Abstract
Purpose
Patients with advanced cancer experience potentially burdensome transitions of care after hospitalizations. We examined predictors of discharge location and assessed the relationship between discharge location and survival in this population.
Methods
We conducted a prospective study of 932 patients with advanced cancer who experienced an unplanned hospitalization between September 2014 and March 2016. Upon admission, we assessed patients’ physical symptoms (Edmonton Symptom Assessment System) and psychological distress (Patient Health Questionnaire-4). The primary outcome was discharge location (home without hospice, postacute care [PAC], or hospice [any setting]). The secondary outcome was survival.
Results
Of 932 patients, 726 (77.9%) were discharged home without hospice, 118 (12.7%) were discharged to PAC, and 88 (9.4%) to hospice. Those discharged to PAC and hospice reported high rates of severe symptoms, including dyspnea, constipation, low appetite, fatigue, depression, and anxiety. Using logistic regression, patients discharged to PAC or hospice versus home without hospice were more likely to be older (odds ratio [OR], 1.03; 95% CI, 1.02 to 1.05; P < .001), live alone (OR, 1.95; 95% CI, 1.25 to 3.02; P < .003), have impaired mobility (OR, 5.08; 95% CI, 3.46 to 7.45; P < .001), longer hospital stays (OR, 1.15; 95% CI, 1.11 to 1.20; P < .001), higher Edmonton Symptom Assessment System physical symptoms (OR, 1.02; 95% CI, 1.003 to 1.032; P < .017), and higher Patient Health Questionnaire-4 depression symptoms (OR, 1.13; 95% CI, 1.01 to 1.25; P < .027). Patients discharged to hospice rather than PAC were more likely to receive palliative care consultation (OR, 4.44; 95% CI, 2.12 to 9.29; P < .001) and have shorter hospital stays (OR, 0.84; 95% CI, 0.77 to 0.91; P < .001). Patients discharged to PAC versus home had lower survival (hazard ratio, 1.53; 95% CI, 1.22 to 1.93; P < .001).
Conclusion
Patients with advanced cancer who were discharged to PAC facilities and hospice had substantial physical and psychological symptom burden, impaired physical function, and inferior survival compared with those discharged to home. These patients may benefit from interventions to enhance their quality of life and care.
INTRODUCTION
The Institute of Medicine report “Dying in America”1 underscores the critical need to optimize the quality of end-of-life (EOL) care for patients in the United States. Burdensome care transitions—particularly hospitalizations or transfers in place of care2—may lead to poor quality care at the EOL and increase health care costs.3-9 For patients with advanced cancer, hospitalizations represent the largest share of health care spending, with significant variations in hospital use at the EOL in the United States.6,10 Although studies have focused on potential drivers of hospital use at the EOL, data are lacking on posthospital transitions of care for patients with advanced cancer, especially discharge to postacute care (PAC) facilities.
PAC facilities such as skilled nursing facilities (SNFs) or long-term acute care hospitals are typically used for patients who are no longer acutely ill but require ongoing nursing care, physical therapy, or additional recovery time. In the Medicare population, 33% of beneficiaries use the postdischarge SNF benefit in the last 6 months of life. Notably, one in 11 Medicare beneficiaries die while using the SNF benefit.11 The increased financial incentives to reduce hospital length of stay (LOS) have contributed to pressure on hospitals to discharge patients to PAC facilities.12 However, these discharges may represent a burdensome transition at the EOL for patients with advanced cancer who have intensive supportive care needs.13-17 Additionally, these facilities generally do not have specialized services in symptom management that are comparable to hospice services.14,18 Therefore, studies are needed to examine the clinical characteristics of patients with advanced cancer who are discharged to PAC facilities and assess the factors contributing to PAC facility use.6
Although investigators have focused on identifying predictors of PAC facility use in the general medical population,19,20 data on predictors of their use in the population with advanced cancer are lacking. By better understanding the characteristics of patients being discharged to PAC facilities, we will be able to identify a population at risk for these potentially burdensome transitions of care and inform development of alternate care-delivery models to prevent unnecessarily burdensome transitions. In this study, we sought to describe the clinical characteristics and symptom burden of patients with advanced cancer who were discharged to PAC facilities, hospice (at home or at a facility), or home without hospice after an unplanned hospitalization. We also explored predictors of discharge location, the relationship between discharge location and survival, and risk factors for hospital readmission.
METHODS
Study Procedures
This study was approved by the Dana Farber Harvard Cancer Center Institutional Review Board. From September 2, 2014, to March 31, 2016, we enrolled 932 patients with advanced cancer who experienced an unplanned hospitalization at Massachusetts General Hospital. We recruited consecutive patients with their first unplanned hospital admission during the study period by screening the inpatient oncology census. We focused on patients with unplanned hospitalizations, because this is a symptomatic population at high risk for further disease progression and complications. Study staff obtained written, informed consent after admission (within 2 to 5 days). After consent, participants completed symptom-burden questionnaires.
Participants
Patients were eligible for participation if they were > 18 years old and admitted to Massachusetts General Hospital with a known diagnosis of advanced cancer. We defined patients with advanced cancer as those not being treated with curative intent; they were identified on the basis of chemotherapy order entry and treatment-intent designation or clinical documentation. We excluded patients who were unable to respond to questionnaires in English, and patients admitted for an elective or planned hospitalization (eg, hospitalization for chemotherapy, planned surgeries or other elective procedures, or chemotherapy desensitization). We excluded patients who did not survive to hospital discharge or those missing an initial nursing assessment.
Study Measures
Sociodemographic, clinical, and functional factors.
We conducted a medical record review to collect demographic information and to determine Charlson Comorbidity Index (CCI) score, date of diagnosis with advanced cancer, cancer type, and reason for admission. We also reviewed a questionnaire completed by nurses within 1 day of admission to assess whether the patient lived alone, used a mobility assistive device, and ambulated independently. These functional status measures have been shown to be comparable to validated functional measures in predicting discharge location.21 We reviewed the inpatient hospitalization record to determine LOS and whether the patient had a palliative care consultation during their hospitalization.
Patient-reported symptom burden.
We used the revised Edmonton Symptom Assessment System-Revised (ESAS-r) to assess patients’ physical symptoms, including nausea, dyspnea, lack of appetite, pain, drowsiness, well-being, and fatigue.22,23 We also included constipation, because this is a highly prevalent symptom in patients with cancer.24 Patients rated their symptoms on a scale of 0 to 10, with 0 reflecting absence of the symptom and 10 reflecting the worst possible symptom. We defined severe symptoms as scores from 7 to 10.25 We computed ESAS-r physical scores including pain, fatigue, drowsiness, nausea, appetite, dyspnea, and constipation.26
To assess patients’ psychological symptoms, we used the Patient Health Questionnaire-4 (PHQ-4).27,28 The PHQ-4 is a four-item tool that contains two subscales that assess depression and anxiety. Both subscales and the composite PHQ-4 can be evaluated continuously, with subscores ≥ 3 out of 6 indicating clinically significant depression or anxiety.27 We added the PHQ-4 to the study questionnaires on November 15, 2014.
Discharge location.
We obtained discharge location from patients’ medical records. We categorized discharge location as home without hospice, PAC facility, hospice (whether provided at home, hospice facility, or general inpatient hospice), or other. Because of the small sample size (n = 1), we excluded patients in the “other” category.
Survival and readmission.
We calculated survival time from the date of discharge to the date of death using the Kaplan-Meier method. We censored data from patients who were alive at the last follow-up date (September 9, 2016). To account for mortality, because patients who die after their index hospitalization have less time at risk for readmission, we used time to first unplanned admission within 90 days of hospital discharge as an outcome measure, censoring patients without a readmission at 90 days and censoring those who died within 90 days of their discharge at their death date. In addition, we created a composite dichotomous outcome categorizing patients as dead and/or readmitted within 90 days versus those alive and with no readmission within 90 days to account for early mortality. This composite outcome has been used previously in the literature for patients at high risk of mortality.29-32 Importantly, recent studies suggest that the use of this composite outcome is a more accurate metric for determining the quality of care.33
Statistical Analysis
To compare participants’ characteristics and symptom burden by discharge location, we used χ2 tests for categorical variables and analysis of variance or Kruskal-Wallis tests for continuous variables that were normally distributed or skewed, respectively. We used χ2 tests to compare symptom burden across discharge locations. To explore predictors of discharge location, we used logistic regression models incorporating the following variables: age, sex, CCI score, cancer type, months since advanced diagnosis, living alone, impaired mobility, hospital LOS, palliative care consultation, and physical and psychological symptoms during hospitalization. We chose these variables a priori on the basis of a review of the literature on clinical predictors of discharge location.19-21,34-41 We first determined predictors of discharge to sites other than home (PAC facility or hospice), using logistic regression models with the dichotomous outcome of discharge home without hospice (reference) versus other site (PAC facility or hospice). We then used similar logistic regression models to determine predictors of discharge to PAC facilities versus hospice. Given collinearity between physical and psychological symptoms, we created separate models to assess the relationship between these symptom assessments (ie, ESAS-r, PHQ-4 depression, and PHQ-4 anxiety) and discharge location.
We used Kaplan-Meier curves to assess survival by discharge location and Cox proportional hazards models, adjusted for age, sex, CCI score, cancer type, and months since advanced cancer diagnosis, to assess the relationship between discharge location and survival. Similarly, we used Cox proportional hazards models to assess the relationship between discharge location and time to readmission within 90 days. We used logistic regression adjusted for the same variables to assess the relationship between discharge location and the composite outcome readmission or death within 90 days. Less than 1% of patients had missing data for each individual symptom, precluding the need for missing data imputations. All reported P values are two-sided with a P < .05 considered statistically significant.
RESULTS
Participant Sample
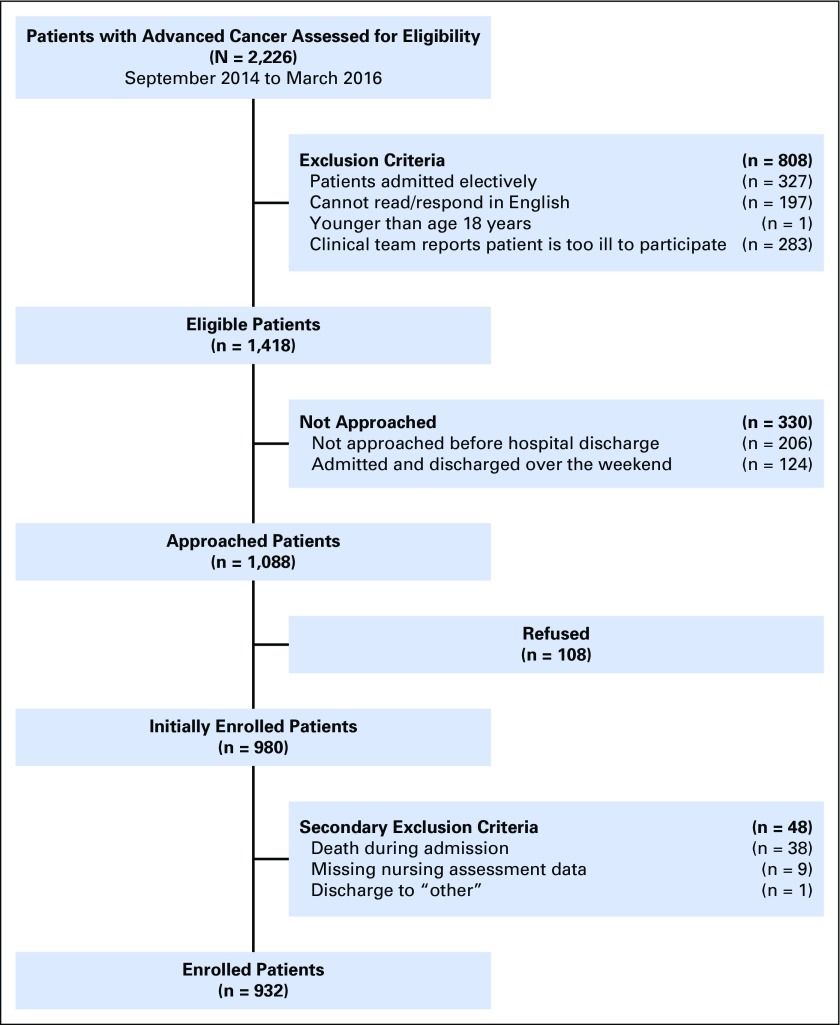
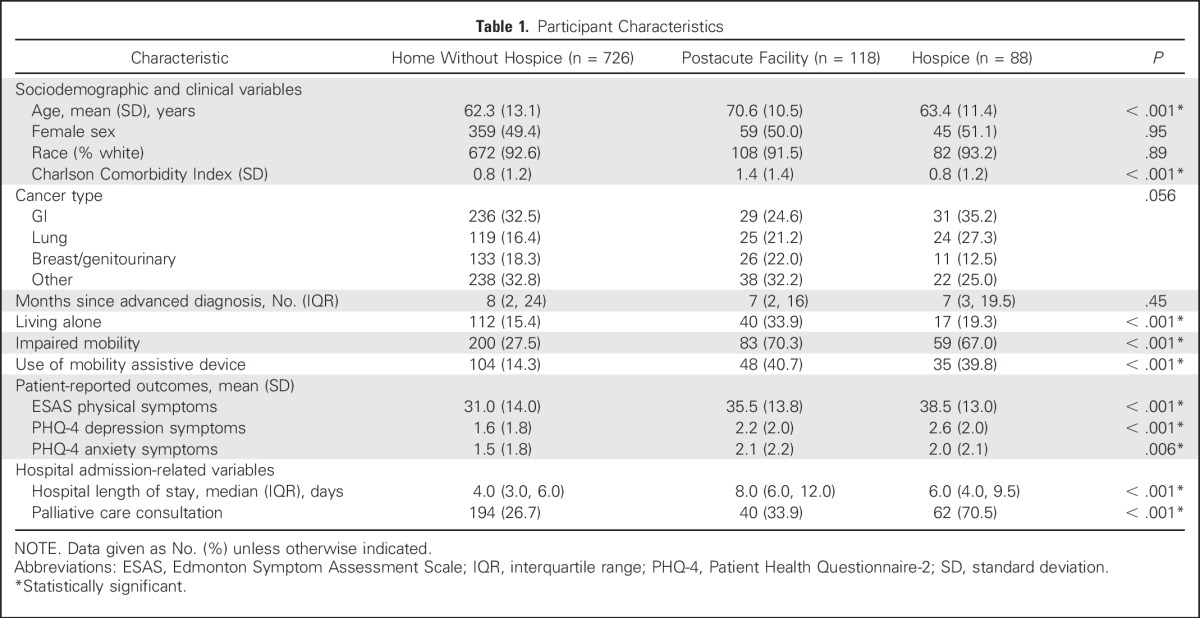
Of the 2,226 patients screened for eligibility, we approached 1,088 eligible patients and enrolled 980 participants (90.0%). We excluded 48 patients from this analysis because of death during admission (n = 38), missing nursing assessment (n = 9), and discharge to “other” (n = 1; Appendix Fig A1, online only). The reason for unplanned admission among the 932 patients included in this analysis was symptom management (55.7%; 519 of 932); fevers or infections (24.1%; 225 of 932); failure to thrive, weakness, or fatigue (14.1%; 131 of 932); and metabolic disarray or laboratory abnormalities (6.1%; 57 of 932). Among the 932 patients, 726 (77.9%) were discharged home without hospice, 118 (12.7%) to PAC facilities and 88 (9.4%) to hospice at home or at an inpatient facility (Table 1). We observed no differences between the groups in terms of sex, race, cancer type, or months since advanced cancer diagnosis. Patients discharged to PAC facilities were more likely to be older, have a higher CCI score, and live alone compared with those discharged to home or hospice. Patients discharged to PAC facilities or hospice were more likely than patients discharged to home without hospice to have a longer LOS, impaired mobility, and use a mobility assistive device. Rates of palliative care consultations varied across the three groups, with a considerably higher rate in the group discharged to hospice.
Table 1.
Participant Characteristics
Patient-Reported Symptom Burden
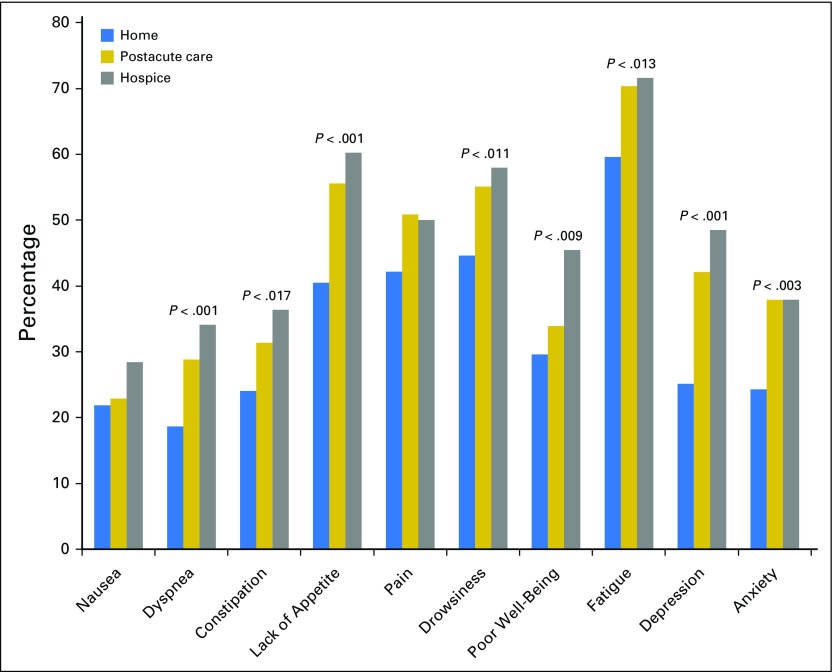
Figure 1 depicts the proportion of patients experiencing severe symptoms by discharge location. Compared with patients discharged to home without hospice, a higher proportion of those discharged to a PAC facility or hospice reported severe physical symptoms, including dyspnea (home v PAC v hospice: 18.6%, 28.8%, and 34.1%, respectively; P < .001), constipation (home v PAC v hospice: 24.0%, 31.4%, 36.4%, respectively; P < .017), lack of appetite (home v PAC v hospice: 40.5%, 55.6%, 60.2%, respectively; P < .001), drowsiness (home v PAC v hospice: 44.6%, 55.1%, 58.0%, respectively; P < .011), poor well-being (home v PAC v hospice: 29.6%, 33.9%, 45.5%, respectively; P < .009), and fatigue (home v PAC v hospice: 59.6%, 70.3%, 71.6%, respectively; P < .013). Notably, more than half of patients discharged to PAC facilities or hospice reported severe lack of appetite, pain, drowsiness, and fatigue. In addition, a higher proportion of patients discharged to a PAC facility or hospice reported clinically significant depression and anxiety (home v PAC v hospice: PHQ-4 depression: 25.1%, 42.1%, 48.5%, respectively, P < .001; PHQ-4 anxiety: 24.3%, 37.9%, 37.9%, respectively, P < .003) compared with those discharged to home without hospice.
Fig 1.
Participants’ symptom severity by discharge location. Only statistically significant P values are shown.
Predictors of Discharge Location
Table 2 lists predictors of discharge to a location other than home without hospice (ie, a PAC facility, hospice provided at home, or an inpatient facility). Older age, living alone, impaired mobility, and longer hospital LOS (measured in days) were all significantly associated with discharge to a PAC facility or hospice. Notably, higher ESAS-r and PHQ-4 scores for physical symptoms and depression symptoms, respectively, were significantly associated with a higher likelihood of being discharge to a PAC facility or hospice. In a separate model, depression symptoms were also associated with a higher likelihood of being discharged to a PAC facility or hospice. Anxiety symptoms were not associated with discharge location. We obtained similar findings when examining predictors of discharge to a PAC facility versus home without hospice. Older age, living alone, impaired mobility, longer hospital LOS, and higher depression and anxiety symptom scores were associated with a higher likelihood of being discharged to a PAC facility compared with being discharged to home without hospice.
Table 2.
Predictors of Patients’ Discharge Location: Home Without Hospice (reference group) v Location Other Than Home (postacute care facility or hospice)
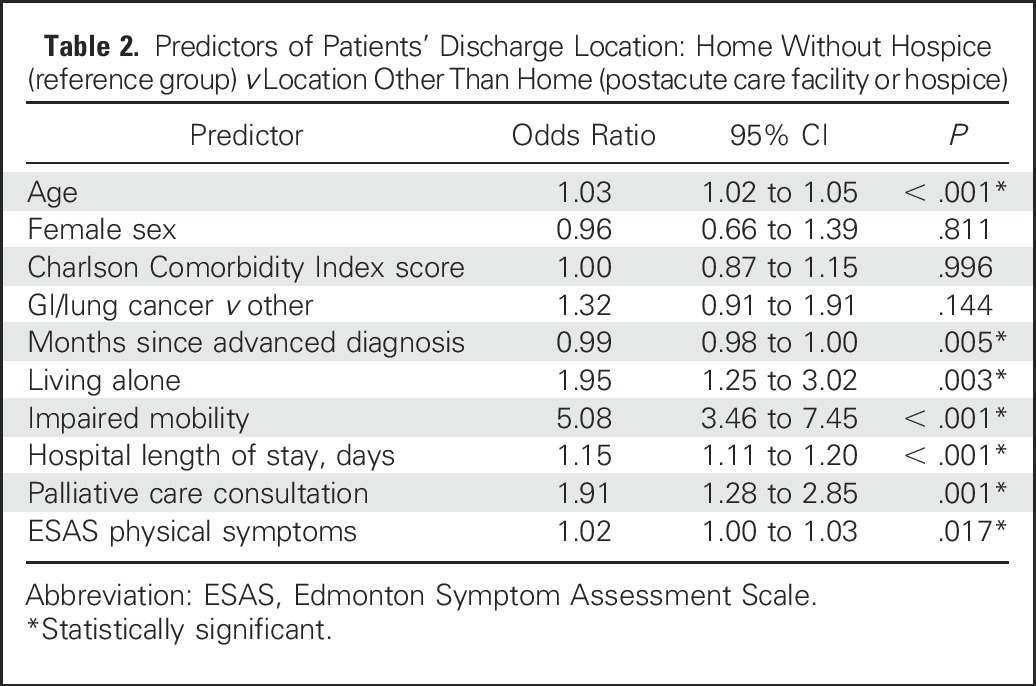
We explored predictors of discharge to hospice versus PAC facility (with the latter serving as the reference). The only predictors of discharge to hospice versus PAC facility were younger age (OR, 0.95; 95% CI, 0.91 to 0.98; P < .001), shorter hospital LOS (OR, 0.84; 95% CI, 0.77 to 0.91; P < .001), and palliative care consultation (OR, 4.44; 95% CI, 2.12 to 9.29; P < .001).
Relationship Between Discharge Location and Survival
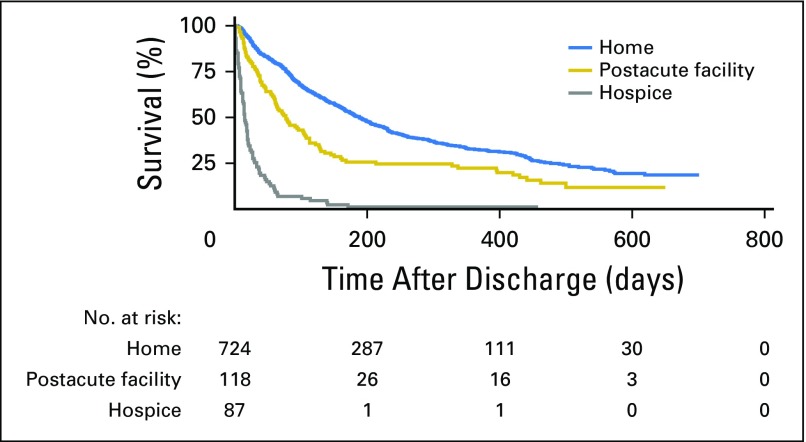
Figure 2 depicts the Kaplan-Meier survival curves for patients by discharge location. Discharge to a PAC facility (HR, 1.53; 95% CI, 1.22 to 1.93; P < .001) and discharge to hospice (HR, 7.92; 95% CI, 6.19 to 10.14; P < .001) were both associated with lower overall survival compared with discharge to home without hospice. Patients discharged home without hospice had a median survival of 188 days (95% CI, 168 to 208 days), whereas patients discharged to a PAC facility had a median survival of 77 days (95% CI, 61 to 105 days) and those discharged to hospice had a mean survival of 15 days (95% CI, 12 to 19 days).
Fig 2.
Overall survival by discharge location.
Discharge Location and Readmission
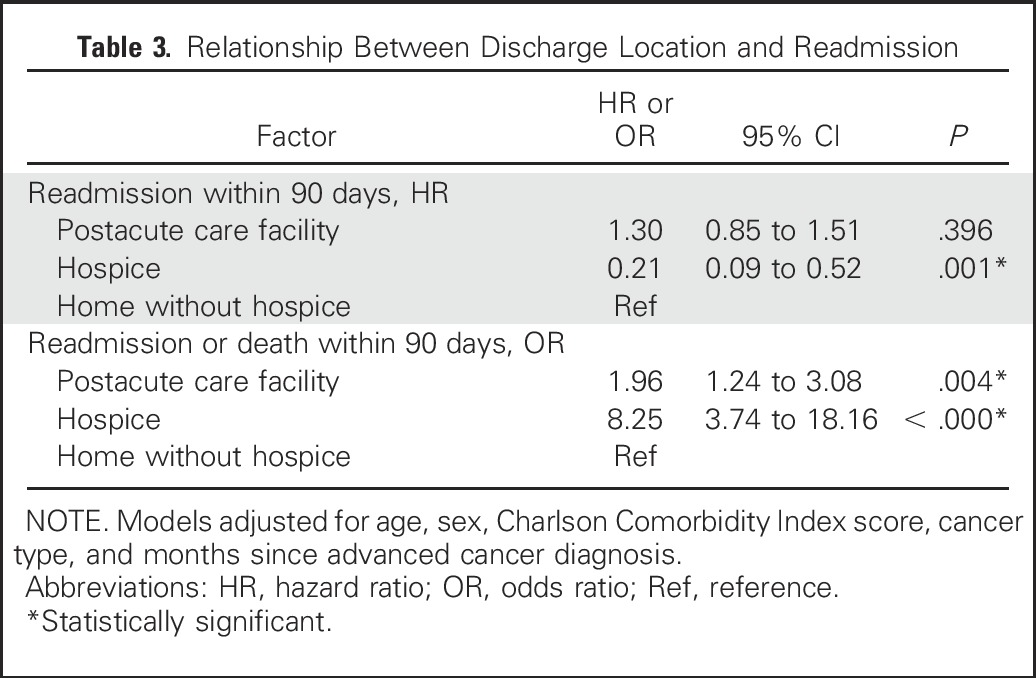
In Cox regression models examining time to readmission within 90 days, discharge to hospice (HR, 0.21; 95% CI, 0.09 to 0.52; P < .001) was associated with a lower likelihood of readmission; there was no association between discharge to PAC facilities and likelihood of readmission within 90 days (HR, 1.30; 95% CI, 0.85 to 1.51; P < .396; Table 3). However, discharge to a PAC facility (OR, 1.96; 95% CI, 1.24 to 3.08; P < .004) and discharge to hospice (OR, 8.25; 95% CI, 3.74 to 18.16; P < .001) were both associated with a higher likelihood of readmission or death within 90 days.
Table 3.
Relationship Between Discharge Location and Readmission
DISCUSSION
In this study of patients with advanced cancer and unplanned hospital admission, we demonstrated that patients discharged to PAC facilities have substantial physical and psychological symptoms and impaired mobility, which are all strikingly similar to the characteristics of patients discharged to hospice. Despite their symptoms, patients discharged to PAC facilities often do not have access to high-quality palliative care services.14,18 Notably, similar to patients discharged to hospice, those discharged to PAC facilities have a longer LOS and worse overall survival compared with those discharged to home without hospice. Although it is clear that patients discharged to PAC facilities and those discharged to hospice have a different symptom profile and outcomes compared with patients discharged to home, we may have lacked statistical power to detect meaningful differences between patients discharged to PAC facilities and those discharged to hospice. Nonetheless, these findings underscore that patients discharged to a PAC facility may be better served by a different postdischarge care setting that can better address their substantial symptom burden and poor prognosis. These findings have several implications and identify several potential areas for interventions to enhance patients’ quality of care.
First, these findings can aid clinicians in identifying patients with advanced cancer early during their hospitalization who are at risk for discharge to PAC facilities. With the exception of LOS, all factors predicting discharge location in this vulnerable population can be identified on admission. Specifically, older patients with functional decline, impaired mobility, and those living alone were more likely to be discharged to a PAC facility or hospice. Because patients discharged to PAC facilities reported a high symptom burden, integrating symptom screening on admission may also help identify a population at risk for discharge to PAC facilities. With high symptom burden and substantial risk of mortality, patients discharged to PAC facilities may experience a potentially burdensome transition of care. By identifying this population early during hospitalization, we can develop supportive care interventions to enhance their quality of care. Interventions such as early assessment of patients’ functional status may help address their impaired mobility and identify those with more intense discharge-planning needs.42-44 Interestingly, we found that consulting palliative care was an important predictor of discharge to hospice versus a PAC facility. Studies have shown that early integrated palliative care improves patient-reported quality of life and mood, and enhances the delivery of EOL care such as length of hospice to patients with advanced cancer.45-47 Thus, early palliative care involvement may help manage patients’ physical and psychological symptoms, as well as enhance goals-of-care conversations to optimize EOL care and potentially decrease the number of burdensome transitions at the EOL.48
However, even with identifying and intervening for patients at risk for PAC facility discharge, many patients with advanced cancer have limited social supports and experience numerous barriers to a home and/or hospice discharge.49,50 Lack of social support at home and high level of care needs may make it impossible to transition to home with hospice services—the only fully funded means for older adults to receive hospice care through Medicare. Patients who desire to pursue supportive care alone but do not have adequate support at home face steep fees for facility-based hospice care. Consequently, the Medicare PAC facility benefit is often the only financially feasible option for a substantial proportion of patients.51 Because PAC facilities lack significant palliative and EOL care expertise,14,18 these patients are thus potentially subject to poor-quality care at the EOL. Alternative care models such as providing more intensive home services may facilitate patients receiving adequate assistance in activities of daily living alongside high-quality hospice care.
This study has several limitations. First, we conducted this study at a single tertiary cancer care center in a patient sample with limited racial or ethnic diversity, which may limit the generalizability of the findings. Second, we examined predictors of discharge location based on the available data. However, other unmeasured factors could confound the relationship between these predictors and discharge location. Third, confounding by indication is an important limitation when examining survival and readmission outcomes for patients discharged to hospice. This was an observational study; therefore, we are unable to comment on patients’ preferences for discharge location. Nonetheless, given the lack of data on survival and readmission outcomes in patients with advanced cancer discharged to a PAC facility, our findings underscore their poor prognosis and need for interventions to address their goals of care and minimize burdensome transitions at the EOL. Last, only a small percentage of our study cohort was discharged to hospice, thereby limiting our ability to conduct more extensive analyses of predictors of discharge to hospice versus a PAC facility. This may have also limited our statistical power to detect meaningful differences in symptom profile and outcomes of patient discharge to a PAC facility versus hospice.
In conclusion, hospitalized patients with advanced cancer who are discharged to PAC facilities and hospice have substantial physical and psychological symptom burdens, impaired physical function, and worse survival compared with those discharged home without hospice. Notably, the physical and psychological symptom burden of patients discharged to a PAC facility was strikingly similar to that of those discharged to hospice, yet these facilities lack the palliative and supportive care infrastructure to optimize the quality of EOL care for this population. Future research should focus on developing targeted interventions to address the functional, social, and symptomatic needs of this population.
Appendix
Fig A1.
Participant flow diagram.
Footnotes
Supported by the Scullen Family Center for Cancer Data Analysis, Conquer Cancer Foundation (D.E.L.), Ghiso Fellowship in Compassionate Care (D.E.L.), and National Institutes of Health Grant No. K24 CA 181253 (J.S.T.).
Presented at the ASCO Annual Meeting, Chicago, IL, June 5, 2017.
AUTHOR CONTRIBUTIONS
Conception and design: Daniel E. Lage, Ryan D. Nipp, P. Connor Johnson, William F. Pirl, Ephraim P. Hochberg, Lara N. Traeger, Vicki A. Jackson, Barbara J. Cashavelly, Joseph A. Greer, David P. Ryan, Jennifer S. Temel, Areej El-Jawahri
Collection and assembly of data: Daniel E. Lage, Ryan D. Nipp, Sara M. D'Arpino, Samantha M. Moran, Risa L. Wong, Holly S. Martinson, Jennifer S. Temel, Areej El-Jawahri
Data analysis and interpretation: Daniel E. Lage, Ryan D. Nipp, Vicki A. Jackson, Joseph A. Greer, Jennifer S. Temel, Areej El-Jawahri
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Predictors of Posthospital Transitions of Care in Patients With Advanced Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Daniel E. Lage
No relationship to disclose
Ryan D. Nipp
No relationship to disclose
Sara M. D'Arpino
No relationship to disclose
Samantha M. Moran
No relationship to disclose
P. Connor Johnson
No relationship to disclose
Risa L. Wong
No relationship to disclose
William F. Pirl
No relationship to disclose
Ephraim P. Hochberg
Stock or Other Ownership: Flatiron Health
Consulting or Advisory Role: Flatiron Health, Intervention Insights
Lara N. Traeger
No relationship to disclose
Vicki A. Jackson
No relationship to disclose
Barbara J. Cashavelly
No relationship to disclose
Holly S. Martinson
No relationship to disclose
Joseph A. Greer
Research Funding: Pfizer (Inst)
David P. Ryan
Stock or Other Ownership: MPM Capital
Honoraria: UpToDate, Research to Practice
Consulting or Advisory Role: MPM Capital
Patents, Royalties, Other Intellectual Property: McGraw Hill Chapter Royalties, Johns Hopkins University Press
Jennifer S. Temel
Research Funding: Pfizer (Inst)
Areej El-Jawahri
No relationship to disclose
REFERENCES
- 1.Institute of Medicine: Dying in America. Washington, DC, The National Academies Press, 2015. [Google Scholar]
- 2.Gozalo P, Teno JM, Mitchell SL, et al. : End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med 365:1212-1221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teno JM, Gozalo PL, Bynum JPW, et al. : Change in end-of-life care for Medicare beneficiaries: Site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA 309:470-477, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright AA, Zhang B, Ray A, et al. : Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 300:1665-1673, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright AA, Keating NL, Balboni TA, et al. : Place of death: Correlations with quality of life of patients with cancer and predictors of bereaved caregivers’ mental health. J Clin Oncol 28:4457-4464, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks GA, Li L, Uno H, et al. : Acute hospital care is the chief driver of regional spending variation in Medicare patients with advanced cancer. Health Aff (Millwood) 33:1793-1800, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks GA, Li L, Sharma DB, et al. : Regional variation in spending and survival for older adults with advanced cancer. J Natl Cancer Inst 105:634-642, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morden NE, Chang CH, Jacobson JO, et al. : End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff (Millwood) 31:786-796, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Earle CC, Landrum MB, Souza JM, et al. : Aggressiveness of cancer care near the end of life: Is it a quality-of-care issue? J Clin Oncol 26:3860-3866, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wennberg JE, Fisher ES, Stukel TA, et al. : Use of hospitals, physician visits, and hospice care during last six months of life among cohorts loyal to highly respected hospitals in the United States. BMJ 328:607, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aragon K, Covinsky K, Miao Y, et al. : Use of the Medicare posthospitalization skilled nursing benefit in the last 6 months of life. Arch Intern Med 172:1573-1579, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ackerly DC, Grabowski DC: Post-acute care reform—Beyond the ACA. N Engl J Med 370:689-691, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Wang S-Y, Aldridge MD, Gross CP, et al. : Transitions between healthcare settings of hospice enrollees at the end of life. J Am Geriatr Soc 64:314-322, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boling PA: Aligning prognosis, patient goals, policy, and care models for palliative care in nursing homes. Arch Intern Med 172:1580-1581, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Baldwin MR, Wunsch H, Reyfman PA, et al. : High burden of palliative needs among older intensive care unit survivors transferred to post-acute care facilities. A single-center study. Ann Am Thorac Soc 10:458-465, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanson LC, Ersek M: Meeting palliative care needs in post-acute care settings: “To help them live until they die”. JAMA 295:681-686, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Johnson VMP, Teno JM, Bourbonniere M, et al. : Palliative care needs of cancer patients in U.S. nursing homes. J Palliat Med 8:273-279, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Ersek M, Carpenter JG: Geriatric palliative care in long-term care settings with a focus on nursing homes. J Palliat Med 16:1180-1187, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burke RE, Juarez-Colunga E, Levy C, et al. : Patient and hospitalization characteristics associated with increased postacute care facility discharges from US hospitals. Med Care 53:492-500, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jette DU, Stilphen M, Ranganathan VK, et al. : AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther 94:1252-1261, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Pavon JM, Sloane R, Morey MC, et al. : Inpatient mobility measures as useful predictors of discharge destination in hospitalized older adults. J Am Geriatr Soc 65:224-226, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe SM, Nekolaichuk C, Beaumont C, et al. : A multicenter study comparing two numerical versions of the Edmonton Symptom Assessment System in palliative care patients. J Pain Symptom Manage 41:456-468, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Bruera E, Kuehn N, Miller MJ, et al. : The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care 7:6-9, 1991 [PubMed] [Google Scholar]
- 24.Rhondali W, Nguyen L, Palmer L, et al. : Self-reported constipation in patients with advanced cancer: A preliminary report. J Pain Symptom Manage 45:23-32, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selby D, Cascella A, Gardiner K, et al. : A single set of numerical cutpoints to define moderate and severe symptoms for the Edmonton Symptom Assessment System. J Pain Symptom Manage 39:241-249, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Hui D, Shamieh O, Paiva CE, et al. : Minimal clinically important difference in the physical, emotional, and total symptom distress scores of the Edmonton Symptom Assessment System. J Pain Symptom Manage 51:262-269, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL, Williams JBW, et al. : An ultra-brief screening scale for anxiety and depression: The PHQ-4. Psychosomatics 50:613-621, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Löwe B, Wahl I, Rose M, et al. : A 4-item measure of depression and anxiety: Validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. J Affect Disord 122:86-95, 2010 [DOI] [PubMed] [Google Scholar]
- 29.El-Jawahri A, Chen Y-B, Brazauskas R, et al. : Impact of pre-transplant depression on outcomes of allogeneic and autologous hematopoietic stem cell transplantation. Cancer 123:1828-1838, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown JR, Conley SM, Niles NW II: Predicting readmission or death after acute ST-elevation myocardial infarction. Clin Cardiol 36:570-575, 2013 . [DOI] [PMC free article] [PubMed]
- 31.Ballen KK, Joffe S, Brazauskas R, et al. : Hospital length of stay in the first 100 days after allogeneic hematopoietic cell transplantation for acute leukemia in remission: Comparison among alternative graft sources. Biol Blood Marrow Transplant 20:1819-1827, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown PM, Ezekowitz JA: Composite end points in clinical trials of heart failure therapy: How do we measure the effect size? Circ Heart Fail 10:e003222, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Glance LG, Li Y, Dick AW: Impact on hospital ranking of basing readmission measures on a composite endpoint of death or readmission versus readmissions alone. BMC Health Serv Res 17:327, 2017 . [DOI] [PMC free article] [PubMed]
- 34.Dombrowski W, Yoos JL, Neufeld R, et al. : Factors predicting rehospitalization of elderly patients in a postacute skilled nursing facility rehabilitation program. Arch Phys Med Rehabil 93:1808-1813, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Manzano J-GM, Gadiraju S, Hiremath A, et al. : Unplanned 30-day readmissions in a general internal medicine hospitalist service at a comprehensive cancer center. J Oncol PR act 11:410-415, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coleman EA, Min S-J, Champak A, et al. : Posthospital care transitions: Patterns, complications, and risk identification. Health Serv Res 39:1449-1465, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasan O, Meltzer DO, Shaykevich SA, et al. : Hospital readmission in general medicine patients: A prediction model. J Gen Intern Med 25:211-219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kansagara D, Englander H, Salanitro A, et al. : Risk prediction models for hospital readmission: A systematic review. JAMA 306:1688-1698, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Donzé J, Lipsitz S, Schnipper JL: Risk factors for potentially avoidable readmissions due to end-of-life care issues. J Hosp Med 9:310-314, 2014 . [DOI] [PubMed]
- 40.Stitzenberg KB, Chang Y, Smith AB, et al. : Exploring the burden of inpatient readmissions after major cancer surgery. J Clin Oncol 33:455-464, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Jawahri A, Keenan T, Abel GA, et al. : Potentially avoidable hospital admissions in older patients with acute myeloid leukaemia in the USA: A retrospective analysis. Lancet Haematol 3:e276-e283, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Laine J, D’Souza A, Siddiqui S, et al. : Rehabilitation referrals and outcomes in the early period after hematopoietic cell transplantation. Bone Marrow Transplant 50:1352-1357, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jette DU, Warren RL, Wirtalla C: The relation between therapy intensity and outcomes of rehabilitation in skilled nursing facilities. Arch Phys Med Rehabil 86:373-379, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Jette DU, Grover L, Keck CP: A qualitative study of clinical decision making in recommending discharge placement from the acute care setting. Phys Ther 83:224-236, 2003 [PubMed] [Google Scholar]
- 45.Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363:733-742, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Temel JS, Greer JA, El-Jawahri A, et al. : Effects of early integrated palliative care in patients with lung and GI cancer: A randomized clinical trial. J Clin Oncol 35:834-841, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greer JA, Pirl WF, Jackson VA, et al. : Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. J Clin Oncol 30:394-400, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Brody AA, Ciemins E, Newman J, et al. : The effects of an inpatient palliative care team on discharge disposition. J Palliat Med 13:541-548, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Barclay JS, Kuchibhatla M, Tulsky JA, et al. : Association of hospice patients’ income and care level with place of death. JAMA Intern Med 173:450-456, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jarosek SL, Shippee TP, Virnig BA: Place of death of individuals with terminal cancer: New insights from Medicare hospice place-of-service codes. J Am Geriatr Soc 64:1815-1822, 2016 [DOI] [PubMed] [Google Scholar]
- 51.Barr P: In the preferred setting? Reimbursement’s role in hospice vs. skilled nursing. Mod Healthc 43:30-31, 2013 [PubMed] [Google Scholar]