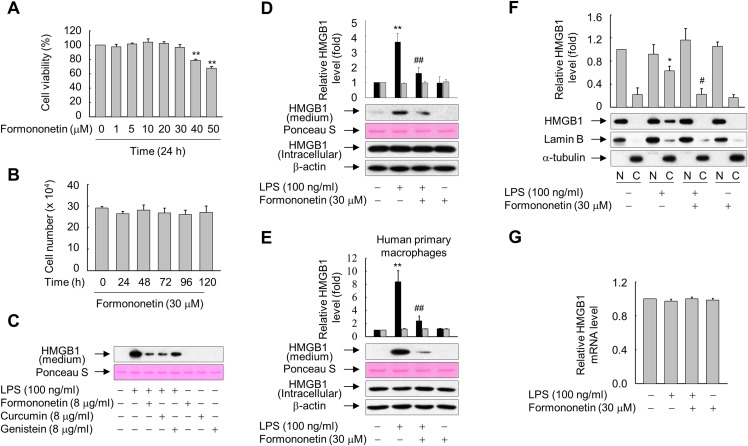
Figure 1. Effects of formononetin on the LPS-induced release and translocation of HMGB1.
(A, B) RAW264.7 cells cultured in serum-free medium for 16 h were treated with the indicated concentrations of formononetin for 24 h (A) or with 30 mM formononetin for the indicated durations (B). Cell viability was determined by the MTT (A) and trypan blue exclusion (B) assays. (C) RAW264.7 cells maintained in serum-free medium for 16 h were stimulated with LPS in the presence or absence of indicated herbal compound for 24 h. Equal volumes of conditioned media were analyzed by immunoblotting. Ponceau S staining was used as the loading controls. (D, E) RAW264.7 cells (D) or human primary macrophages (E) cultured in serum-free medium for 16 h were stimulated with LPS in the presence or absence of formononetin for 24 h. Equal volumes of conditioned media or aliquots of whole-cell lysates were analyzed by immunoblotting. Ponceau S staining and β-actin were used as the loading controls. Black and gray bars indicate secreted HMGB1 and intracellular HMGB1, respectively. (F) RAW264.7 cells treated with LPS in the presence or absence of formononetin for 24 h were fractionated into nuclear (N) and cytosolic (C) fractions. The localization of HMGB1 was determined by Western blot analysis with the indicated antibodies. (G) RAW264.7 cells were treated with LPS in the presence or absence of formononetin. Following incubation for 24 h, total RNA was isolated and the levels of SIRT1 mRNA were analyzed by real-time PCR. The results are plotted as the mean ± SE (n = 3 or 4). *p < 0.05, **p < 0.01 compared with the untreated group; #p < 0.05, ##p < 0.01 compared with the LPS-treated group.