Abstract
Purpose
Racial/ethnic disparities in cancer survival in the United States are well documented, but the underlying causes are not well understood. We quantified the contribution of tumor, treatment, hospital, sociodemographic, and neighborhood factors to racial/ethnic survival disparities in California.
Materials and Methods
California Cancer Registry data were used to estimate population-based cancer-specific survival for patients diagnosed with breast, prostate, colorectal, or lung cancer between 2000 and 2013 for each racial/ethnic group (non-Hispanic black, Hispanic, Asian American and Pacific Islander, and separately each for Chinese, Japanese, and Filipino) compared with non-Hispanic whites. The percentage contribution of factors to overall racial/ethnic survival disparities was estimated from a sequence of multivariable Cox proportional hazards models.
Results
In baseline models, black patients had the lowest survival for all cancer sites, and Asian American and Pacific Islander patients had the highest, compared with whites. Mediation analyses suggested that stage at diagnosis had the greatest influence on overall racial/ethnic survival disparities accounting for 24% of disparities in breast cancer, 24% in prostate cancer, and 16% to 30% in colorectal cancer. Neighborhood socioeconomic status was an important factor in all cancers, but only for black and Hispanic patients. The influence of marital status on racial/ethnic disparities was stronger in men than in women. Adjustment for all covariables explained approximately half of the overall survival disparities in breast, prostate, and colorectal cancer, but it explained only 15% to 40% of disparities in lung cancer.
Conclusion
Overall reductions in racial/ethnic survival disparities were driven largely by reductions for black compared with white patients. Stage at diagnosis had the largest effect on racial/ethnic survival disparities, but earlier detection would not entirely eliminate them. The influences of neighborhood socioeconomic status and marital status suggest that social determinants, support mechanisms, and access to health care are important contributing factors.
INTRODUCTION
Cancer mortality rates in the United States are declining, but one in four deaths is still attributable to cancer,1 and the burden on the population is not equal. Disparities in survival by race and ethnicity have been well documented,2,3 but the underlying causes are not well understood. Various factors have been implicated as contributors toward racial/ethnic survival disparities; these include differences in tumor characteristics at presentation,4,5 disease management and treatment,6,7 factors that relate to the health care institution,8 and sociodemographic and neighborhood characteristics.9-11 The influence of these factors varies for different types of cancer and may vary across racial/ethnic groups.
The persistent disparity in survival between non-Hispanic white (NHW) and black patients is particularly stark. For breast and colorectal cancers, this disparity is most commonly attributed to differences in tumor characteristics at diagnosis. Black patients are more likely than NHW patients to have later-stage disease, higher nodal involvement, hormone receptor–negative breast tumors, and cancers of the proximal colon.4,5,12-14 Differences in management and treatment also influence racial/ethnic disparities in survival,6,7,15-17 but these disparities are greatly reduced,18,19 or even eliminated,20 in equal-access health care systems, which suggests that access to care and delivery of recommended treatments are important contributors to racial/ethnic disparities in cancer survival.
More recently, attention has focused on the contribution of social determinants and neighborhood characteristics to racial/ethnic disparities. Survival is known to increase with higher neighborhood socioeconomic status (SES), but it is among those with the highest neighborhood SES that the biggest disparities exist.9 Disparities in survival by health insurance status also differ according to a patient’s racial/ethnic group. Some of the largest black-white survival disparities are found among patients with private insurance, but these disparities are absent among the uninsured, which suggests that the benefits of private health insurance are not experienced equally for all racial/ethnic groups.10 Married cancer patients have consistently lower mortality than unmarried patients,21 but the magnitude of this marriage-related survival advantage also varies: NHW patients gain the greatest benefit, and Asian Americans, the least.11
So far, to our knowledge, no study has systematically considered the relative influence of a defined set of prognostic factors on racial/ethnic disparities in survival across different types of cancer. We investigated racial/ethnic disparities in cancer-specific survival for the four most common cancers in California, and we quantified the contribution of various tumor, sociodemographic, institutional, treatment, and neighborhood factors to disparities in survival.
PATIENTS AND METHODS
Data
California Cancer Registry (CCR) data were used to estimate population-based survival by racial/ethnic group. Analyses included all patients diagnosed in California between 2000 and 2013 with female breast, prostate, colorectal, or lung cancer as a first, primary malignancy. Of the 897,833 cases eligible for inclusion, those diagnosed at autopsy or from death certificates (n = 7,887) and those with unknown follow-up time (n = 5,276) or unknown cause of death (n = 7,008) were excluded.
The vital status of the patient and cause of death were determined by routine linkage to state and national mortality files. Follow-up was computed as the number of days between diagnosis and either the date of death, the date of last known contact, or the end date of follow-up (December 31, 2013). Survival was estimated by using cancer-specific survival,22 and follow-up was censored at the date of death for those who died of a cause other than the primary cancer. Among the 877,662 included cancer cases, there were 222,042 cancer-specific deaths (25%). The validity of cancer-specific survival estimates was confirmed in a sensitivity analysis by using Fine and Gray competing risks regression models.23
Race/ethnicity was classified as NHW, non-Hispanic black (black), Hispanic, or Asian American and Pacific Islander (AAPI).24 The three largest AAPI subgroups by population (Chinese, Japanese, and Filipino) also were examined separately. Approximately 1% of patients had unknown race/ethnicity; results for these patients were not reported. A number of patient characteristics were considered as potential explanatory factors in the relationship between race/ethnicity and survival, and these were classified as either tumor or treatment related, sociodemographic, institutional, or contextual neighborhood characteristics (Table 1).
Table 1.
Covariables Included as Explanatory Factors in Racial/Ethnic Disparities in Survival

Neighborhood factors from the California Neighborhoods Data System25 were based on the census block group of patients’ residence at diagnosis. Neighborhood SES is a composite index developed with principal components analysis of 2000 census or 2007 to 2011 American Community Survey data on education, occupation, employment, household income, poverty, and rent and house values.26 The components are summed, and the composite SES score is categorized according to quintiles of the state-wide distribution.
Statistical Analysis
Multivariable Cox proportional hazards models were used to examine cancer-specific survival by racial/ethnic group (compared with NHW patients) for each cancer site and by sex. Proportionality of hazards for key covariates was tested by examining the correlation between time and scaled Schoenfeld residuals, and by graphically assessing the log-log plots of survival. Because the assumption of proportional hazards was violated for age, Cox models were age-stratified to allow the baseline hazards to vary.
Mediation analysis was conducted to estimate the relative contribution of each covariable to racial/ethnic disparities in survival. The baseline model was defined as race/ethnicity plus age. The influence of each covariable on racial/ethnic survival disparities first was tested in a base model: race/ethnicity plus age plus covariable. Covariables then were ranked in order of their significance of influence on racial/ethnic survival disparities (ie, by how much the hazard ratio [HR] decreased when included in the model). The process was performed separately for each cancer site and sex. As the influence of each covariable on survival disparities differed by racial/ethnic group, a previously developed summary measure was used to describe the relative influence of a covariable on survival disparities across all racial/ethnic groups combined.27 The derived summary measure is the standard deviation of log HR estimates (Cox regression coefficients) for the racial/ethnic groups from the base model, and it is independent of which group is chosen as the reference group.
Covariables then were added to the baseline model in a sequence of multivariable models, in the order of their significance of influence. With each addition to the multivariable model, the change in HR was assessed as a measure of the relative change in disparity (ie, the proportion of the total disparity contributed by that covariable, after accounting for previously added covariables). The model was defined simply as (D− − D+ ÷ D0) × 100, in which D0 is the HR from the baseline model, D− is the HR from the model without the covariable of interest, and D+ is the HR from the model with the covariable of interest. The change in HR was assessed both for each racial/ethnic group and by using the summary measure for all racial/ethnic groups, as described above. All analyses were performed in STATA 14 (STATA, College Station, TX).
RESULTS
The cohort included 264,681 breast cancers, 270,101 prostate cancers, 181,060 lung cancers, and 161,820 colorectal cancers (Table 2 and Appendix Table A1). The racial/ethnic distribution generally was similar for each cancer: 62% to 71% were NHW, 6% to 9% were black, 10% to 17% were Hispanic, and 7% to 13% were AAPI. The majority of NHW and AAPI patients lived in high-SES neighborhoods, and the majority of black and Hispanic, in low-SES neighborhoods. Black, Hispanic, and AAPI patients had a younger age profile than NHW patients, a correspondingly lower proportion had Medicare insurance, and a higher proportion had public or no health insurance.
Table 2.
Distribution of Key Demographic and Clinical Characteristics of Patients With Cancer by Race/Ethnicity and Cancer Site: California, 2000 to 2013

Breast Cancer
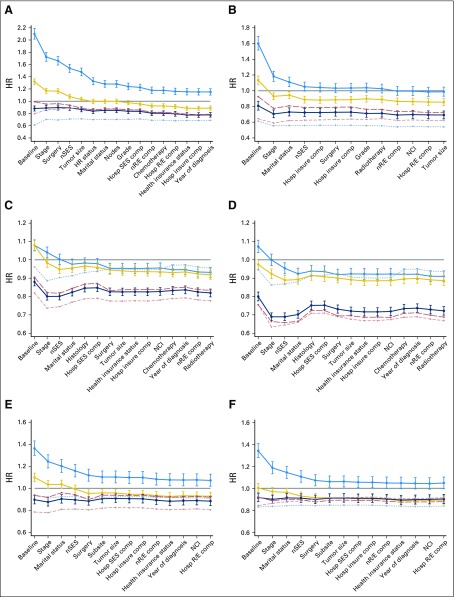
The largest racial/ethnic disparities in survival were among women with breast cancer; cancer-specific mortality in black women was two-fold higher than in NHW women in the baseline model (HR, 2.10; 95% CI, 2.02 to 2.19; Fig 1A). Stage at diagnosis had the greatest influence on overall survival disparities—accounting for 24% of disparities (Table 3)—but its influence varied by racial/ethnic group. Stage explained 11% to 18% of survival disparities for Hispanic and black women relative to NHW women, but it had no effect on the survival advantage experienced by AAPI women. Similarly, neighborhood SES influenced survival disparities in black and Hispanic women (by 6% and 7%, respectively), but not in AAPI women. Hormone receptor status reduced the HR for black and Hispanic women compared with NHW women (by 7% and 2.5%, respectively) but increased the survival advantage for AAPI women (Appendix Table A2, online only). After stage at diagnosis, hormone receptor status had the second largest influence on racial/ethnic disparities in breast cancer survival; it accounted for 9% of the overall disparity (Table 3). In total, adjustment for all covariables explained 54% of the overall disparities in breast cancer survival across all racial/ethnic groups.
Fig 1.
Change in hazard ratios (HRs) with addition of covariables into multivariable models for each racial/ethnic group, by cancer site and sex: (A) breast cancer, (B) prostate cancer, (C) men with lung cancer, (D) woman with lung cancer, (E) men with colorectal cancer, and (F) women with colorectal cancer. Solid gray line, non-Hispanic white (reference); blue line with 95% CIs, non-Hispanic black; yellow line with 95% CIs, Hispanic; dark blue line with 95% CIs, Asian American and Pacific Islander; light red dashed line, Chinese; light gray dotted line, Japanese; red long-dashed line, Filipino. Comp, composition; Hosp insure, hospital insurance composition; Hosp R/E, hospital racial/ethnic composition; Hosp SES, hospital SES composition; HR, hormone receptor; NCI, National Cancer Institute cancer center; nR/E, neighborhood racial/ethnic composition; nSES, neighborhood socioeconomic status.
Table 3.
Percentage Contribution of Covariables to Overall Disparities in Survival Across All Racial/Ethnic Groups by Cancer Site and Sex: California, 2000 to 2013
Prostate Cancer
Cancer-specific mortality among black men with prostate cancer was 60% higher than among NHW men in the baseline model (HR, 1.60; 95% CI, 1.52 to 1.69; Fig 1B). A large proportion of this survival disparity was attributable to differences in stage at diagnosis, which accounted for almost a quarter of overall survival disparities across all racial/ethnic groups (Table 3). An additional 14% was explained by differences in marital status, and 7% was explained by neighborhood SES, though this largely was due to the influence of these factors on survival disparities in black and Hispanic men relative to NHW men (Appendix Table A2). Adjustment for differences in stage at diagnosis, marital status, and neighborhood SES reduced the survival disparity between black and NHW men with prostate cancer to nonsignificant levels. Adjustment for all covariables explained 48% of the overall disparities in prostate cancer survival across all racial/ethnic groups.
Lung Cancer
Racial/ethnic disparities in lung cancer survival were more pronounced in women than in men (Figs 1C and 1D). AAPI patients had significantly lower cancer-specific mortality than NHW patients; this survival advantage was evident for Chinese and Filipino patients but not for Japanese patients, whose cancer-specific mortality was similar to that of NHW patients (Appendix Table A3, online only). Adjusting for differences in stage at diagnosis increased the AAPI survival advantage by 9% to 14% and increased overall survival disparities across all racial/ethnic groups by 17% in men and 30% in women. Differences in neighborhood SES and marital status were the largest contributors to overall survival disparities across all racial/ethnic groups accounting for 18% and 21%, respectively, in men, and 17% and 14%, respectively, in women (Table 3). Tumor histology influenced survival disparities in women to a much greater extent than in men, and it accounted for 19% of the overall survival disparity.
Colorectal Cancer
Cancer-specific mortality among patients with colorectal cancer was 36% higher in black men (HR, 1.36; 95% CI, 1.30 to 1.43) and 34% higher in black women (HR, 1.34; 95% CI, 1.28 to 1.41) in the baseline model compared with NHW patients (Figs 1E and 1F). In women, sequential adjustment for all covariables reduced the black-white survival disparity to nonsignificant levels. AAPI patients had 8% to 10% lower cancer-specific mortality than NHW patients in the baseline model, and adjustment for covariables had little additional effect on this survival advantage in men (HR, 0.88; 95% CI, 0.84 to 0.92) or women (HR, 0.90; 95% CI, 0.86 to 0.94). Of the AAPI subgroups, Chinese men had the lowest cancer-specific mortality (HR, 0.80; 95% CI, 0.74 to 0.87). Stage at diagnosis explained 16% of overall survival disparities across all racial/ethnic groups in men and 28% in women (Table 3). Marital status had a slightly stronger influence on overall survival disparities in men, explaining 16% of disparities compared with 13% in women. Smaller contributions were made by differences in neighborhood SES (5% to 6%), surgery (5%), and tumor subsite (5% to 9%). In total, adjustment for all covariables explained 52% to 55% of overall disparities in colorectal cancer survival across all racial/ethnic groups.
HRs from baseline models estimated by using competing risks regression as an alternative to Cox regression differed by less than 1% for Hispanics and blacks for each of the cancer sites examined. They differed by less than 2% for AAPI patients for each of the cancer sites examined.
DISCUSSION
In a diverse, contemporary, population-based sample of 877,662 patients with cancer, we found continued disparities in survival for breast, prostate, lung, and colorectal cancer across racial/ethnic groups. By using mediation analysis as a novel approach to quantify the contribution of patient and tumor characteristics to racial/ethnic survival disparities, we found that stage at diagnosis, neighborhood SES, and marital status were the most influential factors.
The substantial influence of stage at diagnosis on racial/ethnic survival disparities likely results from differences in both stage distribution and stage-specific survival across racial/ethnic groups. Black patients with breast, prostate, and colorectal cancer were all more likely than NHW patients to be diagnosed with late-stage tumors, and, among those with late-stage tumors, survival was lower (data not shown). The contribution of stage at diagnosis, therefore, is multifaceted. For the most part, stage can be seen as a modifiable risk factor for cancer prognosis, particularly given that established early-detection modalities exist for most of the sites evaluated in this study. However, differences in stage-specific survival suggest that access to recommended treatment, especially for late-stage tumors, also may influence racial/ethnic survival disparities.
Interestingly, the contribution of stage to survival disparities in colorectal cancer was considerably larger in women than in men, and this finding is consistent with previous findings.28 The influence of stage on survival disparities for lung cancer also was greatest in women, but the direction of the effect was different. AAPI patients had a substantial survival advantage, as has been reported previously,29,30 especially for late-stage cancers,31 and adjustment for this increased the overall disparity across all racial/ethnic groups. Stage at diagnosis, therefore, does not explain the racial/ethnic survival disparities in lung cancer reported here.
Hormone receptor status was the second largest contributor to overall racial/ethnic survival disparities for breast cancer, which adds to the evidence base that tumor characteristics at diagnosis are significant mediators of survival disparities, especially among black women.32 Racial/ethnic disparities in breast cancer survival vary considerably according to tumor subtype, however, and are likely to be explained by intrinsic biologic differences in tumors (eg, lymph node involvement, distant metastasis, and triple-negative tumors) rather than simply by earlier detection.33,34
Tumor histology was an important contributing factor to racial/ethnic survival disparities in lung cancer, especially in women, and may reflect a differential distribution of tumor subtypes across racial/ethnic groups. AAPI women had a higher proportion of adenocarcinomas, which have a more favorable prognosis, and a lower proportion of small-cell tumors, for which prognosis is poor. The distribution of tumor subtypes in men did not differ across racial/ethnic groups to the same extent. Only a small percentage of overall racial/ethnic disparities in colorectal cancer survival was explained by anatomic location; black patients had a notably higher proportion of proximal tumors, which are associated with poorer survival than tumors in the distal colon or rectum.35 The receipt of surgery, radiation, or chemotherapy had little additional effect on survival disparities, because these high-level treatment variables are likely to be highly correlated with stage at diagnosis.
Marital status had one of the biggest effects on racial/ethnic survival disparities, and, consistent with the literature, this was most notable among men.11,21 The survival benefit associated with being married often is attributed to increased social support; higher psychological well-being; and instrumental support, such as help in navigation of the health care system.36,37 Interestingly, health insurance status was not a significant contributor of racial/ethnic survival disparities, despite evidence that black-white disparities differ by type of health insurance.10
In this study, neighborhood SES was an important explanatory factor, but its effect was limited exclusively to disparities in survival for black and Hispanic patients relative to NHW patients. AAPI patients have a neighborhood socioeconomic profile similar to NHW patients, and the survival advantage experienced by this group is unlikely to be explained by factors related to SES and more likely to be related to underlying genetic and biologic differences.29 The characteristics of a patient’s neighborhood has the potential to affect cancer survival disparities through a number of mechanisms, including its influence on behavioral risk factors, social support, and access to health care.9,38
Conversely, neighborhood racial/ethnic composition had only negligible effects on racial/ethnic survival disparities. Prior research has shown that residence in ethnic-concordant neighborhoods may confer protective survival effects,39-42 although other studies found opposite effects of residence in high-minority neighborhoods.43-45
The influence of hospital characteristics on racial/ethnic survival disparities also was negligible after analysis had been adjusted for individual tumor and sociodemographic factors. Hospital characteristics often contributed less than 1% toward overall racial/ethnic survival disparities, and this finding is consistent with previous findings.8
After adjustment for a wide variety of patient and tumor characteristics, a large proportion of the overall racial/ethnic survival disparities remained unexplained. The survival advantage experienced by AAPI patients in particular was largely unaffected by the factors investigated. This suggests that we lacked potentially important genetic and tumor information, such as molecular markers known to be prognostic and/or used to determine treatment (eg, EGFR for lung cancer or KRAS for colorectal cancer). We also lacked detailed clinical information about treatment, as well as information about recurrence or disease progression. This study also may be affected by limitations inherent to cancer registry data, such as misclassification of race/ethnicity, although prior research has shown this to be minimal46,47 and validated algorithms are used to improve the classification of Hispanic ethnicity and AAPI race/ethnicity.48,49 Despite these limitations, we were able to leverage a large, population-based data set to quantify the relative contribution of multilevel factors—clinical, patient, hospital, and neighborhood—to racial/ethnic disparities in cancer survival and to demonstrate the importance of modifiable factors and targets for intervention to reduce survival disparities.
In conclusion, stage at diagnosis had the largest effect on racial/ethnic disparities in survival for breast, prostate, and colorectal cancers. Stage is itself influenced by a myriad of factors, which include socioeconomic status, health insurance, uptake of screening, and access to health care. Although earlier detection alone will not entirely eliminate these disparities, strategies to address the low uptake of cancer screening among black and Hispanic populations could make an important contribution. The effect of differences in care after diagnosis was limited, but a more nuanced investigation into the contribution of treatment differences across racial/ethnic groups, with more detailed information than was available in this study, is required. The considerable influence of neighborhood SES and marital status on racial/ethnic disparities in survival, even after analysis is controlled for stage at diagnosis, suggests that social determinants, support mechanisms, and access to health care cannot be overlooked. SES and marital status are not themselves modifiable, but more equitable access to care for underserved groups could substantially reduce racial/ethnic disparities in cancer outcomes. In clinical settings, ensuring that these social determinants are assessed, and that barriers to care for vulnerable populations are addressed, may go a long way toward the reduction of cancer survival disparities.21,36,50,51
ACKNOWLEDGMENT
We thank Richard Sposto (Keck School of Medicine, University of Southern California, Los Angeles, CA) for sharing his STATA code for mediation analysis. The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute SEER program under contract No. HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract No. HHSN261201000035C awarded to the University of Southern California, and contract No. HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention National Program of Cancer Registries under agreement No. U58DP003862-01 awarded to the California Department of Public Health.
Appendix
Table A1.
Distribution of Additional Demographic and Clinical Characteristics of Patients With Cancer by Race/Ethnicity and Cancer Site: California, 2000 to 2013
Table A2.
HRs With Addition of Covariables Into Multivariable Models, by Cancer Site, Sex, and Racial/Ethnic Group

Table A3.
HRs With Addition of Covariables Into Multivariable Models, by Cancer Site, Sex, and AAPI Subgroup

Footnotes
Supported by a 2016 Translational Research Award from the Stanford Cancer Institute and by the National Cancer Institute SEER program under contract No. HHSN261201000140C awarded to the Cancer Prevention Institute of California.
The funders had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The ideas and opinions expressed herein are those of the author(s), and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
See accompanying Editorial on page 1
AUTHOR CONTRIBUTIONS
Conception and design: Libby Ellis, David Spiegel, Uri Ladabaum, Robert Haile, Scarlett Lin Gomez
Collection and assembly of data: Libby Ellis, Alison J. Canchola, Scarlett Lin Gomez
Financial support: Scarlett Lin Gomez
Administrative support: Scarlett Lin Gomez
Provision of study materials or patients: Scarlett Lin Gomez
Data analysis and interpretation: Libby Ellis, Alison J. Canchola, Scarlett Lin Gomez
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Racial and Ethnic Disparities in Cancer Survival: The Contribution of Tumor, Sociodemographic, Institutional, and Neighborhood Characteristics
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Libby Ellis
No relationship to disclose
Alison J. Canchola
Employment: Roche Molecular Diagnostics (I)
David Spiegel
No relationship to disclose
Uri Ladabaum
Consulting or Advisory Role: Covidien/Medtronic, Quorum
Research Funding: Exact Sciences
Robert Haile
No relationship to disclose
Scarlett Lin Gomez
No relationship to disclose
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al.: SEER Cancer Statistics Review (CSR) 1975-2013. http://seer.cancer.gov/csr/1975_2013/
- 2.Clegg LX, Li FP, Hankey BF, et al. : Cancer survival among US whites and minorities: A SEER Program population-based study. Arch Intern Med 162:1985-1993, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Aizer AA, Wilhite TJ, Chen MH, et al. : Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer 120:1532-1539, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Wu AH, Gomez SL, Vigen C, et al. : The California Breast Cancer Survivorship Consortium (CBCSC): Prognostic factors associated with racial/ethnic differences in breast cancer survival. Cancer Causes Control 24:1821-1836, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai Y, Wang C, Civan JM, et al. : Effects of cancer stage and treatment differences on racial disparities in survival from colon cancer: A United States population-based study. Gastroenterology 150:1135-1146, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris AM, Rhoads KF, Stain SC, et al. : Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg 211:105-113, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Shavers VL, Brown ML: Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst 94:334-357, 2002 [DOI] [PubMed] [Google Scholar]
- 8.White A, Vernon SW, Franzini L, et al. : Racial disparities in colorectal cancer survival: To what extent are racial disparities explained by differences in treatment, tumor characteristics, or hospital characteristics? Cancer 116:4622-4631, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kish JK, Yu M, Percy-Laurry A, et al. : Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in Surveillance, Epidemiology, and End Results (SEER) registries. J Natl Cancer Inst Monogr 2014:236-243, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan HY, Walker GV, Grant SR, et al. : Insurance status and racial disparities in cancer-specific mortality in the United States: A population-based analysis. Cancer Epidemiol Biomarkers Prev 26:869-875, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martínez ME, Anderson K, Murphy JD, et al. : Differences in marital status and mortality by race/ethnicity and nativity among California cancer patients. Cancer 122:1570-1578, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silber JH, Rosenbaum PR, Clark AS, et al. : Characteristics associated with differences in survival among black and white women with breast cancer. JAMA 310:389-397, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Silber JH, Rosenbaum PR, Ross RN, et al. : Racial disparities in colon cancer survival: A matched cohort study. Ann Intern Med 161:845-854, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Mitchell EP, Topham A, Singla R, et al: Colorectal cancer in African American and Caucasian patients: A comparison of an urban, university hospital with the National Cancer Institute SEER database. J Clin Oncol 29, 2011 (suppl 15; abstr 3631) [Google Scholar]
- 15.Pietro GD, Chornokur G, Kumar NB, et al: Racial differences in the diagnosis and treatment of prostate cancer. Int Neurourol J 20:S112-S119, 2016 (suppl 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bach PB, Cramer LD, Warren JL, et al. : Racial differences in the treatment of early-stage lung cancer. N Engl J Med 341:1198-1205, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Taioli E, Flores R: Appropriateness of surgical approach in black patients with lung cancer: 15 Years later, little has changed. J Thorac Oncol 12:573-577, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Optenberg SA, Thompson IM, Friedrichs P, et al. : Race, treatment, and long-term survival from prostate cancer in an equal-access medical care delivery system. JAMA 274:1599-1605, 1995 [PubMed] [Google Scholar]
- 19.Graham-Steed T, Uchio E, Wells CK, et al. : ‘Race’ and prostate cancer mortality in equal-access healthcare systems. Am J Med 126:1084-1088, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams CD, Salama JK, Moghanaki D, et al. : Impact of race on treatment and survival among US veterans with early-stage lung cancer. J Thorac Oncol 11:1672-1681, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Aizer AA, Chen MH, McCarthy EP, et al. : Marital status and survival in patients with cancer. J Clin Oncol 31:3869-3876, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marubini E, Valsecchi MG: Analysing Survival Data From Clinical Trials and Observational Studies. New York, John Wiley and Sons, 1995 [Google Scholar]
- 23.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496-509, 1999 [Google Scholar]
- 24. https://www.whitehouse.gov/omb/fedreg_1997standards Office of Management and Budget: Revisions to the standards for the classification of federal data on race and ethnicity.
- 25.Gomez SL, Glaser SL, McClure LA, et al. : The California Neighborhoods Data System: A new resource for examining the impact of neighborhood characteristics on cancer incidence and outcomes in populations. Cancer Causes Control 22:631-647, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Schupp CW, Harrati A, et al. Developing an Area-Based Socioeconomic Measure From American Community Survey Data. Fremont, CA, Cancer Prevention Institute of California, 2014 [Google Scholar]
- 27.Sposto R, Keegan THM, Vigen C, et al. : The effect of patient and contextual characteristics on racial/ethnic disparity in breast cancer mortality. Cancer Epidemiol Biomarkers Prev 25:1064-1072, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valeri L, Chen JT, Garcia-Albeniz X, et al. : The role of stage at diagnosis in colorectal cancer black-white survival disparities: A counterfactual causal inference approach. Cancer Epidemiol Biomarkers Prev 25:83-89, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trinh QD, Nguyen PL, Leow JJ, et al: Cancer-specific mortality of Asian Americans diagnosed with cancer: A nationwide population based assessment. J Natl Cancer Inst 107(6):djv054, 2015 [Google Scholar]
- 30.Ou SH, Zell JA, Ziogas A, et al. : Prognostic factors for survival of stage I nonsmall cell lung cancer patients: A population-based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer 110:1532-1541, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Zell JA, Ou SH, Ziogas A, et al. : Survival improvements for advanced stage nonbronchioloalveolar carcinoma-type nonsmall cell lung cancer cases with ipsilateral intrapulmonary nodules. Cancer 112:136-143, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Warner ET, Tamimi RM, Hughes ME, et al. : Racial and ethnic differences in breast cancer survival: Mediating effect of tumor characteristics and sociodemographic and treatment factors. J Clin Oncol 33:2254-2261, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao L, Gomez SL, Keegan TH, et al. : Breast cancer mortality in African-American and non-Hispanic white women by molecular subtype and stage at diagnosis: A population-based study. Cancer Epidemiol Biomarkers Prev 24:1039-1045, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iqbal J, Ginsburg O, Rochon PA, et al. : Differences in breast cancer stage at diagnosis and survival by race/ethnicity in the US. JAMA 313:165-173, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Wray CM, Ziogas A, Hinojosa MW, et al. : Tumor subsite location within the colon is prognostic for survival after colon cancer diagnosis. Dis Colon Rectum 52:1359-1366, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Gomez SL, Hurley S, Canchola AJ, et al. : Effects of marital status and economic resources on survival after cancer: A population-based study. Cancer 122:1618-1625, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinquart M, Duberstein PR: Associations of social networks with cancer mortality: A meta-analysis. Crit Rev Oncol Hematol 75:122-137, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parise CA, Caggiano V: Disparities in race/ethnicity and socioeconomic status: Risk of mortality of breast cancer patients in the California Cancer Registry, 2000-2010. BMC Cancer 13:449, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warner ET, Gomez SL: Impact of neighborhood racial composition and metropolitan residential segregation on disparities in breast cancer stage at diagnosis and survival between black and white women in California. J Community Health 35:398-408, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schupp CW, Press DJ, Gomez SL: Immigration factors and prostate cancer survival among Hispanic men in California: Does neighborhood matter? Cancer 120:1401-1408, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomez N, Guendelman S, Harley KG, et al. : Nativity and neighborhood characteristics and cervical cancer stage at diagnosis and survival outcomes among Hispanic women in California. Am J Public Health 105:538-545, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel MI, Schupp CW, Gomez SL, et al. : How do social factors explain outcomes in non-small-cell lung cancer among Hispanics in California? Explaining the Hispanic paradox. J Clin Oncol 31:3572-3578, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell E, Kramer MR, Cooper HL, et al. : Residential racial composition, spatial access to care, and breast cancer mortality among women in Georgia. J Urban Health 88:1117-1129, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russell EF, Kramer MR, Cooper HL, et al. : Metropolitan area racial residential segregation, neighborhood racial composition, and breast cancer mortality. Cancer Causes Control 23:1519-1527, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Pruitt SL, Lee SJ, Tiro JA, et al. : Residential racial segregation and mortality among black, white, and Hispanic urban breast cancer patients in Texas, 1995 to 2009. Cancer 121:1845-1855, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gomez SL, Glaser SL: Misclassification of race/ethnicity in a population-based cancer registry (United States). Cancer Causes Control 17:771-781, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Clegg LX, Reichman ME, Hankey BF, et al. : Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: Implications for health disparity studies. Cancer Causes Control 18:177-187, 2007 [DOI] [PubMed] [Google Scholar]
- 48.North American Association of Central Cancer Registries Race and Ethnicity Work Group: NAACCR Asian/Pacific Islander Identification Algorithm [NAPIIA v1.2.1]: Enhancing the Specificity of Identification. Springfield, IL, North American Association of Central Cancer Registries 2011 [Google Scholar]
- 49.North American Association of Central Cancer Registries Race and Ethnicity Work Group : NAACCR Guideline for Enhancing Hispanic-Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2.2.1]. Springfield, IL, North American Association of Central Cancer Registries, 2011 [Google Scholar]
- 50.Kissane DW: Marriage is as protective as chemotherapy in cancer care. J Clin Oncol 31:3852-3853, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Paskett ED: The new vital sign: Where do you live? Cancer Epidemiol Biomarkers Prev 25:581-582, 2016 [DOI] [PubMed] [Google Scholar]