Abstract
Background
Data about the burden of extended-spectrum beta-lactamase (ESBL)-producing microorganisms in Africa are limited. Our study aimed to estimate the prevalence of human faecal ESBL carriage in the community of an informal urban settlement in Dar es Salaam (Tanzania, East Africa) by using environmental contamination of household latrines with ESBL as a surrogate marker.
Methods
Within the context of a large survey in February 2014 assessing 636 randomly selected household latrines for faecal contamination by the detection of growth of E. coli and total faecal coliform bacteria, a randomly selected subset of the samples were screened for ESBL.
Results
Seventy latrines were screened for ESBL. An average of 11.4 persons (SD ±6.5) were sharing one latrine. Only three (4.3%) latrines had hand-washing facilities and 50 showed faeces on the floor. ESBL-producing Enterobacteriaceae were confirmed in 17 (24.3%) of the 70 latrine samples: 16 E. coli and 1 Klebsiella pneumoniae. Five ESBL E. coli strains were detected on door handles. The most prevalent ESBL type was CTX-M-1 group (76.5%). Pulsed-field gel electrophoresis typing of a subset of ESBL-producing E. coli isolates revealed both diverse singular types and a cluster of 3 identical isolates. There was no significant difference of the latrine and household characteristics between the group with ESBL (n = 17) and the group with non-ESBL E. coli (n = 53) (p > 0.05).
Conclusions
Almost a quarter of private and shared latrines in an informal urban settlement in Tanzania are contaminated with ESBL-producing microorganisms, suggesting a high prevalence of human ESBL faecal carriage in the community. Shared latrines may serve as a reservoir for transmission in urban community settings in Tanzania.
Keywords: Extended-spectrum beta-lactamase ESBL, Community, Carriage, Prevalence, Latrines, Sub-Saharan Africa
Background
Extended-spectrum beta-lactamase (ESBL)-producing gram-negative bacteria have become an emerging global health threat and have been associated with high mortality [1, 2]. Whereas ESBL infections were initially associated with nosocomial outbreaks, there is now increasing recognition of high rates of faecal carriage and the importance of community-acquired infections due to ESBL-producing Escherichia coli in industrialized countries [3, 4].
Data about the prevalence of ESBL-producing microorganisms in Africa are limited. A wide variation from 0.6% up to 77.8% has been reported in hospital-based surveys of clinical isolates [5–11]. There is little published data on the magnitude of the community carriage of ESBL in African countries like Tanzania.
Our study aimed to estimate the prevalence of human faecal ESBL carriage in the community in an urban setting in Tanzania, East Africa, by using environmental contamination of household latrines with ESBL as a surrogate marker.
Methods
The study was performed in February 2014 in an urban study site in Keko Machungwa, part of the largest unplanned and under-serviced settlement in Temeke district, Dar es Salaam, Tanzania.
Within the context of a large survey assessing the improvement of sanitation facilities [12], 636 randomly selected household latrines were screened for the presence and concentration of faecal contamination (E. coli and total faecal coliform bacteria) [13]. Surface swipe swabs from high frequency contact points (10 cm2) like door handle and footrest were taken and analysed using direct membrane filtration technique (Merck Millipore, Billerica, MA, USA) with commercial medium m-ColiBlue® (HACH, Loveland, CO, USA) [14]. In this survey, 492 latrines showed growth of E. coli or coliform bacteria either at the footrest, the door handle or both (unpublished data, Fig. 1).
Fig. 1.
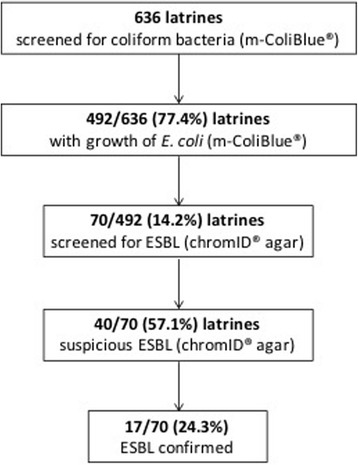
ESBL screening algorithm. ESBL, extended-spectrum beta-lactamase-producing microorganisms
From latrines with E. coli growth, samples were selected using a calculator based random number generator and further screened for ESBL-producing Enterobacteriaceae using the chromogenic selective culture medium chromID® ESBL (bioMérieux, Marcy-l’Étoile, France) according to the manufacturer’s instructions. ESBL or carbapenemase production was confirmed with standard microbiological techniques following EUCAST guideline [15]. ESBL molecular types (specifically CTX-M-1 and CTX-M-9 groups) were determined by isothermal amplification (eazyplex® SuperBug CRE Assay [Amplex Biosystems, Gießen, Germany] for use on Genie® II platform [Optigene, Horsham, UK]). Antimicrobial susceptibility testing and interpretation was done using Vitek® 2 automated system (bioMérieux) or Etest® (bioMérieux) according to EUCAST clinical breakpoints (version 5.0, 2015; http://www.eucast.org/clinical_breakpoints). Molecular typing was performed by pulsed-field gel electrophoresis (PFGE) as described previously [16].
Data about household and latrine characteristics were collected by visual inspection and questionnaire.
Univariable analysis was performed by the chi-square test or Fisher’s exact test, where appropriate, for categorical variables and two-tailed Student’s t test for continuous variables. Two-tailed p-values of <0.05 were considered statistically significant.
The study was approved by the national ethics committee of the National Institute for Medical Research Tanzania (NIMR/HQ/R.8a/Vol.IX/1632).
Results
From the 492 latrines with growth of E. coli, 70 latrines were randomly selected for ESBL screening. All 70 latrines had either a cement, tile or brick floor and met the WHO/UNICEF Joint Monitoring Program for Water Supply and Sanitation definitions for improved latrines [12]. Only 3 (4.3%) latrines had hand-washing facilities and 50 showed faeces on the floor. An average of 11.4 persons (SD ±6.5) were sharing one latrine.
Twenty-four (34.3%) latrines were private (i.e. each of these latrines were used by only one household), the remaining latrines were shared by ≥2 households. The latrines were not accessible for the public but restricted to individuals living in the respective household (Table 1).
Table 1.
Baseline characteristics of latrines screened for ESBL (n = 70)
| Characteristics | ||
|---|---|---|
| Screened latrines (n, %) | 70 | 100% |
| Households sharing one latrine (n, %) | ||
| 1 houshold (private latrine) | 24 | 34.3% |
| 2 households | 26 | 37.1% |
| 3 households | 13 | 18.6% |
| 4–10 households | 4 | 5.7% |
| 10–23 households | 3 | 4.3% |
| Persons sharing one latrine (mean, ±SD) | 11.4 | 6.5 |
| Household leader male (n, %) | 23 | 32.9% |
| Household leader’s educational level (n, %) | ||
| none | 5 | 7.1% |
| primary school | 43 | 61.4% |
| secondary school | 22 | 31.4% |
| Household leader’s monthly income in US$ (mean, ±SD) | 102.7 | 78.9 |
| Latrines used by children <5 years (n, %) | 58 | 82.9% |
| Latrines with stored bucket for anal cleaning (n, %) | 26 | 37.1% |
| Handwashing facilities <1 m (n, %) | 3 | 4.3% |
| Soap available in latrine (n, %) | 6 | 8.6% |
| Age of latrines in years (mean, ±SD) | 4.6 | 3.4 |
| Latrine floor material (n, %) | ||
| brick | 60 | 85.7% |
| cement | 10 | 14.3% |
| Cracked or broken slab (n, %) | 54 | 77.1% |
| Condition of latrine (n, %) | ||
| clean | 20 | 28.6% |
| Dirty - faeces on the floor | 50 | 71.4% |
| Lid available (n, %) | 3 | 4.3% |
| Latrine separation (n,%) | ||
| no door | 6 | 8.6% |
| curtain | 5 | 7.1% |
| wood door | 59 | 84.3% |
| Flies in latrine (n, %) | 40 | 57.1% |
| Animals in the compound (n, %) | 14 | 20.0% |
SD standard deviation
Forty samples showed ESBL suspected colonies on chromID® ESBL culture medium. ESBL-producing Enterobacteriaceae were finally confirmed in 17 (24.3%) of the 70 latrine samples: 16 E. coli and 1 Klebsiella pneumoniae (Table 2). Five ESBL E. coli strains were detected on door handles. Antimicrobial resistance to ciprofloxacin and trimethoprim/sulfamethoxazole was detected in 94.1% and 82.4% of the ESBL isolates, respectively. No carbapenem resistance or indication for carbapenemase production was detected. The most prevalent ESBL type was CTX-M-1 group (76.5%) (Table 2). Microbiological and household/latrine characteristics of the 17 detected ESBL microorganisms are summarized in Table 3. PFGE typing of a subset of ESBL-producing E. coli isolates revealed both diverse singular types and a cluster of 3 identical isolates (Fig. 2). Ten isolates were nontypable by PFGE as observed in other studies [17].
Table 2.
Microbiological results of the screened latrines (n = 70)
| ESBL-producing bacteria total (n, %) | 17 | 24.3% |
| ESBL E. coli | 16 | |
| ESBL K. pneumoniae | 1 | |
| Screening sites positive (n, %) | 17 | |
| Footrests | 12 | 70.6% |
| Door handles ± footrests | 5 | 29.4% |
| Antibiotics resistant/intermediatea (n, %) | ||
| Ampicillin | 17 | 100% |
| Amoxicillin/Clavulanic acid | 16 | 94.1% |
| Piperacillin/Tazobactam | 4 | 23.5% |
| Cefoxitin | 11 | 64.7% |
| Ceftazidime | 16 | 94.1% |
| Ceftriaxone | 17 | 100% |
| Cefepime | 16 | 94.1% |
| Ertapenem | 0 | 0% |
| Meropenem | 0 | 0% |
| Ciprofloxacin | 16 | 94.1% |
| Tobramycin | 15 | 88.2% |
| Amikacin | 6 | 35.3% |
| Trimethoprim/Sulfamethoxazole | 14 | 82.4% |
| Nitrofurantoin | 1 | 5.9% |
| Fosfomycin | 0 | 0% |
| Colistin | 0 | 0% |
| ESBL types (n, %) | 17 | |
| CTX-M-1 group | 13 | 76.5% |
| CTX-M-9 group | 1 | 5.9% |
| Other than CTX-M-1/9 group | 3 | 17.6% |
ESBL extended-spectrum beta-lactamase-producing microorganisms
aInterpretation according to EUCAST breakpoints version 5.0 (2015)
Table 3.
Microbiological and household characteristics of the 17 detected ESBL microorganisms
| No | Sample ID | ESBL organism | ESBL type | Amoxicillin/Clavulanic acid | Piperacillin/Tazobactam | Ceftriaxone | Meropenem | Amikacin | TMP/SMX | Nitrofurantoin | Ciprofloxacin | Fosfomycin | Colistin | Screening site | Age of latrine in years | Persons sharing 1 latrine | Children <5y using 1 latrine | Latrine floor material | Condition of the slab | Condition of the latrine | Lid available | Latrines with stored bucket for anal cleaning | Handwashing facilities | Soap available in latrine | Latrine separation | Flies in latrine | Animals in the compound |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 91BH | E. coli | CTX-M-1 | R | S | R | S | S | S | S | R | S | S | handle | 6 | 14 | 3 | brick | broken | clean | no | no | no | no | door | yes | yes |
| 2 | 124CM | E. coli | CTX-M-1 | R | R | R | S | S | R | S | R | S | S | handle | 2 | 6 | 1 | brick | broken | dirty | no | yes | no | no | door | no | no |
| 3 | 107AH | E. coli | CTX-M-1 | R | R | R | S | S | R | S | R | S | S | handle | 3 | 2 | 0 | cement | broken | dirty | no | yes | no | yes | door | yes | yes |
| 4 | 115AH | E. coli | CTX-M-1 | R | R | R | S | S | R | S | R | S | S | handle | 1 | 5 | 1 | brick | broken | dirty | no | yes | no | no | door | no | no |
| 5 | 106CH | E. coli | other | R | R | R | S | S | R | S | R | S | S | handle | 6 | 6 | 1 | brick | broken | dirty | no | no | no | no | curtain | no | yes |
| 6 | 104DS | E. coli | other | R | S | R | S | S | R | S | R | S | S | floor | 0.5 | 15 | 1 | brick | no cracks | dirty | no | no | no | no | door | yes | no |
| 7 | 102DS | E. coli | CTX-M-1 | R | S | R | S | R | R | S | R | S | S | floor | 4 | 15 | 1 | brick | broken | dirty | no | no | no | no | door | yes | no |
| 8 | 90S | E. coli | other | R | S | R | S | S | R | S | R | S | S | floor | 4 | 15 | 1 | brick | broken | dirty | no | no | no | no | door | no | no |
| 9 | 86BS | E. coli | CTX-M-1 | R | S | R | S | S | R | S | R | S | S | floor | 0.5 | 5 | 1 | cement | broken | dirty | yes | yes | yes | yes | door | no | no |
| 10 | 84BS | E. coli | CTX-M-1 | R | S | R | S | R | R | S | R | S | S | floor | 0.5 | 23 | 1 | brick | broken | clean | no | no | no | no | door | yes | no |
| 11 | 83BS | E. coli | CTX-M-1 | R | S | R | S | R | R | S | R | S | S | floor | 0.5 | 12 | 2 | brick | no cracks | clean | no | no | no | no | door | yes | no |
| 12 | 97DS | E. coli | CTX-M-1 | R | S | R | S | R | R | S | R | S | S | floor | 2 | 9 | 1 | cement | broken | dirty | no | yes | no | no | door | no | yes |
| 13 | 93AS | E. coli | CTX-M-1 | R | S | R | S | R | R | S | R | S | S | floor | 0.5 | 10 | 1 | brick | broken | dirty | no | yes | no | no | door | no | yes |
| 14 | 70DS | E. coli | CTX-M-1 | R | S | R | S | S | S | S | R | S | S | floor | 1 | 15 | 4 | brick | broken | dirty | no | no | no | no | door | yes | no |
| 15 | 67BS | E. coli | CTX-M-9 | R | S | R | S | S | R | S | R | S | S | floor | 2 | 4 | 1 | brick | broken | clean | no | no | no | no | no | yes | no |
| 16 | 94AS | E. coli | CTX-M-1 | R | S | R | S | R | R | S | R | S | S | floor | 9 | 7 | 0 | cement | broken | dirty | yes | no | no | no | curtain | yes | no |
| 17 | 64BA | K. pneumoniae | CTX-M-1 | S | S | R | S | S | S | R | S | S | S | floor | 6 | 10 | 1 | brick | no cracks | clean | no | no | no | no | door | yes | yes |
Susceptibility testing according to EUCAST: R resistant or intermediate, S susceptible, TMP/SMX Trimethoprim/Sulfamethoxazole
Fig. 2.
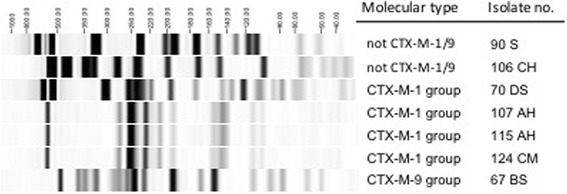
PFGE of seven ESBL-producing E. coli isolates. PFGE, pulsed-field gel electrophoresis; ESBL, extended-spectrum beta-lactamase-producing microorganisms. PFGE shows a cluster of 3 isolates (107AH, 115AH, 124CM) with identical PFGE band pattern. Other isolates show different PFGE pattern
There was no significant difference of the latrine and household characteristics between the group with ESBL (n = 17) and the group with non-ESBL E. coli (n = 53) (p > 0.05).
Discussion
In this study, we found that a quarter of the E. coli from contaminated latrines in an urban informal settlement in Dar es Salaam express ESBL, suggesting a high prevalence of human ESBL faecal carriage in the community, and that shared latrines may serve as a reservoir for transmission in urban areas of Tanzania.
In a recent meta-analysis, the median proportion of ESBL-producing Enterobacteriaceae in patients of Tanzanian healthcare facilities was 39% (range 14.2–75.9%) [11]. Only little is known about the ESBL burden in a community setting in Tanzania. Tellevik et al. reported an ESBL faecal carriage rate in healthy community children in Dar es Salaam of 11.6% [18]. An elderly survey from 2004 found ESBL in 16% of Enterobacteriaceae causing community-acquired urinary tract infections [19]. Studies from other African countries like Senegal, Niger and Madagascar have reported ESBL carriage rates in the community ranging as high as 10% to 31% [3]. In cases where they have been identified, CTX-M enzymes were predominantly of CTX-M-1 group as in our isolates.
Community-onset infections with ESBL-producing pathogens are now increasingly reported. Empirical and targeted antibiotic treatment of such infections is challenging particularly in resource-limited countries. ESBL producing gram-negative bacteria are by definition resistant to extended-spectrum cephalosporins and frequently carry additional resistance genes conferring reduced susceptibility to many other antibiotics like e.g. fluoroquinolones. In many cases the carbapenems remain the only choice for treatment of infections caused by these resistant bacteria. However, the access to antimicrobial agents active against ESBL is limited in many regions of East Africa, thus common infections with multidrug-resistant pathogens cannot be treated adequately.
Intestinal colonization with ESBL-producing microorganisms may last several weeks, months or even years and is a potential source for human-to-human transmission. Precarious hygienic conditions of latrines, which are commonly shared amongst different households and household members, and the presence of ESBL-producing microorganisms on door handles may enable further spread. The cluster of 3 identical E. coli strains from 3 different latrines detected by PFGE typing in our study points to the potential of ESBL transmission in the community.
Our study has limitations: 1) Due to ethical and logistical reasons rectal swabs were not feasible. However, environmental samples of the latrines may give a valuable estimate of the frequency and distribution of ESBL-producing microorganisms. In addition, there is a lower risk of selection bias in the community and the results provide information about potential transmission pathways of multidrug-resistant pathogens in the community, similar to a recent study from airport toilet door handles [20]. 2) Our study was restricted to one informal urban settlement in Dar es Salaam and might differ from other urban and rural settings in Tanzania and East Africa. 3) Due to financial constraints only 70 latrines could be screened for ESBL.
Conclusions
Our study provides evidence of a high prevalence of human ESBL faecal carriage in the community of a resource-limited country such as Tanzania. Further larger surveillance studies are needed in Africa to better describe the epidemiology of ESBL, to raise awareness of the need of strategies to prevent further dissemination, and to improve the access and responsible use of appropriate antimicrobial agents in the empirical treatment of infections caused by otherwise deadly multidrug-resistant microorganisms.
Acknowledgements
We thank Danica Nogarth, Elisabeth Schultheiss and Judith Shipili for performing the special microbiological analyses.
Funding
Division of Clinical Microbiology, University Hospital Basel, Switzerland.
SHARE Research Consortium, London School of Hygiene and Tropical Medicine, UK.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
All authors have seen and approved the manuscript and have significantly contributed to the work.
Ethics approval and consent to participate
The study was approved by the national ethics committee of the National Institute for Medical Research Tanzania (NIMR/HQ/R.8a/Vol.IX/1632).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Jeroen Ensink is deceased. This paper is dedicated to his memory.
Contributor Information
Stefan Erb, Phone: +41-61-556 52 50, Email: stefan.erb@usb.ch.
Lauren D’Mello-Guyett, Email: laurendmelloguyett@gmail.com.
Hamisi M. Malebo, Email: malebo@hotmail.com
Robert M. Njee, Email: robertmussa@gmail.com
Fatuma Matwewe, Email: fmatwewe@ihi.or.tz.
Vladimira Hinic, Email: vladimira.hinic@usb.ch.
Andreas Widmer, Email: andreas.widmer@usb.ch.
Reno Frei, Email: reno.frei@usb.ch.
References
- 1.WHO. Antimicrobial resistance: Global report on surveillance. Geneva: World Health Organization; 2014.
- 2.Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum b-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother. 2007;60:913–920. doi: 10.1093/jac/dkm318. [DOI] [PubMed] [Google Scholar]
- 3.Woerther PL, Burdet C, Chachaty E, Andremont A. Trends in human fecal carriage of extended-spectrum beta-lactamases in the community: toward the globalization of CTX-M. Clin Microbiol Rev. 2013;26:744–758. doi: 10.1128/CMR.00023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ny S, Lofmark S, Borjesson S, et al. Community carriage of ESBL-producing 253 Escherichia coli is associated with strains of low pathogenicity: A Swedish 254 Nationwide Study. J Antimicrob Chemother. 2017;72(2):582–588. doi: 10.1093/jac/dkw419. [DOI] [PubMed] [Google Scholar]
- 5.Frank T, Arlet G, Gautier V, Talarmin A, Bercion R. Extended-spectrum beta-lactamase-producing Enterobacteriaceae, Central African Republic. Emerg Infect Dis. 2006;12:863–865. doi: 10.3201/eid1205.050951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moyo SJ, Aboud S, Kasubi M, Lyamuya EF, Maselle SY. Antimicrobial resistance among producers and non-producers of extended spectrum beta-lactamases in urinary isolates at a tertiary hospital in Tanzania. BMC Res Notes. 2010;3:348. doi: 10.1186/1756-0500-3-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mshana SE, Matee M, Rweyemamu M. Antimicrobial resistance in human and animal pathogens in Zambia, Democratic Republic of Congo, Mozambique and Tanzania: an urgent need of a sustainable surveillance system. Ann Clin Microbiol Antimicrob. 2013;12:28. doi: 10.1186/1476-0711-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tansarli GS, Poulikakos P, Kapaskelis A, Falagas ME. Proportion of extended-spectrum beta-lactamase (ESBL)-producing isolates among Enterobacteriaceae in Africa: evaluation of the evidence - systematic review. J Antimicrob Chemother. 2014;69:1177–1184. doi: 10.1093/jac/dkt500. [DOI] [PubMed] [Google Scholar]
- 9.Sangare SA, Maiga AI, Guindo I, et al. Prevalence of extended-spectrum beta-lactamase-producing Enterobacteriaceae isolated from blood cultures in Africa. Med Mal Infect. 2015;45:374–382. doi: 10.1016/j.medmal.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Storberg V. Esbl-producing Enterobacteriaceae in Africa - a non-systematic literature review of research published 2008–2012. Infect Ecol Epidemiol. 2014;4. 10.3402/iee.v4.20342. eCollection 2014. [DOI] [PMC free article] [PubMed]
- 11.Sonda T, Kumburu H, van Zwetselaar M, et al. Meta-analysis of proportion estimates of extended-spectrum-beta-lactamase-producing Enterobacteriaceae in East Africa hospitals. Antimicrob Resist Infect Control. 2016;5:18. doi: 10.1186/s13756-016-0117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bain RE, Gundry SW, Wright JA, Yang H, Pedley S, Bartram JK. Accounting for water quality in monitoring access to safe drinking-water as part of the millennium development goals: lessons from five countries. Bull World Health Organ. 2012;90:228–235A. doi: 10.2471/BLT.11.094284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Exley JL, Liseka B, Cumming O, Ensink JH. The sanitation ladder, what constitutes an improved form of sanitation? Environ Sci Technol. 2015;49:1086–1094. doi: 10.1021/es503945x. [DOI] [PubMed] [Google Scholar]
- 14.Rusin P, Orosz-Coughlin P, Gerba C. Reduction of faecal coliform, coliform and heterotrophic plate count bacteria in the household kitchen and bathroom by disinfection with hypochlorite cleaners. J Appl Microbiol 1998; 85: 819-828. [DOI] [PubMed]
- 15.EUCAST. Eucast guideline for the detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. Version 1.0. European Committee on Antimicrobial Susceptibility Testing .2013. http://www.eucast.org/resistance_mechanisms/.
- 16.Pfaller M, Hollis R, Sader H, editors. Chromosomal restriction fragment analysis by pulsed-field gel electrophoresis. Washington, D.C.: American Society for Microbiology; 1992. [Google Scholar]
- 17.Oethinger M, Conrad S, Kaifel K, et al. Molecular epidemiology of fluoroquinolone-resistant Escherichia Coli bloodstream isolates from patients admitted to European cancer centers. Antimicrob Agents Chemother. 1996;40:387–392. doi: 10.1128/aac.40.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tellevik MG, Blomberg B, Kommedal O, Maselle SY, Langeland N, Moyo SJ. High prevalence of faecal carriage of ESBL-producing Enterobacteriaceae among children in Dar es Salaam, Tanzania. PLoS One. 2016;11:e0168024. doi: 10.1371/journal.pone.0168024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manyahi J, Moyo SJ, Tellevik MG, et al. Detection of CTX-M-15 beta-lactamases in Enterobacteriaceae causing hospital- and community-acquired urinary tract infections as early as 2004, in Dar es Salaam, Tanzania. BMC Infect Dis. 2017;17:282. doi: 10.1186/s12879-017-2395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaumburg F, Kock R, Leendertz FH, Becker K. Airport door handles and the global spread of antimicrobial-resistant bacteria: a cross sectional study. Clin Microbiol Infect. 2016;22:1010–1011. doi: 10.1016/j.cmi.2016.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.


