Abstract
Premature ovarian insufficiency (POI) is a major complication of cytotoxic treatments due to extreme ovarian sensitivity to chemotherapy and radiation. In pediatric cancer patients modern therapy has improved the long-term survival to over 80% in the United States. However, these cancer survivors face long-term health problems related to treatment toxicity. In female cancer survivors POI leads to sterility, along with the consequences of estrogen deficiency such as premature osteopenia, muscle wasting, accelerated cardiovascular diseases and a vast array of other health and developmental problems. These long-lasting effects are particularly significant for young girls reaching puberty. As such, restoring ovarian endocrine function is paramount in this population. In the present study, we evaluated the feasibility of restoring ovarian endocrine function in ovariectomized mice by transplanting syngeneic and allogeneic ovarian tissue encapsulated in alginate capsules or TheraCyte®. Histological analysis of the implants retrieved after 7 and 30 days' post implantation showed follicular development up to the secondary and antral stages in both syngeneic and allogeneic implants. Implantation of syngeneic and allogeneic ovarian grafts encapsulated in TheraCyte devices restored ovarian endocrine function, which was confirmed by decreased serum FSH levels from 60 to 70 ng/mL in ovariectomized mice to 30–40 ng/mL 30 days after implantation. Absence of allo-MHC—specific IgG and IgM antibodies in the sera of implanted mice with allogeneic ovarian tissue encapsulated in TheraCyte indicate that the implants did not evoke an allo-immune response, while the allogeneic controls were rejected 21 days after implantation. Our results show that TheraCyte effectively isolates the graft from immune recognition but also supports follicular growth.
Keywords: Ovariectomy, Alginate, TheraCyte®, Follicular growth, Endocrine function
Introduction
Premature ovarian insufficiency (POI) is a common outcome of cytotoxic treatments in pediatric cancer patients9 affecting both fertility and endocrine ovarian function. Pediatric patients experience delayed puberty as well as side effects of artificial hormonal replacement.10 Puberty is the most important physiological transformative event in girls' development after birth and it requires healthy ovaries to respond to the signals in the hypothalamus-pituitary-gonad axis. Pharmaceutical options involving increasing doses of estrogen followed with progesterone address some aspects of puberty, but are far from matching the physiological complexity of hormonal milieu during puberty and cause potentially significant side effects.8
The lost ovarian endocrine function can be restored with fresh or cryopreserved and auto transplanted ovarian tissue.20 Patient may also choose to save the autologous ovarian tissue for later to achieve live birth of biologically related offspring. Unfortunately, the cryopreserved ovarian tissue may carry the risk of reintroducing malignant cells harbored in the transplant, particularly in case of hematologic malignancies.6,18 Using donor ovarian tissue could avoid this risk; yet provide the physiological complex hormonal milieu needed during puberty. In the last two decades, the concept of allo-transplantation has been studied in donor pancreatic islets encapsulated in an immuno-isolating device for treatment of Type 1 diabetes.1,16,19,25 An immuno-isolating device creates a barrier between the implanted allogeneic cells or tissue and the host and eliminates the need for systemic immunosuppression.19 The requirements from an efficient immuno-isolation device are two-fold. First, it must provide a supportive environment for the implanted cells allowing sufficient diffusion of nutrients and oxygen. Second, the device must serve as a barrier between the host and the graft to prevent rejection caused by infiltration of the host immune cells. These goals can be achieved by using semisolid hydrogels, such as alginate5,24 encapsulating the graft, or durable pouches made of semipermeable membrane.1,4,11,22
Figure 6.
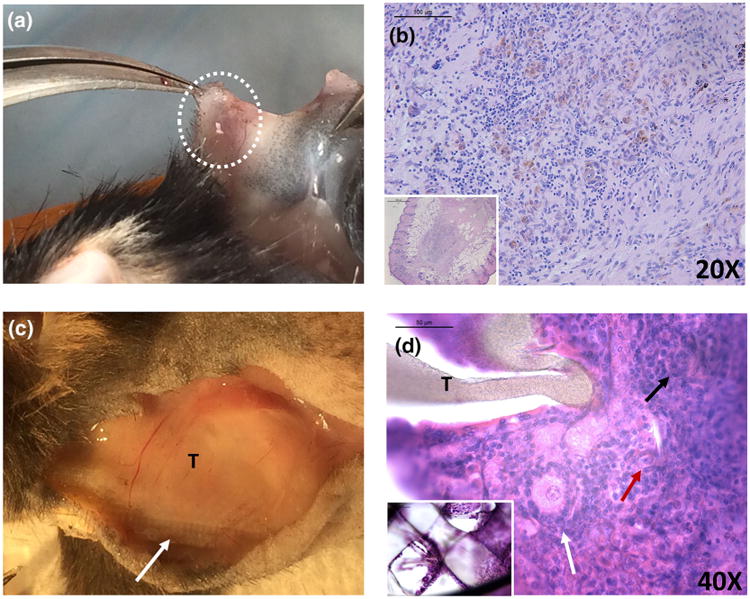
(a) Macroscopic image of control allogeneic ovarian tissue implanted subcutaneously. (b) Histological analysis of the allogeneic control revealed no follicles 28 days after the implantation. (c) Macroscopic image of TheraCyte® demonstrated a capsule around the device 21 days post transplantation. A white arrow points to the mesh structure of the device in the macroscopic image (arrow) and in the inset in (d). Primordial (red arrow), primary (black arrow) and secondary (white arrow) follicles observed after implantation of allogeneic ovarian tissue encapsulated in TheraCyte® (T) for 21 days. Insets (b, d, 5×).
In the present study, we tested two immuno-isolators, alginate and TheraCyte®, for their ability to support the survival and function of the ovarian tissue. Alginate is a naturally derived polysaccharide produced by brown algae. TheraCyte® is an FDA approved device made of polytetrafluoroethylene (PTFE) membrane, which is impermeable to cells but allows diffusion of soluble molecules through its 0.4 μm pores.
Ovarian follicles, the functional units of the ovary, have different physiology, growth pattern and nutritional demands compared to pancreatic islets. Yet, our knowledge of ovarian follicles suggests that the immuno-isolation approach can successfully protect and support the function of the allograft. To prove this hypothesis, we implanted encapsulated ovarian tissue in ovariectomized mice and assessed implant survival and function after implantation. We used syngeneic implants to study graft support and allogeneic grafts to study immunoisolation.
Materials and Methods
Experimental Design
Cycling adult female mice (B6CBAF1) underwent bilateral ovariectomy to induce menopause. In the first part of the study, ovaries from syngeneic B6CBAF1 6–8 days old mice were encapsulated in alginate or TheraCyte and subcutaneously implanted in the adult ovariectomized mice for a period of 7 days (n =9 mice for TheraCyte, n =7 mice for alginate) and 30 days (n = 7 mice for TheraCyte, n = 9 mice for alginate). In the second part, ovaries from allogeneic BALB/c 6–8 days old mice were encapsulated in TheraCyte only, because alginate proved to be inferior, and then subcutaneously implanted in ovariectomized cycling adult female mice (C57BL/6) for 21 days (n = 4 recipient mice). In control group C57BL/6 mice (n = 2) were implanted with ovaries from 6 to 8 days old BALB/c mice without encapsulation for 28 days. The number of donor mice equals to the number of recipient mice, because each recipient mouse received 2 ovaries.
Ethics
The UCUCA guidelines for survival surgery in rodents and the UCUCA Policy on Analgesic Use in Animals Undergoing Surgery were followed for all the procedures. Animal experiments for this work were performed in accordance with the protocol approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Michigan (PRO00005750).
Ovariectomies in Recipient Mice
Bilateral ovariectomies were performed on adult female mice (B6CBAF1: syngeneic mice, C57BL/6: allogeneic mice) aged 12–16 weeks. The females were anesthetized by isoflurane. Preemptive analgesics were administered before the first cut was made. Using aseptic techniques and procedures, a midline incision was made in the abdominal wall. The intraperitoneal space was exposed with an abdomen retractor. The ovaries were removed and the remaining reproductive tract was gently reinserted into the body cavity. The muscle layer and the skin of the abdominal wall were closed with 5/0 absorbable sutures (AD Surgical, USA) in two separate layers. The animal was then placed in a clean warmed cage for recovery. Following recovery, the animal was housed in the animal facility. Mice received Carprofen (Rimadyl, Zoetis, USA) for analgesia for at least 48 h after surgery or as needed.
Collection of Donor Ovaries
Ovaries from 6 to 8 days old B6CBAF1 female pups genetically matching the receiving host were collected and transferred to Leibovitz L-15 media (Sigma-Aldrich, USA). The ovaries were dissected into 2–4 pieces and transferred in the maintenance media (α-MEM, Gibco, USA) in the CO2 incubator for further manipulation. Similarly, ovaries from 6 to 8 days old BALB/c were used as donor ovarian tissue for allogeneic implants.
Ovarian Tissue Encapsulation in 1% Alginate
The ovarian pieces were encapsulated in 1% w/v alginate (Carrageenan, Xanthan, Alginate, Ingredients Solution, Inc., Waldo, ME, USA) and purified as described by Xu et al.26, dissolved in phosphate buffered saline (PBS; Gibco, USA). The ovarian tissue pieces were transferred to alginate solution and pipetted in 10 μL droplets of alginate into the cross-linking solution (50 mM CaCl2 and 140 mM NaCl). The beads were allowed to crosslink for 5 min and after that the beads were transferred to maintenance media (α-MEM; Gibco), kept at 37°C and 5% CO2 for 5–20 min awaiting implantation. Each bead contained ovarian tissue from one ovary, and each recipient received two alginate beads.
Ovary Encapsulation in TheraCyte®
The port of the TheraCyte® (TheraCyte Inc., USA) was widened using a Hamilton syringe without compromising the integrity of the TheraCyte® wall. Using a mouth pipette, the ovarian pieces (3–4) were picked up in a pipette and inserted into the TheraCyte® (Fig. 1d). To ensure that all the ovarian pieces were in the TheraCyte®, the transparent plastic port and the pipette were inspected under the stereo microscope. The long port tube was shortened and sealed by melting the plastic to prevent leakage of the ovarian pieces and invasion of the host cells. The loaded devices were placed in the maintenance media (α-MEM; Gibco), kept at 37°C and 5% CO2 for 5–20 min awaiting implantation in mice. Each TheraCyte device contained ovarian tissue from two ovaries, and each recipient was implanted with one TheraCyte device.
Figure 1.
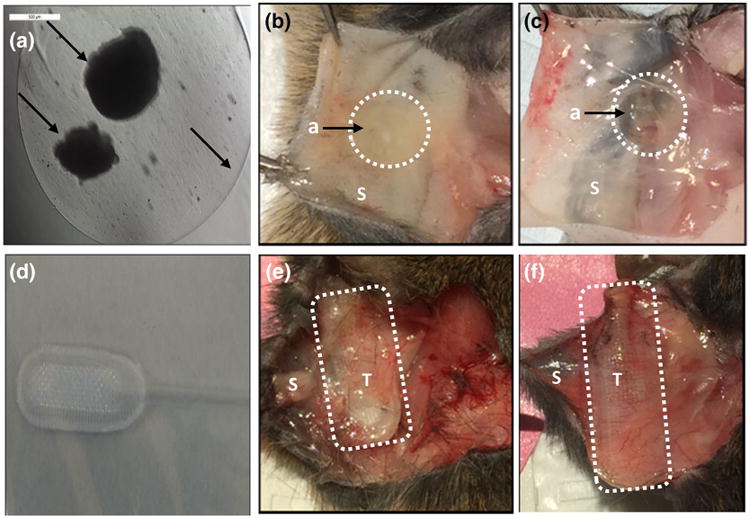
Alginate and TheraCyte before and after implantation. Microscopic encapsulation of ovarian pieces in 1% alginate (a), alginate beads attached to the skin after 7 and 30 days of subcutaneous implantation (b, c). Macroscopic image of TheraCyte (d), TheraCyte attached to the skin after 7 and 30 days of subcutaneous implantation (e, f). Arrows show alginate and ovarian tissue encapsulated in alginate. a: Alginate, T: TheraCyte, S: Skin.
Subcutaneous Transplantation
Briefly, a small incision was made on the dorsal side of the anesthetized mice and the immunoisolator (alginate or TheraCyte®) containing the ovaries was implanted subcutaneously. The skin was then closed using 5/0 absorbable sutures. The mice were placed in a clean warmed cage for recovery and monitored post-operatively for 10 days. The mice received Carprofen for analgesia for at least 48 h after surgery or as needed. After transplantation, the mice were euthanized at pre-determined time points (7 and 30 days). Nine TheraCyte® in 9 mice and two alginate beads per mouse (7 mice) were implanted for 7 days; seven TheraCyte® in 7 mice and two alginate beads per mouse (9 mice) were implanted for 30 days.
Similarly, for allogeneic implantation, four TheraCyte® containing BALB/c ovaries were implanted subcutaneously in B6 mice for a period of 21 days. Ovaries from BALB/c mice without immunoisolation were implanted subcutaneously in immune competent B6 mice for 28 days as controls.
Blood Collection for Hormone Analysis
Blood from the lateral tail vein was collected at set time points, up to 1% of the body weight of blood was taken in a 53/4” glass Pasteur pipette. At the time of sacrifice, blood was collected via cardiac puncture. All blood samples were kept at 4°C overnight, then centrifuged for 10 min, and the collected serum was stored at −20°C. The samples were analyzed for mouse FSH using radio-immunoassay (Ligand Assay and Analysis Core Facility, University of Virginia Center for Research in Reproduction). The core used mouse FSH reference preparation AFP5308D for assay standards and mouse FSH antiserum (guinea pig; AFP-1760191) diluted to a final concentration of 1:400,000 as primary antibody. The secondary antibody is purchased from Equitech-Bio, Inc. and diluted to a final concentration of 1:25. The assay has a sensitivity of 2.0 ng/mL, reportable range 3–75 ng/mL and less than 0.5% cross-reactivity with other pituitary hormones. The samples were diluted 5–10 fold when necessary.
Vaginal Cytology in the Allogeneic Group
The absence of estrous cycle after ovariectomies and the restoration of ovarian endocrine function were assessed with daily vaginal cytology. A persistent presence of leukocytes served as evidence for successful ovariectomy further confirmed with raised levels of follicular stimulating hormone (FSH). A cyclical and interchanging appearance of leukocytes, cornified epithelium and nucleated epithelial cells served as evidence for normal estrus cycle. The resumption of ovarian endocrine function was further confirmed with serum FSH levels.
Histological Analysis
Retrieved immunoisolating devices with encapsulated ovarian tissue in alginate or TheraCyte® were fixed in Bouin's fixative at 4°C overnight and transferred to 70% ethanol at 4°C. After processing, samples were embedded in paraffin, serially sectioned at 5 μm thickness, and stained with hematoxylin and eosin. Every sixth section was analyzed for the presence of primordial, primary, secondary and antral follicles.
Primordial follicles were characterized by one layer of squamous granulosa cells around the oocyte, primary follicles by a single layer of cuboidal granulosa cells, secondary follicles by two or more layers of granulosa cells, and antral follicles by the presence of an antral cavity. Follicular proportion was expressed as percentage of follicles in both alginate and TheraCyte implants.
Flow Cytometry
Measurements of serum allo-antibody titers were carried out by flow cytometry before and after implantation. Briefly, allogeneic thymocytes were incubated with serially diluted recipient serum. Antibody bound to the thymocytes were revealed by goat anti mouse IgG, FITC labeled, or goat anti-mouse IgM, PE-labeled. Titers were determined by the highest dilution that gives fluorescence detectable above background (non-immune serum- from a non-implanted mouse).
Statistics
Statistical analysis was performed using the R software. Analysis of follicular proportion was carried out with a χ2 test for proportions, and Welch Two-Sample t-tests were used to evaluate changes in hormone levels. The results were considered statistically significant when p < 0.05. Non-parametric unpaired t test was carried out for the immunological study.
Results
Macroscopic Evaluation of the Syngeneic Ovarian Grafts
Ovarian tissue was encapsulated in the center of the alginate bead (Fig. 1a) with the diameter of the bead measuring 5 mm and ovarian tissues 400 μm—1 mm. Upon retrieval 7 and 30 days after implantation, ovarian tissue was visible and macroscopically surrounded with alginate (Figs. 1b, 1c). The encapsulating volume for TheraCyte® with tissue inside is 4 μL and the tissue is physically entrapped inside the PTFE membrane (Figs. 1e, 1f). The physical properties of the outer membrane of TheraCyte promote non-specific adsorption of cells and blood vessel regrowth around the device.2,3 Indeed, at the retrieval, the TheraCyte device presented with visible capsule and blood vessel regrowth around the device (Figs. 1e, 1f). Alginate, on the contrary, is an inert plant-derived biomaterial that does not actively promote interactions with animal cells. The retrieved alginate hydrogels showed weak attachment with the host tissues and were easily detached from the implantation site.
Histological Evaluation of Syngeneic Mice Ovarian Grafts Encapsulated and Implanted in Alginate and TheraCyte
The majority of the implanted alginate capsules (80% after 7 days and 90% after 30 days) disintegrated into smaller fragments of a hydrogel. As the result the alginate capsule no longer surrounded the encapsulated ovarian tissue. The fragments of alginate hydrogels were scattered around the partially engrafted ovarian tissue with the host. Only 20% of the alginate grafts maintained the initial integrity 7 days' post implantation where the encapsulated ovarian tissue was fully surrounded by the alginate capsule (Fig. 3f).
Figure 3.
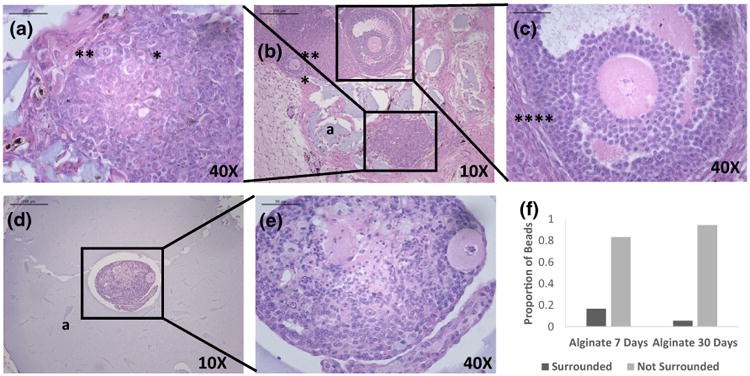
Healthy primordial (*), primary (**) and secondary (***) follicles (a–c) observed 30 days after implantation of syngeneic ovarian tissue pieces encapsulated in alginate (a). (a–c) demonstrates the histology of the syngeneic ovarian tissue that escaped the alginate capsule (non-surrounded), (d, e) shows when the ovarian tissue is completely surrounded, (f) demonstrates the proportion of ovarian tissue recovered from surrounded versus non surrounded alginate beads after 7 and 30 days of implantation.
The state of the alginate capsule (surrounded versus non-surrounded) affected follicle growth and development. In the majority of the cases the expanding ovarian tissue caused deformation and eventual failure of alginate capsule. In the non-surrounded grafts', the ovarian tissue contained multiple primordial follicles 7 days' post implantation and we did not observe necrotic regions (Figs. 2a and 2b). In the non-surrounded grafts retrieved 30 days' post implantation the ovarian tissue expanded and contained multiple follicles at different development stages, including primordial, primary and antral follicles (Figs. 3a–3c). On the contrary, in the 10–20% of the cases where the alginate capsule remained intact (e.g. surrounded) follicle growth and development were uneven (e.g. surrounded) and we identified only a small number of follicles in the grafts retrieved after 7 and 30 days' post implantation (Figs. 3d and 3e). Notably, the core of the encapsulated tissue looks devoid of follicles and has abnormal stroma, while the outer layers that were closer to the host presented healthier tissue morphology, but with a decreased follicular density.
Figure 2.
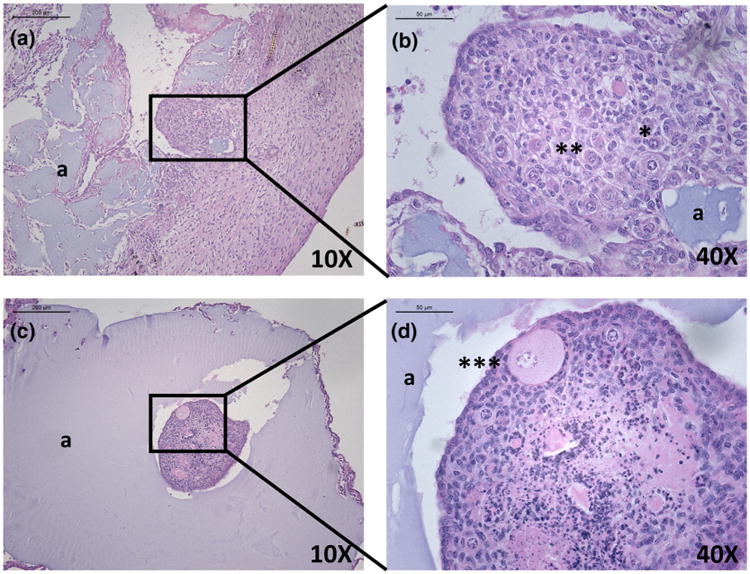
Healthy primordial (*), primary (**) and secondary (***) follicles observed 7 days after implantation of syngeneic ovarian tissue pieces encapsulated in alginate (a). (a, b) a typical histology of syngeneic ovarian tissue in a “non-surrounded” alginate capsule that disintegrated in vivo. (c, d) a typical histology of the ovarian tissue completely surrounded by the alginate capsule.
In contrast to ovarian tissue implants in alginate hydrogels, all the implants in TheraCyte® were completely surrounded by the device and tightly attached to the host. Multiple primordial and primary follicles were observed in the grafts retrieved after 7 days (Figs. 4a–4d), and antral follicles appeared in the grafts retrieved after 30 days (Figs. 4e–4h). No signs of necrosis were observed.
Figure 4.
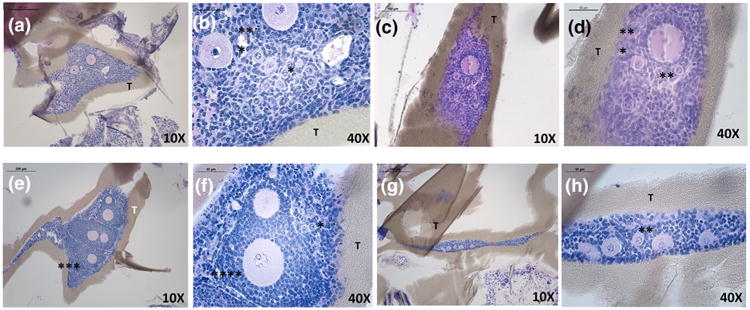
Healthy primordial (*), primary (**) and secondary (***) follicles observed after implantation of syngeneic ovarian tissue pieces encapsulated in TheraCyte® (T) for 7-day period (a–d) and 30-day period (e–h). The ovarian tissue is tightly surrounded by the device and no interaction with the host tissue was observed.
Follicular Development in Syngeneic Ovarian Grafts Implanted in Alginate and TheraCyte®
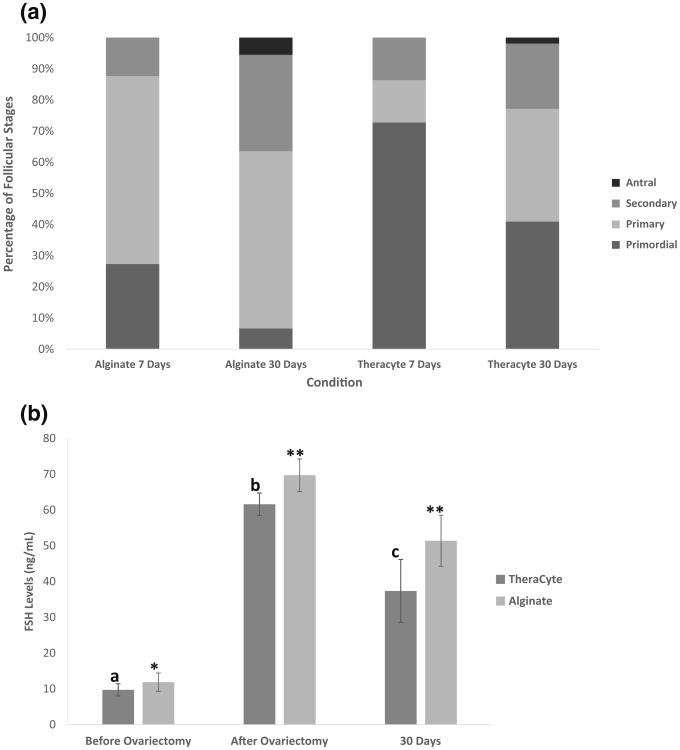
Serial paraffin sections were evaluated for follicular proportion in the retrieved alginate and TheraCyte® implants. Presence of healthy ovarian follicles and their distribution between different developmental stages of folliculogenesis following transplantation were subsequently investigated. The follicles observed after 7 and 30 days (Fig. 5a) were classified according to Myers's criteria for mouse follicle classification.15 The total number of follicles identified and counted in the surrounded alginate grafts was the lowest compared to other groups. 78 follicles were observed in surrounded alginate after 7 days and 7 follicles were observed after 30 days. The distribution of the stages after 7 days was 42.8% primordial, 28.6% primary and 28.6% secondary follicles; and after 30 days the distribution of the follicles in the grafts was 12.8% primordial follicles, 84.6% primary follicles and 2.6% secondary follicles.
Figure 5.
(a) Proportion of follicles at different developmental stages recovered from alginate (non surrounded) and TheraCyte and after 7 and 30 days of syngeneic transplantation. (b) Serum FSH levels in the ovariectomized mice after implantation of ovaries encapsulated in TheraCyte and alginate for a period of 30 days. FSH levels significantly increased after ovariectomy (p = 0.0002) and significantly decreased by day 30 (p = 0.03) following implantation of ovarian tissue encapsulated in TheraCyte. The decrease in FSH levels following implantation of ovarian tissue encapsulated in alginate was not significant by day 30.
The greatest number of follicles was found after 7 days in the non-surrounded alginate: 684 follicles with a distribution of 26.6% primordial follicles, 60.7% primary follicles, and 12.7% secondary follicles. However, the total follicle number decreased significantly after 30 days' post transplantation: 269 follicles with a distribution of 8.2% primordial follicles, 47.6% primary follicles, 36.8% secondary and 7.4% antral follicles.
Because of the difficulties associated with the sectioning of TheraCyte® devices we recovered only a portion of sections from the implanted devices. We counted only 88 and 105 follicles after 7 and 30 days, which underestimates the total number of the follicles present in the grafts. We evaluated the distribution of the follicles at various developmental stages by evaluating the recovered sections. The distribution of the follicle stages after 7 days was 72.8% primordial follicles, 13.6% primary follicles, and 13.6% secondary follicles. The distribution of the follicle stages after 30 days of implantation in TheraCyte was 41.0% primordial, 36.1% primary, 21.0% secondary and 1.9% antral follicles (Fig. 5a).
The proportion of follicle stages was not significantly different between 7-day non- surrounded alginate and 7-day TheraCyte® (p = 0.774) and 30-day non- surrounded alginate and 30-day TheraCyte® (p = 0.958). At the time of retrieval, new blood vessels were clearly observed around the TheraCyte® at both 7 and 30 days of implantation.
Endocrine Function in Mice Implanted with Syngeneic Grafts
We measured serum FSH levels before ovariectomy to establish the baseline levels in mice with functioning ovaries, two weeks' post ovariectomy to ensure complete removal of gonads, and 7 and 30 days after implantation of ovarian tissue encapsulated in alginate or TheraCyte®. The serum levels of FSH in all healthy mice prior to ovariectomy (n = 32) measured at or below 10 ng/mL. The FSH levels increased after bilateral ovariectomy to 60–70 ng/mL, which confirmed that the mice were completely ovariectomized. The decrease in the serum levels of FSH after implantation of ovarian tissue in alginate was not statistically significant compared to pre-implantation levels. The levels of FSH decreased from 70 to 51 ng/mL after 30 days (p = 0.058), which indicates that the amount of estradiol that reached the circulation from the implanted ovarian tissue was not sufficient to restore the negative feedback in the HPG axis. Whereas for TheraCyte® implants, FSH levels significantly decreased from an average of 62 to 37 ng/mL (p = 0.03) 30 days' post implantation (Fig. 5b). However, FSH levels were still greater in the TheraCyte group after 30 days compared to the levels observed before ovariectomy.
Endocrine Function and Histological Evaluation of Allogeneic Grafts Implanted in TheraCyte
The allogeneic ovarian tissue encapsulated in TheraCyte® and implanted in immune-competent B6 ovariectomized females for 21 days contained healthy primary and secondary follicles (Fig. 6d). The presence of healthy ovarian tissue was further confirmed with the serum levels of FSH at 34.06 ng/mL. We performed daily vaginal smears in mice implanted with allogeneic ovarian tissue encapsulated in TheraCyte beginning at day 7 post implantation till the retrieval of the implant at 21 days. Three out of four mice (75%) exhibited resumption of estrus activity as indicated by the transition from diestrus to the other stages of the cycle after implantation of TheraCyte in the allogeneic group. The mice had 2 cycles from first estrus appearing on day 14. One mouse exhibited one estrus followed by diestrus till the day of sacrifice. Control mice, which were implanted with allogeneic tissue with no device, rejected the tissue. Figure 6b shows the absence of functional ovarian tissue with no surviving follicles and abundant mononuclear cell infiltrates consistent with cellular mediated graft rejection.
Sensitization of the Host by the Implanted Allogeneic Ovarian Tissue
Flow cytometry analysis of allogeneic antibodies produced in the sera of control recipients transplanted with allogeneic ovarian tissue without immuno-isolating device revealed a strong allo-specific IgG response (MFI = 717) indicative of sensitization at 21 days after transplantation (Fig. 7). Figure 7 shows that mice transplanted with ovarian tissue enclosed within the TheraCyte have no allo-specific IgG (MFI = 39–51) in the blood, and thus are not sensitized 21 days after transplantation. This finding is consistent with an immune-isolating function of the TheraCyte device. IgG–IgM levels at day 21 was not significant to the IgG–IgM levels observed before transplantation (p = 0.4857).
Figure 7.
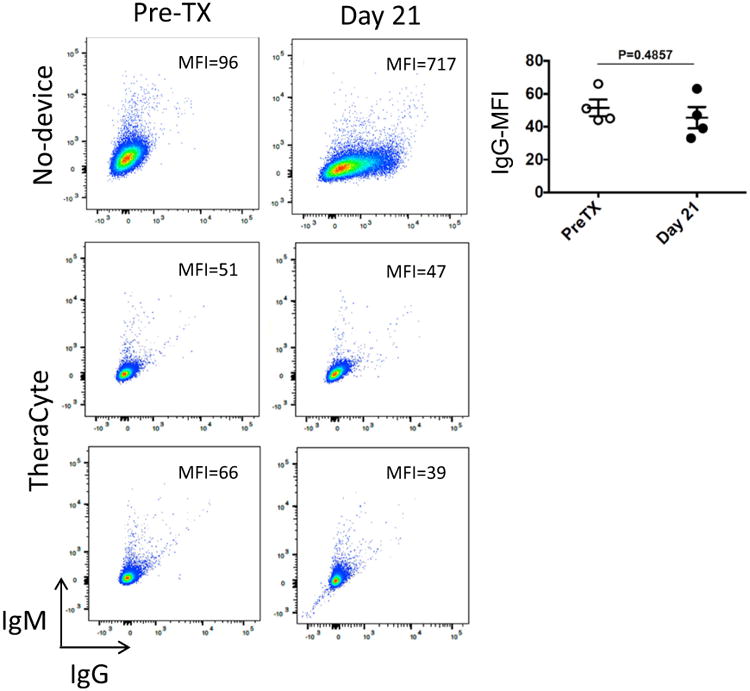
Figure shows that the mice transplanted with ovarian tissue enclosed within the TheraCyte are not sensitized 21 days after transplantation. Plots show flow cytometry analysis of allogeneic antibodies produced overtime, in recipients transplanted with naked full allogeneic ovarian tissue (no-device), or transplanted with ovarian tissue contained in TheraCyte (n = 4). Plots were obtained and mean fluorescence intensities (MFI) in the APC-channel (measuring bound IgG) determined with FlowJo software. Graph depicts the MFIs of bound IgG obtained from individual recipients before and after transplantation. Comparison of the means was by non-parametric unpaired t test.
Discussion
In this work, we studied two immuno-isolating devices, alginate and TheraCyte®, for their ability to support and protect ovarian grafts. In the past, alginate and TheraCyte® were used for encapsulation of allogeneic pancreatic islet transplants for restoring glucose levels in animal diabetic models.24,27 Here we report the first application of these devices for restoring ovarian endocrine function in ovariectomized mice. First, we used a syngeneic mouse model to test whether alginate and TheraCyte provide a supportive environment for the encapsulated and implanted ovarian follicles by allowing diffusion of nutrients and tissue expansion. Second, TheraCyte that proved more successful in supporting graft survival, was tested in an allogeneic model for its ability to serve as a barrier between the host and the graft. Immuno-isolating devices such as TheraCyte, prevent rejection by blocking antigens leaking from the graft and sensitization, and/or the infiltration of the effector host immune cells. Overall, only a device that supports growth and development of ovarian tissue and prevents rejection can potentially assist in restoring lost ovarian function through allogeneic transplant.
Previously13 reported that alginate supports the development of the encapsulated cortical ovarian tissue. They concluded that early follicular development of human follicles requires the support of native ovarian cortex. The inert but biomimetic nature of alginate makes it a strong candidate as supportive matrix for both ovarian follicle culture in vitro and transplantation in vivo. Alginate has been used to create artificial ovaries in a murine model23 where autologous isolated preantral follicles developed to antral stages. Alginate also proved to prevent rejection of islets in allogeneic mice model,24,27,28 and thus presented a compelling choice for ovarian tissue. An important distinction between ovarian tissue and islets is that ovarian follicles expand hundred-fold during folliculogenesis. While alginate supports the follicular development of isolated follicles in vivo23 as well as in vitro,12,17,21 in this study we found that the pressure exerted by the ovarian tissue with multiple growing follicles during the developmental phase seems to challenge the integrity of the alginate. The release of divalent ions in the environment resulting in the degradation of alginate hydrogels,14 may be another contributing factor which could compromise the long-term mechanical properties and immunoisolation efficiency of the hydrogel. Furthermore, alginate hydrogels used in the current study were not viscoelastic in nature. A study by Zhao et al.30 has shown that alginate gels with ionic crosslinks behave more like a plastic, hence these ionically crosslinked alginate gels are prone to breaking compared to those formed with covalent bonds and capable of elastic deformation. We observed that after in vivo implantation, the majority of alginate gels were not able to maintain their structural integrity, and disintegrated under the pressure of the encapsulated expanding ovarian tissue. As a result, the implanted ovarian tissue escaped from the alginate capsule and attached to the host tissue. On the other hand, in the alginate hydrogels that maintained the integrity during the experiment, e.g. surrounded, we observed significantly smaller numbers of follicles compared to the disintegrated alginate beads and the encapsulated tissue appeared necrotic. Lastly, the levels of FSH in the mice that received alginate transplants did not decrease significantly compared to ovariectomized mice 30 days' post implantation. This indicates that the number of surviving follicles was not sufficient to initiate negative feedback of the HPG axis. A small number of surviving follicles in fully encapsulated alginate beads indicates that, in contrast to islets, alginate is a less suitable candidate to be used for encapsulation of ovarian tissue due to its restrictive impact on growth and expansion of the ovarian tissue followed with eventual failure of the isolating layer. While syngeneic implant benefited from receiving blood supply, exposure of allogeneic tissue leads to immune rejection. Thus it is reasonable to suggest that in allogeneic model alginate will not provide sufficient shielding from the immune system and the grafts will be rejected. On the other hand, syngeneic ovarian tissue encapsulated in TheraCyte® maintained physical integrity, which was confirmed histologically in the sections of the retrieved grafts. The ovarian tissue inside the device was viable, with no signs of necrosis and contained multiple follicles at different developmental stages. Most importantly, the serum FSH levels decreased after the implantation of ovarian tissue in TheraCyte® and were significantly lower than the levels after ovariectomy, which demonstrates that the ovarian tissue in the device responded to the circulating gonadotropins.
Allogeneic ovary tissue transplantation was previously attempted by Donnez et al.7 who reported restoration of ovarian function after allografting ovarian tissue between genetically non-identical sisters. In all these cases the recipient had received bone-marrow from her human leucocyte antigen (HLA)-compatible sister and was not administered immunosuppression. The implanted ovarian tissue functioned for a year. Since bone marrow transplantation is a high-risk procedure and may not be feasible for non-HLA compatible individuals, as it would require immunosuppression for extended function of the implanted tissue, a fundamental advance in the field would be to determine the means to avoid immune-mediated destruction of ovary allografts.
After confirming that syngeneic ovarian tissue encapsulated in TheraCyte remained viable and functioning, we investigated whether TheraCyte is able to sustain the function of allogeneic ovarian tissue. The mechanism of immuno-isolation provided by TheraCyte could be either creating a site of immune-privilege blocking sensitization of the host or blocking immune effectors from reaching the graft, or both. The absence of allo-specific IgG in the host sera confirmed that encapsulated ovarian tissue within the TheraCyte did not sensitize the host. In contrast to the non-encapsulated allograft control, TheraCyte enclosed ovarian allografts restored ovarian endocrine function, confirmed by the transition of stages observed during vaginal cytology, presumably through estrogen restoration, and revealed healthy ovarian tissue in biopsy. These results suggest absence of immune-mediated rejection. How long absence of sensitization lasts will be addressed by long term implantations to determine the feasibility of TheraCyte to sustain long-lived function of ovarian tissue allografts.
Our study is the first step to evaluate the concept of immuno-isolation for restoring ovarian endocrine function in females with premature ovarian failure. It is our goal to develop the means to maintain implants beyond 30 days to determine whether the implants with ovarian tissue rich in primordial follicles (including allografts) are a viable option for long-term restoration of ovarian endocrine function. Our results suggest that the TheraCyte type devices can successfully support the function of allografts and avoid immune sensitization of the host.
Furthermore, a study by Zhang et al.29 reported the use of alginate-chitosan-alginate (ACA) shell for cell encapsulation which has shown to reduce inflammatory and immune responses. Yet for the purpose of implantation of ovarian tissue this approach may not address the brittleness of alginate beads around the expanding ovarian follicles.
Acknowledgments
The hormone assays were performed at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core and was supported by grant from the Hartwell Foundation (14-PAF00981).
Funding: This work is supported by Grants from the Hartwell Foundation (14-PAF00981) to AS to the National Science Foundation Graduate Research Fellowship (DGE 1256260) to JD and by the NIH (R56AI123262) to MC.
Footnotes
Author Contributions: This research was conceived and designed by AS and AD. MC designed the immunological study. AD did all the animal surgeries. AD did the ovarian encapsulation in alginate and AS did the ovarian encapsulation in TheraCyte®. JRD assisted with animal work and did the statistical analysis. AD and ALC were involved with histological and morphological analysis. AL, AD and JRD carried out the flow cytometry work. AD wrote the first draft. MC and AS revised the manuscript. All authors approved the final manuscript.
Conflict Of Interest: There is no conflict of interest.
References
- 1.Boettler T, Schneider D, Cheng Y, Kadoya K, Brandon EP, Martinson L, Herrath MV. Pancreatic tissue transplanted in TheraCyte™ encapsulation devices are protected and prevent hyperglycemia in a mouse model of immune-mediated diabetes. Cell Transpl. 2015;25:609–614. doi: 10.3727/096368915X688939. [DOI] [PubMed] [Google Scholar]
- 2.Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res. 1995;29:1517–1524. doi: 10.1002/jbm.820291208. [DOI] [PubMed] [Google Scholar]
- 3.Brauker JH, Martinson LA, Young SK, Johnson RC. Local inflammatory response around diffusion chambers containing xenografts. Nonspecific destruction of tissues and decreased local vascularization. Transplantation. 1996;61:1671–1677. doi: 10.1097/00007890-199606270-00002. [DOI] [PubMed] [Google Scholar]
- 4.D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 5.De Vos P, De Haan BJ, Wolters GH, Strubbe JH, Van Schilfgaarde R. Improved biocompatibility but limited graft survival after purification of alginate for microencapsulation of pancreatic islets. Diabetologia. 1997;40:262–270. doi: 10.1007/s001250050673. [DOI] [PubMed] [Google Scholar]
- 6.Dolmans MM, Marinescu C, Saussoy P, Van Langendonckt A, Amorim C, Donnez J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood. 2010;116:2908–2914. doi: 10.1182/blood-2010-01-265751. [DOI] [PubMed] [Google Scholar]
- 7.Donnez J, Squifflet J, Pirard C, Jadoul P, Dolmans MM. Restoration of ovarian function after allografting of ovarian cortex between genetically non-identical sisters. Hum Reprod. 2010;25:2489–2495. doi: 10.1093/humrep/deq186. [DOI] [PubMed] [Google Scholar]
- 8.Fish JD. Part 1: Hormone replacement for survivors of childhood cancer with ovarian failure–when is it worth the risk? J Pediatr Adolesc Gynecol. 2011;24:98–101. doi: 10.1016/j.jpag.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Ginsberg JP. New advances in fertility preservation for pediatric cancer patients. Curr Opin Pediatr. 2011;23:9–13. doi: 10.1097/MOP.0b013e3283420fb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gore L, DeGregori J, Porter CC. Targeting developmental pathways in children with cancer: what price success? Lancet Oncol. 2013;14:e70–e78. doi: 10.1016/S1470-2045(12)70530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly OG, Chan MY, Martinson LA, Kadoya K, Ostertag TM, Ross KG, Richardson M, Carpenter MK, D'Amour KA, Kroon E, Moorman M, Baetge EE, Bang AG. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol. 2011;29:750–756. doi: 10.1038/nbt.1931. [DOI] [PubMed] [Google Scholar]
- 12.Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27:714–723. doi: 10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laronda MM, Duncan FE, Hornick JE, Xu M, Pahnke JE, Whelan KA, Shea LD, Woodruff TK. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J Assist Reprod Genet. 2014;31:1013–1028. doi: 10.1007/s10815-014-0252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee KY, Mooney DJ. Alginate: properties and biomedical properties. Prog Polym Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myers M, Britt KL, Wreford NGM, Ebling FJP, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127:569–580. doi: 10.1530/rep.1.00095. [DOI] [PubMed] [Google Scholar]
- 16.Nabavimanesh MM, Hashemi-Najafabadi S, Vasheqhani-Farahani E. Islets immunoisolation using encapsulation and PEGylation, simultaneously, as a novel design. J Biosci Bioeng. 2015;119:486–491. doi: 10.1016/j.jbiosc.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues JK, Navarro PA, Zelinski MB, Stouffer RL, Xu J. Direct actions of androgens on the survival, growth and secretion of steroids and anti-Mülle-rian hormone by individual macaque follicles during three-dimensional culture. Hum Reprod. 2015;30:664–674. doi: 10.1093/humrep/deu335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosendahl M, Andersen MT, Ralfkiær E, Kjeldsen L, Andersen MK, Andersen CY. Evidence of residual disease in cryopreserved ovarian cortex from female patients with leukemia. Fertil Steril. 2010;94:2186–2190. doi: 10.1016/j.fertnstert.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 19.Schweicher J, Nyitray C, Desai TA. Membranes to achieve immunoprotection of transplanted islets. Front Biosci (Landmark Ed) 2014;19:49–76. doi: 10.2741/4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silber S, Pineda J, Lenahan K, DeRosa M, Melnick J. Fresh and cryopreserved ovary transplantation and resting follicle recruitment. Reprod Biomed Online. 2015;30:643–650. doi: 10.1016/j.rbmo.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Skory RM, Xu Y, Shea LD, Woodruff TK. Engineering the ovarian cycle using in vitro follicle culture. Hum Reprod. 2015;30:1386–1395. doi: 10.1093/humrep/dev052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarantal AF, Lee CC, Itkin-Ansari P. Real-time bioluminescence imaging of macroencapsulated fibroblasts reveals allograft protection in rhesus monkeys (Macaca mulatta) Transplantation. 2009;88:38–41. doi: 10.1097/TP.0b013e3181a9ee6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanacker J, Dolmans MM, Luyckx V, Donnez J, Amorim CA. First transplantation of isolated murine follicles in alginate. Regen Med. 2014;9:609–619. doi: 10.2217/rme.14.33. [DOI] [PubMed] [Google Scholar]
- 24.Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader AR, Li J, Langan E, Wyckoff J, Loo WS, Jhunjhunwala S, Chiu A, Siebert S, Tang K, Hollister-Lock J, Aresta-Dasilva S, Bochenek M, Mendoza-Elias J, Wang Y, Qi M, Lavin DM, Chen M, Dholakia N, Thakrar R, Lacík I, Weir GC, Oberholzer J, Greiner DL, Langer R, Anderson DG. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat Mater. 2015;14:643–651. doi: 10.1038/nmat4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber LM, Cheung CY, Anseth KS. Multifunctional pancreatic islet encapsulation barriers achieved via multilayer PEG hydrogels. Cell Transpl. 2008;16:1049–1057. [PubMed] [Google Scholar]
- 26.Xu M, Banc A, Woodruff TK, Shea LD. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol Bioeng. 2009;103:378–386. doi: 10.1002/bit.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yakhnenko I, Wong WK, Katkov II, Itkin-Ansari P. Cryopreservation of human insulin expressing cells macro-encapsulated in a durable therapeutic immunoisolating device. TheraCyte Cryo Lett. 2012;33:518–531. [PubMed] [Google Scholar]
- 28.Yin N, Han Y, Xu H, Gao Y, Yi T, Yao J, Dong L, Cheng D, Chen Z. VEGF-conjugated alginate hydrogel prompt angiogenesis and improve pancreatic islet engraftment and function in type 1 diabetes. Mater Sci Eng C Mater Biol Appl. 2016;59:958–964. doi: 10.1016/j.msec.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Zhao S, Rao W, Snyder J, Choi JK, Wang J, Khan IA, Saleh NB, Mohler PJ, Yu J, Hund TJ, Tang C, He X. A novel core-shell microcapsule for encapsulation and 3D culture of embryonic stem cells. J Mater Chem B Mater Biol Med. 2013;7:1002–1009. doi: 10.1039/C2TB00058J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao X, Huebsch N, Mooney DJ, Suo Z. Stress-relaxation behavior in gels with ionic and covalent crosslinks. J Appl Phys. 2010;15(107):63509. doi: 10.1063/1.3343265. [DOI] [PMC free article] [PubMed] [Google Scholar]