the real nexus for enhancing bioavailable micronutrients in food crops. J Trace Elem Med Biol. 2005;18(4):299–307. doi: 10.1016/j.jtemb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Weng HX, Weng JK, Yan AL, Hong CL, Yong WB, Qin YC. Increment of iodine content in vegetable plants by applying iodized fertilizer and the residual characteristics of iodine in soil. Biol Trace Elem Res. 2008;123(1–3):218–228. doi: 10.1007/s12011-008-8094-y. [DOI] [PubMed] [Google Scholar]
- Weng HX, Yan AL, Hong CL, Qin YC, Pan L, Xie LL. Biogeochemical transfer and dynamics of iodine in a soil–plant system. Environ Geochem Health. 2009;31(3):401–411. doi: 10.1007/s10653-008-9193-6. [DOI] [PubMed] [Google Scholar]
- Weng HX, Hong CL, Xia T, Bao L, Liu H, Li D. Iodine biofortification of vegetable plants—an innovative method for iodine supplementation. Chin Sci Bull. 2013;58(17):2066–2072. doi: 10.1007/s11434-013-5709-2. [DOI] [Google Scholar]
- World Health Organization (WHO) Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. 3. Geneva: World Health Organization; 2007. [Google Scholar]
- Yu X. Distribution characteristics of iodine in humic acid-high underground water in Inner Mongolia and their relations to iodine defect disease. J Environ Sci China. 2000;21(2):56–59. [Google Scholar]
- Yu YX, Han SY, Jia WL. Character and stable carbon isotope effect of DDTs during digestion in simulating gastrointestinal tract. Chin Sci Bull. 2009;54(4):441–447. [Google Scholar]
- Zheng J, Takata H, Tagami K, Aono T, Fujita K, Uchida S. Rapid determination of total iodine in Japanese coastal seawater using SF-ICP-MS. Microchem J. 2012;100:42–47. doi: 10.1016/j.microc.2011.08.007. [DOI] [Google Scholar]