Abstract
Purpose
We aimed to predict individual risk of ischemic heart disease and stroke in 5-year survivors of childhood cancer.
Patients and Methods
Participants in the Childhood Cancer Survivor Study (CCSS; n = 13,060) were observed through age 50 years for the development of ischemic heart disease and stroke. Siblings (n = 4,023) established the baseline population risk. Piecewise exponential models with backward selection estimated the relationships between potential predictors and each outcome. The St Jude Lifetime Cohort Study (n = 1,842) and the Emma Children’s Hospital cohort (n = 1,362) were used to validate the CCSS models.
Results
Ischemic heart disease and stroke occurred in 265 and 295 CCSS participants, respectively. Risk scores based on a standard prediction model that included sex, chemotherapy, and radiotherapy (cranial, neck, and chest) exposures achieved an area under the curve and concordance statistic of 0.70 and 0.70 for ischemic heart disease and 0.63 and 0.66 for stroke, respectively. Validation cohort area under the curve and concordance statistics ranged from 0.66 to 0.67 for ischemic heart disease and 0.68 to 0.72 for stroke. Risk scores were collapsed to form statistically distinct low-, moderate-, and high-risk groups. The cumulative incidences at age 50 years among CCSS low-risk groups were < 5%, compared with approximately 20% for high-risk groups (P < .001); cumulative incidence was only 1% for siblings (P < .001 v low-risk survivors).
Conclusion
Information available to clinicians soon after completion of childhood cancer therapy can predict individual risk for subsequent ischemic heart disease and stroke with reasonable accuracy and discrimination through age 50 years. These models provide a framework on which to base future screening strategies and interventions.
INTRODUCTION
There are > 400,000 survivors of childhood cancer in the United States.1 Cardiovascular disease is the leading noncancer contributor to early morbidity and mortality in this population.2-7 On average, survivors of childhood cancer have been shown to have a ≥ 10-fold increased risk of ischemic heart disease and stroke compared with siblings.3,8 However, these averages mask significant variation, with some groups experiencing cumulative incidences ≥ 10% of having one of these events by middle age and other groups having incidences not dissimilar to those of siblings without a history of childhood cancer (< 2%).8-10 This variation is likely attributable to cancer treatment exposures such as radiotherapy and chemotherapy, genetic predisposition, and conventional cardiovascular risk factors such as hypertension, dyslipidemia, and diabetes.
Cardiovascular disease risk predictors play a prominent role in clinical decision making in the general population among older adults.11 Given the increased incidence of cardiovascular disease among young adult survivors of childhood cancer, this high-risk population may benefit from early assessment with validated risk prediction models that include cancer treatment exposures. Therefore, our goal was to use the Childhood Cancer Survivor Study (CCSS) cohort to create customized models that incorporate demographic and cancer treatment information available at the end of therapy to predict subsequent ischemic heart disease and stroke risk among 5-year survivors and then to externally validate the resulting risk scores. We recently applied this approach to develop models that predict cardiomyopathy and heart failure among survivors of childhood cancer.12 The development of additional disease prediction models for ischemic heart disease and stroke in this population may further help clinicians refine surveillance strategies to better identify and counsel patients at higher risk of these future events and refine the choices for new cancer treatment protocols.
PATIENTS AND METHODS
Primary Study Population
The CCSS methodology and participant accrual have been reported previously.13 For this analysis, we included individuals diagnosed with the most common types of childhood cancer before age 21 years from 26 institutions in the United States and Canada between 1970 and 1986 and who survived at least 5 years after diagnosis. Our analytic cohort excluded patients who did not provide consent for medical records abstraction (n = 1,110) and those who experienced a major cardiovascular event (ischemic heart disease, stroke, heart failure, or any cardiovascular death) within 5 years of their initial cancer diagnosis (n = 188), leaving 13,060 patients (91%) from the original cohort available for analysis. A random sample of siblings of participating CCSS survivors was recruited and served as a comparison population (n = 4,023). The protocol was approved by the human subjects committee at each institution. Participants provided informed consent.
Cancer Therapy Exposures in the CCSS
Chemotherapy, surgery, and radiotherapy information for the first 5 years after initial cancer diagnosis was abstracted from medical records. This includes conditioning regimen exposures for patients treated with hematopoietic stem-cell transplantation. Radiotherapy records were centrally reviewed, and exposures to the brain, neck, chest, and abdomen were categorized as yes or no (yes if at least part of the region was in the direct treatment field), with field-specific maximum total doses calculated for the brain, chest, and abdomen separately.14 Patients treated with total-body irradiation had all body regions defined as exposed, with the total-body irradiation dose included as part of each field-specific maximum dose. Chest fields included any abdominal treatment that extended to the lower part of the chest (ie, above the diaphragm) and also treatments directed at the thorax (eg, shoulders, ribs, and/or supraclavicular areas), even if the central chest was not a target. In defining dose-specific exposures for each region, radiation scatter from adjacent fields also was noted; these exposures were categorized as < 5 Gy. Heart-specific absorbed doses were estimated by applying water phantom measurements to three-dimensional mathematical phantoms that simulate a patient of any age or size.14
Outcome Definitions in the CCSS
CCSS participants completed a baseline questionnaire covering demographic characteristics, health care utilization, health conditions, and health-related behaviors and were prospectively observed with periodic questionnaires (available at the CCSS Web site). Proxy responses from family members were used for 5-year survivors who had subsequently died, were younger than age 18, or were unable to complete the questionnaires. The cohort was also linked with the National Death Index to ascertain deaths and causes of deaths, from which we identified deaths caused by ischemic heart disease (International Classification of Diseases [ICD], 9th revision, codes 410 to 411, 413 to 414, 427.5, and 440) and stroke (ICD-9 codes 430 to 434, 436, 437 to 438, and 444); equivalent ICD-10 codes also were used.
Using previously described methodology to define any self-reported ischemic heart disease and stroke,3,9 baseline and subsequent questionnaire items related to these outcomes, including information on medications, were classified and graded using the Common Terminology Criteria for Adverse Events (version 4.03).15 Only those outcomes graded as severe or disabling (grade 3: ischemic heart disease requiring antianginal medications), life threatening (grade 4: ischemic heart disease requiring revascularization; any stroke or cerebrovascular accident), or fatal (grade 5) were included. If insufficient information was available to distinguish between grades, the lower grade was applied. Outcomes were limited to those occurring by age 50 years given the limited number of events after that age. Information on related cardiovascular conditions such as obesity, hypertension, dyslipidemia, diabetes, and smoking status was generally unavailable at the prediction time point (5 years from cancer diagnosis).
Statistical Analysis
Similar to our previous cardiomyopathy prediction modeling,12 exposures selected a priori to be examined in our prediction models included sex, age at cancer diagnosis (5-year increments), alkylating agents, anthracyclines, platinum agents, vinca alkaloids, and cranial, neck, chest, and abdominal radiotherapy. The following prediction models were created for different clinical scenarios: a simple model where cancer therapy–related exposures are categorized as yes or no only; and a standard model where clinical dose information is known. For ischemic heart disease only, we also examined the performance of a model on the basis of average radiation dose to the heart in lieu of chest field dose because some contemporary radiation plans provide heart-specific dosimetry.
Piecewise exponential models, adjusted for current age, estimated the relationships between the selected exposures listed earlier and ischemic heart disease and stroke. Backward selection using the likelihood ratio test determined a set of influential predictors with P < .05 adjusted for sex and age at cancer diagnosis.16 Regression coefficient estimates of covariates that remained, and those associated with sex and diagnosis age, were converted to integer risk scores for ease of summing in subsequent risk models (rate ratios of < 1.3, 1.3 to 1.9, 2.0 to 2.9, 3.0 to 4.9, and ≥ 5.0 corresponded to risk scores of 0, 1, 2, 3, and 4, respectively).17 Cox regression models estimated a model’s discriminatory or predictive power on the basis of the area under the receiver operating characteristic curve (AUC) at age 50 years and the concordance (C) statistic (representing the weighted average AUC from study start through age 50 years), with values close to 0.5 suggesting a model does no better than chance and values approaching 1 equating to perfect discrimination or prediction.18,19 Values ≥ 0.7 are considered reasonable, and those ≥ 0.8 are considered excellent. To minimize overfitting, the backward selection process and the reported C-statistics and AUCs were internally cross-validated using 10 random partitions of the CCSS cohort.20
Risk scores were then summed to create low-, moderate-, and high-risk groups for each outcome on the basis of the absolute risks (cumulative incidence at age 50 years)21 and rate ratios compared with siblings (piecewise exponential models)22 associated with each integer risk score value. The risk groupings were designed such that each group ideally was significantly distinct from both siblings and the adjacent risk group (P < .05). Further methodologic details, including software used, are provided in the Appendix (online only).
External Validation Cohorts
We used two well-established childhood cancer cohorts to validate the CCSS prediction models (Table 1; Appendix). The St Jude Lifetime Cohort Study (SJLIFE) data set featured 1,842 survivors and was used to validate the ischemic heart disease and stroke prediction models.6,23,24 The Emma Children’s Hospital and Academic Medical Center (EKZ/AMC) data set featured 1,362 survivors and was used to validate the stroke models only.25,26 Only six ischemic heart disease events were ascertained in the EKZ/AMC data set, which was insufficient for validation.5 In contrast to CCSS, both of these cohorts ascertained targeted outcomes via medical records supplemented by death records. SJLIFE participants and most EKZ/AMC participants also had prospective clinical assessments. For each validation group, outcomes were restricted to Common Terminology Criteria for Adverse Events grade ≥ 3 conditions.
Table 1.
Characteristics of the Study Cohorts
AUCs and C-statistics (at or through age 50 years) for the relevant outcomes were estimated for each validation cohort on the basis of the CCSS risk scores. Each individual in these cohorts was then categorized into the appropriate CCSS-based risk groupings, and the resulting cumulative incidence of the applicable outcome was plotted and compared against those derived from the CCSS.
RESULTS
Within the CCSS cohort, 35.1% of participants received cranial radiotherapy, whereas 25.9% received chest radiotherapy excluding scatter < 5 Gy (Table 2). After a median follow-up time of 19 years (range, 0 to 34 years upon cohort entry), by age 50 years, 265 CCSS participants had ischemic heart disease (7.7% cumulative incidence; 95% CI, 6.3% to 9.1%), and 295 had a stroke (6.3% cumulative incidence; 95% CI, 5.1% to 7.5%). In comparison, siblings had cumulative incidences at age 50 years of 1.2% (95% CI, 0.4% to 2.0%) for ischemic heart disease (n = 26) and 1.1% (95% CI, 0.4% to 1.7%) for stroke (n = 19). Notably, when conventional cardiovascular risk factors such as obesity, hypertension, diabetes, dyslipidemia, and current smoking status were first assessed among CCSS survivors (n = 13,060; median of 15 years after cancer diagnosis and at age 23 years), they were generally uncommon, with rates of 12.1%, 3.4%, 0.8%, 0.2%, and 13.2%, respectively.
Table 2.
Demographic and Clinical Characteristics of ≥ 5-Year Survivors of Childhood Cancer
Piecewise exponential modeling using backward selection identified a set of influential predictors available at the 5-year cancer survival time point for each risk model, from which corresponding scoring tables were created (Table 3; Appendix Table A1, online only). The resulting AUCs for CCSS-derived integer risk scores at or through age 50 years ranged from 0.68 to 0.70 for ischemic heart disease and 0.63 to 0.66 for stroke, with the standard models performing modestly better than the simple models (Table 3). If average radiation dose to the heart was substituted for clinical dose to the chest, the AUC and C-statistic associated with ischemic heart disease were similar, at 0.71 and 0.70, respectively (data not shown). Platinum agent exposure was selected as a significant predictor for stroke in the simple model but not in the stroke standard model. Platinum agents were used more commonly to treat CNS tumors compared with other histologies (8.5% v 4.7%, respectively; P < .001). Finally, because alkylator exposure was also predictive for stroke, we investigated the presence of statistical interaction between alkylator exposure and cranial radiation for stroke but did not find any meaningful effect modification (P = .34 to .77).
Table 3.
Integer Risk Scores Associated With Each Cardiovascular Outcome and Corresponding Prediction Model
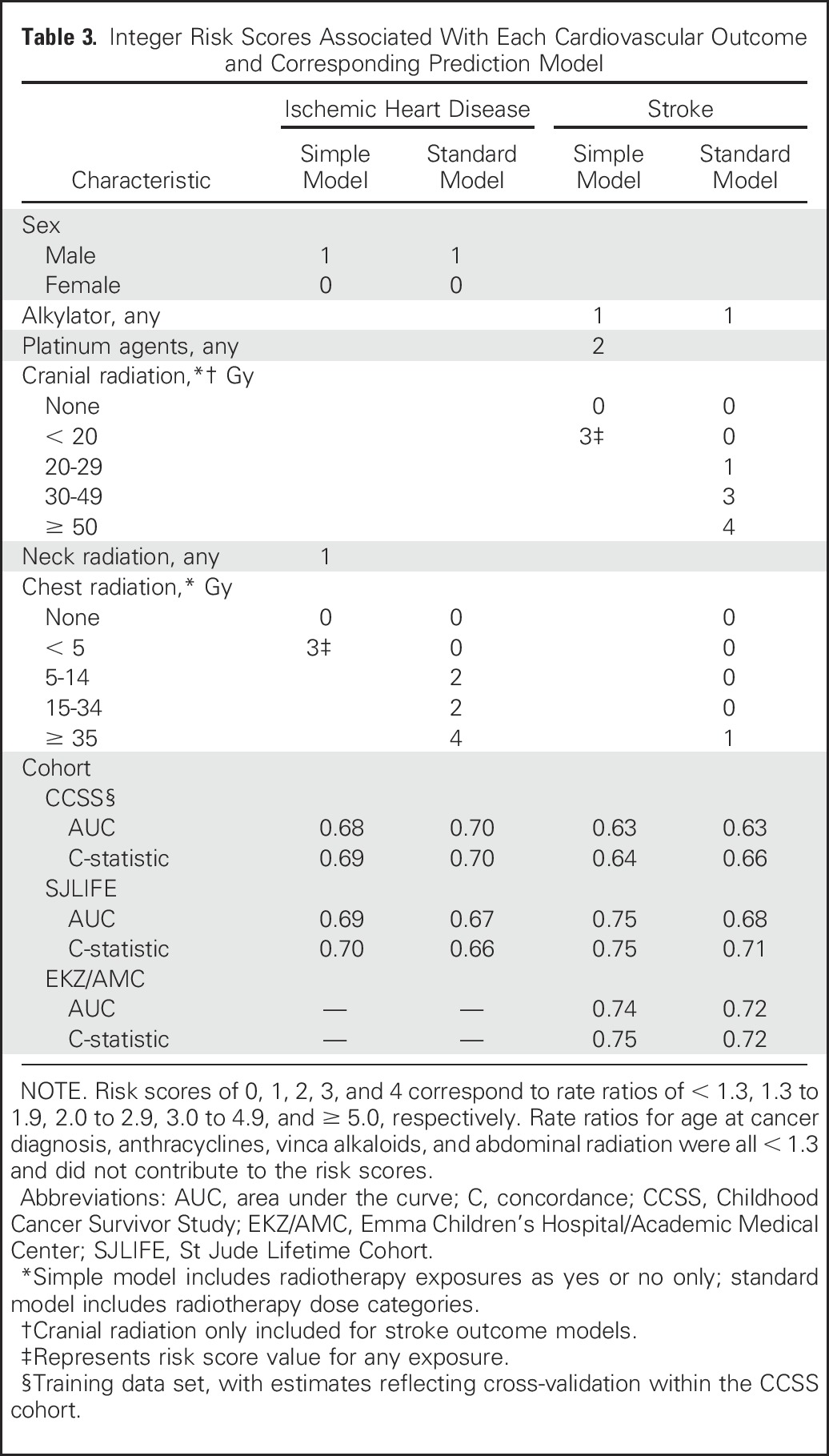
In general, AUCs and C-statistics were comparable, suggesting that estimates were stable at least through age 50 years (Table 3). As part of this process, we also updated our previously published models for cardiomyopathy and heart failure through age 40 years12 to now show estimates through age 50 years (Appendix Table A2, online only). Application of CCSS-based risk scores to the external cohorts showed that when compared with the CCSS results, the AUCs and C-statistics were similar, if not slightly better, when applied to the SJLIFE and EKZ/AMC cohorts.
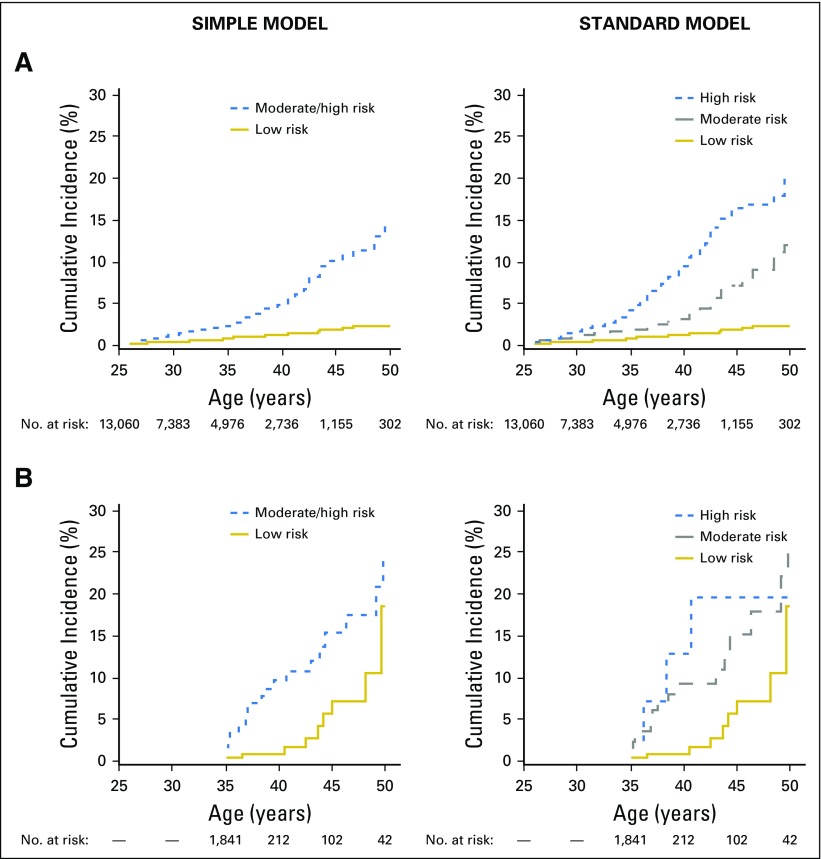
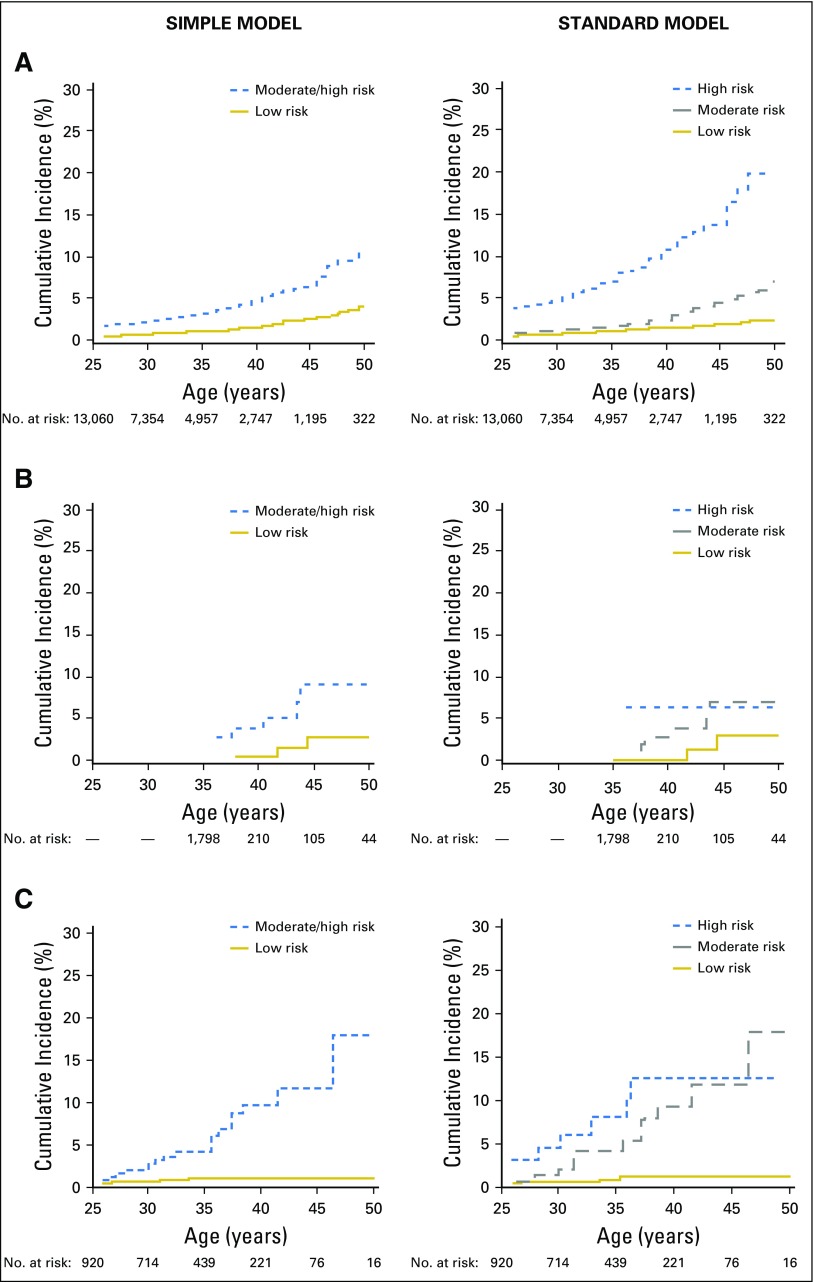
Risk scores were then summed for each individual, and the corresponding cumulative incidence and rate ratio associated with each risk score value were estimated using rates among siblings as the reference group. Summed risk scores that shared similar relative and absolute rates were then grouped together to form low-, moderate-, and high-risk groups (Table 4; similar results for cardiomyopathy and heart failure extended to age 50 years are listed in Appendix Table A3, online only). The low-risk groups tended to have cumulative incidences at age 50 of < 5%, whereas the high-risk groups tended to have cumulative incidences of ≥ 15%. For the ischemic heart disease and stroke simple models, given the narrower range of possible scores, only two groups were defined—a low-risk group and a combined moderate-/high-risk group. For all comparisons, the lowest survivor risk group still had a significantly greater rate ratio relative to siblings (P < .001). In addition, all survivor risk groups were statistically distinct from each other (P < .01). The CCSS risk groupings were able to segregate low versus higher risk groupings within the SJLIFE and EKZ/AMC cohorts (Figs 1 and 2; Appendix Table A4, online only). However, possibly because of the small numbers of survivors who remained at risk at older ages, moderate- and high-risk curves in the SJLIFE and EKZ/AMC cohorts tended to overlap after age 40 years.
Table 4.
Classification of Cardiovascular Event Risk and Empirical Cumulative Incidence and Rate Ratios Within the Childhood Cancer Survivor Study Cohort Based on Summed Risk Scores
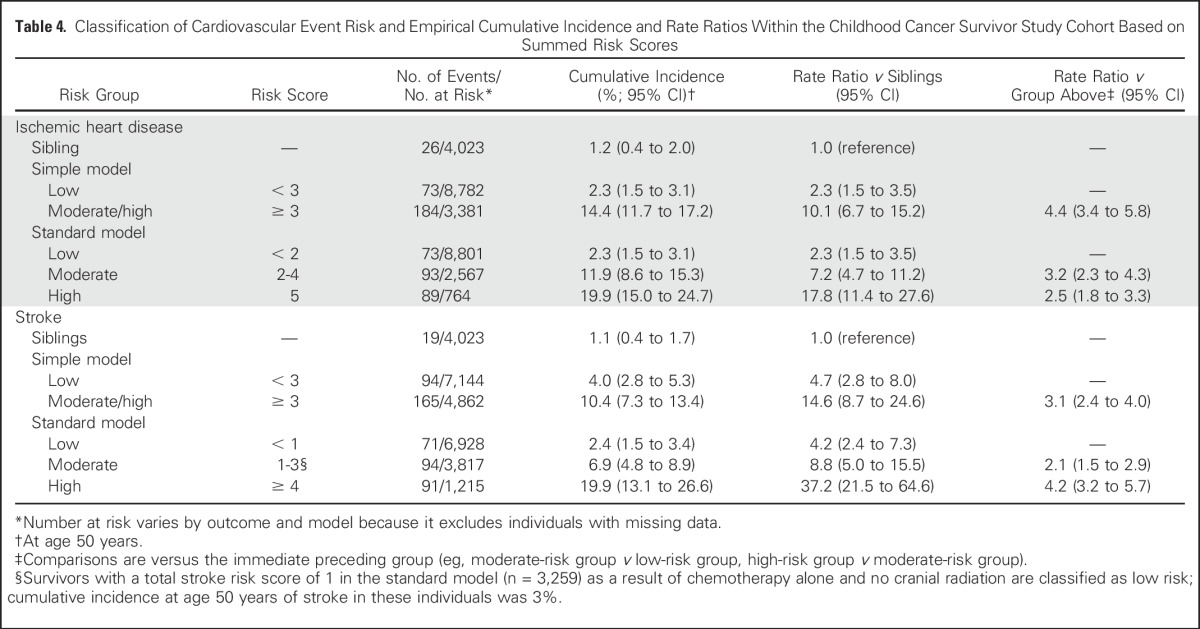
Fig 1.
Cumulative incidence of ischemic heart disease by risk group for the (A) Childhood Cancer Survivor Study (n = 13,060 at baseline) and (B) St Jude Lifetime (n = 1,842 at baseline) cohorts. Curves start when all eligible cohort members have entered follow-up (Childhood Cancer Survivor Study at age 26 years; St Jude Lifetime at age 35 years). As such, initial values shown may be > 0%.
Fig 2.
Cumulative incidence of stroke by risk group for the (A) Childhood Cancer Survivor Study (n = 13,060 at baseline), (B) St Jude Lifetime (n = 1,842 baseline), and (C) Emma Children’s Hospital/Academic Medical Center (n = 1,362 at baseline) cohorts. Curves start when all eligible cohort members have entered follow-up (age 26 years, except for St Jude Lifetime Cohort, which starts at age 35 years). As such, initial values shown may be > 0%.
DISCUSSION
We used data from some of the largest, best-characterized cohorts of survivors of childhood cancer available internationally to develop reasonably robust ischemic heart disease and stroke risk prediction models for 5-year survivors of childhood cancer through age 50 years. The treatment combinations we examined continue to be widely used in current pediatric oncology protocols.27,28 The data needed to produce these estimates are all elements of basic cancer survivorship care plans recommended by the Institute of Medicine.29 As was shown in our prior heart failure models, even our simple models, which require only knowing the presence or absence of exposures, were able to segregate survivors into lower and higher risk groups. Several hypothetical patient scenarios are provided in the Appendix; an online calculator also is available at the CCSS Web site (ccss.stjude.org/cvcalc).
Prior research, now incorporated into national guidelines, has identified significant clinical risk factors for cardiovascular disease after cancer therapy.2,30 These primarily feature radiation to the heart as a risk factor for ischemic heart disease, heart failure, and stroke; radiation to the brain and neck as a risk factor for stroke; and exposure to anthracyclines and related chemotherapy as risk factors for cardiomyopathy and heart failure. Risk may also vary slightly by sex, with men known to have an increased risk of ischemic heart disease in the general population (also seen in our analysis of survivors of cancer).11 In addition, some research suggests that female survivors may be at greater risk of anthracycline-related cardiomyopathy.12
Although the purpose of our analysis was not to explore potential etiologic associations, but to identify those exposures predictive of future events, alkylating and similar DNA interstrand cross-linking (ie, platinum-based) agents were identified as additional predictors for stroke. Agents such as cisplatin have been associated with acute ischemic stroke, possibly as a result of release of prothrombotic complexes after administration.30a However, it is more likely that cisplatin may be acting as an additional proxy for brain tumor treatment, which is more likely to feature these agents compared with treatment of other cancers in our cohort. Survivors of brain tumors have a well-established increased risk of stroke compared with survivors of other cancers.9,10 As with exposure to cisplatin, exposure to alkylators could also be a marker of more aggressive therapy for patients with brain tumor during the CCSS treatment era.27,32 Alkylators can also enhance the risk for hypogonadism, which has been associated with an increased risk of stroke.31,33 However, data from studies of survivors of cancer are mixed.34,35
In general, the predictive influence of chemotherapy agents in our models pales in comparison with radiotherapy exposure. Newer radiotherapy delivery methods may allow more sparing of tissue outside the tumor field, which may reduce cardiovascular and other organ toxicity.36 Although many of the historic treatment combinations featured in CCSS are still being used,27,28 our models may need to be revised as newer radiotherapy technologies and newer, hopefully less cardiotoxic, chemotherapeutic agents become more widely available. Inclusion of robust genetic markers may also further improve discrimination, although at present, these have not become common, even for the general population.37
Finally, cancer treatment may also precipitate the development of conditions that predispose toward poorer cardiovascular health (eg, neuropathy that leads to decreased physical activity and obesity and eventually manifesting as hypertension and insulin resistance).2 This may explain the increased risk observed among our low-risk group compared with siblings as well as in other studies of survivors of cancer.38 The distribution of these conditions also varies regionally and internationally, which may partially explain the variable cumulative incidences of ischemic heart disease and stroke among our different study cohorts, particularly with increasing age.39,40 Because many of these conditions may take years, if not decades, to manifest, we cannot account for them when our prediction time point is based at 5-year cancer survival, when most survivors are still young and rarely affected. Assessing the added discriminatory power of conventional cardiovascular risk factors such as hypertension, dyslipidemia, and diabetes across a range of age time points after cancer treatment may further refine prediction as survivors age. Most general population cardiovascular risk predictors in common use only provide estimates for individuals who are at least 30 or 40 years of age.11,41
There are several other important considerations when interpreting our results. CCSS outcomes, aside from death, are based on self-report. However, prediction was similar, if not slightly better, in our validation cohorts, which both featured outcomes ascertained via medical records and prospective clinical assessment, supporting the CCSS phenotype and methodology. Compared with our previously published heart failure models12 and our ischemic heart disease models, discrimination was more modest for our stroke models (AUCs and C-statistics of 0.63 to 0.66). However, in general, most other cardiovascular and other outcome prediction models used in clinical practice have discriminatory power similar to that of our models (AUCs and C-statistics of 0.6 to 0.8).41-45 In addition, we tested models only through age 50 years. More follow-up will be important to determine whether childhood cancer treatment exposures remain influential as survivors age further. Thus far, available data from CCSS and other cohorts do not suggest a plateau in the risks of late effects among survivors of cancer compared with the general population, and if anything, the data suggest further continued increased risk.
In conclusion, the major contribution of these models is that they combine established risk factors in a rational manner that allows individualized risk prediction and go beyond the single risk factor–based approach used currently.30,46 We believe these validated models can be useful tools for counseling survivors of childhood cancer who have recently completed therapy, particularly among survivors with potentially modifiable cardiovascular risk factors. These models also provide a stronger evidence base upon which to develop and test potential screening and intervention strategies for high-risk patients, including assessing the cost effectiveness of various surveillance schedules. Such models can also aid the future generation of clinical trials in determining what potential reductions in late toxicity could be gained by using less toxic combinations of therapy.
Appendix
Application of the Prediction Models (Three Patient Scenarios)
Patient 1.
An 18-year-old woman who was diagnosed with Hodgkin lymphoma at age 13 years is seen for follow-up. As part of her cancer therapy, she received mantle radiotherapy (21 Gy to the chest; same average dose to the heart). She was also treated with doxorubicin (200 mg/m2), bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide. On the basis of the scoring tables (Tables 3 and 4), her risk scores and corresponding risk groups for each outcome and each model can then be calculated (Appendix Table A5). Note that some exposures (eg, bleomycin, vincristine, etoposide, and prednisone) do not contribute to any of the risk scores, whereas other exposures (eg, cyclophosphamide) may only contribute in some but not all models.
Patient 1’s risk of having ischemic heart disease by age 50 years is moderate or moderate/high, corresponding to a cumulative incidence ranging from 12% to 14% depending on which model is used (rate ratio relative to siblings, approximately 7 to 10). Her risk of stroke is low, corresponding to a cumulative incidence ranging from 2% to 4% (rate ratio relative to siblings, < 5). Note that for the stroke standard model, because patient 1’s risk score value of 1 is a result of chemotherapy exposure only, without any cranial radiation, she is classified as low risk and not moderate risk. Finally, using the information provided in Appendix Tables A2 and A3, her risk of heart failure by age 50 years is high regardless of model used, corresponding to a cumulative incidence of approximately 12% to 14% (rate ratio relative to siblings, > 30).
Patient 2.
A 10-year-old boy was diagnosed with medulloblastoma at age 5 years and treated with whole-brain and spine radiotherapy (23 Gy) plus a boost up to a total of 56 Gy to the primary tumor (posterior fossa). On the basis of dosimetry, the average dose to the heart was estimated to be 15 Gy. He also received vincristine, cisplatin, and cyclophosphamide chemotherapy. The results of the prediction models for this patient are listed in Appendix Table A6.
Patient 2’s risk of having ischemic heart disease by age 50 years is moderate or moderate/high, similar to patient 1. His risk of having stroke by age 50 years is moderate/high to high, corresponding to a cumulative incidence ranging from approximately 10% to 20% depending on which model is used (rate ratio relative to siblings, approximately 15 to 40). Finally, his risk of heart failure by age 50 years is moderate, corresponding to a cumulative incidence of approximately 9% (rate ratio relative to siblings, approximately 10 to 15).
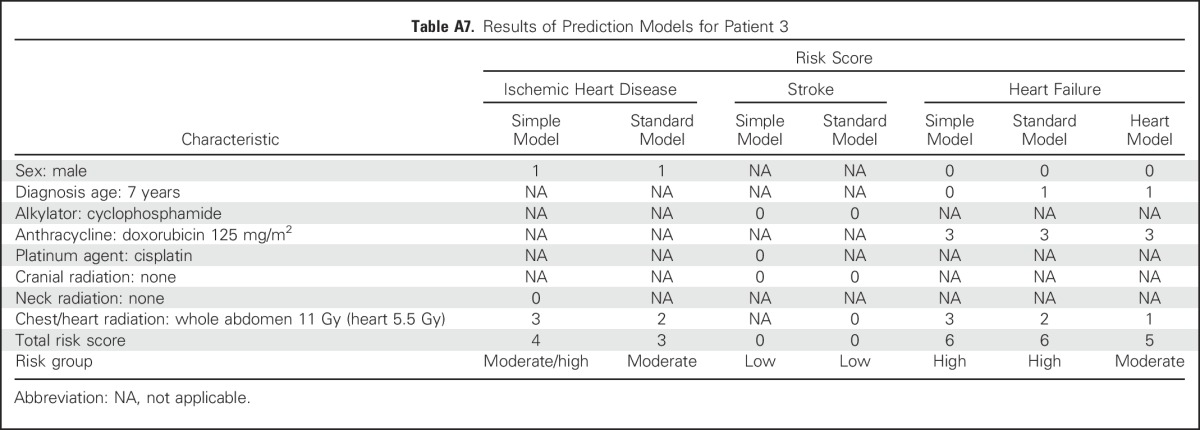
Patient 3.
A 12-year old boy was treated for Wilms tumor at age 7 years. He received doxorubicin (125 mg/m2), vincristine, and dactinomycin. He also received whole-abdomen radiation (11 Gy). Note that the chest radiation exposure includes abdominal treatments that included the lower part of the chest (ie, above the diaphragm); this is also consistent with the Children’s Oncology Group guidelines,30 which classify whole-abdomen radiation as cardiotoxic. Given the original radiation oncology treatment plan, the estimated dose to the heart itself was approximately 5.5 Gy. The results of the prediction models for patient 3 are listed in Appendix Table A7.
Overall, patient 3’s estimated risk of ischemic heart disease is similar to that of patients 1 and 2, and his risk of stroke is similar to that of patient 1. His risk of heart failure is predicted to be high on the basis of the simple and standard models, with a cumulative incidence of approximately 12% to 13% by age 50 years (rate ratio relative to siblings, > 30). However, if heart radiation dosimetry information is available, the predicted risk of heart failure is reduced to the moderate range, with a cumulative incidence of < 10% and a rate ratio < 15.
Statistical Approach
Model selection.
The following variables were selected a priori for statistical testing in our models: sex; age at cancer diagnosis (5-year increments); and exposure (yes or no) to anthracyclines, alkylating agents, platinum agents, vinca alkaloids, and cranial, neck, chest, and abdominal radiotherapy. Cranial radiotherapy was included a priori for the stroke models only. For the standard and heart dose models, exposure doses were substituted for chest or heart radiotherapy (none, < 5, 5 to 14, 15 to 34, and ≥ 35 Gy). Individuals with missing data relevant to each model were excluded. Using piecewise exponential regression adjusted for current age as a cubic spline, models were built to examine the relationships between these independent variables and the outcomes (ischemic heart disease and stroke). Current age was handled by splitting the records at each age (as an integer) during follow-up. Backward selection was then used to determine the most influential treatment predictors accounting for sex and age at cancer diagnosis.16 The least statistically significant variable with P ≥ .05 (as determined by the likelihood ratio test) was dropped and the reduced model refitted using the same rule until all remaining exposure variables were statistically significant (P < .05). To estimate the performance of the prediction models, we performed 10-fold internal cross-validation of the variable selection process.20 Exploratory analyses examining potential interaction between therapeutic exposures and sex or diagnosis age did not reveal any consistent relationships.
Risk score creation.
Estimates of the regression coefficients associated with predictors that remained after backward selection plus those associated with sex and diagnosis age were then converted to integer risk scores for ease of summing in subsequent risk models (rate ratios < 1.3, 1.3 to 1.9, 2.0 to 2.9, 3.0 to 4.9, and ≥ 5.0, corresponding to risk scores of 0, 1, 2, 3, and 4, respectively) on the basis of previously published methods (Sorror ML, et al: Bone Marrow Transplant 46:464-466, 2011).12,17
Risk score discriminatory and predictive power.
Cox regression with age as its time scale was used to estimate our risk scores’ discriminatory and predictive power.18 Specifically, we examined the area under the curve (AUC) at age 50 years and the concordance (C) statistic through age 50 years.19 The AUC(t) is the probability that a classifier will rank a randomly chosen case higher than a randomly chosen noncase on a given time t. C(t) statistics represents the weighted average of AUC from the study start time to time t. Similar to the initial model-building process, the reported AUCs and C-statistics also reflected estimates from 10-fold internal cross-validation.20
Risk group creation.
Although other general population predictors are often based on the sum of individual risk scores (Kannel WB, et al: Arch Intern Med 159:1197-1204, 1999; Conroy RM, et al: Eur Heart J 24:987-1003, 2003; McGill HC Jr, et al: Circulation 117:1216-1227, 2008),41 given the relatively smaller number of participants we had available, estimates associated with individual risk scores were not always precise. Therefore, we collapsed risk scores into several risk groups predictive of low, moderate, and high risk of ischemic heart disease or stroke.17 To determine the most appropriate groupings, the sums of individual risk scores were examined based on their absolute risks (cumulative incidence at age 50 years treating death from other causes as a competing risk event)21 and rate ratios compared with siblings (piecewise exponential regression, incorporating a generalized estimating equation modification to account for potential within-family correlation).22 The resulting low-, moderate-, and high-risk groups corresponded in general to cumulative incidence rates of approximately < 5%, 5% to 15%, and > 15% at age 50 years, respectively. For the simple models, only a low-risk group and a combined moderate/high-risk group were able to be defined. The risk groupings were designed such that each group ideally would be significantly distinct from both siblings and the immediate lower group (P < .05) per our regression models.
External validation.
AUCs and C-statistics (at or through age 50 years) for ischemic heart disease and stroke were estimated for each of the validation cohorts based on the Childhood Cancer Survivor Study (CCSS) risk scoring described earlier. Each individual in these validation cohorts was then categorized into the appropriate CCSS-based risk group, and the empirical cumulative incidences of ischemic heart disease and stroke were plotted and compared against those derived from the CCSS cohort. We then assessed the difference of the AUCs and C-statistics of each model between the external cohorts and the CCSS using 1,000 bootstrap iterations (Good PI: Permutation, Parametric and Bootstrap Tests of Hypotheses [ed 3]. New York, NY, Springer, 2005).
Software.
R (version 3.3.2; https://www.r-project.org/), specifically the function risksetROC (version 1.0.4), was used to calculate the AUCs and C-statistics. SAS (version 9.3; SAS Institute, Cary, NC) was used for the piecewise exponential regression analyses. The codes used are available from the authors upon request.
External Validation Cohorts
St Jude Lifetime Cohort Study.
The St Jude Children’s Research Hospital (SJCRH) is a National Cancer Institute–designated comprehensive cancer center based in Memphis, Tennessee. St Jude Lifetime Cohort Study eligibility criteria at the time that the data set used in this analysis was frozen were as follows: diagnosis of any childhood cancer treated at SJCRH; survival ≥ 10 years from diagnosis; and age ≥ 18 years at time of enrollment.23 In 2015, eligibility was expanded to ≥ 5-year survivors of any age.24 The cohort was started in 2007 and features a retrospective component, where medical record data on surviving patients dating back to 1962 were collated, as well as a prospective component, where participants are invited for comprehensive in-person health evaluations at the SJCRH campus. Recruitment onto the St Jude Lifetime Cohort Study is ongoing. For the data set used in this analysis, the overall participation rate was approximately 65% (Hudson MM, et al: JAMA 309:2371-2381, 2013).
Data obtained from the medical records include demographic characteristics, cumulative doses of selected chemotherapeutic agents, the fields and doses of any radiotherapy, and information on surgeries, cancer recurrence, new cancers, and acute and large organ-specific toxicities. Health and vital status also are monitored by the St Jude Cancer Registry and supplemented by periodic National Death Index searches. In-person assessments feature a core battery of standard evaluations (including 12-lead ECGs), supplemented by additional risk-based testing per the Children’s Oncology Group’s Long-Term Follow-Up Guidelines (eg, echocardiogram for those exposed to chest radiation and/or anthracycline chemotherapy) (Hudson MM, et al: JAMA 309:2371-2381, 2013; Landier W, et al: J Clin Oncol 22:4979-4990, 2004). Organ toxicities are then classified and graded using a system adapted from the National Cancer Institute’s Common Terminology Criteria for Adverse Events.24
Emma Children’s Hospital and Academic Medical Center childhood cancer survivor cohort.
Emma Children’s Hospital (EKZ) is part of the University of Amsterdam’s Academic Medical Center (AMC), based in Amsterdam, the Netherlands. Eligibility criteria for the EKZ/AMC cohort consist of individuals who were age < 18 years at time of cancer diagnosis, were diagnosed in the Netherlands from 1966 onward, were treated primarily at EKZ/AMC, and survived ≥ 5 years from diagnosis.25 The cohort started in 1966 with information on patient demographic characteristics, original cancer diagnosis, treatment (including chemotherapeutic agents used and radiotherapy fields and doses), recurrences, and vital status collected by the EKZ/AMC hospital registry.
In 1996, prospective in-person clinical assessments began, with eligible survivors reassessed on a 1- to 5-year basis, depending on their prior cancer treatment and expected burden of late effects. Assessments are risk based per available Dutch national guidelines (Jaspers MW, et al: Int J Med Inform 76:297-305, 2007). Late outcomes information is obtained from these assessments and from a prospective review of medical records from both EKZ/AMC and outside medical providers. Classification and grading of outcomes were based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 3.0) (Geenen MM, et al: JAMA 297:2705-2715, 2007).15
Although the EKZ/AMC cohort is also ongoing and open to enrollment, for this particular analysis, survivors were limited to those diagnosed and treated from 1966 to 1996, with follow-up to 2010. For this analysis, the symptomatic strokes ascertained, including transient ischemic attacks, were all diagnosed by neurologists and clinically validated.26
Table A1.
Multivariable Piecewise Exponential Regression Results for Each Cardiovascular Outcome Model in the Childhood Cancer Survivor Study Cohort
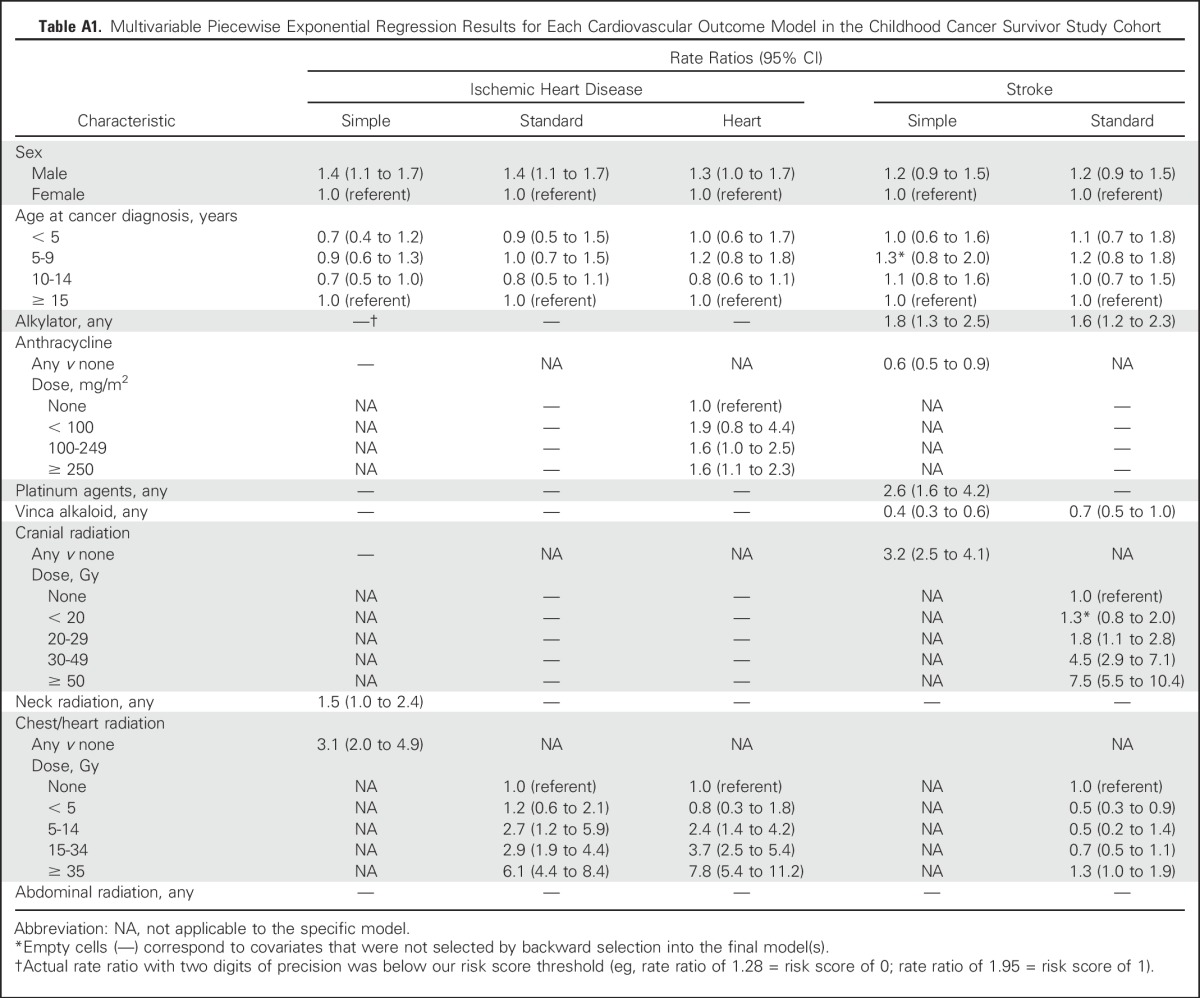
Table A2.
Integer Risk Scores Associated With Cardiomyopathy or Heart Failure and Corresponding Prediction Models Through Age 50 Years
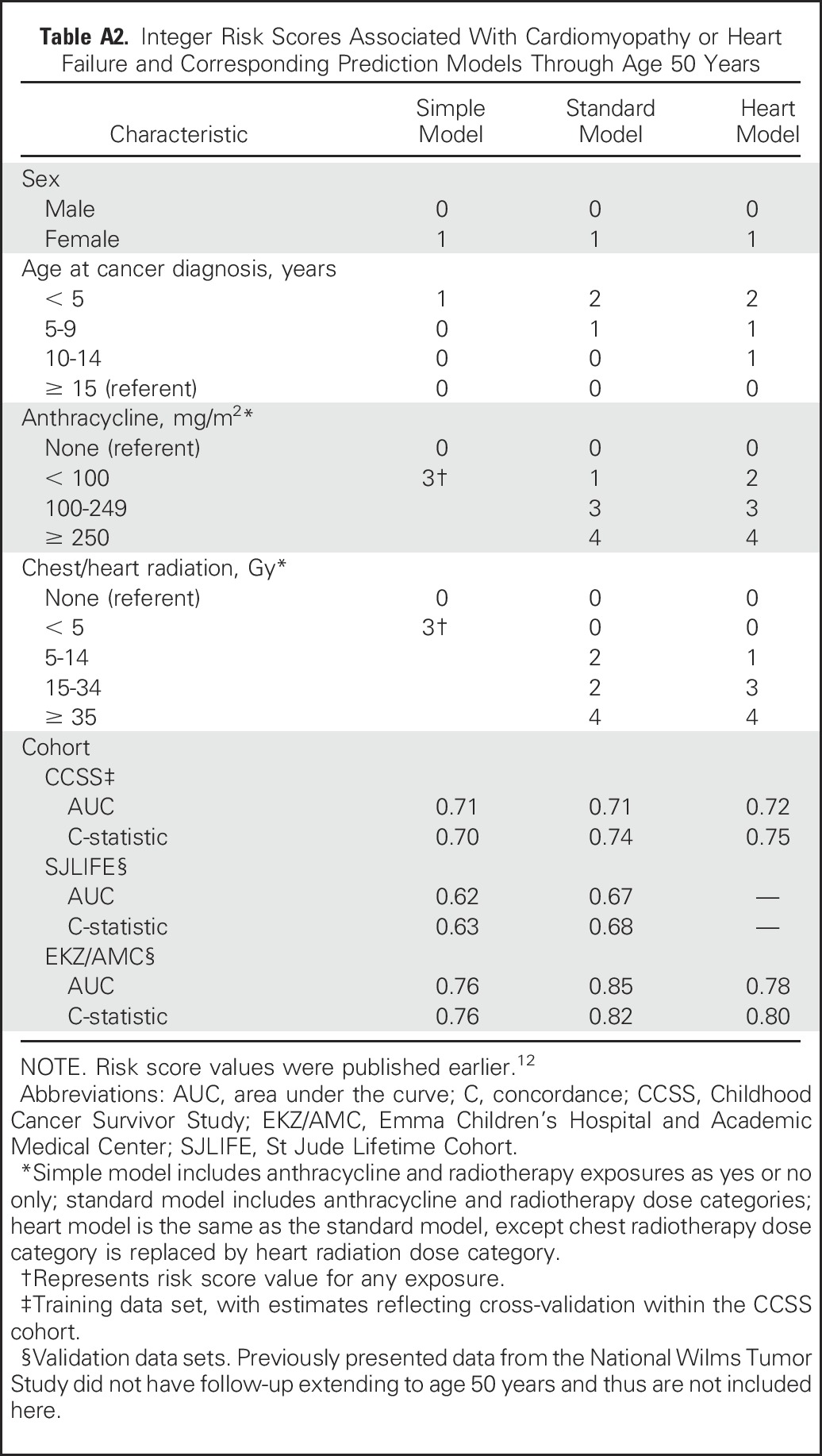
Table A3.
Classification of Cardiomyopathy or Heart Failure Risk and Empirical Cumulative Incidence and RRs Within the CCSS Cohort Based on Summed Risk Scores Through Age 50 Years
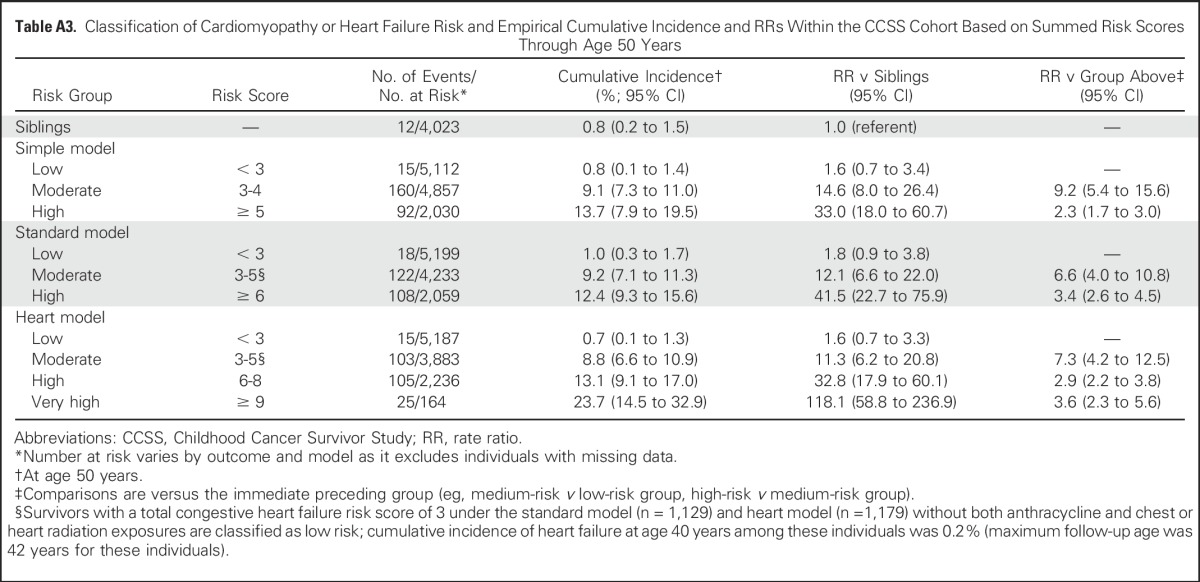
Table A4.
Classification of Cardiovascular Outcome Risk Groups Within External Validation Cohorts Based on CCSS-Derived Risk Scores
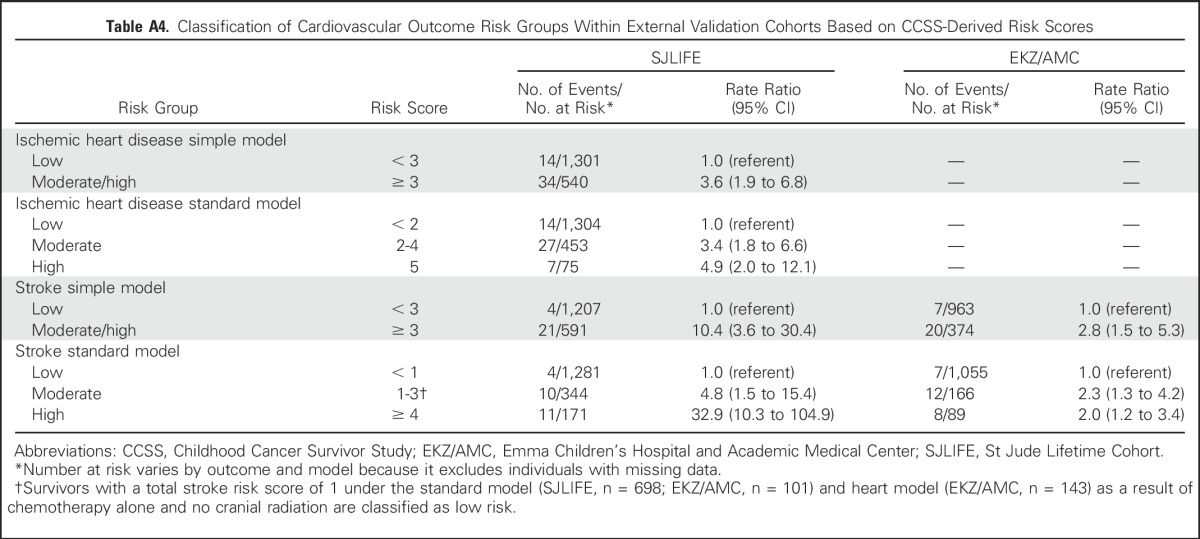
Table A5.
Results of Prediction Models for Patient 1
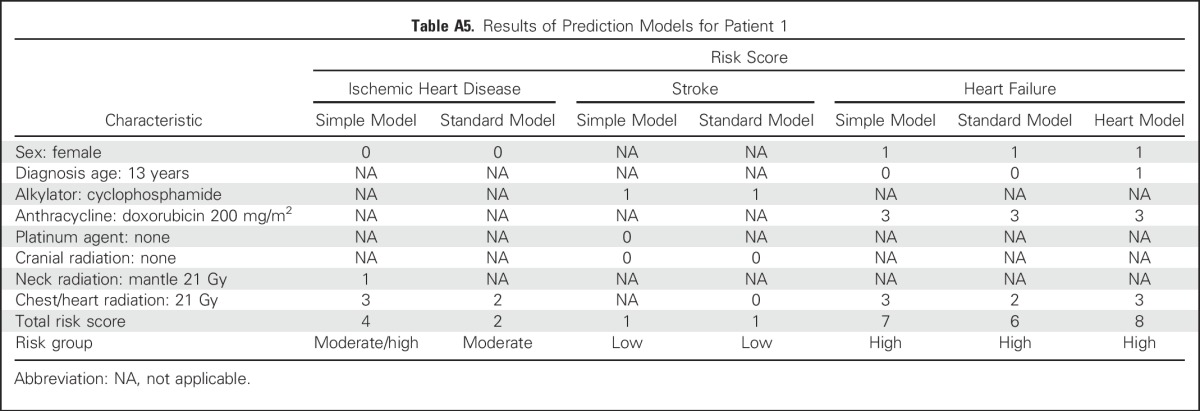
Table A6.
Results of Prediction Models for Patient 2
Table A7.
Results of Prediction Models for Patient 3
Footnotes
Supported by Grants No. U24 CA55727, U01 CA195547, K07 CA151775, R01 CA204378, and P30 CA21765 from the National Institutes of Health; Grant No. 257505 from PanCareSurFup EU; the American Lebanese Syrian Associated Charities; the Dutch Cancer Society; the Foundation for Pediatric Cancer Research (the Netherlands); and the Leukemia and Lymphoma Society. E.J.C. and Y.C. contributed equally to this work.
Presented in part at the 50th Annual Meeting of the American Society of Clinical Oncology Annual Meeting, Chicago, IL, May 29-June 2, 2014.
AUTHOR CONTRIBUTIONS
Conception and design: Eric J. Chow, Charles A. Sklar, Yutaka Yasui
Financial support: Eric J. Chow, Melissa M. Hudson, Leontien C. Kremer, Flora E. van Leeuwen, Leslie L. Robison, Gregory T. Armstrong
Administrative support: Melissa M. Hudson, Leontien C. Kremer, Marilyn Stovall, Flora E. van Leeuwen, Leslie L. Robison, Gregory T. Armstrong
Provision of study materials or patients: Melissa M. Hudson, Leontien C. Kremer, Lillian R. Meacham, Cécile M. Ronckers, Marilyn Stovall, Helena J. van der Pal, Flora E. van Leeuwen, Rita E. Weathers, Leslie L. Robison, Gregory T. Armstrong
Collection and assembly of data: Melissa M. Hudson, Elizabeth A.M. Feijen, Leontien C. Kremer, Kirsten K. Ness, Cécile M. Ronckers, Marilyn Stovall, Helena J. van der Pal, Irma W.E.M. van Dijk, Flora E. van Leeuwen, Rita E. Weathers, Leslie L. Robison, Gregory T. Armstrong
Data analysis and interpretation: Eric J. Chow, Yan Chen, Melissa M. Hudson, Elizabeth A.M. Feijen, Leontien C. Kremer, William L. Border, Daniel M. Green, Lillian R. Meacham, Daniel A. Mulrooney, Kirsten K. Ness, Kevin C. Oeffinger, Charles A. Sklar, Marilyn Stovall, Yutaka Yasui
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prediction of Ischemic Heart Disease and Stroke in Survivors of Childhood Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Eric J. Chow
No relationship to disclose
Yan Chen
No relationship to disclose
Melissa M. Hudson
Consulting or Advisory Role: Coleman Supportive Oncology Initiative for Children With Cancer, Oncology Research Information Exchange Network, Pfizer Genotropin Advisory Board 2016, Princess Máxima Center
Elizabeth A.M. Feijen
No relationship to disclose
Leontien C. Kremer
No relationship to disclose
William L. Border
No relationship to disclose
Daniel M. Green
No relationship to disclose
Lillian R. Meacham
No relationship to disclose
Daniel A. Mulrooney
No relationship to disclose
Kirsten K. Ness
No relationship to disclose
Kevin C. Oeffinger
No relationship to disclose
Cécile M. Ronckers
No relationship to disclose
Charles A. Sklar
No relationship to disclose
Marilyn Stovall
No relationship to disclose
Helena J. van der Pal
No relationship to disclose
Irma W.E.M. van Dijk
No relationship to disclose
Flora E. van Leeuwen
No relationship to disclose
Rita E. Weathers
No relationship to disclose
Leslie L. Robison
No relationship to disclose
Gregory T. Armstrong
No relationship to disclose
Yutaka Yasui
No relationship to disclose
REFERENCES
- 1.Robison LL, Hudson MM: Survivors of childhood and adolescent cancer: Life-long risks and responsibilities. Nat Rev Cancer 14:61-70, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipshultz SE, Adams MJ, Colan SD, et al. : Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association. Circulation 128:1927-1995, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Oeffinger KC, Mertens AC, Sklar CA, et al. : Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355:1572-1582, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Reulen RC, Winter DL, Frobisher C, et al. : Long-term cause-specific mortality among survivors of childhood cancer. JAMA 304:172-179, 2010 [DOI] [PubMed] [Google Scholar]
- 5.van der Pal HJ, van Dalen EC, van Delden E, et al. : High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol 30:1429-1437, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Mulrooney DA, Armstrong GT, Huang S, et al. : Cardiac outcomes in adult survivors of childhood cancer exposed to cardiotoxic therapy: A cross-sectional study. Ann Intern Med 164:93-101, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haddy N, Diallo S, El-Fayech C, et al. : Cardiac diseases following childhood cancer treatment: Cohort study. Circulation 133:31-38, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Armstrong GT, Oeffinger KC, Chen Y, et al. : Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol 31:3673-3680, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowers DC, Liu Y, Leisenring W, et al. : Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: A report from the Childhood Cancer Survivor Study. J Clin Oncol 24:5277-5282, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Mueller S, Fullerton HJ, Stratton K, et al. : Radiation, atherosclerotic risk factors, and stroke risk in survivors of pediatric cancer: A report from the Childhood Cancer Survivor Study. Int J Radiat Oncol Biol Phys 86:649-655, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. : 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129:S49-S73, 2014. (suppl 2) [DOI] [PubMed] [Google Scholar]
- 12.Chow EJ, Chen Y, Kremer LC, et al. : Individual prediction of heart failure among childhood cancer survivors. J Clin Oncol 33:394-402, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robison LL, Armstrong GT, Boice JD, et al. : The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol 27:2308-2318, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stovall M, Weathers R, Kasper C, et al. : Dose reconstruction for therapeutic and diagnostic radiation exposures: Use in epidemiological studies. Radiat Res 166:141-157, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Cancer Therapy Evaluation Program: Common Terminology Criteria for Adverse Events (CTCAE). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
- 16.Draper NR, Smith H: Applied Regression Analysis (ed 3). New York, NY, Wiley, 1998 [Google Scholar]
- 17.Sorror ML, Maris MB, Storb R, et al. : Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 106:2912-2919, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heagerty PJ, Zheng Y: Survival model predictive accuracy and ROC curves. Biometrics 61:92-105, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Harrell FE, Jr, Lee KL, Mark DB: Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361-387, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Stone M: Cross-validatory choice and assessment of statistical predictions. J R Stat Soc Ser A 36:111-147, 1974 [Google Scholar]
- 21.Kalbfleisch JD, Prentice RL: The Statistical Analysis of Failure Time Data (ed 2). New York, NY, Wiley, 2002 [Google Scholar]
- 22.Zeger SL, Liang KY: Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42:121-130, 1986 [PubMed] [Google Scholar]
- 23.Hudson MM, Ness KK, Nolan VG, et al. : Prospective medical assessment of adults surviving childhood cancer: Study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer 56:825-836, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudson MM, Ehrhardt MJ, Bhakta N, et al. : Approach for classification and severity grading of long-term and late-onset health events among childhood cancer survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev 26:666-674, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sieswerda E, Mulder RL, van Dijk IW, et al. : The EKZ/AMC childhood cancer survivor cohort: Methodology, clinical characteristics, and data availability. J Cancer Surviv 7:439-454, 2013 [DOI] [PubMed] [Google Scholar]
- 26.van Dijk IW, van der Pal HJ, van Os RM, et al. : Risk of symptomatic stroke after radiation therapy for childhood cancer: A long-term follow-up cohort analysis. Int J Radiat Oncol Biol Phys 96:597-605, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Green DM, Kun LE, Matthay KK, et al. : Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr Blood Cancer 60:1083-1094, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudson MM, Neglia JP, Woods WG, et al. : Lessons from the past: Opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer 58:334-343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hewitt M, Greenfield S, Stovall E (eds): Improving care and quality of life, in From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC, Institute of Medicine and National Research Council, National Academies Press, 2006. [Google Scholar]
- 30.Shankar SM, Marina N, Hudson MM, et al. : Monitoring for cardiovascular disease in survivors of childhood cancer: Report from the Cardiovascular Disease Task Force of the Children’s Oncology Group. Pediatrics 121:e387-e396, 2008 [DOI] [PubMed] [Google Scholar]
- 30a.Periard D, Boulanger CM, Eyer S, et al. : Are circulating endothelial-derived and platelet-derived microparticles a pathogenic factor in the cisplatin-induced stroke? Stroke 38:1636-1638, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Rocca WA, Grossardt BR, Miller VM, et al. : Premature menopause or early menopause and risk of ischemic stroke. Menopause 19:272-277, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gajjar A, Bowers DC, Karajannis MA, et al. : Pediatric brain tumors: Innovative genomic information is transforming the diagnostic and clinical landscape. J Clin Oncol 33:2986-2998, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeppesen LL, Jørgensen HS, Nakayama H, et al. : Decreased serum testosterone in men with acute ischemic stroke. Arterioscler Thromb Vasc Biol 16:749-754, 1996 [DOI] [PubMed] [Google Scholar]
- 34.De Bruin ML, Dorresteijn LD, van’t Veer MB, et al. : Increased risk of stroke and transient ischemic attack in 5-year survivors of Hodgkin lymphoma. J Natl Cancer Inst 101:928-937, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Koppelmans V, Vernooij MW, Boogerd W, et al. : Prevalence of cerebral small-vessel disease in long-term breast cancer survivors exposed to both adjuvant radiotherapy and chemotherapy. J Clin Oncol 33:588-593, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Maraldo MV, Jørgensen M, Brodin NP, et al. : The impact of involved node, involved field and mantle field radiotherapy on estimated radiation doses and risk of late effects for pediatric patients with Hodgkin lymphoma. Pediatr Blood Cancer 61:717-722, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Natarajan P, O’Donnell CJ: Reducing cardiovascular risk using genomic information in the era of precision medicine. Circulation 133:1155-1159, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipshultz SE, Landy DC, Lopez-Mitnik G, et al. : Cardiovascular status of childhood cancer survivors exposed and unexposed to cardiotoxic therapy. J Clin Oncol 30:1050-1057, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang J, Yang Q, Hong Y, et al. : Status of cardiovascular health among adult Americans in the 50 States and the District of Columbia, 2009. J Am Heart Assoc 1:e005371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roth GA, Johnson C, Abajobir A, et al. : Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 70:1-25, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. : General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 117:743-753, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Wilson PW, D’Agostino RB, Levy D, et al. : Prediction of coronary heart disease using risk factor categories. Circulation 97:1837-1847, 1998 [DOI] [PubMed] [Google Scholar]
- 43.McMahan CA, Gidding SS, Fayad ZA, et al. : Risk scores predict atherosclerotic lesions in young people. Arch Intern Med 165:883-890, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Rockhill B, Spiegelman D, Byrne C, et al. : Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst 93:358-366, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Park Y, Freedman AN, Gail MH, et al. : Validation of a colorectal cancer risk prediction model among white patients age 50 years and older. J Clin Oncol 27:694-698, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armenian SH, Hudson MM, Mulder RL, et al. : Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 16:e123-e136, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]