Abstract
In the present study, polyphenols from green tea (GTP), oolong tea (OTP) and black tea (BTP) were prepared by extraction with hot water and polyamide column chromatography. In antioxidant assay in vitro, each tea polyphenols exhibited potential activity; the intestinal absorption of GTP, OTP and BTP was investigated individually by Caco-2 transwell system, and each sample was poorly transported, illustrating a low transport rate for tea polyphenols through cell monolayers. The effects of GTP, OTP and BTP on human intestinal microbiota were also evaluated, and each sample induced the proliferation of certain beneficial bacteria and inhibited Bacteroides–Prevotella and Clostridium histolyticum. Moreover, the short-chain fatty acids (SCFA) produced in cultures with tea polyphenols were relatively higher. Together, these results suggested GTP, OTP and BTP may modulate the intestinal flora and generate SCFA, and contribute to the improvements of human health.
Keywords: Tea, Polyphenols, Intestinal microbiota, Prebiotic, Modulatory effect
Introduction
Containing numerous bioactive compounds, tea is one of the most popularly beverages, and has been viewed as a potential health-promoting natural resource worldwide (Kumar et al. 2011; Jobu et al. 2013). Tea polyphenols are important bioactive ingredients in tea, as its main ingredient, tea catechins accounted to 60–80% and (−)-epigallocatechin gallate (EGCG) was reported as the most abundant components. Furthermore, epigallocatechin-3-O-(3″-O-methyl)-gallate (EGCG3″Me,) in tea have exhibited significant health benefits (Fei et al. 2014; Zhang et al. 2013). Accordingly, tea polyphenols have attracted great attentions for their potential applications in the prevention and alleviation of human diseases (Pinto 2013).
In human gastrointestinal tract, the vast majority of microbial residents play a critical role for the maintenance of host health (Gill et al. 2006), and the disturbance of gut microbiota is associated with metabolic syndromes (Turnbaugh et al. 2009). The gut microbiota can balance the nutrient acquisition and energy regulation by processing the indigestible components from our daily diet (Consortium HMP 2012). Tea polyphenols have exhibited multiple beneficial effects in vitro; however, for the low bioavailability, those absorbed directly by the small intestine are only a small fraction, more than 90% are metabolized to simpler compounds by the bacteria in the colon (Zhou et al. 2016), which may positively affect intestinal micro-ecology.
In our previous studies, the modulatory effect of oolong tea catechins on intestinal flora was investigated by high-throughput sequencing, the decrease in the Firmicutes/Bacteroidetes ratio indicated tea polyphenols may be used as functional food components with potential therapeutic utility in manipulating human intestinal microbiota (Cheng et al. 2017). However, the prebiotic-like activity of different tea polyphenols need to be further studied. Therefore, in the present study, we investigated the transepithelial transport of polyphenols from different teas through a Caco-2 cell monolayer model, a widely used model for human intestinal drug absorption, and then the effects of tea polyphenols on the composition of gut microbiota and SCFA production were evaluated.
Materials and methods
Materials
Polyamide resin was purchased from Ocean Chemical (Qingdao, China). Standards of EGCG and EGCG3″Me were prepared according to our reported methods (Zhang et al. 2013). Pepsin, pancreatin, bile salt, penicillin and streptomycin were purchased from Sigma Co. (St. Louis, MO, USA). Fructo-oligosaccharides (FOS, > 90%) were purchased from Qibang Biological Co. (Shanghai, China). All other chemicals and reagents were analytical grade.
Preparation of GTP, OTP and BTP
Green tea, oolong tea and black tea were purchased from a local tea plantation in Ningbo, China. GTP, OTP and BTP were prepared according to our reported method (Zhang et al. 2013). Briefly, 100 g of tea powder was extracted with 1600 mL of distilled water at 96 °C for 40 min. The supernatants were concentrated and purified by a polyamide column. As results, the fractions containing tea polyphenols were concentrated and lyophilized.
HPLC analysis and characterization of tea catechins and their metabolites
The contents of tea catechins were determined by HPLC (Agilent, CA, USA). The separation was achieved on a TSKgel ODS-100Z column (4.6 × 150 mm, 5 μm, Tosoh, Tokyo, Japan) (Zhang et al. 2014). The molecular weight of the metabolites of tea catechins after anaerobic fermentation was analyzed by ESI-MS/MS, which was performed by TSQ Quantum Ultra triple quadrupole mass spectrometer (Thermo Scientific) in positive mode (Zhang et al. 2013).
Determination of the antioxidant activity
The DPPH and ABTS free radical scavenging activity was determined according to the reported method (Stratil et al. 2006; Zhang et al. 2012), and it was expressed by IC50 value which is the concentration of samples required to decrease the absorbance at 517, 734 nm by 50%, respectively. The ability to reduce ferric ions was measured by our previous reported method (Zhang et al. 2014).
In vitro cytotoxicity assay
Caco-2 cells were cultured in DMEM maintained in a humidified incubator at 37 °C with 5% CO2. Cell viability was measured by MTT assay with slightly modifications (Zhang et al. 2014). Caco-2 cells were pipetted into 96-well flat-bottom plate, after 12 h, non-adherent cells were removed by washing with serum free culture medium. Then fresh medium (100 μL/well, control group) or samples (100 μL/well, GTP, OTP and BTP at a final concentration of 25, 50, 100 and 200 μg/mL) were added to each well, and incubated for 24, 48 and 72 h, respectively. After the incubation, the cell viability was measured by the MTT assay and expressed by CTC50 values.
For the transport experiment, Caco-2 cells were seeded on transwell polycarbonate insert filters in 12-well plates. After seeding, cells were fed with culture medium in the apical (AP) and basolateral sides (BL) (Willenberg et al. 2015). Firstly, PSPAs and PSPAs-PC samples at a final concentration of PSPAs at 50 μg/mL was added to AP and HBSS was added to BL. Then 20 μL of solution from BL were withdrawn at 30, 60, 90, 120 min, respectively. For the efflux study, both samples at the same concentration as mentioned above were added to BL, which acted as the donating side, while AP filled with HBSS worked as the receiver chamber. The operation was opposite with the above mentioned absorption procedure. The apparent permeability coefficient (P app) was calculated according to:
where ΔC is the concentration (μg/mL) in the receiver chamber, V is the volume of the receiver chamber (mL), Δt is the duration of the transport experiment (s), C0 is the initial concentration in the donor chamber (μg/mL), and A is the surface area of filter (cm2), which is 4.67 cm2 in our study. The drug was measured from AP → BL and BL → AP, respectively. The efflux ratio (ER) was calculated according to:
In vitro fermentation of tea polyphenols
Fecal samples were obtained from 6 healthy volunteers (3 females and 3 males, age 25–30) without antibiotic treatment over the preceding 6 months and without gastrointestinal disorders. The fecal slurries were prepared by mixing fresh fecal samples with autoclaved phosphate-buffered saline (PBS, 0.1 M, pH 7.2) to yield 10% (w/v) suspensions. GTP, OTP, BTP and FOS (positive control) were mixed with autoclaved nutrient basal growth medium at a final concentration of 1% (w/v). Fermentation was initiated by adding 150 μL of fecal slurry to 1350 μL of culture medium with a manual homogenizer in an anaerobic atmosphere of 10% H2, 10% CO2 and 80% N2 at 37 °C (Zhang et al. 2013).
To investigate the structural changes of tea catechins during the anaerobic fermentation in vitro, fermentation was also initiated by adding 150 μL of fecal slurry to 1350 μL of culture medium containing EGCG and EGCG3″Me and incubated at 37 °C. Samples were taken from the inoculum at 36 h for the identification of the corresponding metabolites.
Enumeration of bacteria by fluorescent in situ hybridization (FISH)
Briefly, culture samples (50 μL) were taken at 0, 12, 24 and 36 h, added to 150 μL of a filtered paraformaldehyde solution (4%, w/v), and fixed overnight at 4 °C. Hybridization was performed using 16S rRNA-targeted oligonucleotide probes labeled with cyanine dye-3 (Cy3) fluorescent dye to enumerate specific bacterial groups (Zhang et al. 2013). An Axio Imager A1 epifluorescence microscope was used to count bacterial cells, which were expressed as log10 cells per milliliter ± standard deviation (SD).
Determination of SCFA content
The content of SCFA was analyzed by an Agilent 1100 series HPLC system with a Beckman Ultrasphere column (4.6 × 250 mm, 5 μm, Beckman Instruments Inc., Brea, CA, USA) according to reported method (Li et al. 2015). The concentrations of SCFA were calculated according to calibration curves of respective authentic compounds, including formic, acetic, propionic and butyric acids.
Statistical analysis
Data were analyzed by SPSS and expressed as mean ± standard deviation (SD) for at least three replicates. Significance was determined at P < 0.05 by ANOVA followed by Duncan’s multiple-comparison tests.
Results and discussion
Preparation of GTP, OTP and BTP
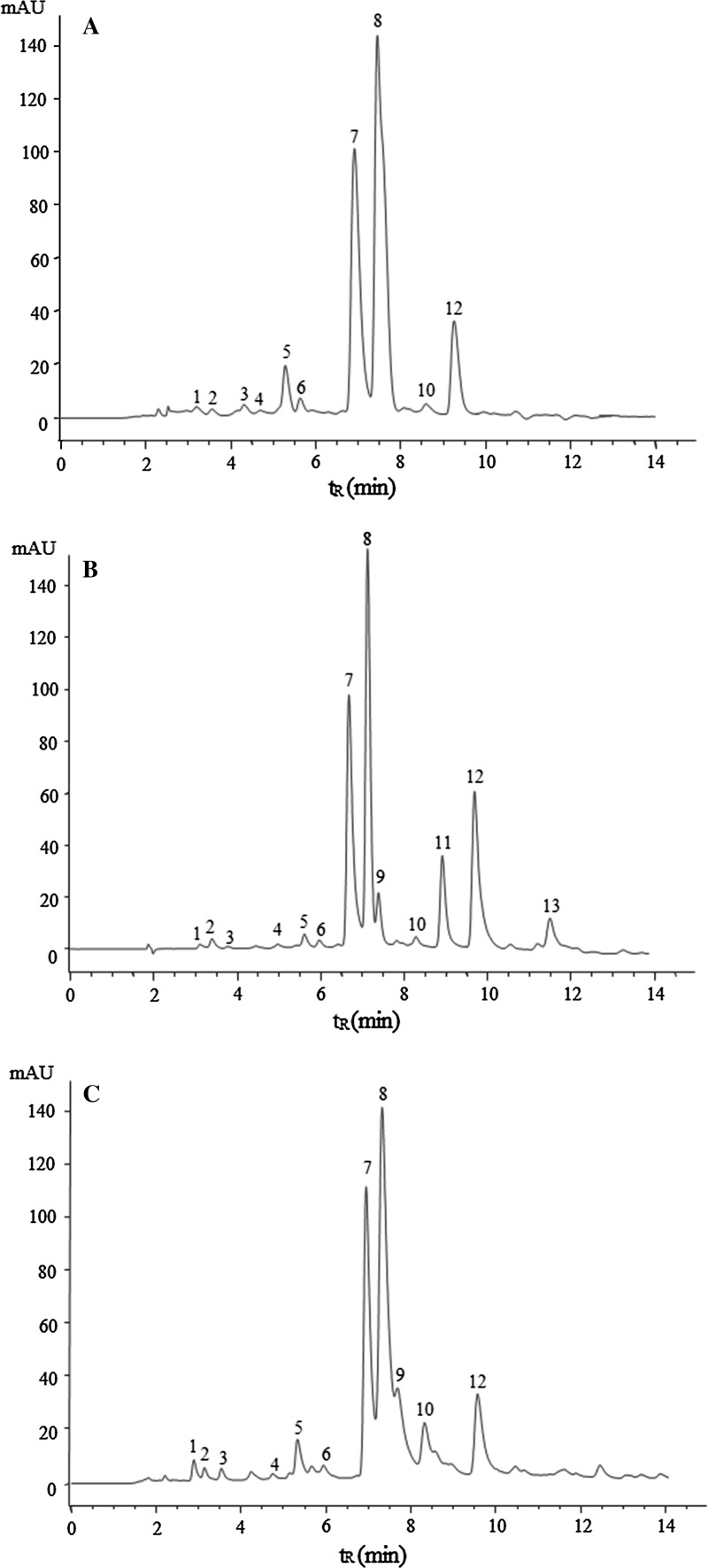
In the present study, GTP, OTP and BTP were prepared by column chromatography according to our reported method. As shown in Fig. 1, peaks 1–13 in the HPLC chromatogram of oolong tea infusion were identified. In addition, it was found that Ningbo oolong tea contained relative high content of EGCG3″Me, which amounted to 10.42 ± 0.47 mg/g (Table 1), while it was not detected in green tea and black tea.
Fig. 1.
Representative elution profiles of tea catechins, gallic acid, caffeine, theobromine and theophylline from green tea (a), oolong tea (b), black tea (c) (1 gallic acid, 2 (−)-gallocatechin (GC), 3 theobromine, 4 EGC, 5 (−)-catechin (C), 6 theophylline, 7 EGCG, 8 caffeine, 9 EC, 10 (−)-gallocatechin-3-gallate (GCG), 11 EGCG3″Me, 12 ECG, 13 (−)-catechin gallate (CG))
Table 1.
Contents of tea catechins, gallic acid, caffeine, theobromine and theophylline in green tea, oolong tea and black tea
| Components | Green tea | Oolong tea | Black tea |
|---|---|---|---|
| Gallic acid | 0.01 ± 0.00a | 0.02 ± 0.00b | 0.02 ± 0.00b |
| GC | 3.46 ± 0.24c | 2.55 ± 0.21a | 3.08 ± 0.16b |
| Theobromine | 0.35 ± 0.02c | 0.23 ± 0.02a | 0.28 ± 0.01b |
| EGC | 19.01 ± 0.87c | 16.62 ± 0.57a | 17.61 ± 0.66b |
| C | 0.54 ± 0.03c | 0.35 ± 0.02a | 0.47 ± 0.02b |
| Theophylline | 0.01 ± 0.00a | 0.01 ± 0.00a | 0.01 ± 0.00a |
| EGCG | 51.08 ± 3.27c | 48.71 ± 2.64b | 43.25 ± 3.03a |
| Caffeine | 45.96 ± 3.36c | 43.29 ± 4.06b | 38.64 ± 2.65a |
| EC | – | 3.17 ± 0.24a | 4.02 ± 0.22b |
| GCG | 2.25 ± 0.16a | 2.44 ± 0.18b | 3.62 ± 0.23c |
| EGCG3″Me | – | 10.42 ± 0.47a | – |
| ECG | 12.89 ± 0.86b | 13.03 ± 0.97b | 8.73 ± 0.65a |
| CG | – | 0.77 ± 0.38a | – |
Different lowercase letters indicate significant differences (P < 0.05) for each tea sample
Antioxidant activity of GTP, OTP and BTP
At the range from 0.2 to 0.8 mg/mL, the scavenging activity increased rapidly with the concentration, and GTP showed the highest free radical scavenging activity with an IC50 of 0.35 mg/mL, followed by OTP and BTP. The antioxidant abilities determined by the ABTS method and ferric ion reducing activities of GTP, OTP and BTP were similar to the above results, GTP exhibited highest antioxidant activity, and OTP exhibited considerably higher activity than BTP. In our previous study, the antioxidant activity of EGCG3″Me is weaker than EGCG in vitro, which may be related to the substitution of 3-OH by methoxy group in D ring (Zhang et al. 2014). The antioxidant capacity of tested tea is green tea > oolong tea > black tea, which was consistent with the previous study (Richelle et al. 2001). Unfermented tea showed the best antioxidant capacity, while semi-fermented tea was relatively better than fermented tea, which indicated that the anti-oxidative property of tea is affected by the processing technology.
Tea polyphenols accounted for more than one third of the dry weight of tea. Tea catechins were the most biologically active part of tea polyphenols, during the fermentation, they were oxidized to quinones, and then bisflavanol, thearubigen, theaflavin and other high molecular substances were formed (Higdon and Frei 2003). With the deepening of fermentation degree, the content of tea catechins decreased rapidly. In this experiment, the antioxidant capacity of tea and phenolic compounds were significantly correlated, which consistent with previous reports, indicating that the higher the degree of fermentation, the lower antioxidant capacity.
Transepithelial transport study
The intestinal absorption of each tea polyphenols was investigated individually by Caco-2 transwell system. The cytotoxicity of each sample was studied by in vitro cytotoxicity assay, the CTC50 value of GTP, OTP and BTP was 173, 164 and 166 μg/mL for 72 h, respectively, which showed tea polyphenols we prepared had no cytotoxicity for Caco-2 cell line in this study.
As shown in Table 2, the absorption (P app (AP → BL)) and excretion (P app (BL → AP)) of OTP and BTP were relatively higher than GTP. As the low efflux ratios would result in high bioavailability, it revealed that of GTP was relatively lower. Compounds which are completely absorbed in human intestine typically exhibit P app values of > 70 × 10−6 cm/s in Caco-2 transwell system. Therefore it could be concluded that tea polyphenols will unlikely to be absorbed from human intestinal epithelium after dietary intake. Several studies have been attempted to assess the bioavailability of polyphenols, including the specific sites where it exerts biological actions (Singh et al. 2011). The low transport rate for tea polyphenols was confirmed in our study, might be due to their instability at near neutral or slightly alkaline conditions during intestinal digestion (Xie et al. 2013). Moreover, catechins undergo metabolism by phase II enzymes during the absorption across intestinal epithelial cells, these derivatives are likely to show higher biological activity in vivo (Huo et al. 2010). In our study, although OTP and BTP showed relatively low antioxidant activity, their bioavailability was relatively higher. For the health effects of tea polyphenols, this crucial issue encompasses dissolution, absorption, distribution and disposition in target tissues, and numerous factors may contribute to the variation of the metabolism of tea polyphenols (Duda-Chodak et al. 2015).
Table 2.
The bilateral Papp values of GTP, OTP and BTP and their efflux ratios
| Sample | P app (AP → BL) (×10−6 cm/s) | P app (BL → AP) (×10−6 cm/s) | Efflux ratio |
|---|---|---|---|
| GTP | 0.31 ± 0.02a | 0.86 ± 0.06a | 2.77 |
| OTP | 0.46 ± 0.02b | 0.94 ± 0.04b | 2.04 |
| BTP | 0.47 ± 0.03b | 0.97 ± 0.04b | 2.06 |
Different lowercase letters indicate significant differences (P < 0.05) among different samples
Effect of GTP, OTP and BTP on bacterial populations
The bacterial populations present during the fermentation of different tea polyphenols were determined by FISH. As shown in Table 3, the numbers of Bifidobacterium in broth containing GTP, OTP and BTP gradually increased during the fermentation, which was maintained for 36 h. Each tea polyphenols samples exerted proliferative effects on Bifidobacterium spp., moreover, OTP and BTP showed better effects than GTP during the fermentation, and OTP showed the best effect when cultivated for 24 and 36 h, followed by BTP. For Lactobacillus/Enterococcus spp., tea polyphenols samples also positively affect their proliferation.
Table 3.
Numbers (log10 cell/mL) of Bifidobacterium and Lactobacillus/Enterococcus spp. and Bacteroides–Prevotella and Clostridium histolyticum groups in anaerobic fermentation broth containing GTP, OTP, BTP and FOS at 0, 12, 24 and 36 h
| Probe type | Sample | Anaerobic fermentation time (h) | |||
|---|---|---|---|---|---|
| 0 | 12 | 24 | 36 | ||
| Bif 164 | Control | 8.03 ± 0.02A | 8.17 ± 0.02a,B | 8.22 ± 0.02a,C | 8.25 ± 0.02a,D |
| FOS | 8.46 ± 0.03d,B | 8.58 ± 0.03e,C | 8.71 ± 0.02e,D | ||
| GTP | 8.23 ± 0.03b,B | 8.40 ± 0.03b,C | 8.46 ± 0.03b,D | ||
| OTP | 8.28 ± 0.02c,B | 8.47 ± 0.02d,C | 8.55 ± 0.02d,D | ||
| BTP | 8.27 ± 0.02c,B | 8.44 ± 0.01c,C | 8.52 ± 0.02c,D | ||
| Lab 158 | Control | 7.91 ± 0.02A | 8.02 ± 0.03a,B | 8.13 ± 0.02a,C | 8.18 ± 0.03a,C |
| FOS | 8.26 ± 0.02c,B | 8.44 ± 0.01e,C | 8.68 ± 0.02e,D | ||
| GTP | 8.18 ± 0.02b,B | 8.27 ± 0.01b,C | 8.43 ± 0.03b,D | ||
| OTP | 8.19 ± 0.02b,B | 8.39 ± 0.02d,C | 8.56 ± 0.03d,D | ||
| BTP | 8.19 ± 0.01b,B | 8.35 ± 0.03c,C | 8.50 ± 0.02c,D | ||
| Bac 303 | Control | 7.43 ± 0.02A | 7.84 ± 0.02a,B | 8.02 ± 0.02a,C | 8.19 ± 0.02a,D |
| FOS | 7.62 ± 0.02c,B | 7.71 ± 0.03c,C | 7.76 ± 0.02e,D | ||
| GTP | 7.67 ± 0.03b,B | 7.79 ± 0.03b,C | 7.88 ± 0.03e,C | ||
| OTP | 7.68 ± 0.02b,B | 7.79 ± 0.03b,C | 7.88 ± 0.03e,C | ||
| BTP | 7.67 ± 0.02b,B | 7.77 ± 0.03b,C | 7.87 ± 0.03e,C | ||
| His 150 | Control | 7.31 ± 0.02A | 7.53 ± 0.02a,A | 7.72 ± 0.02a,A | 7.98 ± 0.03a,C |
| FOS | 7.38 ± 0.03c,B | 7.46 ± 0.02c,C | 7.51 ± 0.02c,D | ||
| GTP | 7.43 ± 0.03b,B | 7.53 ± 0.02b,B | 7.65 ± 0.03b,B | ||
| OTP | 7.44 ± 0.02b,B | 7.55 ± 0.03b,B | 7.66 ± 0.02b,B | ||
| BTP | 7.44 ± 0.02b,B | 7.55 ± 0.02b,B | 7.65 ± 0.02b,B | ||
Different lowercase letters indicate significant differences (P < 0.05) in the total number of bacteria (i.e., within column) among different compounds. Different capital letters indicate significant differences (P < 0.05) in the total number of bacteria (i.e., within line) among different time points
As shown in Table 3, Bacteroides–Prevotella and Clostridium histolyticum in the cultures containing GTP, OTP and BTP were lower than that of the control. FOS showed the best inhibitory effect at each time point, but there was no significant difference between different tea polyphenols during the fermentation (P > 0.05). When cultivated for 36 h, tea polyphenols showed similar inhibitory effects, which indicated that GTP, OTP and BTP can effectively inhibit the proliferation of Bacteroides–Prevotella and Clostridium histolyticum in the gut.
Prebiotics can alter the composition of microbiota in human gut, and protect against obesity-induced inflammation (Chang et al. 2015). Dietary consumption of non-digestible FOS has been confirmed to exert a positive impact on bifidobacteria, and phenolic compounds in tea may affect the relative abundance of different human intestinal bacteria, including repressing potential pathogens, whereas selective promoting the growth of probiotics (Kemperman et al. 2010; Tuohy et al. 2012). In our previous studies, the modulatory effect of EGCG3″Me on human intestinal microbiota in vivo was investigated by high-throughput sequencing: the decrease in the Firmicutes/Bacteroidetes ratio, which may be considered as a representative parameter of health status, contributing to the prevention of gut dysbiosis (Cheng et al. 2017). Therefore, different strains of intestinal bacteria may have varying degrees of sensitivity to tea polyphenols and their metabolites, and tea polyphenols may exert modulatory effects on intestinal environment by acting as metabolic prebiotics.
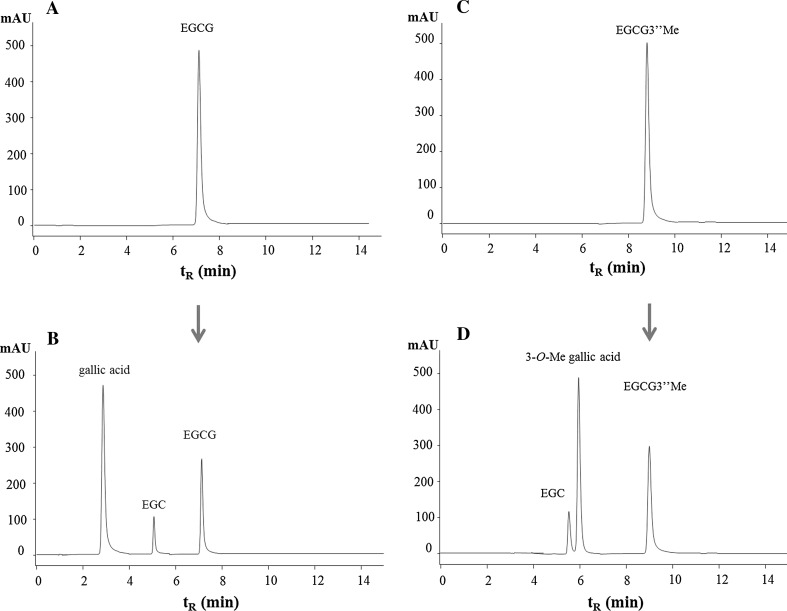
HPLC chromatograms of changes of EGCG (A), EGCG3″Me (B) and their metabolites during the fermentation was shown in Fig. 2. It can be seen that after 36 h of anaerobic fermentation in vitro, the ester bonds of tea catechins were hydrolyzed by intestinal microbiota. The corresponding metabolites of gallic acid and EGC were produced from EGCG, while EGCG3″Me was metabolized to EGC and unknown compound X. Positive-mode MS gave molecular ion signals at m/z 182.97 for [M + H]+, which indicated its molecular weight is 184, corresponding to that of 3-O-Me gallic acid.
Fig. 2.
HPLC chromatograms showing changes of EGCG (a), EGCG3″Me (b) and their metabolites during the fermentation
The bioconversion capacity of polyphenols has been reported to be associated with Clostridia and Actinobacteria including Bifidobacterium and Eggertella spp., and their degradation degree is significantly influenced by individual variations in the composition of intestinal microbiota (Selma et al. 2009; Velzen et al. 2014). Certain intestinal bacteria can metabolize tea polyphenols to different aromatic metabolites, the majority of which have shown the features to inhibit pathogens and promote the growth of commensal anaerobes and probiotics, and those released from one bacterial species would be freely available to be substrate for other bacterial species and further metabolized (Kutschera et al. 2011). The microbiota in human gut has extensive hydrolytic activities which can increase the bioavailability of polyphenols when they reach the colon, and breaks down them into smaller phenolic acids. For the microbe-derived phenolic metabolites excreted represented the largest part of intake, it indicated that the amount of non-absorbable polyphenols reached the colon is rather high (Monagas et al. 2010). Therefore, attentions should be paid to colonic ring-fission products and their contribution to the bioavailability of dietary phytochemicals.
Effect of GTP, OTP and BTP on the production of SCFA
As shown in Table 4, the production of SCFA was significantly influenced by the presence of GTP, OTP and BTP during the fermentation. The SCFA concentrations in cultures containing tea polyphenols were significantly increased and relatively higher than the control (P < 0.05) at each time. For FOS, it produced most abundant of formic acid, acetic acid and butyric acid, however, the propionic acid produced was the least.
Table 4.
Effects of GTP, OTP, BTP and FOS on production of SCFA at 0, 12, 24, and 36 h
| Acid type | Sample | Anaerobic fermentation time (h) | |||
|---|---|---|---|---|---|
| 0 | 12 | 24 | 36 | ||
| Formic acid (mM) | Control | 8.12 ± 0.35A | 11.32 ± 0.74a,B | 13.08 ± 1.01 a,C | 14.69 ± 0.87a,D |
| FOS | 20.78 ± 1.45e,B | 27.42 ± 1.33 e,C | 35.01 ± 1.18e,D | ||
| GTP | 15.67 ± 1.03b,B | 19.96 ± 0.64 b,C | 22.53 ± 0.76b,C | ||
| OTP | 17.68 ± 0.82d,B | 23.44 ± 1.85 d,C | 28.44 ± 0.89d,C | ||
| BTP | 16.35 ± 0.97c,B | 21.18 ± 1.27 c,C | 26.21 ± 0.64c,C | ||
| Acetic acid (mM) | Control | 16.55 ± 1.12A | 17.89 ± 1.02a,A | 18.66 ± 0.87 a,C | 19.52 ± 0.87a,D |
| FOS | 27.19 ± 0.82d,B | 37.64 ± 1.45 e,C | 44.16 ± 1.86e,D | ||
| GTP | 21.93 ± 1.32b,B | 28.27 ± 1.05 b,C | 33.77 ± 1.53b,D | ||
| OTP | 25.47 ± 0.99c,B | 32.76 ± 1.56 d,C | 38.69 ± 1.82d,D | ||
| BTP | 22.44 ± 1.01b,B | 30.15 ± 0.87 c,C | 35.49 ± 1.27c,D | ||
| Propionic acid (mM) | Control | 0.00 ± 0.00A | 3.59 ± 0.77a,B | 5.27 ± 0.74 a,C | 6.94 ± 0.88a,D |
| FOS | 4.38 ± 0.23b,B | 6.46 ± 0.32 b,C | 8.21 ± 0.32b,D | ||
| GTP | 4.56 ± 0.34b,B | 7.82 ± 0.42 b,C | 10.56 ± 0.63d,D | ||
| OTP | 4.47 ± 0.28b,B | 7.69 ± 0.57 b,C | 9.32 ± 0.56c,D | ||
| BTP | 4.63 ± 0.32b,B | 7.73 ± 0.49 b,C | 11.88 ± 0.95e,D | ||
| Butyric acid (mM) | Control | 0.00 ± 0.00A | 4.78 ± 0.22a,B | 6.66 ± 0.02 a,C | 7.69 ± 0.51a,D |
| FOS | 9.38 ± 0.67c,B | 12.01 ± 0.88 c,C | 14.55 ± 1.13d,D | ||
| GTP | 6.93 ± 0.54b,B | 9.66 ± 0.42 b,C | 11.44 ± 0.53b,D | ||
| OTP | 7.24 ± 0.62b,B | 9.89 ± 0.53 b,C | 12.81 ± 0.88c,D | ||
| BTP | 6.99 ± 0.32b,B | 9.57 ± 0.72 b,C | 11.97 ± 0.77b,D | ||
Different lowercases indicate significant differences (P < 0.05) for each SCFA (i.e., within column) among different compounds. Different capital letters indicate significant differences (P < 0.05) for each SCFA (i.e., within line) among different time points
The production of SCFA is influenced by diet and intestinal microbiota dramatically (Zhang et al. 2016). It has been reported that dietary polyphenols may cause the variation in specific microorganism populations, whereas microbial conversion of polyphenols affects other colonic pathways and processes, such as SCFA production (Zhu et al. 2013). The concentrations of formic and propionic acids increased significantly in cultures containing GTP, OTP and BTP. Formic acid is the end product of the reaction in which pyruvate is converted to formic acid, and high concentration of propionate in the colon has great potential for hypocholesterolemia prevention (Kang et al. 2000). In addition, Bacteroides–Prevotella is a dominant group of intestinal microbial communities and is the known producer of propionic acid (Gullón et al. 2014), and the results were consistent with our results about the variation of intestinal microbiota population. In our experiment, acetic acid was in the greatest proportion for all treatment. Some SCFA including acetic acid could stimulate the secretion of mucin, and transported to other body sites for further utilization (Wong et al. 2006). The high level of acetic acid may be correlated with metabolic activities of Bifidobacterium and Lactobacillus (Sanz et al. 2005a, b), which was also consistent with the results of intestinal microbiota. The production of butyric acid was correlated with the transformation of other bacterial metabolites (Dominika et al. 2011). It has been reported acetic and butyric acids were produced by almost all intestinal bacteria, acetic acid was considered typical fermentation end-products of the bifidus pathway, whereas butyric acid was a major product of Clostridia and Eubacteria (Sanz et al. 2005a, b). Increased production of SCFA in the gut is considered desirable as acidifying the colonic environment can promote absorption of minerals and protect against pathogenic bacteria; therefore, tea polyphenols might be beneficial for gut ecosystems.
Conclusion
The present study indicated that tea polyphenols including GTP, OTP and BTP could significantly increase the Bifidobacterium and Lactobacillus–Enterococcus spp. and SCFA production, while restrained the proliferation of Bacteroides–Prevotella and Clostridium histolyticum groups. Therefore, the consumption of tea rich in polyphenols may be beneficial for intestinal microecology and contribute to host health. The bioavailability and effects of polyphenols greatly depend on their transformation in the gut, and the modulatory effect of tea polyphenols on intestinal microbiota was also explored to understand the two-way interaction. In particular, the health benefits of tea polyphenols may be attributed to their bioactive metabolites and the modulatory effect of human intestinal microbiota.
Acknowledgements
This work was sponsored by National Natural Science Foundation of China (31501473), Key Research and Development Project of Zhejiang Province (2017C02039), People-benefit Project of Ningbo (2015C10061) and K.C. Wong Magna Fund in Ningbo University.
References
- Chang CJ, Lin CS, Lu CC, Martel J, Ko YF, Ojcius DM, Tseng SF, Wu TR, Chen YY, Young JD, Lai HC. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 2015;6:7489. doi: 10.1038/ncomms8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M, Zhang X, Miao YJ, Cao JX, Wu ZF, Weng PF. The modulatory effect of (-)-epigallocatechin 3-O-(3-O-methyl) gallate (EGCG″Me) on intestinal microbiota of high fat diet-induced obesity mice model. Food Res Int. 2017;92:9–16. doi: 10.1016/j.foodres.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Consortium HMP Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominika S, Arjan N, Karyn RP, Henryk K. The study on the impact of glycated pea proteins on human intestinal bacteria. Int J Food Microbiol. 2011;145:267–272. doi: 10.1016/j.ijfoodmicro.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Duda-Chodak A, Tarko T, Satora P, Sroka P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: a review. Eur J Nutr. 2015;54:325–341. doi: 10.1007/s00394-015-0852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei QQ, Gao Y, Zhang X, Sun Y, Hu B, Zhou L, Jabbar S, Zeng XX. Effects of oolong tea polyphenols, EGCG, and EGCG3″Me on pancreatic α-amylase activity in vitro. J Agric Food Chem. 2014;62:9507–9514. doi: 10.1021/jf5032907. [DOI] [PubMed] [Google Scholar]
- Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullón B, Gullón P, Tavaria F, Pintado M, Gomes AM, Alonso JL, Parajó JC. Structural features and assessment of prebiotic activity of refined arabinoxylooligosaccharides from wheat bran. J Funct Food. 2014;6:438–449. doi: 10.1016/j.jff.2013.11.010. [DOI] [Google Scholar]
- Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- Huo CD, Yang HJ, Cui QZ, Dou QP, Chan TH. Proteasome inhibition in human breast cancer cells with high catechol-O-methyltransferase activity by green tea polyphenol EGCG analogs. Bioorg Med Chem Lett. 2010;18:1252–1258. doi: 10.1016/j.bmc.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobu K, Yokota J, Yoshioka S, Moriyama H, Murata S, Ohishi S, Ukeda H, Miyamura M. Effects of Goishi tea on diet-induced obesity in mice. Food Res Int. 2013;54:324–329. doi: 10.1016/j.foodres.2013.07.037. [DOI] [Google Scholar]
- Kang MH, Naito M, Sakai K, Uchida K, Osawa T. Mode of action of sesame lignans in protecting low-density lipoprotein against oxidative damage in vitro. Life Sci. 2000;66:161–171. doi: 10.1016/S0024-3205(99)00574-3. [DOI] [PubMed] [Google Scholar]
- Kemperman RA, Bolca S, Roger LC, Vaughan EE. Novel approaches for analysing gut microbes and dietary polyphenols: challenges and opportunities. Microbiology. 2010;156:3224–3231. doi: 10.1099/mic.0.042127-0. [DOI] [PubMed] [Google Scholar]
- Kumar PV, Basheer S, Ravi R, Thakur MS. Comparative assessment of tea quality by various analytical and sensory methods with emphasis on tea polyphenols. J Food Sci Technol. 2011;48:440–446. doi: 10.1007/s13197-010-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera M, Engst W, Blaut M, Braune A. Isolation of catechin-converting human intestinal bacteria. J Appl Microbiol. 2011;111:165–175. doi: 10.1111/j.1365-2672.2011.05025.x. [DOI] [PubMed] [Google Scholar]
- Li W, Wang KQ, Sun Y, Ye H, Hu B, Zeng XX. Influences of structures of galactooligosaccharides and fructooligosaccharides on the fermentation in vitro by human intestinal microbiota. J Funct Food. 2015;13:158–168. doi: 10.1016/j.jff.2014.12.044. [DOI] [Google Scholar]
- Monagas M, Urpi-Sarda M, Sánchez-Patán F, Llorach R, Garrido I, Gómez-Cordovés C, Andres-Lacueva C, Bartolomé B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010;1:233–253. doi: 10.1039/c0fo00132e. [DOI] [PubMed] [Google Scholar]
- Pinto MDS. Tea: a new perspective on health benefits. Food Res Int. 2013;53:558–567. doi: 10.1016/j.foodres.2013.01.038. [DOI] [Google Scholar]
- Richelle M, Tavazzi I, Offord E. Comparison of the antioxidant activity of commonly consumed polyphenolic beverages (coffee, cocoa, and tea) prepared per cup serving. J Agric Food Chem. 2001;49:3438–3442. doi: 10.1021/jf0101410. [DOI] [PubMed] [Google Scholar]
- Sanz ML, Côté GL, Gibson GR, Rastall RA. Prebiotic properties of alternansucrase maltose-acceptor oligosaccharides. J Agric Food Chem. 2005;53:5911–5916. doi: 10.1021/jf050344e. [DOI] [PubMed] [Google Scholar]
- Sanz ML, Polemis N, Morales V, Corzo N, Drakoularakou A, Gibson GR, Rastall RA. In vitro investigation into the potential prebiotic activity of honey oligosaccharides. J Agric Food Chem. 2005;53:2914–2921. doi: 10.1021/jf0500684. [DOI] [PubMed] [Google Scholar]
- Selma MV, Espín JC, Tomásbarberán FA. Interaction between phenolics and gut microbiota: role in human health. J Agric Food Chem. 2009;57:6485–6501. doi: 10.1021/jf902107d. [DOI] [PubMed] [Google Scholar]
- Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82:1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratil P, Klejdus B, Kubán V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables evaluation of spectrophotometric methods. J Agric Food Chem. 2006;54:607–616. doi: 10.1021/jf052334j. [DOI] [PubMed] [Google Scholar]
- Tuohy KM, Conterno L, Gasperotti M, Viola R. Up-regulating the human intestinal microbiome using whole plant foods, polyphenols, and/or fiber. J Agric Food Chem. 2012;60:8776–8782. doi: 10.1021/jf2053959. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6–14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velzen EJJV, Westerhuis JA, Grün CH, Jacobs DM, Eilers PHC, Mulder TP, Foltz M, Garczarek U, Kemperman R, Vaughan EE, Duynhoven JPMV, Smilde AK. Population-based nutrikinetic modeling of polyphenol exposure. Metabolomics. 2014;10:1059–1073. doi: 10.1007/s11306-014-0645-y. [DOI] [Google Scholar]
- Willenberg I, Michael M, Wonik J, Bartel LC, Empl MT, Schebb NH. Investigation of the absorption of resveratrol oligomers in the Caco-2 cellular model of intestinal absorption. Food Chem. 2015;167:245–250. doi: 10.1016/j.foodchem.2014.06.103. [DOI] [PubMed] [Google Scholar]
- Wong JM, De SR, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- Xie YL, Kosińska A, Xu H, Andlauer W. Milk enhances intestinal absorption of green tea catechins in in vitro, digestion/Caco-2 cells model. Food Res Int. 2013;53:793–800. doi: 10.1016/j.foodres.2012.07.063. [DOI] [Google Scholar]
- Zhang X, Xu F, Gao Y, Wu J, Sun Y, Zeng XX. Optimising the extraction of tea polyphenols, (-)-epigallocatechin gallate and theanine from summer green tea by using response surface methodology. Int J Food Sci Tech. 2012;47:2151–2157. doi: 10.1111/j.1365-2621.2012.03082.x. [DOI] [Google Scholar]
- Zhang X, Zhu XL, Sun Y, Hu B, Sun Y, Jabbar S, Zeng XX. Fermentation in vitro of EGCG, GCG and EGCG3″Me isolated from Oolong tea by human intestinal microbiota. Food Res Int. 2013;54:1589–1595. doi: 10.1016/j.foodres.2013.10.005. [DOI] [Google Scholar]
- Zhang X, Wu ZF, Weng PF. Antioxidant and hepatoprotective effect of (-)-epigallocatechin 3-O-(3-O-methyl) gallate (EGCG3″Me) from Chinese oolong tea. J Agric Food Chem. 2014;62:10046–10054. doi: 10.1021/jf5016335. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yang Y, Wu ZF, Weng PF. The modulatory effect of anthocyanins from purple sweet potato on human intestinal microbiota in vitro. J Agric Food Chem. 2016;64:2582–2590. doi: 10.1021/acs.jafc.6b00586. [DOI] [PubMed] [Google Scholar]
- Zhou L, Wang W, Huang J, Ding Y, Pan Z, Zhao Y, Zhang R, Hu B, Zeng XX. In vitro extraction and fermentation of polyphenols from grape seeds (Vitis vinifera) by human intestinal microbiota. Food Funt. 2016;7:1959–1967. doi: 10.1039/C6FO00032K. [DOI] [PubMed] [Google Scholar]
- Zhu XL, Zhang X, Sun Y, Su D, Sun Y, Hu B, Zeng XX. Purification and fermentation in vitro of sesaminol triglucoside from sesame cake by human intestinal microbiota. J Agric Food Chem. 2013;61:1868–1877. doi: 10.1021/jf304643k. [DOI] [PubMed] [Google Scholar]